Abstract
Humans express six highly conserved actin isoforms, which differ the most at their N-termini. Actin’s N-terminus undergoes co- and post-translational processing unique among eukaryotic proteins. During translation, the initiator methionine of the two cytoplasmic isoforms is N-terminally acetylated (Nt-acetylated) and that of the four muscle isoforms is removed and the exposed cysteine is Nt-acetylated. Then, an unidentified acetylaminopeptidase post-translationally removes the Ac-Met (or Ac-Cys), and all six isoforms are re-acetylated at the N-terminus. Despite the vital importance of actin for cellular processes ranging from cell motility to organelle trafficking and cell division, the mechanism and functional consequences of Nt-acetylation remained unresolved. Two recent studies significantly advance our understanding of actin Nt-acetylation. Drazic et al. (2018) identify actin’s dedicated N-terminal acetyltransferase (NAA80/NatH), and demonstrate that Nt-acetylation critically impacts actin assembly in vitro and in cells. NAA80 knockout cells display increased filopodia and lamellipodia formation and accelerated cell motility. In vitro, the absence of Nt-acetylation leads to a decrease in the rates of filament depolymerization and elongation, including formin-induced elongation. Goris et al. (2018) describe the structure of Drosophila NAA80 in complex with a peptide-CoA bi-substrate analog mimicking the N-terminus of β-actin. The structure reveals the source of NAA80’s specificity for actin’s negatively-charged N-terminus. Nt-acetylation neutralizes a positive charge, thus enhancing the overall negative charge of actin’s unique N-terminus. Actin’s N-terminus is exposed in the filament and influences the interactions of many actin-binding proteins. These advances open the way to understanding the many likely consequences and functional roles of actin Nt-acetylation.
N-terminal acetylation
N-terminal acetylation (Nt-acetylation) is a widespread protein modification catalyzed by a family of enzymes called N-terminal acetyltransferases (NATs) that affects 80–90% of human proteins (Aksnes et al. 2016; Arnesen et al. 2009). For most proteins, the role of Nt-acetylation is still unknown, but recent studies demonstrate a link between Nt-acetylation and protein stability, subcellular localization and protein-protein interaction (Shemorry et al. 2013; Scott et al. 2011; Behnia et al. 2004; Setty et al. 2004; Forte et al. 2011; Holmes et al. 2014). Animals express at least six NATs (named NatA–NatF), whose catalytic subunits (named NAA10 to NAA60) are relatively well conserved and share a common fold (Aksnes et al. 2016; Neuwald and Landsman 1997). Several NATs contain auxiliary subunits, which may modulate their catalytic activities and mediate their recruitment to the ribosome (Aksnes et al. 2016; Gautschi et al. 2003; Polevoda et al. 2008; Liszczak et al. 2013). The five ribosome-associated NATs (NatA–NatE) act co-translationally on specific subsets of substrates, recognized through their N-terminal amino acid sequences as the first ~30–50 amino acids of the nascent polypeptide chain emerge from the ribosome (Aksnes et al. 2016; Palmiter et al. 1978). NatA, NatB, NatC and NatE act on a larger number of substrates (Arnesen et al. 2009; Van Damme et al. 2012; Van Damme et al. 2015), whereas NatD Nt-acetylates only two substrates – histones H2A and H4 (Song et al. 2003; Hole et al. 2011). NatD is also the only NAT that associates with the ribosome directly, without the need for auxiliary subunits (Polevoda et al. 2009; Magin et al. 2015). NatF also lacks auxiliary subunits, and is the only known human NAT that is not associated with the ribosome(Van Damme et al. 2011; Aksnes et al. 2015). It binds directly to the Golgi membrane and Nt-acetylates the cytosolic N-termini of transmembrane proteins. Finally, NatG Nt-acetylates proteins in plant chloroplasts (Dinh et al. 2015).
