Abstract
Cardiovascular disease (CVD) is a major cause of morbidity and mortality worldwide. Compared to traditional therapeutic strategies, three-dimensional (3D) bioprinting is one of the most advanced techniques for creating complicated cardiovascular implants with biomimetic features, which are capable of recapitulating both the native physiochemical and biomechanical characteristics of the cardiovascular system. The present review provides an overview of the cardiovascular system, as well as describes the principles of, and recent advances in, 3D bioprinting cardiovascular tissues and models. Moreover, this review will focus on the applications of 3D bioprinting technology in cardiovascular repair/regeneration and pharmacological modeling, further discussing current challenges and perspectives.
Keywords: 3D bioprinting, Cardiovascular, Regeneration, Pharmacology, Cardiac tissue, Tissue model, Myocardium, Valves, Vasculature
1. Introduction
As a major cause of morbidity and mortality worldwide, cardiovascular disease (CVD) currently remains a huge challenge for clinical treatments. CVDs, such as congenital heart disease, acute coronary syndrome, hypertension, and arrhythmias, account for >17.5 million deaths per year, and will predictably increase to 23.6 million by 2030 [1–3]. Cardiovascular tissue is fully differentiated and load bearing, and constitutes the myocardium, heart valves, and vasculature [4]. Adult cardiovascular tissue exhibits an inability to repair itself or self-renew after injury, due to the limited regenerative capacity of cardiomyocytes (CMs). In the later stages of cardiovascular disease, transplantation or replacement is usually the only therapeutic option [5]. Annually, it is reported that >80,000 heart valve replacements, and over 600,000 vascular implantations are performed in the United States alone, resulting in an approximate expenditure of $200 billion [3,6–8]. These clinical implantations include autografts, allografts, xenografts, and artificial prostheses [7]. Cardiac implantation methods are majorly limited by the shortage of available donors and immune rejection. Additionally, blood coagulation, mechanical mismatch, and limited durability are other major concerns. As a promising alternative, cardiovascular tissue engineering is being explored to restore cardiac functionality, and replace abnormal or necrotic cardiovascular tissues [4,9]. Based on an understanding of the pathogeneses of various CVDs, engineered cardiac tissue constructs can also serve as in vitro micro-physiological models for drug screening and disease detection [2,10–12]. Compared to the inconsistencies of two-dimensional (2D) cell-culture and animal models, the engineering of 3D microenvironments is better suited to replicate the substantial cell-cell and cell-matrix interactions of native human tissues [13,14]. The engineering of tissue for utility in cardiovascular regeneration is extremely challenging due to the inherent structural complexity of the associated in vivo tissue and as such, requires several considerations in the design process. Therein, the key elements for consideration include: (i) the cardiac tissue component (appropriate cell sources and biomaterial selection), (ii) structural characteristics (oriented myofiber and perfusable vascularization), (iii) mechanical properties, and (iv) physiologically relevant functionalities (electro-mechanical coupling and synchronous contractility) [9,14,15]. Although many advances have been made in tissue culturing methodologies, current approaches fail to achieve precise control of tissue structure, especially in a physiologically relevant manner [4,15].
Among the innovative manufacturing techniques that have been developed, 3D printing enables precise control over multiple compositions, spatial distributions, and architectural accuracy/complexity [16]. It is this notable control over the printing process that allows for the effective replication of native structural features, mechanical properties, and even functions of targeted tissues [16–19]. 3D scanners, computed tomography (CT), magnetic resonance imaging (MRI) systems, and other imaging technologies, as well as computer-aided design (CAD) software, are employed to collect, draw, and digitize the complex structural information of native tissues in order to create 3D printable files, (typically stereolithography (STL) files) [16,20]. Based on a highly precise, layer-by-layer building process, 3D printing techniques have been utilized to create patient-specific models for cardiovascular surgeons to visualize anatomical structures, thus facilitating a more comprehensive understanding of tissue abnormalities and promoting better surgical procedures [21–23]. For engineered active tissues/organs, 3D bioprinting is able to fabricate complex tissue architecture with spatiotemporal distribution of bioactive substances (cells, growth factors, and others) to better guide tissue regeneration [16,19,24]. It has been widely used to create bone, cartilage, neural, and vascularized tissues, cancer models, and, even 4D transformative constructs [25–38]. In addition to applications in cardiovascular repair/regeneration, 3D bioprinted cardiovascular models are better able sues compared to other engineered tissue products. As such, it is able to facilitate the study of the molecular basis of cardiac function, and explore related signaling pathways, thus leading to more accurate predictions of therapeutic/toxicity responses [39–41]. Although the bioprinting technique is still in its early stages, we believe it would be a feasible approach to produce a robust, and physiologically relevant, cardiac model by replicating in vivo tissue composition, geometry, and complexity.
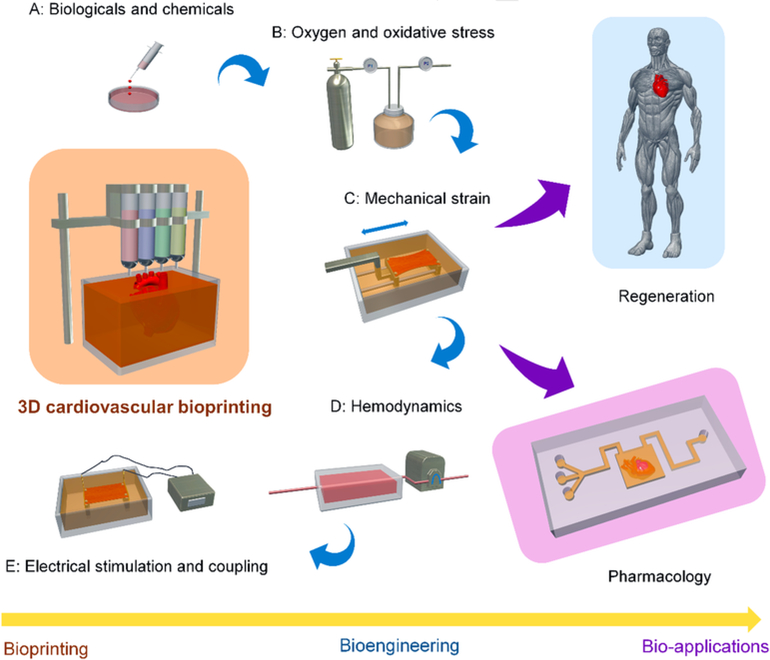
In this review, we present an overview of the cardiovascular system, as well as describe the principles and techniques of 3D cardiovascular bioprinting. We also discuss the bioprinting strategies used for creating functional cardiovascular tissues, including: cell sources, bioink selection, structural designs, and bioengineered approaches. Furthermore, we outline the recent advances in 3D bioprinting cardiovascular tissues/models for regeneration and pharmacological modeling applications. We conclude with a discussion of the current challenges and perspectives of cardiovascular bioprinting in both basic research and for clinical applications (Fig. 1).
Fig. 1.
Schematic diagram outlined in this review, including the techniques of 3D cardiovascular bioprinting, bioengineering methods, and bio-applications in regeneration and pharmacology.
2. Cardiovascular system and tissue models
2.1. In vivo cardiovascular system, cardiac tissue and disease
The cardiovascular system (Fig. 2), includes the heart, blood vessels (arteries, veins, arteriovenous shunts, and capillaries), and lymphatic vessels [42]. It is a closed loop transport system that carries blood and lymph for circulation throughout the body. The constitutive elements of the circulating bulk include nutrients (such as amino acids and electrolytes), waste products, oxygen, carbon dioxide, antibodies, hormones, and blood cells [42]. This circulation of blood bulk works to provide nourishment and assists in combating diseases, stabilizing temperature and pH, and maintaining homeostasis [43,44]. The heart is the most crucial organ of cardiovascular system, as it provides the means to circulate blood throughout the body by connecting the blood vessels (both arteries and veins) [16]. Functional contraction (beating) of the heart is critical for life from the early stages of embryogenesis through >100years of life (several billion beats) [45]. The healthy adult human heart weighs 200–350g and beats 60–80times/min, which allows for a sustained average blood pressure of 120mmHg systolic and 80mmHg diastolic [46,47]. The average cardiac output is 5L/min at rest with a 60% ejection fraction, which increases with exercise to 15L/min with up to an 85% ejection fraction [46,48].
Fig. 2.
Schematic diagram of cardiovascular system structure. Micro-physiological models of the 3D bioprinted heart have focused mostly on generating the myocardium, valve, and vessels.
The heart consists of several distinct anatomical units, including four chambers (the left/right atrium and the left/right ventricle), four valves and a heart wall [43]. The four heart chambers and associated valves control the unidirectional flow of blood through the heart, where the blood is pumped out of the semilunar/outflow valves (aortic and pulmonary) to the blood vessels of the systemic and pulmonary circulatory systems, and then returns through atrioventricular/ inflow valves (mitral and tricuspid) [16,44]. Each valve consists of three leaflets and a fibrous annulus wall (root wall), mainly containing valve interstitial cells (VICs), smooth muscle cells (SMCs), and valvular endothelial cells (VECs). The heart wall contains three layers: (i) the inner endocardium, which is primarily composed of endothelial cells (ECs) and acts as a blood–heart barrier; (ii) the middle myocardium, which contains 2–4 billion CMs associating with ECs and is the thick muscular layer responsible for contraction and relaxation of the heart; and (iii) the outer epicardium/pericardium, which is a double-walled fibroserous sac that serves to protect the heart tissue [42,49]. In the myocardium, there is a high-density capillary network (3000/mm2) which exists to ensure the metabolic activity of myocardium contraction, and the distance between CMs and ECs is no >2–3μm [12]. Adjacent CMs form intercalated disks, whereby intercellular junctions promote synchronous contractions via electrical coupling [12]. Further, the spiral arrangement of ventricular myocardial fibers allows coordinated force generation adequate to pump blood to the rest of the body. The involuntary contraction process is actuated by electrical impulses (or action potentials), which are generated spontaneously at the sinoatrial node to further transmit through the whole heart. These impulses propagate via the electrical conduction system (based on extracellular calcium ions flux), and the frequency (or the rate of potential) is modulated by the nervous system [50]. The blood vessels have complex unique structures in multi-scale and multilayer arrangements in this complex tissue. The inner diameters of blood vessels range from microscopic size, ~5–10μm for the smallest capillaries, to 30mm, for the largest artery (aorta) [16,51]. On the cellular level, a mammalian heart is composed of ≈20% to 30% CMs (roughly 75% of the heart volume) and 70% to 80% nonmyocytes (ECs, SMCs, and fibroblasts (FBs)) [2,15,52]. The multiple cell components of the cardiovascular system are intricately organized in a 3D architecture, which connects to each other through physical interactions, chemical bonds, and biological signals, in order to achieve synergic functionality in both a mechanical and electrical manner [53]. Extracellular matrix (ECM) networks of the heart, primarily in the form of twisted perimysial collagen fibers (but can also include fibrous proteins (e.g. collagen, elastin), adhesive glycoproteins (e.g. laminin, fibronectin, proteoglycans) orients in parallel with the long axis of cardiac muscles, and works to guide the anisotropic alignment of CMs as well as contributes to its non-linear passive stiffness [2].
