INTRODUCTION
Adherence to tenofovir disoproxil fumarate and emtricitabine is critical to the efficacy of these agents for human immunodeficiency virus (HIV) pre-exposure prophylaxis (PrEP). Non-objective adherence measures were initially used in HIV PrEP studies, but they correlated poorly with trial outcomes. Objective pharmacologic-based measures in a number of biomatrices were key to interpreting adherence to and efficacy of PrEP interventions in these trials, and are commonplace for understanding PrEP adherence and outcomes today.
BACKGROUND
Tenofovir (TFV) disoproxil fumarate (TDF) with emtricitabine (FTC) is an oral fixed dose combination tablet that is FDA-approved for human immunodeficiency virus (HIV) pre-exposure prophylaxis (PrEP), as once daily therapy. PrEP responses depend upon adequate drug exposure, which is governed by variable pharmacokinetics and drug interactions, but most importantly for the PrEP field, variable adherence. In early PrEP studies, the most common methods of assessing medication adherence were pill counts, review of medication refill histories, and self-report. However, these approaches did not confirm medication ingestion and were ultimately inaccurate, mostly due to over-reporting associated with social desirability bias. When drug concentrations were used to assess adherence in PrEP trials, a strong correlation was observed with trial efficacy.1 Drug concentrations can be measured in plasma, urine, saliva, hair, and intracellular tenofovir-diphosphate and emtricitabine-triphosphate in various cell types. The purpose of this communication is to discuss advantages, disadvantages, and adherence interpretations for these measures.
PHARMACOLOGIC ADHERENCE MONITORING
Although “on-demand” TDF/FTC dosing is recommended in some countries for men who have sex with men, daily TDF/FTC is the only FDA-approved therapy to prevent HIV infection. This discussion will focus on pharmacologic considerations for assessing adherence to daily TDF/FTC. Importantly, drug concentrations for adherence assessments are untimed relative to the last dose, unlike carefully timed collections in pharmacokinetic studies. TDF and FTC are nucleos(t)ide reverse transcriptase inhibitors that are phosphorylated by cells to their triphosphate anabolites, TFV-diphosphate (TFV-DP) and FTC-triphosphate (FTC-TP) (TFV is a monophosphate analog, so the diphosphate is the triphosphate anabolite). The parent forms of these agents can be assayed in numerous matrices such as plasma, dried blood, urine, saliva, and hair. Intracellular TFV-DP and FTC-TP become trapped in cells, conferring longer half-lives. These moieties can be assayed from red blood cells (RBCs), peripheral blood mononuclear cells (PBMCs), and other cell types. The half-lives of these moieties vary by matrix, providing a variety of options that can be used alone or in combination to assess medication adherence. These will be summarized below under recent dosing markers and cumulative dosing markers. Table 1 provides an overview of existing techniques.
Table 1.
Comparison of pharmacologic−based adherence measures in HIV prevention
| Matrix | Adherence Interpretation |
Advantages | Disadvantages |
|---|---|---|---|
| Recent dosing Markers | |||
| Plasma | Dose in preceding 2−7 days, depending on assay sensitivity |
• Experience in trials • Commonly collected matrix • Detects dose as far back as 7 days |
• Requires venipuncture/blood processing • Dichotomous (yes/no) interpretation • Cannot distinguish white coat adherence |
| Urine | Dose in preceding 7 days |
• Non−invasive • Commonly collected matrix • Detects dose 7 days back |
• Dichotomous (yes/no) interpretation • Cannot distinguish white coat adherence |
| Saliva | Dose in preceding 24 hours |
• Non−invasive | • Low TFV penetration into oral cavity • Dose detects only 24 hours back • Dichotomous (yes/no) interpretation • Cannot distinguish white coat adherence |
| FTC−TP in DBS |
Dose in preceding 2 days |
• Measured simultaneously with TFV−DP • in DBS • Can distinguish white coat dosing if TFV−DP is low |
• Requires phlebotomy or fingerstick • Dichotomous (yes/no) interpretation |
| Cumulative dosing Markers | |||
| TFV−DP PBMCs |
Gradients of cumulative dosing over 1−2 weeks |
• Cumulative adherence over previous weeks • Measure obtained within target cells |