Actin N-terminal acetylation
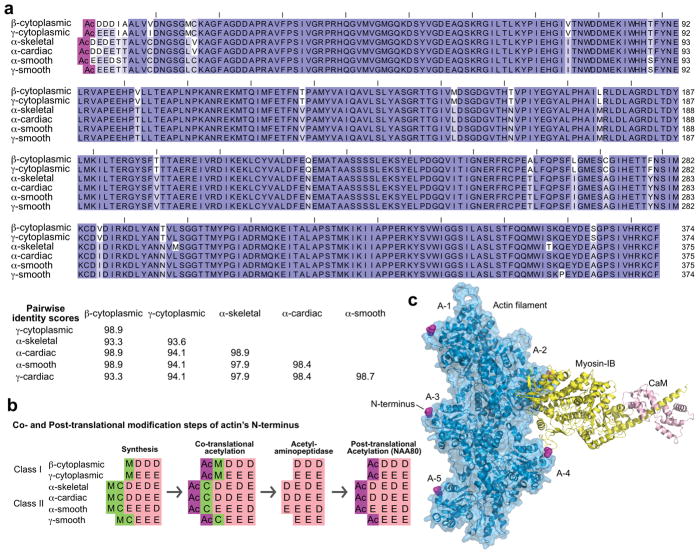
Humans express six actin isoforms, sharing 93–99% sequence identity (Perrin and Ervasti 2010) (Figure 1a). Actin isoforms undergo several types of post-translational modifications, including phosphorylation, methylation, ADP-ribosylation, arginylation and acetylation (Terman and Kashina 2013). In all the species investigated, actin is Nt-acetylated. In yeast and plants, actin Nt-acetylation follows the general co-translational mechanism of most eukaryotic proteins, without further post-translational processing (Van Damme et al. 2012; Cook et al. 1991). In contrast, actin Nt-acetylation in animals proceeds through a multi-step mechanism that is unique among eukaryotic proteins and involves both co-translational and post-translational modifications. During translation, the initiator methionine (iMet) of the two cytoplasmic isoforms (class I actins) is Nt-acetylated (Sheff and Rubenstein 1989), whereas the iMet of the four muscle isoforms (class II actins) is removed and the resulting exposed cysteine is Nt-acetylated (Rubenstein and Martin 1983a; Solomon and Rubenstein 1985) (Figure 1b). NatB is thought to be responsible for the initial Nt-acetylation step of the iMet of class I actins, whereas for class II actins the N-terminal cysteine is most likely acetylated by NatA, based on its substrate specificity (Van Damme et al. 2012; Arnesen et al. 2009; Polevoda and Sherman 2003). A still unidentified acetylaminopeptidase removes the N-terminal Ac-Met of the two cytoplasmic isoforms or the Ac-Cys of the four muscle isoforms (Rubenstein and Martin 1983a; Sheff and Rubenstein 1989). All six isoforms, which at this point carry either three (cytoplasmic and γ-smooth isoforms) or four (other muscle isoforms) negatively-charged amino acids at their N-termini, are subsequently post-translationally Nt-acetylated. While the sequence of these events has been known since the early 1980s (Berger et al. 1981; Redman and Rubenstein 1981; Rubenstein and Martin 1983a; Rubenstein and Martin 1983b; Sheff and Rubenstein 1989; Solomon and Rubenstein 1985), the identity of the NAT responsible for the last acetylation step had remained a mystery, which also impeded addressing the physiological role of actin Nt-acetylation. Two recent studies reveal the identity of actin’s specific NAT, named NatH (catalytic subunit NAA80) and the structural basis for NAA80’s specificity (Goris et al. 2018; Drazic et al. 2018). NAA80 is so far the only eukaryotic NAT acting post-translationally on a single dedicated substrate, actin.
Figure 1. Nt-acetylation of actin isoforms.