CVD includes coronary artery diseases (such as angina, and myocardial infarction (MI)), stroke, valvular heart disease, carditis, cardiomyopathy, aortic aneurysms, artery disease, thromboembolic disease, and venous thrombosis, among others, all of which have various underlying disease mechanisms. Therein, atherosclerosis is a common cause for the formation of blood clots (thrombosis) which can lead to stroke if they completely block the vasculature of the brain, or can cause heart attacks (MI) if they obstruct the coronary artery [54,55]. More seriously, without the supplementation of oxygenated blood (ischemia), hypoxia (oxygen deprivation) of the cardiac cells can set off a series of complicated processes which can have considerably detrimental long-term effects on the heart and other tissues. These hypoxia-induced processes can include cell death, scar formation, and ventricular dysfunction that alter the overall cellular, structural, and mechanical properties of the heart, and can ultimately lead to heart failure [54,56,57]. In the treatment of acute CVD, current therapeutic approaches, including various drug and surgical interventions, have several notable disadvantages. Namely, these approaches have limited durability and efficacy, boast a high risk for drug toxicity and surgical complications, suffer from donor tissue shortages and immune rejection, and are plagued by the necessity for prolonged anticoagulation therapy. An alternative interventional approach involves stem cell therapy, such as cell injection into the myocardium. This approach is surgically less invasive, but has been shown to have low viability and weak host cell integration [51,58–60]. Therefore, there is an urgent need to address these challenges to effective CVD treatment through the development of new therapeutic strategies.
2.2. 3D cardiac tissue models
Currently, recreating the entirety of the cardiovascular system in vitro is formidable, cost-prohibitive, and largely superfluous for drug screening, disease treatment, and tissue repair applications. Alternatively, micro-physiological models of the heart have targeted the myocardium, the source of electrical propagation and force generation in the ventricles, as the minimal functional unit that could successfully model drug responses, disease states, and potential disease treatments [12]. 3D bioprinting offers a unique approach for generating this minimal cardiovascular functional unit, which can recapitulate the complex structure and functions of in vivo cardiac tissues with considerable fidelity. As such, a robust bioprinted cardiac model could be utilized for drug discovery and screening, pathophysiological and anatomical studies, cardiac surgery simulation, cardiac device development, and even advanced engineered tissue studies [2,13,14,61].
Compared to animal cardiac models, which display notable anatomical and physiological dissimilarities to that of man, the ability to outline clinically pertinent tissue architectures, and predict various physiological/biological responses, is an outstanding advantage for the development of 3D nonliving/living tissue models. The current approach for preclinical drug screening is through the utilization of animal models (primarily murine). However, it is these significant anatomical and physiological differences between the murine and human cardiovascular systems, such as in tissue structure, heart beating rate, and electrophysiological properties, which often leads to inaccurate predictions of the human cardiac tissue's response to various drug formulations [2,12]. Additionally, increasing ethical concerns for animal usage in cardiovascular therapeutics research should be considered as well [12]. Therefore, developing in vitro models of human cardiac physiology is a promising solution to current limitations.
As mentioned above, the process of recreating physiological or pathological environments of the human heart in vitro is considerably challenging and involves various considerations pertaining to multicellular arrangement, anisotropic myofibers, orientation of the extracellular matrix, nerve supply, vascularization, circulation, as well as various mechanical properties, biological signals, and electrical conduction. The native myocardium requires coordinated electrical and mechanical activities of spatially-arranged CMs to perform its function [12]. As such, dysregulation of CMs can lead to pathological conditions such as reentry ventricular arrhythmia [12]. Current studies on 2D models focus on cellular alignments or patterns, ion channels, monolayer cell responses, and other aspects of cardiovascular tissue culture [12]. Although some additional techniques such as biomechanical stimulation and/or electrical stimulation have been applied to improve 2D cultures, they lack many essential anatomical and physiological features of cardiovascular micro-environments. Thereby, these 2D culturing methodologies fail to offer an effective strategy for regenerating cardiac tissue and by extension, are not suitable for developing disease model/drug screening platforms [2,12,51].
Within tissue engineering approaches, fabricating 3D cellularized scaffolds is the most common method for creating 3D architectures. Although native tissues are devoid of exogenous materials, the limitations of current bioengineering and fabrication techniques constrain the development of scaffold-free 3D models [13,62,63]. One technique to model 3D tissues without scaffolding is through the generation of cell spheroids, which rely on the direct self-assembly of cells or cell aggregation to form 3D architectures, as is reported in many studies. However, the aggregation of cellular spheroids alone is not enough to replicate the mechanical and functional properties of native tissues. By comparison, the ordered construction of nonliving tissue scaffold material requires considerations for its structural and mechanical properties, as well as its processability, and biological activity [12,13]. These tissue engineering scaffolds work to establish regenerative micro-environments within tissue constructs by providing 3D living space for CMs, leading to a more physiologically relevant model. Additionally, many standardized microfluidic−/microarray-based organ-on-chip systems have been reported for high-throughput drug discovery or toxicity testing [10,64–70]. However, there are considerable challenges to the development of microfluidic or chip cardiac models, stemming from their limited ability to fully recapitulate cardiac niche elements (especially for complex architecture and biological characteristics) as well as to precisely organize multiple-cell distributions and regulate cell behaviors/functions.
Advancements in biomaterials, engineering designs, and fabrication techniques, (particularly with emerging 3D printing techniques), provide an unprecedented opportunity to create anatomically relevant 3D tissues. 3D printed models created from imaging data offer the advantage of visual and haptic display, thereby enhancing the understanding of both tissue anatomy and pathologies [21,71–73]. The applications of 3D printed cardiac models are vast in scope and include, but are not limited to: (i) assisting in diagnostics, (ii) optimizing therapeutic outputs, (iii) testing novel devices, (iv) assessing patient-specific suitability for treatments, (v) providing visualized insight for surgical procedures, (vi) allowing for performance of parametric studies on hydrodynamics, and (vii) validating computational simulations. Additionally, 3D printed cardiac models present a robust didactic and communication medium for students, patients, and the greater medical public [21,23,74]. So far, considering the requisite precision, formability, and stability of 3D printed models, current strategies for cardiovascular 3D printing in clinical practices have relied on robust, non-living manufacturing technologies. These technologies include fused deposition modeling, selective laser sintering, stereolithography and ink jetting. Whereas non-living printable materials have been used to 3D print cardiac models for surgical planning purposes, these non-living products do not fully represent the tissue properties and functions of the living myocardium to the same degree which is possible with living materials, and therefore, have a limited ability to assist in the accurate evaluation of the feasibility of surgical procedures. These non-living 3D printable products also have limited utility for the testing of medical devices, as well as for tissue implantation/replacement purposes.
With regard to functional living tissue, 3D bioprinting enables the production of 3D cellularized tissue constructs with accurate architecture and integration of various cell types. The resulting micro-environment of the printed construct more precisely resembles the native milieu, in turn promoting complex natural tissue formation in vitro.
3. 3D bioprinting of the cardiovascular system
3D bioprinting is a set of rapid prototyping and additive manufacturing techniques, used to create functional living constructs, for achieving complex 3D architectures with high-precision, high-throughput, and high reproducibility as well as repeatability [16]. It provides precise control on placement of cells, and other bioactive factors to accurately mimic native physiological niches for pharmaceutical study, and to guide tissue regeneration for patient-specific therapy [16]. Specifically, in terms of bioprinting the tissue of the cardiovascular system, a printed construct must account for complex anisotropic structure (heart beating/contraction), perfusion (vascularization and blood pumping), mechanical adaptability, and electrical signal propagation.
3.1. 3D bioprinting techniques of vessel and cardiac tissue
Currently, cellular bioprinting techniques, including droplet (inkjet), stereolithography, and extrusion bioprinting, have been developed to create cardiovascular tissues [6,7,16,24,75–77]. Table 1 summarizes the features of the common cardiovascular bioprinting techniques.
Table 1.
Comparison of common 3D cardiovascular bioprinting technique.
| Inkjet | Extrusion | Stereolithography | ||
|---|---|---|---|---|
| Principle | Mechanism | Generating bioink droplets for patterned deposition | Extruding bioink filaments through needles | Solidifying bioink in a reservoir via laser energy |
| Modeling | Thermal-, electric-, laser-, acoustic- or pneumatic- | Pneumatic-, mechanical(piston or screw), or solenoid- | Beam scanning or image projection modeling | |
| Gelation methods | Physical, or chemical | Physical, or chemical, | Photo-crosslinking | |
| Specification | Speed | Medium | Slow, medium | Fast, medium |
| Resolution | High, medium | Low, medium | High | |
| Bioink | Types | Hydrogels | Cell aggregates, hydrogels, micro-carriers, decellularized matrices, and synthetic polymer fibers | Cell aggregates, hydrogels, micro-carriers, decellularized matrices |
| Viscosity | Low<12mPa·s | High 6×107mPa·s | High, medium <2000mPa·s | |
| Cell | Density | Low<106cells/mL | High, medium No clogging | High crosslinkable |
| Viability | >85% | 40–80% | 65–85% | |
| Other features | Advantage | Wide ink availability, low cost | Wide printable material, mild condition, medium cost | High cost, no any force application |
| Disadvantage | Clogging; applicable to low viscosity ink only; post-chemical crosslinking request | Low cell viability due to shear stress and pressure; postchemical crosslinking request | Applicable to photopolymers only; harmful effect from laser and residual toxic photoinitiators |
3.1.1. Inkjet based bioprinting
Inkjet based bioprinting (also called drop-on-demand bioprinting) implements various (thermal-, electric-, laser-) energy sources to generate ink droplets for patterned deposition [16]. This technique ensures relatively rapid fabrication, and is more suitable to generate structures with high resolution for soft tissue regeneration. Fluid drop densities, shapes, and sizes are controlled and can be ejected to predefined locations by adjusting energy parameters, to form the 3D construct with different concentration gradients. Multi-jet bioprinters consisting of multiple cartridges or reservoirs, enable different types of cells and biological components to be deposited synchronously. Moreover, inkjet bioprinting remarkably maintains over 80% cell viability. However, the application of inkjet bioprinters is also limited by material viscosity, cell density, and mechanical strength. To achieve droplet ejection, diminish nozzle clogging, and avoid high shear stresses, relatively low cell densities and low-viscosity bioinks (printable biomaterials) are required for this approach, leading to a lack of structural integrity and strength. Although additional crosslinking chemistry can address this disadvantage concerning mechanical strength, this approach may be difficult when printing high cell densities, and thus remains a challenge for creating viable cardiac constructs.