• Expensive • Specialized, time−consuming processing • Pharmacokinetic/sample variability |
| TFV−DP DBS |
Gradients of cumulative dosing over 6−8 weeks |
• Can be collected via finger−stick or venipuncture • No cell count needed • Relatively easy processing prior to storage • Measured simultaneously with FTC−TP in DBS |
• Expensive • Specialized equipment to process and analyze results • Cold−chain needed for analyte stability • Pharmacokinetic variability |
| Hair | Gradients of cumulative dosing over 4−6 weeks |
• Non−invasive • Relatively easy to collect • Stable at room temperature • Segmental analysis possible • Can be used for multiple ARV medications |
• Requires individuals have hair • Possible appearance of bald spot with sample collection • Variability specific to hair – eg curliness, coloring,etc • Pharmacokinetic variability |
Key: ARV = antiretroviral, DBS = dried blood spots, FTC-TP = emtricitabine-triphosphate, PBMCs = peripheral blood mononuclear cells, TFV = tenofovir, TFV-DP = tenofovir-diphosphate
RECENT DOSING MARKERS
“Recent dosing” drug concentrations indicate a recent dose was ingested if the moiety is detectable/quantifiable, but cannot ascertain whether multiple doses were taken. This is because these moieties have relatively short half-lives, generally less than 24 hours, and thus little accumulation. Therefore, drug concentrations following a single dose almost mirror those at steady-state, following repeated doses. Moieties with these characteristics are TFV and FTC in plasma, urine, and saliva, as well as FTC-TP in RBCs (measured using dried blood spots [DBS]). The most significant advantage of these assays are the relative ease of collection and processing, whereas key limitations are the dichotomous (yes/no) interpretation and inability to detect “white coat” adherence, where patients/participants are non-adherent but take a dose just before their study or clinic visits.
Plasma testing was the first assay used in PrEP studies, and powerful associations were found between drug detection and PrEP efficacy.2 Plasma assays vary in sensitivity with lower limits of quantitation (LLOQ) ranging from 0.1 to 10 ng/mL, which is one factor for determining how long ago the most recent dose can be detected (e.g., variable pharmacokinetics also has an impact). The most sensitive assays can detect the most recent dose as long ago as 7 days, whereas higher LLOQ assays detect the most recent dosing in the preceding 2 days. This informs the duration of a recent dosing holiday.
Urine TFV concentrations represent an emerging approach to monitor recent dosing adherence.3 This is appealing as urine would be acceptable to patients who dislike needles and urine samples can also be used for sexually transmitted infection testing and renal safety monitoring. Promising point of care urine TFV assays are under development. Urine concentrations generally mirror those in plasma, but are 3–4 log10 concentrations higher, so quantifiable for 7 days or longer.3 Urine TFV concentrations of ~1000 ng/mL and higher correspond to quantifiable TFV plasma concentrations over 10 ng/mL, and thus indicate recent dosing within the previous 2–3 days. Urine concentrations between 10–1000 ng/mL and less than 10 ng/mL is suggestive of dosing ~3–7 days and over 7 days prior to collection, respectively.
TFV and FTC concentrations in saliva were examined in limited PrEP studies with the primary goal of determining whether adequate drug concentrations were permeating the oral cavity to protect against HIV through this route, but concentrations could also be used as a way to assess adherence.4 FTC demonstrates a penetration ratio of 0.17 in saliva versus plasma, but this ratio is much lower for TFV at 0.02. Given these lower concentrations, a dose can only be detected from the preceding day or two. Thus, there is likely a limited role for using salivary concentrations as an adherence marker.
FTC-TP in DBS exhibits a relatively short half-life (35 hours), and low concentrations such that concentrations in the quantifiable range indicate a dose in the preceding 48 hours. One advantage of FTC-TP in DBS in comparison to other recent dosing measures is it can be measured simultaneously with TFV-DP in DBS, a cumulative dosing moiety.