(a) Humans express six actin isoforms, sharing 94–99% sequence identity. Most of the differences among isoforms concentrate near the N-terminus, which is additionally acetylated in all the isoforms. Dark blue to white background coloring indicates amino acids conservation scores of 100%, 67%, 50% and <50%. The UniProt accession codes are (in the order of the alignment): P60709, P63261, P68133, P68032, P62736, P63267. (b) The acetylation of actin’s N-terminus proceeds through a multi-step process, unique among all eukaryotic proteins, which involved both co- and post-translational modification. Muscle (class II) actins contain Met-Cys at the N-terminus after synthesis, and the initial step of co-translational acetylation (likely catalyzed by NatA) is preceded by the removal of the initiator methionine. Cytoplasmic (class I) actins are co-translationally Nt-acetylated directly on the initiator methionine, a reaction catalyzed by NatB (Van Damme et al. 2012; Arnesen et al. 2009; Polevoda and Sherman 2003). A still non-identified acetylaminopeptidase removes the acetylated N-terminal residues, which is followed by the last step of post-translational Nt-acetylation catalyzed by NatH (NAA80) (Goris et al. 2018; Drazic et al. 2018) (c) Actin’s N-terminus is prominently exposed in the actin filament, such that most F-actin-binding protein likely “see” the difference between acetylated and non-acetylated actin, as well as differences among actin isoforms. Actin subunits along the short-pitch helix of the actin filament are named A1–5 (from the filament pointed to barbed ends). Of particular interest is myosin, which binds in a cleft between two actin subunits along the long-pitch helix of the actin filament, within contact distance to the N-termini of both actin subunits. The figure shows the recently determined cryo-EM structure of ADP-myosin-IB bound to the actin filament (Mentes et al. 2018).
Source of NAA80’s specificity for actin
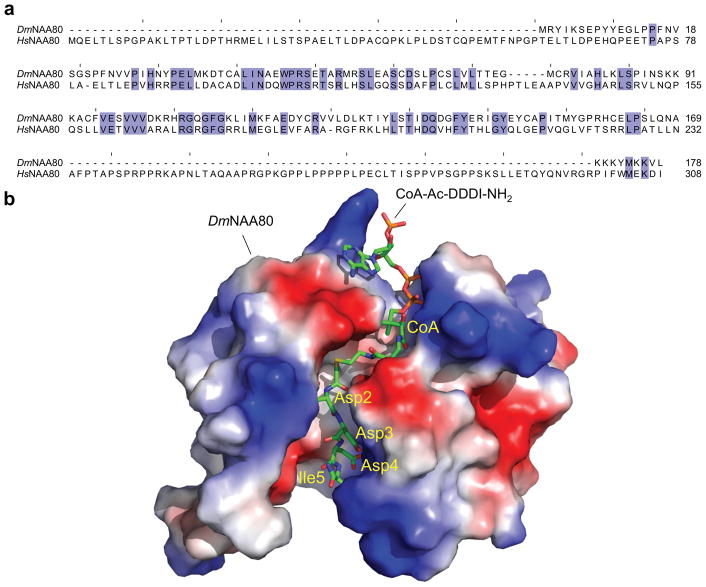
Goris et al. (Goris et al. 2018) describe the 1.76 Å resolution structure of Drosophila melanogaster NAA80 (DmNAA80) in complex with an actin peptide-CoA bisubstrate analog (Figure 2a, b), mimicking the N-terminus of β-actin (CoA-Ac-DDDI-NH2). Despite low sequence identity with other NATs, DmNAA80 shares the typical Gcn5-related Nt-acetyltransferase (GNAT) fold found in many acetyltransferases (Neuwald and Landsman 1997). As the structure reveals, several positively-charged arginine residues line the substrate-binding groove of DmNAA80, making direct and water-mediated contacts with actin’s negatively-charged N-terminus, specifically with the side chains of Asp-3 and Asp-4 of β-actin (actin is conventionally numbered from Met-1). As observed with other NATs, the backbone amide and carbonyls of actin’s N-terminal residues also make extensive contacts with the enzyme, which in DmNAA80 extend to the fourth actin residues (Ile-5 in β-actin), instead of the more typical third residue in other NATs (Liszczak et al. 2013). DmNAA80 also differs from the other NATs in that it has an open substrate- and Ac-CoA-binding groove. Of note, the catalytic subunit of NatB (NAA20), which acetylates the iMet of β-actin (Van Damme et al. 2012), is unsuited to acetylate processed actin, as it has a closed groove lined by hydrophobic amino acids near the acetylation site, which can accommodate a methionine residue at position-1, but not an acidic amino acid.