3.1.2. Extrusion based bioprinting
Extrusion based bioprinting (also called dispensing or direct writing bioprinting) utilizes pneumatic-, mechanical-, or solenoid induced- forces to extrude bioinks (including cell aggregates, cell-laden hydrogels, micro-carriers, and decellularized matrices) through a syringe needle in a controlled, filamentous manner to fabricate a 3D architecture [16]. To ensure printability and minimize cell damage, the viscosities of bioinks can range from 30 to >6×107mPa·s [19]. More advanced features have been developed for extrusion printer cartridges and stages in an effort to improve the printing process. These advancements include the introduction of temperature-controlled cartridge (nozzle) or stage systems, multiple independently controlled nozzles or chambers, multiple direction-controlled nozzle or stage systems, and coaxial nozzle systems [16]. Parameters controlling the extruded filament dimension and resolution include: (i) the geometry of nozzle orifice (i.e., needle type and gauge number), (ii) printing speed, (iii) extrusion speed, (iv) printing temperature, (v) solidification method/time, and (vi) the physiochemical properties of the bioink in interaction with substrate (e.g., polymer concentration, viscosity, shear modulus and surface tension) [16]. The most utilized bioprinting method for fabricating cardiac tissue constructs thus far has been extrusion-based bioprinting. This is largely due to the notable capability of extrusion bioprinting to deposit physiologically relevant CMs densities in the engineered tissue construct (108–109cells/mL). Other major advantages for extrusion based bioprinting for cardiac tissue engineering include a wide selection of usable bioinks, fast bioprinting times, ease of operation, and greater affordability in comparison to other bioprinting methods. One major challenge, however with this printing method, is that only high-viscosity bioinks are suitable for printing, which often results in nozzle clogging, poor initial cell survival, and other damages to cell morphology/functions. Regarding cardiac bioprinting, the high-viscosity materials may hinder the gap junctions among bioprinted cells, electrical conduction, and even contractile functions [16].
3.1.3. Stereolithography based bioprinting
Stereolithography based bioprinting (vat photopolymerization) implements laser energy (UV, visible light or near-infrared light) to crosslink cell-laden bioinks in a reservoir, allowing the molding of high-precision patterns in order to form 3D constructs [16,76,78]. The beam-scanning technique (also called laser direct writing), uses a laser beam to scan photocurable bioinks for solidification of a 2D patterned layer, thus creating 3D structures [16]. In Mask-image-projection printing, another stereolithography printing system, a defined mask image is dynamically generated and projected onto the surface of photocurable bioinks using a digital light procession (DLP) technique [16]. The projection can solidify an entire 2D patterned layer simultaneously, and thereby produce complex 3D constructs [16]. The print resolution is largely dependent on irradiant exposure conditions, and the selection of a photo-initiator or any UV absorbers [16]. Generally, the main advantage of this technique is its ability to fabricate complex designs with high resolution (10–100μm, depending upon the type of photocrosslinkable polymers), and to print numerous biological materials with a wide range of viscosities (1–2000mPa·s). However, this technique is only suitable for photopolymerizable bioinks. Compared to other bioprinting techniques, especially for cardiac bioprinting, the nozzle-free approach avoids possible clogging and cell damage by shear stress, and facilitates the deposition of high cardiomyocyte densities. Considering that the free radicals induced by photopolymerization or UV light can damage resin encapsulated cells, the utilization of a cytocompatible photo-initiator and optimized printing parameters are necessary for maintaining cell viability, phenotypes, and function. Additionally, other challenges for stereolithographic bioprinting include: high equipment costs, large time expenditure in printing, difficulties in multicell/multi-material constructs, and the uneven mechanical strength or undesired 3D structures/patterns of constructs associated with repeated laser exposure [16].
3.2. Elements and strategies of 3D bioprinting cardiovascular system
Regeneration of a tissue or organ, including its cellular and extracellular components, requires accurate recapitulation of specific cellular structures and functions. As such, a complete understanding of tissue components, structures, and micro-environments is fundamental to the construct design process. This process includes considerations for the specific organization of various cell types, composition of the ECM, placement and arrangement of gradients of biological molecules, as well as the integration of native biophysical information [16]. Therefore, appropriate bioink selection (i.e. various cell types, biomaterials, and biochemical signals), topological/structural characteristics, as well as mechanical and electrical cues are essential for the successful fabrication of engineered cardiovascular constructs.
3.2.1. Cell sources
Prior to 3D cardiovascular bioprinting, cell based regenerative therapies have been considered as an alternative, and useful strategy for the management of patient heart failure. These cell-based therapies have included: (i) direct injection, (ii) cell sheet engineering, (iii) 3D cell organoids, (iv) injectable hydrogels, (v) cardiac patches, and (vi) engineered scaffolds, among others [2,11,12,14,46,60,79]. Compared to these methods, 3D bioprinting can provide essential environmental parameters for expanding cell populations and influencing cell behaviors. In cardiovascular bioprinting, cells (re-)vitalize 3D matrices and build-up extracellular matrix components, as well as associate upon bioink degradation through their proliferative activity and differentiation. As such, these cells effectively regenerate, remodel, and construct new tissues, respectively. An appropriate cell population from a safe source is critical for an effective regenerative therapy; especially for cardiac regeneration [9,59,80–86]. Therefore, some cell source requirements need to be taken into account: (i) a cell source should be easily accessible and ideally of autologous origin; (ii) the harvested cells should quickly proliferate and be easily differentiated into a desired cell type, or—if already differentiated—should preserve their cellular phenotype and function, and (iii) they should not transmit pathogens and should be non-antigenic. Currently, there are few studies related the comparison of different cell types when different printing approaches are utilized.
FBs, ECs, CMs, SMCs, and specialized conducting cells (including pacemaker and Purkinje cells) form the cellular bulk of the heart [53,87]. Differentiated FBs, SMCs, and ECs derived from either immortalized cell lines or primary cells isolated from multiple species have a high proliferative capacity, and are broadly used for cardiovascular regeneration [2]. However, CMs show no, or very limited and slow proliferation, leading to insufficient cell numbers in use. Therefore, stem cells, including embryonic stem cells (ES), adult stem cells (i.e. cardiac stem or progenitor cells, bone marrow-or adipose-derived mesenchymal stem cells), and induced pluripotent stem cells (iPS cells), have widely been used and differentiated into these different cardiac cell types for experimental and clinical applications [9,14,59,81,88]. The majority of these cell types is produced preclinically and is relatively safe and effective in clinical practice.
3.2.1.1. Mesenchymal stem cells (MSCs)
Mesenchymal stem cells (MSCs) or stromal cells traditionally isolated from bone marrow, umbilical cord tissue, adipose tissue, tooth bud, amniotic fluid, and peripheral blood have a high clinical potential. This is attributable to their abundant availability, ease of accessibility, notable safety, and in particular, to their immunosuppressive profile [85]. Several studies have shown that the cardiac differentiation capacity of MSCs is very low, and can only assume cardiomyocyte-like cell fates. Interestingly, the limited differentiation capacity of MSCs seemingly has minimal impact on the therapeutic effects cell therapy confers on the infarcted heart. Therefore, paracrine effects (e.g. secretion of cytokines, angiogenic and antiapoptotic factors) appear to be the major mechanism of action of MSCs after transplantation, while the evidence of MSCs trans-differentiation into functional CMs remains controversial [85]. Therefore, MSC's suboptimal capability for cardiac differentiation has limited the primary use of these cells in cardiac tissue models [2].
3.2.1.2. Cardiac stem cells (CSCs)
Cardiac stem cells are tissue-specific stem progenitor cells, which are harbored within the adult mammalian heart [89]. Since resident cardiac stem cell populations were isolated and identified, there has been accumulating evidence regarding the heart bearing its own regenerative potential. Thereby, this endogenous source of stem cells seemingly can provide for new opportunities and approaches for CVD treatment and myocardial repair [86,90]. Following the associated protocols, CSCs can be isolated, amplified in vitro, and induced to differentiate in response to growth factors, and are able to generate the major cell types of the myocardium including myocytes, smooth muscle cells, and endothelial vascular cells [91]. For clinical use, CSCs may be isolated from the human heart using a minimally invasive biopsy procedure, therefore the autologous approach would minimize the risks of immune rejection and teratoma formation [92]. To further exploit the clinical potential, feasibility, and long-term efficacy of endogenous CSC therapies, their exact mechanistic contributions to myocardial repair and regeneration still needs to be elucidated.
3.2.1.3. Embryonic stem cells (ESCs)
Embryonic stem cells (ESCs) are pluripotent stem cells derived from the blastocyst stage of early mammalian embryogenesis, and are able to differentiate into any cell type [93]. As such, ESCs can differentiate and propagate, devoid of reprogramming induced genomic alterations and mutations [94]. Embryonic stem cell therapies have been utilized largely because of the unlimited self-renewal capacity of ESCs, and as such, their use presents an ideal option for regenerative medicine. Notably, they exhibit a highly-regenerative potential for generating CMs with a complete phenotype [95]. In several studies, ESCs have been verified to improve cardiac function and survival for the treatment of MI [96]. However, several limitations and challenges impede their broader clinical uses, including limited differentiative-controllability in vivo, tumorigenicity, immunogenicity, and ethical implications [96].
3.2.1.4. Induced pluripotent stem cells (iPSCs)
Similar in characteristics to ESCs, induced pluripotent stem cells (iPSCs), also known as pluripotent stem cells, are capable of differentiating into all cell types of the three germ layers (including functional CMs (iPSC-CM)) [97]. Differentiation protocols have been published for CMs, FBs, endothelial, and epicardial cells. Therefore, iPSCs now offer the opportunity to produce all the essential cellular components of the cardiovascular system in vitro [98]. IPSCs can be directly generated from the reversal conversion (reprogramming) of adult mature cells (FBs, keratinocytes, peripheral blood cells, or renal epithelial cells) via retroviral transduction of stemness transduction factors, such as Oct 3/4, Klf4, Sox2 and c-Myc, as well as using chemicals and reprogramming proteins [99,100]. As cardiomyocyte differentiation efficiency increases and new purification techniques develop, in vitro studies may generate a pure population of iPSC-CMs [12]. In addition to in vitro studies, the transplantation of iPSCs in the infarcted myocardium has also shown their ability for differentiation into CMs in preclinical studies [99]. The ability to derive iPSCs from readily available autologous cells of patients, as well as their pluripotency, has made iPSCs culturing a potential solution to the problem of obtaining large numbers of human cells, while avoiding the ethical and immunological issues of ESCs [87]. However, significant utility and safety issues, such as low efficiency, incomplete reprogramming, high tumorigenicity, and genomic insertion, remain unresolved and limit their current clinical applicability [99].