CUMULATIVE DOSING MARKERS
“Cumulative dosing” drug concentrations represent long half-life moieties that accumulate with repeated dosing such that the concentrations represent gradients of cumulative dosing (adherence) over the preceding days to weeks. Current approaches to determine cumulative adherence include intracellular tenofovir-diphosphate in PBMCs and RBCs (measured with DBS), and the parent moieties in hair. A common misconception about long half-life moieties is that their value is in detecting “long ago” dosing events. In fact, “long ago” dosing events that decay over weeks from a high steady-state concentrations cannot be distinguished from a single recent dosing event. Instead, the value is in the dynamic range of drug accumulation with repeated dosing. The main limitations with these measures are that adherence is interpreted as averaged dosing, thus specific patterns of adherence cannot be estimated. Additionally, pharmacokinetic variability will lead to some imprecision in assigning adherence estimates. Directly observed dosing studies are needed to define and account for pharmacokinetic variability for cumulative dosing moieties.
TFV-DP has an intracellular half-life of ~3–5 days in PBMCs, which is approximately 7-fold longer than plasma TFV. This feature confers an ability to estimate adherence gradients over the course of 1–2 weeks, as intracellular concentrations accumulate ~5–8 fold from first dose to steady-state. For instance, a directly observed dosing study provided the following interquartile ranges for TFV-DP associated with 2, 4, and 7 doses per week; 6–13, 25–39, and 31–47 fmol/106 cells.5 However, PBMCs are impractical for widespread use because they require specialized/time-consuming/costly processing, and concentrations depend on consistent and accurate cell counts.
TFV-DP also persists in RBCs with a half-live of ~17 days, and can be quantified in DBS using a 3mm punch from a 10–50 uL spot of whole blood collected via venipuncture or finger-stick. TFV-DP was shown to be dose-proportional in a directly observed dosing study, and concentrations accumulate over a 25-fold range in RBCs from first dose to steady-state, enabling estimation of gradients of adherence over the preceding 1–2 months (Figure 1).6 This measure is analogous to gradients of hemoglobin A1C, where higher concentrations reflect consistently high glucose exposures in the preceding weeks to months. These adherence thresholds were estimated based on 25th percentiles of TFV-DP concentrations. Pre-steady-state concentrations can be adjusted to estimate steady-state concentrations using a 17 day half-life. In the iPrEx open label extension, higher TFV-DP concentrations were associated with a decreased risk of acquiring HIV. A threshold of 700 fmol/punch in DBS, commensurate with ≥4 doses per week, corresponded to 100% (95% CI 86%−100%) risk reduction for HIV infection (Figure 1).7 If FTC-TP is measured in the same DBS punch, it provides complementary recent dosing information. For example, if TFV-DP is in the low range, suggesting <2 doses/week on average, and FTC-TP is quantifiable, together this would suggest white coat adherence. Similarly, the measurement of TFV-DP in DBS and comparisons with the parent form of TFV in plasma or urine could also provide insight into recent and cumulative medication adherence. Specific limitations of DBS testing include the need for cold-chain storage for analyte stability and cost with the labor-intensive intracellular extraction and LC-MS/MS analysis.
Figure 1.
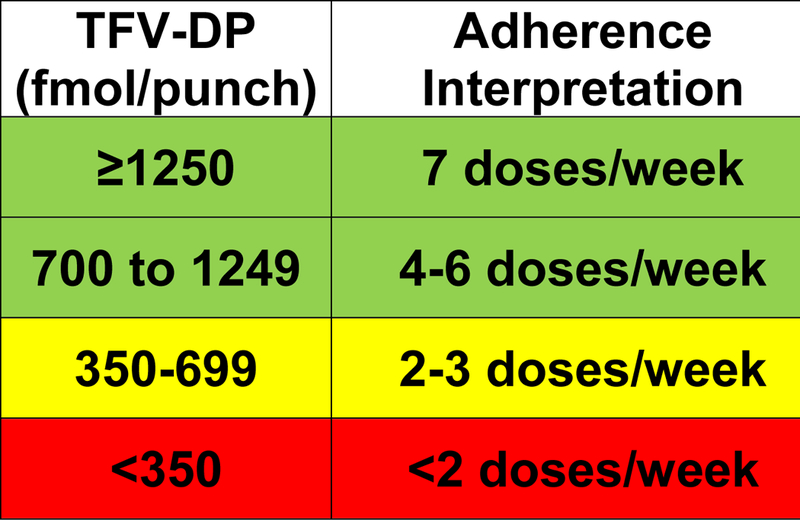
Adherence interpretations in terms of average doses per week over prior 6 to 8 weeks, according to TFV-DP concentrations in DBS
Antiretroviral medications accumulate and are quantifiable within hair. As hair grows at a rate of ~1 centimeter per month, this matrix can provide insight into cumulative dosing over several weeks to months of therapy, depending on the length of hair assayed. Sample collections generally require 50–100 hair strands, and require cutting at the scalp to assess drug concentrations in the most proximal portion of hair. For TFV, hair concentrations were measured in individuals receiving TDF under directly observed therapy at varying dosing levels 2, 4, and 7 doses per week for six-week intervals.8 Median hair concentration were of 0.012, 0.023, and 0.038 ng/mg, respectively, signifying the gradients of adherence. TFV concentrations in hair have been