Figure 2. Source of NAA80’s specificity for actin.
(a) Alignment of the sequences of Drosophila and human NAA80 (UniProt accession codes Q59DX8 and Q93015-2, respectively). The two proteins share only ~29% sequence identity (blue background color indicates identical amino acids). Different from Drosophila NAA80, the human protein has a long extension at the N-terminus and a large, proline-rich insertion near the C-terminus. The N-terminal extension is unique to mammalian NAA80, and precedes the catalytic core that is most highly conserved among all NATs. The N-terminal extension could possible recruit still to be identified auxiliary subunits that might regulate NAA80’s activity in cells. The proline-rich insertion contains a classical profilin-binding site, as observed in many cytoskeletal proteins (Dominguez 2016). (b) Electrostatic surface representation of the structure of Drosophila NAA80 in complex with an actin peptide-CoA bi-substrate analog, mimicking the N-terminus of β-actin (Goris et al. 2018). The positively-charged substrate-binding groove makes direct and water-mediated contacts with the side chains of Asp-3 and Asp-4 of β-actin and appears uniquely adapted for binding actin’s negatively-charged N-terminus, which is unique among all eukaryotic proteins.
Functional consequences of actin Nt-acetylation
Drazic et al. study in parallel wild-type and NAA80 KO human HAP1 cells, revealing dramatic effects in actin cytoskeleton morphology and cell motility. Importantly, actin Nt-acetylation is completely eliminated in NAA80 KO cells, and this phenotype can be rescued by the exogenous expression of NAA80. Moreover, actin appears to be the only protein displaying lack of Nt-acetylation in NAA80 KO cells. Contrary to most NATs, that are ribosome-associated, NAA80 does not pull down with ribosomes during ultracentrifugation, consistent with it being a post-translational NAT. Cells deficient for actin Nt-acetylation display decreased ratios of monomeric (G) to filamentous (F) actin, manifested as an increase in filopodia and lamellipodia formation. These cells also move significantly faster than control cells in wound-healing and chemotaxis assays. The exact source of these whole-cell effects, however, is not fully understood, since Nt-acetylation can potentially impact many actin-based processes. To begin addressing some of the most common actin assembly activities that could be impacted by Nt-acetylation, Drazic et al. purified Nt-acetylated and non-acetylated β/γ-actin from wild-type and KO cells. Thus, actin filaments consisting of Nt-acetylated actin subunits were shown to depolymerize and elongate faster, both alone and driven by formins from profilin-actin. In contrast, actin filament nucleation seemed to be mostly unaffected by Nt-acetylation, whether alone or driven by common nucleators such as the Arp2/3 complex and the formins mDia1 and mDia2.
Unresolved questions and perspectives
While combined these studies represent a major step forward, many questions remain about the role and mechanism of actin Nt-acetylation. For example, Nt-acetylation of a representative library of peptides strongly suggests that NAA80 acetylates all six actin isoforms (Drazic et al. 2018), but the ability of NAA80 to acetylate class II actins remains to be formally demonstrated. Furthermore, if NAA80 turns out to be the NAT of all actin isoforms, this raises an important question – how is NAA80 capable of achieving such a degree of substrate specificity when actin isoforms diverge the most at their N-termini? Also, human and Drosophila NAA80 differ significantly, with the human protein containing a 75-aa extension N-terminal to the catalytic domain (consisting of DmNAA80 residues F16-L178; HsNAA80 residues A76-N232 and P300-I308) and a 67-amino acid proline-rich loop inserted before the C-terminal β-strand (Figure 2a). The proline-rich loop of NAA80 contains a classical profilin-binding site, analogous to many other cytoskeletal proteins that recognize profilin-actin (instead of actin alone) (Dominguez 2016). Therefore, we need to address whether the favorite substrate of human NAA80 is actin or profilin-actin, and whether actin Nt-acetylation occurs prior to polymerization or interaction with other actin-binding proteins (ABPs). The N-terminal extension of human NAA80 may serve to recruit still to be identified auxiliary subunits, which could modulate substrate specificity in cells, for instance distinguishing between G- and F-actin.