3.2.1.5. Endothelial cells (ECs), hematopoietic stem cells (HSCs),and endothelial progenitor cells (EPCs)
ECs line the entire circulatory system, from the heart to the smallest capillaries, comprising up to 40% of the cell population of the heart [101]. They play important roles in barrier functions between the myocardium and blood, as well as buffering against thrombosis, inflammation, angiogenesis, vasoconstriction and vasodilation. The interactions between ECs and CMs play critical roles in cardiomyocyte survival and contractility [12]. Endothelial dysfunction is regarded as a key early event, or main cause in the development of atherosclerosis, MI, and other CVDs [102,103]. In cardiovascular regeneration, vascularization is arguably the most important issue. The primary sources of ECs utilized experimentally are from the aorta and human umbilical vein, however, autologous cell sources such as iPSC-derived ECs or blood/bone marrow-derived endothelial progenitor cells, are more likely to be applied clinically due to their non-immunogenicity [87,93]. The source and phenotype of the ECs may also play an important role in their function, as ECs differ in functionality between different organs, but also within different parts of the heart [12,93].
Thus, further characterization may be necessary to assess the functional phenotypes of ECs in the context of the cardiac micro-environment [12]. Endothelial progenitor cells (EPCs) are a circulating, heterogeneous cell population most likely originating from the bone-marrow. EPCs are mainly considered to support endothelial regeneration and angiogenesis, as well as promote differentiation towards ECs and active cardiomyocytes [104,105]. Hematopoietic stem cells (HSCs) which are defined by the surface markers CD34 and CD133 have been demonstrated to be effective in improving cardiac function [106]. Interestingly, this HSC conferred improvement of cardiac function is also associated with an increased neovascularization and less fibrotic remodeling, as opposed to the trans-differentiation into cardiac myocytes [107,108].
3.2.1.6. Cardiac fibroblasts (FBs)
Cardiac FBs constitute a significant portion of the cells in the adult mammalian heart (45 to 70%), exceeding in number that of CMs [12,109–111]. They occupy the connective tissue space between CMs, playing several key roles in cardiac development and physiology (i.e. ECM remodeling, paracrine signaling, mechano-sensing, and electrical coupling) [12]. Cardiac FBs regulate ECM synthesis and degradation which can be affected by external factors, which involves the deposition and remodeling of collagen, fibronectin, fibrillin, elastin, periostin, proteoglycans, and glycoproteins [12,109,112]. Additionally, cardiac FBs can be reprogrammed into adult cardiomyocytes, and also directly coupled with CMs (even up to 300μm in distance in vitro), utilizing connexins to allow propagation of electrical signals [12,113–116]. The fibroblast-cardiomyocyte coupling largely affects the electrophysiological properties of CMs, affecting electrical conduction speed, resting membrane potential, repolarization, and excitability [12,111,116]. In the future, the mechanisms and behaviors of cardiac FBs in vivo need to be studied for directing in vitro applications.
3.2.1.7. Coculture
To succeed in engineering cardiac tissues with physiological structures and functionality, the full array of cell types and relevant cell quantities that compose the heart is required. The presence of non-CMs, such as MSCs, ECs, and FBs, can improve cardiac tissue formation [12,52,93]. Thereby coculture systems are necessary to develop the complicated physiological features requisite for cardiovascular regeneration and drug screening. Although many functions of non-CMs in the native cardiac tissue have been elucidated, co-culture investigation of non-CMs functionality and interactions has largely been unexplored in vitro.
Most efforts at co-culturing endothelial-cardiomyocytes have focused on the goal of generating vascularized cardiac tissue scaffolds [9]. Importantly, micro-physiological systems cannot only capture paracrine effects in co-culture, but also offer an opportunity to investigate 3D contact-mediated signaling pathways as well [12]. Stromal cells or MSCs have been used in coculture with CMs and ECs to improve cell viability, myogenesis, angiogenesis, cardiac contractility, and other cardiovascular functions [81,85]. Incorporating cardiac FBs in coculture models has also shown a beneficial result for improving cardiac mechanical properties, increasing electrical coupling, and beating contractility [116]. Moreover, neuronal cells should be incorporated into the coculture system in future studies as well. The neuronal cells can release biological compounds such as catecholamines and acetylcholine which directly regulate CM function through the sympathetic innervation of the ventricles in the native tissues [12,53,117–119].
A systematic analysis of the minimal effectiveness of cell source, cell population, and cell ratio is necessary, but is likely difficult to assess in vitro or in animal models. The question of the minimum cell requirements for in vitro cardiac tissue constructs is especially interesting, as there has been no established correlation between cell/graft parameters and functional recovery to date. Additionally, the choice of medium is crucial for the formation of engineering tissue, especially for co-culture systems where different cell types require different nutrients and growth factors [12]. Therefore, the choice and optimization of media composition which improve physiological functions of CMs and other cardiac cells grown in vitro, will be crucial for generating micro-physiological models [12].
3.2.2. Extracellular matrix (ECM) environment
Engineering a 3D cardiac construct with physiological micro-environments and cell morphologies in vitro is a significant challenge, as mentioned previously. The complexity of cardiac construct design is further compounded by considerations that must be made with regards to the intricate ECM in which cultured cells reside. As such, matrices serve as the structural backbone for the tissue constructs, and provide guidance and anchorage for the encapsulated cells [120]. Natively, tissue matrices are subject to dynamic tissue remodeling through both the situation-adapted synthesis and degradation of their components. As such, cell matrices, including those of the developing cardiovascular system, must exhibit biochemical and biomechanical characteristics which promote tissue proliferation and restructuring [121–123]. Therefore, an ideal printed cardiac matrix construct should exhibit the following characteristics: (i) it should be biocompatible, biodegradable, non-antigenic and immunogenic (ii) it should be supportive of cellular growth/arrangement, (iii) have physiologically relevant mechanical properties, and (iv) possess a 3D biomimetic tissue architecture to facilitate efficient vessel networking and successful vascular integration in vivo.
3.2.2.1. Bioinks
Bioinks play a major role in creating 3D tissue models, as they not only support cell growth, but also provide physiologically relevant (biochemical, biomechanical, bioelectrical) cues, transmit cell loads, and can be degraded and replaced over time by cell-secreted ECM proteins [2,16]. Soft biomaterials (mostly hydrogels) are classified as being either naturally occurring or synthetic polymers. Natural materials and their derivatives are often isolated from bacterial, plant, animal, or human cells and tissues, while synthetic materials (water-soluble and solidifiable polymers) are manufactured [16,124,125]. The advantages of natural polymers are in their similarity to human ECM, and their inherent bioactivity and biocompatibility [16]. However, their disadvantages are in their immunogenicity, weak mechanical strength, and lack of control in composition/molecular weight [16,124,125]. Comparatively, synthetic polymers can achieve specific physiochemical properties for targeted tissues requirements, including adjustable molecular weights, chemical structures, and functional groups, as well as bioactive anchorage sites [16,124]. For cardiovascular bioprinting, soft biomaterials can recapitulate the native elastic modulus and components through optimizing the bioink chemistry, bioink concentration, and crosslinking chemistry. As such, soft bioinks are able to reliably mimic the native physical properties of cardiac tissues and vasculature in the body. In turn, several functionalized polymers can be considered in bioink design, including conductive or electroactive materials, nanomaterials, protein and peptide-bearing materials, as well as other bioactive materials [57,126–135].
Although both synthetic and naturally derived engineered cardiac scaffolds or patches have been extensively studied, the majority of 3D cardiovascular bioprinting efforts have focused on natural materials [7,24]. Specifically, ECM proteins such as collagen, fibrin, elastin, laminin, and fibronectin have been found not only to contribute to structural strength and compliance, but also improve vessel formation [121]. Another biomaterial option is decellularization of allogenic or xenogenic tissues, which contain a variety of proteins, proteoglycans, and glycoproteins [16]. As such, these decellularized extracellular matrices (dECMs) have been used as bioinks, and have shown their ability to recapitulate tissue-specific components in 3D printed tissue products [136, 137]. Although the ethical issues of using non-human primate organs have been largely circumvented, the use of dECMs raises other concerns that must be addressed [87]. These concerns include notable dECM elicited immunogenicity (largely due to the presence of the oligosaccharide galactose (α (1,3)-galactose (α-gal) epitope), and toxicity (owing to the retention of the decellularization detergents) [87,136,138]. Additionally, Matrigel can be often used as a supplemental material to increase cell viability and attachment due to its various growth factors and matrix components, however, it cannot be used for in vivo study due to its non-human(mouse) tumor-based origin [2]. Moreover, a high degree of natural material variability associated with Matrigel is of major concern, since it will ultimately affect cellular responses and tissue formation [2]. This variability in cellular response can further alter functional readouts, such as varied contractile force. Therefore, standardized assays for the requirements of bioink consistency, reproducibility, and high-throughput capability need to be established [2]. To overcome the limitations of natural bioink material printability and other physiochemical properties, chemical modifications to bioink composition have succeeded in adjusting ink viscosity, optimizing degradation and swelling behaviors, and increasing mechanical strength, among other improvements [16,124]. Matrix mechanical properties can influence vascular network formation and sprouting density, as well as cardiomyocyte alignment and maturation. Moreover, bioinks can deliver bioactive compounds, small molecules, proteins, or genes in a controlled fashion, for improving cardiac regeneration.
3.2.2.2. Architectural design
Another important prerequisite for the generation of tissues is an appropriate structural design. The fundamental requirements for designing 3D cardiovascular tissue constructs are that they must: (i) recapitulate the native 3D hierarchical fibrillar structure, (ii) possess biomimetic, perfusable vasculature, and (iii) demonstrate electromechanical integrity and conductivity [2].
Within the heart, cardiac muscle fibers are surrounded and coupled by endomysial collagen sheaths that are bundled within a honeycomb-like network of undulated perimysial collagen fibers [139–141]. These structural features yield directionally dependent electrical and mechanical properties, termed “cardiac anisotropy” [139]. The anisotropic architecture enables efficient pumping of blood, as is conferred by the fiber angle and orientation of CMs, achieving the maximal ejection fraction. If the orientations of CMs are not well aligned, the orderly propagation of force generation and electrical signals will not be well established. Therefore, to achieve oriented myocardial fiber distributions, an optimal in vitro model would need to incorporate the cardiomyocyte growth into an in vivo-like cardiac tissue microstructure. This would allow for precise control over cell alignment, cell-cell interactions, ECM composition, and microenvironment geometry [2]. The relevant topographic tissue cues have been widely studied in 2D patterning culture previously [139,142,143]. Significant observations in calcium handling, action potentials, conductional velocities, cardiomyogenesis and myofibrillogenesis have been shown to better resemble the native myocardium in aligned CMs as compared to those grown in randomly oriented cultures [2]. Microfabrication-based patterning techniques (such as surface topography, micromolding/microchannels, microcontact printing, sacrificial template methods, high temperature molding, and laser patterned electrospinning), have been developed in some pioneering studies to confine colony geometry, regulate cell morphology and functions, and support high-throughput analysis [2]. However, these 2D culture systems lack the full architecture and functionality of 3D human tissues and organs [2,139,142,144]. Alternatively, 3D bioprinting not only can accomplish this anisotropy in 3D architecture, but also cells can be directly encapsulated into the constructs to form cellularized tissue.