examined in PrEP studies, corresponding with clinical outcomes such as renal dysfunction, as well as other adherence measures. Assessments of hair concentrations provided perhaps the most objective evidence of poor adherence (less than 1 dose/week on average) in the VOICE study, in which PrEP efficacy was much lower than anticipated despite high levels of medication adherence with self-report.9 Hair offers several advantages. It can be used to quantify numerous antiretroviral drugs, and segmental hair strand analysis could provide insight into varying adherence patterns as proximal to distal segments of hair are analyzed.10 Limitations include patient acceptance, and the need for more studies to understand pharmacokinetic variability unique to hair.
CONCLUSIONS
Pharmacologic-based adherence measures are critical to understanding adherence to PrEP therapy and subsequent associations with efficacy to prevent HIV transmission. Multiple measures have also been used to varying degrees in HIV treatment settings. Adherence measures should be validated with pharmacokinetic studies to define and account for factors affecting inter-individual variability, such as sex, weight, race, and concomitant medications. Though a single, universal adherence measure would ease coordination of adherence assessments across research trials and clinical practice, the ability to use and integrate methods into study designs and clinical practice will vary by geographical region and setting. Early studies suggest improved adherence with drug concentration feedback,11 but more research is needed. Patient preference for certain methods and willingness to provide samples will need to be understood, and how to communicate adherence interpretations between providers and patients requires more study. The combined use of recent adherence measures in combination with cumulative adherence measures can help untangle adherence patterns. This and segmental hair analyses is an active area of research. Additional future research is aimed at point of care assays to provide real time adherence information. Thus, pharmacologic adherence monitoring promises to continue to provide useful information for PrEP clinicians, patients, and researchers.
Acknowledgments
Funding information: NIAID NIH R01 AI122298 (PLA)
Footnotes
Conflicts of Interest: PLA receives grants and contracts from Gilead Sciences, paid to his institution.
REFERENCES
- (1).Fonner VA et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS 30, 1973–83 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Donnell D et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr 66, 340–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Koenig HC et al. Urine assay for tenofovir to monitor adherence in real time to tenofovir disoproxil fumarate/emtricitabine as pre-exposure prophylaxis. HIV Med 18, 412–8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Fonsart J et al. Single-dose pharmacokinetics and pharmacodynamics of oral tenofovir and emtricitabine in blood, saliva and rectal tissue: a sub-study of the ANRS IPERGAY trial. J Antimicrob Chemother 72, 478–85 (2017). [DOI] [PubMed] [Google Scholar]
- (5).Anderson PL et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 4, 151ra25 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Anderson PL et al. Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy. Antimicrob Agents Chemother 62, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Grant RM et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 14, 820–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Liu AY et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One 9, e83736 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Koss CA et al. Differences in Cumulative Exposure and Adherence to Tenofovir in the VOICE, iPrEx OLE, and PrEP Demo Studies as Determined via Hair Concentrations. AIDS Res Hum Retroviruses, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Rosen EP et al. Analysis of Antiretrovirals in Single Hair Strands for Evaluation of Drug Adherence with Infrared-Matrix-Assisted Laser Desorption Electrospray Ionization Mass Spectrometry Imaging. Anal Chem 88, 1336–44 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Landovitz RJ et al. Plasma Tenofovir Levels to Support Adherence to TDF/FTC Preexposure Prophylaxis for HIV Prevention in MSM in Los Angeles, California. J Acquir Immune Defic Syndr 76, 501–11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]