Actin’s N-terminus is conspicuously exposed in the filament, and is believed to participate in interactions with many actin-binding proteins (ABPs), most notably myosin (Figure 1c), which could affect muscle contraction and cellular fusion and fission events. Our newly gained ability to control for actin Nt-acetylation in cellular and in vitro studies opens the way to addressing these and many related questions. The importance of post-translational modifications of cytoskeletal components is an emerging topic, with recent studies highlighting the importance of microtubule and actin post-translational modifications for cellular function (Janke and Bulinski 2011; Kerr et al. 2015; Robison et al. 2016; Kashina 2014). Actin Nt-acetylation constitutes the latest addition to this trend, and a topic that is likely to inspire numerous follow-up studies.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 GM073791 to R.D. and R35 GM118090 to R.M. T.A. was supported by the Research Council of Norway Grants 230865 and 249843
Footnotes
Conflict of Interest
The authors declare not having any conflict of interest to declare
References
- Aksnes H, Drazic A, Marie M, Arnesen T. First Things First: Vital Protein Marks by N-Terminal Acetyltransferases. Trends Biochem Sci. 2016;41(9):746–60. doi: 10.1016/j.tibs.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Aksnes H, Van Damme P, Goris M, Starheim KK, Marie M, Stove SI, Hoel C, Kalvik TV, Hole K, Glomnes N, et al. An organellar nalpha-acetyltransferase, naa60, acetylates cytosolic N termini of transmembrane proteins and maintains Golgi integrity. Cell Rep. 2015;10(8):1362–74. doi: 10.1016/j.celrep.2015.01.053. [DOI] [PubMed] [Google Scholar]
- Arnesen T, Van Damme P, Polevoda B, Helsens K, Evjenth R, Colaert N, Varhaug JE, Vandekerckhove J, Lillehaug JR, Sherman F, et al. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proc Natl Acad Sci U S A. 2009;106(20):8157–62. doi: 10.1073/pnas.0901931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia R, Panic B, Whyte JR, Munro S. Targeting of the Arf-like GTPase Arl3p to the Golgi requires N-terminal acetylation and the membrane protein Sys1p. Nat Cell Biol. 2004;6(5):405–13. doi: 10.1038/ncb1120. [DOI] [PubMed] [Google Scholar]
- Berger EM, Cox G, Weber L, Kenney JS. Actin acetylation in Drosophila tissue culture cells. Biochem Genet. 1981;19(3–4):321–31. doi: 10.1007/BF00504277. [DOI] [PubMed] [Google Scholar]
- Cook RK, Sheff DR, Rubenstein PA. Unusual metabolism of the yeast actin amino terminus. J Biol Chem. 1991;266(25):16825–33. [PubMed] [Google Scholar]
- Dinh TV, Bienvenut WV, Linster E, Feldman-Salit A, Jung VA, Meinnel T, Hell R, Giglione C, Wirtz M. Molecular identification and functional characterization of the first Nalpha-acetyltransferase in plastids by global acetylome profiling. Proteomics. 2015;15(14):2426–35. doi: 10.1002/pmic.201500025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez R. The WH2 Domain and Actin Nucleation: Necessary but Insufficient. Trends Biochem Sci. 2016;41(6):478–90. doi: 10.1016/j.tibs.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazic A, Aksnes H, Marie M, Boczkowska M, Varland S, Timmerman E, Foyn H, Glomnes N, Rebowski G, Impens F, et al. NAA80 is actin’s N-terminal acetyltransferase and regulates cytoskeleton assembly and cell motility. Proc Natl Acad Sci U S A. 2018 doi: 10.1073/pnas.1718336115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte GM, Pool MR, Stirling CJ. N-terminal acetylation inhibits protein targeting to the endoplasmic reticulum. PLoS Biol. 2011;9(5):e1001073. doi: 10.1371/journal.pbio.1001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautschi M, Just S, Mun A, Ross S, Rucknagel P, Dubaquie Y, Ehrenhofer-Murray A, Rospert S. The yeast N(alpha)-acetyltransferase NatA is quantitatively anchored to the ribosome and interacts with nascent polypeptides. Mol Cell Biol. 2003;23(20):7403–14. doi: 10.1128/MCB.23.20.7403-7414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris M, Magin RS, Foyn H, Myklebust LM, Varland S, Ree R, Drazic A, Bhambra P, Stove SI, Baumann M, et al. Structural determinants and cellular environment define processed actin as the sole substrate of the N-terminal acetyltransferase NAA80. Proc Natl Acad Sci U S A. 2018 doi: 10.1073/pnas.1719251115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hole K, Van Damme P, Dalva M, Aksnes H, Glomnes N, Varhaug JE, Lillehaug JR, Gevaert K, Arnesen T. The human N-alpha-acetyltransferase 40 (hNaa40p/hNatD) is conserved from yeast and N-terminally acetylates histones H2A and H4. PLoS One. 2011;6(9):e24713. doi: 10.1371/journal.pone.0024713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes WM, Mannakee BK, Gutenkunst RN, Serio TR. Loss of amino-terminal acetylation suppresses a prion phenotype by modulating global protein folding. Nat Commun. 2014;5:4383. doi: 10.1038/ncomms5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12(12):773–86. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- Kashina A. Protein arginylation, a global biological regulator that targets actin cytoskeleton and the muscle. Anat Rec (Hoboken) 2014;297(9):1630–6. doi: 10.1002/ar.22969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JP, Robison P, Shi G, Bogush AI, Kempema AM, Hexum JK, Becerra N, Harki DA, Martin SS, Raiteri R, et al. Detyrosinated microtubules modulate mechanotransduction in heart and skeletal muscle. Nat Commun. 2015;6:8526. doi: 10.1038/ncomms9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszczak G, Goldberg JM, Foyn H, Petersson EJ, Arnesen T, Marmorstein R. Molecular basis for N-terminal acetylation by the heterodimeric NatA complex. Nat Struct Mol Biol. 2013;20(9):1098–105. doi: 10.1038/nsmb.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magin RS, Liszczak GP, Marmorstein R. The molecular basis for histone H4- and H2A-specific amino-terminal acetylation by NatD. Structure. 2015;23(2):332–41. doi: 10.1016/j.str.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentes A, Huehn A, Liu X, Zwolak A, Dominguez R, Shuman H, Ostap EM, Sindelar CV. High-resolution cryo-EM structures of actin-bound myosin states reveal the mechanism of myosin force sensing. Proc Natl Acad Sci U S A. 2018;115(6):1292–97. doi: 10.1073/pnas.1718316115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwald AF, Landsman D. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem Sci. 1997;22(5):154–5. doi: 10.1016/s0968-0004(97)01034-7. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Gagnon J, Walsh KA. Ovalbumin: a secreted protein without a transient hydrophobic leader sequence. Proc Natl Acad Sci U S A. 1978;75(1):94–8. doi: 10.1073/pnas.75.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin BJ, Ervasti JM. The actin gene family: function follows isoform. Cytoskeleton (Hoboken) 2010;67(10):630–4. doi: 10.1002/cm.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polevoda B, Sherman F. N-terminal acetyltransferases and sequence requirements for N-terminal acetylation of eukaryotic proteins. J Mol Biol. 2003;325(4):595–622. doi: 10.1016/s0022-2836(02)01269-x. [DOI] [PubMed] [Google Scholar]