Moreover, the heart is among the organs with the highest ratio of vessels to CMs, where capillaries sprouting from large vessels are adjacent to every cardiomyocyte, continuously providing oxygen and nutrients to the tissue [16,93]. Although hydrogels have a high capacity of water absorption and exchange, their limited diffusivity affects the cell viability beyond a critical thickness. Therefore, for the engineered tissue constructs, porous or channel design is another structural element that has a significant effect on the potential for vascularization. Pore size and internal pore organization can influence nutrient diffusion, as well as in vitro cell growth, proliferation, and migration. Pore size is also important for biodegradation dynamics in vivo, structural and mechanical stability, cell infiltration, rapid vascular ingrowth, and ECM secretion. For successful cardiomyocyte transplantation, vascularization is essential for the survival and function of larger scale, thick engineered cardiac tissues after implantation [93]. Additionally, successful integration within the host will also depend on rapid initial vascular anastomosis and long-term integration with host vasculature [145]. Surgical connection of fragile engineered tissues to the high-pressure circulation of the in vivo system, is a huge problem for any vascularization approach. However, this issue can be mitigated by incorporating robust, perfusable vessels in the 3D architecture. Currently, most studies on pre-vascularization focus on simple patterns, or capillaries in the cardiac constructs. As such, perfusable vasculature with complex hemodynamic capacity remains to be developed within thick cardiac constructs.
3.2.3. Biochemical and biophysical stimulation
The heart is a mechanically active organ, wherein CMs are subjected to mechanical and electrical signals [146]. Within the heart, the main types of mechanical loading are the wall shear stress caused by blood flow, and the strain caused by blood pressure and myocardial contractions [146]. During heart development, cardiogenic cells undergo a complex series of structural changes, which are significantly affected by hydrodynamic forces and mechanical strain, ultimately resulting in their adult phenotype [2]. Complex loading patterns coordinate the form and function of the heart throughout its development: from the linear tube, through the stages of looping, the establishment of chambers, and the initiation of the pumping function. Therefore, heart function strongly relies on appropriately timed electrical signals, which synchronize mechanical contractions of the muscle and pumping of the blood [146].
In vitro, CMs retain a relatively immature phenotype and exhibit incomplete functions, such as reduced electrical excitability, impaired excitation-contraction coupling (ECC), and incomplete adrenergic sensitivity [2]. To accelerate the maturation process, advanced bioengineering approaches focus on providing relevant environmental motifs via bioreactor or stimulation settings, including: biochemical, topographical, mechanical, and electrical signals [146–148]. Based on biological principles, these engineering designs attempt to recapitulate some of the crucial physiological events to improve the matrix reorganization during cardiovascular development. These relevant physio logical events and characteristics include anisotropic cell alignment, biochemical factors with spatiotemporal distribution, hydrodynamic forces associated with perfusion, rhythmical mechanical stretch, and pulsatile electrical stimulation [146,148,149] (Fig. 1). These in vitro platforms for improving engineering cardiac tissue maturation aim to achieve several special features of the heart: (i) ECC, (ii) synchronous contractions and signal propagation, (iii) effective exchange of nutrients and metabolites, (iv) pumping with sufficient ejection fraction [146]. We expect these biochemical and biophysical stimulation aspects would in turn be considered and applied for current 3D printed cardiovascular research.
3.2.3.1. Biologicals and chemicals
In addition to the matrix composition in the heart, several chemicals or biological agents are employed to improve the maturation of engineered cardiac tissue [52,126,147]. Some factors were identified early as important components for inducing maturation of CMs from different sources such as: supplementation of an oxygen carrier, perfluorocarbon (PFC), media supplementation with L-thyroxine or, replacement of serum, triiodothyronine (T3) and hydrocortisone, as well as B-27 Supplement, cytokines, retinoic acid, and growth factors (such as Activin A, bone morphogenetic protein 4 (BMP4), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), and others) [9,12,58,147,150]. As such, cardiomyocytes can be generated by reprogramming cells. Activin A and BMP4 are applied to induce a primitive streak-like population and mesoderm development; Dickkopf-related protein 1 (DKK1), and VEGF can be applied to enhance differentiation of progenitor cells into cardiomyocytes [46,52,151]. Phenylephrine (α- adrenergic receptor agonist) is thought to sustain the beating rate for CMs in vitro [12]. In the case of cardiac disease models, some drugs can be used to positively or negatively affect the response and development of cardiomyocytes. For instance, phenylephrine and/or angiotensin-II can induce hypertrophic growth of CMs in culture [12]. Proarrhythmic compounds, such as chromanol and erythromycin, can affect repolarization inhibition; E-4031, procainamide, sertindole, quinidine, and cisapride can inhibit repolarization; the cardiotoxic drug doxorubicin can affect force generation of cardiac tissue; Isoprenaline (isoproterenol) and carbachol can affect the spontaneous contractile rate [2,152,153]. Additionally, some drugs have been administrated to establish a tachycardiac model of arrhythmogenesis for in vitro, patient-specific disease modeling [2,11,12]. Moreover, 3D cultures have also exhibited higher IC50 values in drug screening studies, suggesting a better recapitulation of human physiology compared to those of their 2D counterparts. However, while the physiological functions of the secreted factors in the heart are well established, their significance in creating cardiac micro-physiological models remains unclear [12].
3.2.3.2. Oxygen and oxidative stress
Oxygen supplementation is indispensable for supporting cell viability and cardiac functioning in the heart [154–156]. Oxygen plays a crucial role in myocardial gene expression, as well as the generation of NO (nitrous oxide) and reactive oxygen species (ROS). It has been found that oxygen positively determines vascular tone, cardiac contractility, and cell signaling processes, as well as negatively induces irreversible cellular damage and death [155]. Several studies have shown that reduction of oxidative stress can affect cardiomyocyte proliferation and gene expression [157,158]. Hypoxia can prolong the heart's regenerative capacity in newborn mice, and the inhibition of aerobic respiration by inducing systemic hypoxaemia can alleviate oxidative damage to DNA, thereby inducing cardiomyocyte proliferation in adult mammals [159]. Immature CMs are considered to be hypoxia resistant, and are able to develop without an apparent loss of function under anoxic conditions [15]. However, the maturation of CMs increases mechanical and electrical production, which may decrease resistance to hypoxia over time, and can lead to lower cell survival after implantation [148]. Due to the low solubility of oxygen in standard media, oxygen depletion becomes a serious problem in the micro-physiological systems that house 3D cardiac tissues, and may contribute to abnormal tissue function [12,80]. Interestingly, in vitro culture incubated under 40% oxygen can improve the function of engineered cardiac tissue compared with the standard 21%, suggesting oxygen supplement plays a role in cardiac tissue engineering [80,160]. Perfusion of media containing oxygen carriers, such as PFC, can significantly improve the contractility of engineered cardiac tissues in vitro [12,161,162]. However, for in vivo implantation purposes, vascularization is essential for generating and maintaining functional cardiac tissues.
3.2.3.3. Mechanical strain
In previous studies, mechanical strain has been shown to be a significant parameter for cardiomyocyte alignment and tissue maturation in various engineered cardiac tissues [9,80,146,163]. Strain can be employed by controlling the substrate properties of the constructs, such as the modulus and surface stiffness, or transferring external stimuli, such as compressive and tensile forces. Mechanical stretch is the most common method to generate contractile motion in vitro, which imitates the normal conditions of the heart contracting against the blood pressure [148,149]. Additionally, the passive tension is able to induce anisotropic alignment of the CMs along with the force direction, which improves mechano-transduction and facilitates electrical conduction [146]. In previous studies, three major modes of strain loading have been reported: (i) a static model (constant stretch), (ii) a phasic model (cyclic stretch), and (iii) an auxotonic model (proportional resistance on a flexible base) [146]. Although the strain forms are different, they are successful in improving tissue maturation, and also in developing a positive force-frequency relationship for the bioengineered models. The maturation of CMs needs appropriate mechanical loading, whereas excessive loading can cause pathological hypertrophy and apoptosis [146]. The native contractile behaviors are based on binding of calcium to cardiac troponin [146]; the mechanical strain enables the upregulation of cardiac troponin expression, the enhancement of contractile function, and the improvement of cytoskeletal and ECM organization.
3.2.3.4. Hemodynamics
Hemodynamics are an essential epigenetic factor in the development of the electrical conduction (His-Purkinje) system in the heart [51,146,149]. Many studies have compared static and perfusable culture conditions during the generation of various vascularized constructs, especially for engineered cardiac tissue. These studies have shown increased cell viabilities, better tolerance for burst pressure, higher mechanical strength, angiogenic capability, and significant ECM collagen content under dynamic conditions [164–167]. Perfusion allows for continuous nutrient circulation within engineered constructs beyond the diffusional penetration depth. Thus, allowing for the capability to cultivate a large-scale tissue/organ construct by improving cell viability, proliferation, differentiation, and ultimately vascular network formation [168–170]. The ability to perfuse vessel networks in engineered constructs is also critical for in vivo anastomosis upon implantation.
Bioreactor systems, which are used for the generation of blood vessels and engineered heart tissues, enable effective transport of nutrients, metabolites, regulatory molecules, oxygen, and simulate parameters of physiological dynamics, (e.g. pH, blood flow velocity, shear stress and shear rate, temperature and blood pressure), which are crucial for recreating the physiology of tissue being developed [168,169]. Conventional bioreactor design consists of a tissue culture chamber or reservoir connected to a pump, process control unit, temperature controller, waste stream, gas source, pH adjuster, flow and pressure transmitters, and sensors [169]. Considerations for bioreactor design for cardiovascular tissue engineering must: (i) mimic pertinent physiological conditions that control cell growth, maturation, and phenotype; (ii) have controllable shear forces to prevent tissue damage; (iii) enable efficient transfer of homogeneous mixing of nutrients and gases; (iv) possess scalability potential into clinical use; and finally (v) allow development of full laminar flow to prevent downregulation of vascular markers that are often associated with turbulent flows. Additionally, a steady-state mathematical model accounting for diffusion, convection, and oxygen consumption by the cells, should be developed to relate oxygen and nutrient distribution within the tissue to flow parameters, channel specifications, and other parameters [146].
3.2.3.5. Electrical stimulation and coupling
Rhythmic beating of the heart (or synchronous contraction of CMs) is initiated by the pacemaker cells in the sinoatrial node, and is propagated by electrical signals over the whole heart [146,148,149,171,172]. The electrical signals mainly depend on the voltage-gated calcium channels of the cell membrane, and are converted into mechanical responses through an ECC process [146,148]. Engineered cardiac constructs also contract spontaneously, but at varying rates, and with some irregularity over time [173]. This arrhythmia risk can in turn lead to implantation failure [15]. Thus, electric coupling of the engineered cardiac tissue to the host myocardium is an essential component for successful autonomous integration into the physiology of heart contraction [9,80]. The electrophysiological features or electrical properties of engineered cardiac tissues are considered to be an important indicator for cardiac maturation [174,175]. In several studies, electrical stimulation has been used to induce myocyte alignment, promote cardiomyogenesis, increase junction protein expression, increase electrical coupling via Cx43 up-regulation, improve arrhythmia, and improve contractility for cardiac maturation [176–178].
Electrical stimulation devices are able to deliver electrical signals throughout the engineered tissue constructs, such as in neural, bone, and cardiac tissues, helping to overcome the initial spatial heterogeneity and incomplete interconnectivity of cells within engineered tissues, as well as improving cell-cell communication and functions [146,179–186]. Different stimulus devices have been developed and applied in engineered cardiac tissue studies, and as such, the synchronization of electrical stimulation results in tightly coupled ECC mechanisms which are important for integration of the engineered tissue into the host myocardium [146]. It is likely that pulsatile signals enable a biomimetic cue in the developing engineered cardiac construct, and are considered critical for synchronous contractions of heart muscle [146,177,178]. Both echocardiography and electrocadiography analyses are useful in the evaluation of the electrophysiological characteristics of engineered cardiac tissues [87]. A continual monitoring of wave morphology of these analyses indicates the ECC effect, intimating the development of functional cellular contractile signaling. For studies performed in vitro, calcium transients are often quantified using Fluo probe, as it is an effective means for evaluating the electrical coupling of CMs in the engineered constructs. Currently, evaluation of electrical coupling to the host myocardium is technically challenging. Genetically modified cells expressing calcium sensors such as GCAmP (genetically encoded calcium indicator) represent the gold standard for the evaluation of electric coupling. Voltage-sensitive dyes or transgenic voltage sensors may also be good tools to analyze action potential propagation within the transplanted cells or constructs [146,174,175].
4. Regeneration and pharmacological study of 3D bioprintedcardiovascular models
A variety of cardiovascular devices and tissue models have been developed using 3D printing techniques including patient-specific surgical visualization models, engineered cardiac tissue implants (patches or constructs), pharmacological studies (disease models, drug screening platforms or drug delivery systems), bioelectrical or mechanical devices, sensors, and actuators. The numerous advantages and achievements in the biofabrication of tissue constructs accelerate the discipline inexorably towards clinical application. In this portion of the review, we will focus our discussion on the advancements in 3D bioprinting cardiovascular tissue models (i.e. cell laden or supported models) in terms of development towards to regenerative and pharmacological applications.
4.1. Bioprinting cardiovascular tissues for regeneration
Currently, bioprinting research for cardiovascular tissue regeneration largely focuses on the myocardium, heart valves, and vasculature. Whether fabricated from synthetic materials or biological components, a wide variety of cardiovascular implants require the incorporation of biomimetic or physiological characteristics into their structures and subsequent functionalities.
4.1.1. Myocardium
To demonstrate the potential as a tissue printing technique, Laser-Induced-Forward-Transfer (LIFT) based cell bioprinting was first used to fabricate ECs and MSCs laden polyester urethane urea (PEUU) cardiac patches. The patterned patch showed enhanced angiogenesis in the border zone of infarction, and preserved cardiac functions after acute MI, compared to the non-patterned patch. [187] (Fig. 3A–C). After which, extrusion-printing was also used to fabricate cardiac patches, wherein subsequent cell viability, proliferation, and cardiac differentiation was investigated [188,189] (Fig. 3D–J). The results of incorporation of human cardiac-derived cardiomyocyte progenitor cells (hCMPCs) with either alginate or hyaluronic acid/gelatin bioinks, suggested the hCMPCs were more likely to differentiate into CMs in 3D conditions compared to 2D cultures. After implantation into a mouse MI model, the transplanted cells were able to survive for up to one month and improve cardiac function. However, depreciated migration of implanted cells was also observed. Additionally, a dECM bioink was used to print the cardiac tissues [190,191] (Fig. 4A–I). The pre-vascularized patch was developed through spatial organization of cardiac progenitor and mesenchymal stem cells/VEGF. After implantation, promoted vascularization, prolonged cell survival, and tissue remodeling was observed at the injured myocardium. Recently, based on microfluidic 3D technology, an endothelialized myocardium scaffold was fabricated by a two-step method [192]. ECs were encapsulated within the bioprinted anisotropic microfibrous lattices to induce their migration towards the inner surface of the microfibers in order to form the endothelium. After which, CMs were grown onto the fibers' outside surface. When combined with microfluidic perfusion, the resulting endothelialized-myocardium-on-a-chip platform was adopted to screen pharmaceutical compounds for their cardiovascular toxicity [192]. A 3D bioprinted micro-channeled gelatin hydrogel was fabricated to promote MSCs myocardial commitment and support native CMs contractile functionality [193]. This study suggested the effects of topographical control over MSC and CM behaviors, which is beneficial for generating in vitro cardiac model systems. Hibino et al. developed a novel method “free of biomaterials” to deliver stem cells using 3D bioprinted cardiac patches [194]. iPSC-CMs, FBs, and ECs were aggregated to create mixed cell spheroids for the cardiac patch printing. Cardiac tissue patches beat spontaneously, and exhibited ventricular-like action potential waveforms and uniform electrical conduction. Conduction velocities were higher and action potential durations were significantly longer in patches containing a lower percentage of FBs. In vivo implantation also intimated the successful vascularization of 3D bioprinted cardiac patches with engraftment into native rat myocardium [194].
Fig. 3.
(A) Schematic bioprinting setup based on LIFT [187]. (B) Arrangement of transferred cells by LIFT observed after 24h: Human MSC were pre-stained with PKH26 and patches were stained with polyclonal goat anti-Pecam1 24h after LIFT to separate grid patterned HUVEC [187]. (C) Patch implantation in vivo: After LAD-ligation rats received the cardiac patch sutured onto the area of blanched myocardium [187]. (D) Seven rows of Hy-Stem/CMPCs mixture were printed at a distance of 2.5mm from each other, both horizontally and vertically [189]. (E) Live-dead assay performed 2h after printing showed the vast majority of CMPCs to be alive (green) and only a few were dead (red), scale bar 1000mm [189]. (F) In vivo CMPC survival. Assessment of cell survival by bioluminescent imaging showed no significant decrease in luciferase positive signal over 1month [189]. Transplanted scaffolds were visible in all treated mice as a patch on the infarcted area of the ventricular wall both at 1 (G) and 4 (I) weeks [189]. The presence of human transplanted cells was confirmed by immunofluorescence analysis for human-specific b-integrin (H) and human Lamin A/C (J), which reveled robust presence of CMPCs inside the matrix at all time points [189]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
(A) Optical and microscopic images of native and decellularized heart tissue (scale bar, 100mm) [190]. (B) Heart tissue construct was printed with heart dECM (hdECM) [190]. (C) Representative microscopic images of hdECM construct (scale bar, 400mm), Structural maturation of myoblasts in (D) COL and (E) hdECM construct showing Myh7 (red) and cell nuclei (DAPI, blue) (scale bar, 200mm) [190]. (F) Illustration of pre-vascularized stem cell patch including multiple cell-laden bioinks and supporting PCL polymer [191]. (G) Fabricated patch including the two types of cell-laden bioink and PCL supporting layer (Scale bar (left top), 1mm; Scale bar (bottom), 200mm) [191]. (H) Optical image of pre-vascularized stem cell patch implanted into post-MI rat [191]. (I) Vessel formation in pattern C/M patch and migration phenomenon from patch to injured myocardium at week 8 (scale bar, 50mm; S represents the strut of the PCL mesh; BV represents the newly formed blood vessels) [191]. (J) Bioprinting of heart valve conduit with encapsulation of HAVIC within the leaflets [200]. (K) representative image of immunohistochemical staining for α-SMA (green) and vimentin (red), and Draq 5 counterstaining for cell nuclei (blue) [200]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Additionally, multiphoton-excited 3D printing was used to generate a native-like extracellular matrix scaffold with submicron resolution, and was able to achieve the individual cell interaction [98]. CMs, smooth muscle cells, and ECs were differentiated from human iPSCs to fabricate the cardiac patch. After 4weeks post-implantation, cardiac function, infarction size, apoptosis, and vascular density were significantly better in cell-laden patches than in cell-free patches. Currently, the freeform reversible embedding of suspended hydrogels (FRESH) technique (i.e. extrusion into a support hydrogel) offers a potential approach to generate a large-scale and complex, 3D construct of both internal and external anatomical architectures of the heart [195]. It was developed to create a 3D heart construct of a 5-day-old chick embryo in a gelatin slurry supported by the incorporation of a fibrinogen-collagen-Matrigel bioink with C2C12 myoblasts. Thus, this construct effectively demonstrates the unique capability of bioprinting to print the complex trabecular structures of a whole heart through CAD modeling while using remarkably low-viscosity bioinks that would otherwise not be printable [195]. Atala et al. also developed a contractile cardiac tissue construct with cellular organization, uniformity, and scalability through a 3D bioprinting approach [196]. The bioprinted cardiac tissue constructs were fabricated by processing three key components: a primary cardiomyocyte laden fibrin-based hydrogel, a sacrificial hydrogel, and a supporting polycaprolactone (PCL) polymeric frame. The cardiac constructs exhibited a spontaneous synchronous contraction in culture, and also displayed physiological responses to known cardiac drugs in terms of beating frequency and contraction forces [196]. A nano-reinforced hybrid cardiac patch laden with human coronary artery endothelial cells (HCAECs) was fabricated by UV-assisted 3D bioprinting to improved electrical, mechanical, and biological behaviors [197]. A safe UV exposure time with insignificant effects on cell viability was identified for methacrylated collagen (MeCol) micropatterning. Carboxyl functionalized carbon nanotubes (CNTs) were incorporated in alginate framework to increase mechanical strength and electrical behavior. Results showed that CNT-reinforced hybrid implants remarkably improved cell elongation and attachment, and promoted HCAEC proliferation, migration, and differentiation represented by lumen-like formation in the implant within 7–10days in vitro [197]. Although some studies, including in vitro and rodent models, have shown the various advantages of 3D bioprinted myocardium constructs, long-term experiments and large-scale animal models are still necessary to rectify several clinically relevant deficiencies and questions such as mismatched heart features, and potential construct oncogenicity/pathogenicity, as well as overall construct therapeutic efficiency.