- Polevoda B, Hoskins J, Sherman F. Properties of Nat4, an N(alpha)-acetyltransferase of Saccharomyces cerevisiae that modifies N termini of histones H2A and H4. Mol Cell Biol. 2009;29(11):2913–24. doi: 10.1128/MCB.00147-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polevoda B, Brown S, Cardillo TS, Rigby S, Sherman F. Yeast N(alpha)-terminal acetyltransferases are associated with ribosomes. J Cell Biochem. 2008;103(2):492–508. doi: 10.1002/jcb.21418. [DOI] [PubMed] [Google Scholar]
- Redman K, Rubenstein PA. NH2-terminal processing of Dictyostelium discoideum actin in vitro. J Biol Chem. 1981;256(24):13226–9. [PubMed] [Google Scholar]
- Robison P, Caporizzo MA, Ahmadzadeh H, Bogush AI, Chen CY, Margulies KB, Shenoy VB, Prosser BL. Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes. Science. 2016;352(6284) doi: 10.1126/science.aaf0659. aaf0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein PA, Martin DJ. NH2-terminal processing of Drosophila melanogaster actin. Sequential removal of two amino acids. J Biol Chem. 1983a;258(18):11354–60. [PubMed] [Google Scholar]
- Rubenstein PA, Martin DJ. NH2-terminal processing of actin in mouse L-cells in vivo. J Biol Chem. 1983b;258(6):3961–6. [PubMed] [Google Scholar]
- Scott DC, Monda JK, Bennett EJ, Harper JW, Schulman BA. N-terminal acetylation acts as an avidity enhancer within an interconnected multiprotein complex. Science. 2011;334(6056):674–8. doi: 10.1126/science.1209307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty SR, Strochlic TI, Tong AH, Boone C, Burd CG. Golgi targeting of ARF-like GTPase Arl3p requires its Nalpha-acetylation and the integral membrane protein Sys1p. Nat Cell Biol. 2004;6(5):414–9. doi: 10.1038/ncb1121. [DOI] [PubMed] [Google Scholar]
- Sheff DR, Rubenstein PA. Identification of N-acetylmethionine as the product released during the NH2-terminal processing of a pseudo-class I actin. J Biol Chem. 1989;264(19):11491–6. [PubMed] [Google Scholar]
- Shemorry A, Hwang CS, Varshavsky A. Control of protein quality and stoichiometries by N-terminal acetylation and the N-end rule pathway. Mol Cell. 2013;50(4):540–51. doi: 10.1016/j.molcel.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon LR, Rubenstein PA. Correct NH2-terminal processing of cardiac muscle alpha-isoactin (class II) in a nonmuscle mouse cell. J Biol Chem. 1985;260(12):7659–64. [PubMed] [Google Scholar]
- Song OK, Wang X, Waterborg JH, Sternglanz R. An Nalpha-acetyltransferase responsible for acetylation of the N-terminal residues of histones H4 and H2A. J Biol Chem. 2003;278(40):38109–12. doi: 10.1074/jbc.C300355200. [DOI] [PubMed] [Google Scholar]
- Terman JR, Kashina A. Post-translational modification and regulation of actin. Curr Opin Cell Biol. 2013;25(1):30–8. doi: 10.1016/j.ceb.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme P, Hole K, Gevaert K, Arnesen T. N-terminal acetylome analysis reveals the specificity of Naa50 (Nat5) and suggests a kinetic competition between N-terminal acetyltransferases and methionine aminopeptidases. Proteomics. 2015;15(14):2436–46. doi: 10.1002/pmic.201400575. [DOI] [PubMed] [Google Scholar]
- Van Damme P, Hole K, Pimenta-Marques A, Helsens K, Vandekerckhove J, Martinho RG, Gevaert K, Arnesen T. NatF contributes to an evolutionary shift in protein N-terminal acetylation and is important for normal chromosome segregation. PLoS Genet. 2011;7(7):e1002169. doi: 10.1371/journal.pgen.1002169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme P, Lasa M, Polevoda B, Gazquez C, Elosegui-Artola A, Kim DS, De Juan-Pardo E, Demeyer K, Hole K, Larrea E, et al. N-terminal acetylome analyses and functional insights of the N-terminal acetyltransferase NatB. Proc Natl Acad Sci U S A. 2012;109(31):12449–54. doi: 10.1073/pnas.1210303109. [DOI] [PMC free article] [PubMed] [Google Scholar]