Most recently, a novel 4D hierarchical micropattern using a unique photolithographic stereolithographic-tandem strategy (PSTS) was developed for effectively regulating cardiomyogenic behaviors of MSCs [37]. Results showed MSCs actively grew with highly aligned micropatterns for significantly improving cardiomyogenesis. The immediate post-print 4D self-folding of the construct enabled reprogrammable transformation of the cardiac patch for better dynamic integration with damaged heart tissue [37]. This pioneering study demonstrates that the advanced 4D dynamic feature may provide seamless structural integration with damaged tissues or organs, and provides a proof-of-concept 4D patch for cardiac regeneration. With the encouraging results of this bioprinting study, we expect that the revolutionary 4D bioprinting approach can not only perform the dynamic contractile behavior requisite for regulating cardiomyocyte functions, but also can create a wholly-functional artificial heart.
4.1.2. Cardiac valves
In addition to myocardial damage, valvular heart diseases (e.g., valvular stenosis and hardening due to calcification) represent another significant cause of heart failure. 3D valve bioprinting has been explored as an alternative methodology to address the weaknesses (such as durable property, anticoagulation, patient-specific, and physiological characteristics) of traditional mechanical or bio-prosthetic valve replacements. A heterogeneous aortic valve scaffold was 3D photocrosslink-printed by incorporating poly(ethylene glycol)-diacrylate (PEG-DA)/alginate hydrogels with porcine aortic valve interstitial cells (PAVIC) seeding [198]. The native anatomic and axisymmetric aortic valve geometries (root wall and tri-leaflets) with 12–22mm inner diameters were quantified and compared using Micro-CT. The results of the study showed that the scaffolds could achieve an over tenfold range in elastic modulus, considerable shape fidelity, and high cell viability. Additionally, aortic heart valves (constructed with alginate-gelatin bioinks encapsulating human aortic root sinus SMCs (for the root wall) and PAVICs (for the leaflets) along with methacrylated hyaluronic acid (Me-HA) and gelatin methacrylate (GelMA) bioink encapsulating human aortic valvular interstitial cells (HAVICs)), were also developed by extrusion bioprinting [199,200] (Fig. 4J–K). 3D bioprinting enabled an appropriate cell distribution, and through varying bioink components and concentration, the results showed a high ECM (collagen and glycosaminoglycans) deposition, upregulation of muscle actin, and a strong HAVIC phenotype. These early attempts in engineering valves primarily focused on structural, physicochemical, and biological properties, however, physiological functionality is the most important feature for clinical application, which needs to be further recapitulated in future studies.
4.1.3. Vasculature
Considering the limitations in autologous vascular grafts and the shortcomings of early engineered vessel grafts, 3D bioprinted vessels can replicate the anatomical, physiological, and biochemical properties of native analogues (particularly in creating small diameter (<6mm) vessels) [16,24,76]. Vasculature in the circulatory system mainly involves coronary vasculature and microvasculature. Differing from the capillaries that rely on angiogenesis (native sprouting process) stimulated by existing vasculature, perfusable vasculature requires the formation of endothelialized lining over the lumen [76,201–204]. In our previous review paper, we discussed three approaches for, and outlined both the advantages and weakness of manufacturing interconnected vessels, including (i) indirect bioprinting through utilizing sacrificial templates (a fugitive ink) that is removed to create hollow channels in a bulk construct; (ii) directly bioprinting interconnected channels in a construct, and (iii), direct bioprinting of a vasculature network or blood vessel in a tubular shape [16]. These important results have demonstrated the potential to generate tubular structures in small scales, however, several challenges still exist to translate these early successes for the ultimate clinical use [205]; particularly in replicating multilayered (intima, tunica media, and tunica adventitia) and hierarchically scaled (tree like) structures with essential cellular components and physiological characteristics.
4.2. Bioprinting cardiovascular models for drug delivery, drug screening and disease detection
Before launching a new drug for market approval, the identification of suitable drug candidates requires the study of the molecular interactions with an associated biochemical target in the preclinical phase, validation of drug safety and efficacy through trial phases I-III, and long-term study of drug effects phase IV clinical trials. Although there have been many advancements in drug discovery and testing, several challenges still beset pharmaceutical development, such as long cycle times and high testing costs. The major problem is that in vitro toxicity and efficacy assays are challenging and have difficulty in accurately predicting in vivo results. Traditional 2D or 3D cell culture systems are unable to accurately recapitulate the in vivo physiological conditions of complex organ systems, and thus fail to provide accurate evaluations. Considering the numerous advantages for creating biomimetic structural, componential, mechanical, and cellular heterogenic characteristics, 3D bioprinting offers great potential to address the problem of the complexity, specificity, and reproducibility of 3D cellular niches for in vitro studies. Compared to the numerous other 3D tissue disease models that have developed, there are only a few current efforts aimed for the 3D bioprinting of CVD models (largely due to the complexity of cardiovascular system).
Recently, a muscle-powered, biological, microelectromechanical system (Bio-MEMS) was bioprinted to serve as a functional biosensor for efficient analysis of drug molecules [206]. An inkjet printer was modified to precisely align C2C12 cells onto cantilevers (biopaper) for the formation of confluent myotubes after 4days of culture, while non-bioprinted cells were randomly distributed without formation of myofibers after 7days. Moreover, myotubes also showed contraction upon excitation with an electrical pulse. After treatment with veratridine (VTD, an alkaloid neurotoxin), the construct lost synchronous contraction, and no further contractions were detected. The myofibers regained the ability of synchronous contraction upon electric stimulus after removal of VTD. The bioprinted Bio-MEMS demonstrated that this technique had the potential to incorporate functional biosensors, motors, and actuators as needed. As mentioned above, the endothelialized myocardium was created by microfluidic 3D technology [192] (Fig. 5A–H). Researchers further embedded the organoids into a specially designed microfluidic perfusion bioreactor to complete the endothelialized-myocardium-on-a-chip platform for cardiovascular toxicity evaluation. A common anti-cancer drug (doxorubicin), was applied to the test biomolecular deliverability and availability potential of the cardiac model. Both CMs and ECs showed dose-dependent responses towards the doxorubicin application. The beating of the CMs was significantly reduced compared to the control (without doxorubicin treatment) [192]. The results illustrate the endothelialized-myocardium-on-a-chip platform is an effective model for probing drug-induced cardiovascular toxicity, and has translational potential for personalized drug screening in the future. The researchers further reported a fully integrated modular physical, biochemical, and optical sensing platform through a fluidics-routing breadboard, which operates organ-on-a-chip units in a continual, dynamic, and automated manner. The new platform allowed for long-term monitoring of drug-induced organ toxicity in a dual-organ human liver-and-heart-on-a-chip [207].
Fig. 5.
(A) Confocal fluorescence micrograph showing the cross-sectional view of a three-layer scaffold at Day 14, indicating the formation of the endothelium by the HUVECs [192]. (B) Confocal fluorescence micrograph showing the GFP-HUVECs in a single fiber for CD31, GFP, and nuclei [192]. (C) High-resolution confocal fluorescence micrograph showing the distribution of the HUVECs in a single microfiber at Day 14. Left: projection view; Right: 3D rendering of the tubular structure at the position of the dotted line [192]. (D) Immunofluorescence staining of sarcomeric α-actinin (red) and connexin-43 (Cx-43, green) of cardiomyocytes seeded on bioprinted microfibrous scaffolds, showing the sarcomeric banding [192]. (E) Schematic showing a native myocardium containing blood vessels embedded in a matrix of cardiomyocytes [192]. (F) Schematic and high-resolution confocal fluorescence micrograph showing an endothelialized myocardial tissue formed by seeding neonatal rat cardiomyocytes onto the bioprinted endothelialized microfibrous scaffold after 15days of pre-endothelialization [192]. (G, H) Relative beating of the endothelialized myocardial tissues and the levels of vWF expression by the endothelial cells, respectively, upon treatment with different dosages of doxorubicin [192]. (I) The fully printed final device. Insert 1: Confocal microscopy image of immunostained laminar NRVM cardiac tissue on the cantilever surface. Blue, DAPI nuclei stain. White, α-actinin. Scale bar, 10μm. Insert 2: Still images of a cantilever deflecting upon tissue contraction. Insert 3: Example resistance signal [208]. (J) Left, 1: Modified device cantilever containing micro-pin and micro-well to support thicker laminar NRVM tissue. 2: Detail of cantilever with micro-pins. 3: Still images of a cantilever deflecting upon tissue contraction. Right, 1: z-projection of immunostained thicker laminar tissue on the cantilever surface with micro-pin (scale bar, 30μm). 2: x–z line scan of thicker laminar tissue in grooves (scale bar, 10μm). Blue, DAPI nuclei stain. White, α-actinin. Red, actin [208]. (K, L) Dose–response curves for thicker laminar NRVM tissues. Stress values for thicker tissues assumed linear proportional to relative resistance change: σ~ΔR/R0. Stress normalized between maximal and minimal values [208]. (K) Dose–response curve for verapamil (n=4). Error bars are s.e.m. Individual data points included (circles), tissue paced at 1.5Hz. Apparent half maximal effective concentration (EC50) 7.90×10−7 M [208]. (L) Dose–response curve for isoproterenol (n=3). Error bars are s.e.m. Individual data points included (circles), tissue paced at 1.5Hz. Apparent EC50 1.16×10−9M [208]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In another study, a facile route for fabricating an instrumented cardiac micro-physiological device was developed via multi-material 3D printing [208]. Specifically, six functional inks were designed, based on piezo-resistive, high-conductance, and biocompatible soft materials. The design enabled integration of soft strain gauge sensors within micro-architectures that guide the self-assembly of physio-mimetic laminar cardiac tissues. The results showed these embedded sensors provide non-invasive, electronic readouts of contractile tissue stresses inside cell incubator environments [208] (Fig. 5I–L). Moreover, the cumulative drug-dose studies were performed with the L-type calcium channel blocker verapamil and the β-adrenergic agonist isoproterenol. The negative inotropic and chronotropic responses for verapamil were observed, while positive responses were observed for isoproterenol. The expected positive and negative responses with apparent EC50 values were comparable to earlier data from engineered 3D tissues and isolated postnatal whole rat hearts [208]. Results demonstrated the instrumented cardiac micro-physiological devices with the integrated sensors drastically simplify data acquisition and long-term functional studies, enabling new insights into tissue morphogenesis, pathogenesis, and drug-induced structural and functional remodeling for in vitro tissue engineering, toxicology, and drug screening research [208].
5. Conclusions and perspectives
The significant progress in cardiovascular bioprinting includes improved vascularization, cardiomyocyte alignment, control over heterogeneous structure, biomimetics, and proper mechanical properties as well as functionality. This progress thus demonstrates the potential ability to create an entirely large-scale, functional cardiovascular tissue. Bioprinting technologies also offer the possibility to generate miniaturizing tissue arrays in order to create organ-on-a-chip or micro-physiological systems, which can be used to investigate the pharmacological and toxicological effects of drugs.
Cardiovascular constructs (i.e., vasculature constructs, myocardium, and heart valve conduits), have been successfully bioprinted with discrete structure and function. However, these techniques are still in their infancy and several challenges remain for generating cardiovascular analogs with full biological functionality and complex micro-architectures. Considering clinically relevant requirements, 3D bioprinting of cardiac tissue should focus on addressing six core design challenges through future studies. The first challenge is to obtain a sufficient number of human-derived cardiovascular cells and achieve physiological distribution of the associated cell types. Secondly, it is necessary to develop and optimize multiple bioinks to maintain different cell viability, phenotypes, and functionality. The third challenge is to develop new bioprinters with new print modeling, or/and improve the printing speed and resolution. The fourth major challenge is to create perfusable vascular networks in the thick constructs, which would be able to provide enough oxygen and nutrients under physiologic pressures in vitro and in vivo for improving functional and biomechanical integration with the host cardiovascular system. The fifth challenge is to balance among structural stability, degradation rates, flexibility, and proliferative space of the printed myocardial tissue for longer culture periods, ensuring cellular aggregation, intercellular connectivity, ECM deposition, and tissue fusion. Moreover, various bioengineering methods such as stress stretch, hemodynamics, and electric stimulation would be encouraged to promote effective tissue remodeling, ECC, and achieve physiological contraction. The sixth and final challenge is to conduct long-term in vivo evaluation of printed constructs, especially for large animal implantation. As such, long-term study would ensure the biological safety, availability, and fidelity of constructs before clinical trials. After addressing these challenges, we believe the whole functional cardiovascular system with more complicated, anatomical structure will be successfully created. Therefore, more research efforts should be dedi cated to developing comprehensive techniques for cardiovascular bioprinting applications and further clinical use.
Acknowledgment
This work is financially supported by NIH Director's New Innovator Award 1DP2EB020549–01.
☆
This review is part of the Advanced Drug Delivery Reviews theme issue on “3D-Bioprinting and Micro-/Nano-Technology: Emerging Technologies in Biomedical Sciences”.
References
- [1].Simon-Yarza T, Bataille I, Letourneur D, Cardiovascular bio-engineering: current state of the art, J. Cardiovasc. Transl. Res 10 (2017) 180–193. [DOI] [PubMed] [Google Scholar]
- [2].Mathur A, Ma Z, Loskill P, Jeeawoody S, Healy KE, In vitro cardiac tissue models: current status and future prospects, Adv. Drug Deliv. Rev 96 (2016) 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics, S. stroke statistics, heart disease and stroke statistics–2014 update: a report from the American Heart Association, Circulation 129 (2014) e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wilhelmi MH, Haverich A, Cardiovascular Tissue Engineering, Springer-Verlag, Berlin Heidelberg, 2017. [Google Scholar]
- [5].Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, 2017 AHA/ACC focused update of the 2014 AHA/ ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, Circulation 135 (2017) e1159–e1195. [DOI] [PubMed] [Google Scholar]
- [6].Jana S, Lerman A, Bioprinting a cardiac valve, Biotechnol. Adv 33 (2015) 1503–1521. [DOI] [PubMed] [Google Scholar]
- [7].Duan B, State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering, Ann. Biomed. Eng 45 (2017) 195–209. [DOI] [PubMed] [Google Scholar]
- [8].Lundberg MS, Cardiovascular tissue engineering research support at the National Heart, Lung, and Blood Institute, Circ. Res 112 (2013) 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pham PV, Liver, Lung and Heart Regeneration, Springer Nature, Switzerland, 2017. [Google Scholar]
- [10].Sidorov VY, Samson PC, Sidorova TN, Davidson JM, Lim CC, Wikswo JP, I-Wire Heart-on-a-Chip I: three-dimensional cardiac tissue constructs for physiology and pharmacology, Acta Biomater. 48 (2017) 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Eder A, Vollert I, Hansen A, Eschenhagen T, Human engineered heart tissue as a model system for drug testing, Adv. Drug Deliv. Rev 96 (2016) 214–224. [DOI] [PubMed] [Google Scholar]
- [12].Kurokawa YK, George SC, Tissue engineering the cardiac microenvironment: multicellular microphysiological systems for drug screening, Adv. Drug Deliv. Rev 96 (2016) 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Roth A, Singer T, The application of 3D cell models to support drug safety assessment: opportunities & challenges, Adv. Drug Deliv. Rev 69–70 (2014) 179–189. [DOI] [PubMed] [Google Scholar]
- [14].Emmert MY, Hitchcock RW, Hoerstrup SP, Cell therapy, 3D culture systems and tissue engineering for cardiac regeneration, Adv. Drug Deliv. Rev 69–70 (2014) 254–269. [DOI] [PubMed] [Google Scholar]
- [15].Hirt MN, Hansen A, Eschenhagen T, Cardiac tissue engineering: state of the art, Circ. Res 114 (2014) 354–367. [DOI] [PubMed] [Google Scholar]
- [16].Cui H, Nowicki M, Fisher JP, Zhang LG, 3D bioprinting for organ regeneration, Adv. Healthcare Mater 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Derby B, Printing and prototyping of tissues and scaffolds, Science 338 (2012) 921–926. [DOI] [PubMed] [Google Scholar]
- [18].Melchels FPW, Domingos MAN, Klein TJ, Malda J, Bartolo PJ, Hutmacher DW, Additive manufacturing of tissues and organs, Prog. Polym. Sci 37 (2012) 1079–1104. [Google Scholar]
- [19].Murphy SV, Atala A, 3D bioprinting of tissues and organs, Nat. Biotechnol 32 (2014) 773–785. [DOI] [PubMed] [Google Scholar]
- [20].Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor HU, Giesel FL, 3D printing based on imaging data: review of medical applications, Int. J. Comput. Assist. Radiol. Surg 5 (2010) 335–341. [DOI] [PubMed] [Google Scholar]
- [21].Giannopoulos AA, Mitsouras D, Yoo SJ, Liu PP, Chatzizisis YS, Rybicki FJ, Applications of 3D printing in cardiovascular diseases, Nat. Rev. Cardiol 13 (2016) 701–718. [DOI] [PubMed] [Google Scholar]
- [22].Vukicevic M, Mosadegh B, Min JK, Little SH, Cardiac 3D printing and its future directions, JACC Cardiovasc. Imaging 10 (2017) 171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sun Z, Lee SY, A systematic review of 3-D printing in cardiovascular and cerebrovascular diseases, Anatol. J. Cardiol 17 (2017) 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang YS, Yue K, Aleman J, Moghaddam KM, Bakht SM, Yang J, Jia W, Dell'Erba V, Assawes P, Shin SR, Dokmeci MR, Oklu R, Khademhosseini A, 3D bioprinting for tissue and organ fabrication, Ann. Biomed. Eng 45 (2017) 148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhou X, Zhu W, Nowicki M, Miao S, Cui H, Holmes B, Glazer RI, Zhang LG, 3D bioprinting a cell-laden bone matrix for breast cancer metastasis study, ACS Appl. Mater. Interfaces 8 (2016) 30017–30026. [DOI] [PubMed] [Google Scholar]
- [26].Cui H, Zhu W, Nowicki M, Zhou X, Khademhosseini A, Zhang LG , Hierarchical fabrication of engineered vascularized bone biphasic constructs via dual 3D bioprinting: integrating Regional Bioactive Factors into Architectural Design, Adv. Healthcare Mater 5 (2016) 2174–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhou X, Castro NJ, Zhu W, Cui H, Aliabouzar M, Sarkar K, Zhang LG, Improved human bone marrow mesenchymal stem cell osteogenesis in 3D bioprinted tissue scaffolds with low intensity pulsed ultrasound stimulation, Sci. Rep 6 (2016) 32876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee SJ, Nowicki M, Harris B, Zhang LG, Fabrication of a highly aligned neural scaffold via a table top stereolithography 3D printing and electrospinning, Tissue Eng. Part A 23 (2017) 491–502. [DOI] [PubMed] [Google Scholar]
- [29].Zhu W, Castro NJ, Cui H, Zhou X, Boualam B, McGrane R, Glazer RI, Zhang LG, A 3D printed nano bone matrix for characterization of breast cancer cell and osteoblast interactions, Nanotechnology 27 (2016) 315103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Miao S, Zhu W, Castro NJ, Nowicki M, Zhou X, Cui H, Fisher JP, Zhang LG, 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate, Sci. Rep 6 (2016) 27226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhou X, Nowicki M, Cui HT, Zhu W, Fang XQ, Miao SD, Lee SJ, Keidar M, Zhang LJG, 3D bioprinted graphene oxide-incorporated matrix for promoting chondrogenic differentiation of human bone marrow mesenchymal stem cells, Carbon 116 (2017) 615–624. [Google Scholar]
- [32].Cui H, Zhu W, Holmes B, Zhang LG, Biologically inspired smart release system based on 3D bioprinted perfused scaffold for vascularized tissue regeneration, Adv. Sci. (Weinh) 3 (2016) 1600058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Heo DN, Castro NJ, Lee SJ, Noh H, Zhu W, Zhang LG, Enhanced bone tissue regeneration using a 3D printed microstructure incorporated with a hybrid nano hydrogel, Nanoscale 9 (2017) 5055–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Miao S, Castro N, Nowicki M, Xia L, Cui H, Zhou X, Zhu W, Lee SJ, Sarkar K, Vozzi G, Tabata Y, Fisher J, Zhang LG, 4D printing of polymeric materials for tissue and organ regeneration, Mater. Today (Kidlington) 20 (2017) 577–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhou X, Cui H, Nowicki M, Miao S, Lee SJ, Masood F, Harris BT, Zhang LG, Three-dimensional-bioprinted dopamine-based matrix for promoting neural regeneration, ACS Appl. Mater. Interfaces 10 (2018) 8993–9001. [DOI] [PubMed] [Google Scholar]
- [36].Zhu W, Cui H, Boualam B, Masood F, Flynn E, Rao RD, Zhang ZY, Zhang LG, 3D bioprinting mesenchymal stem cell-laden construct with core-shell nanospheres for cartilage tissue engineering, Nanotechnology 29 (2018) 185101. [DOI] [PubMed] [Google Scholar]
- [37].Miao S, Cui H, Nowicki M, Lee SJ, Almeida J, Zhou X, Zhu W, Yao X, Masood F, Plesniak MW, Mohiuddin M, Zhang LG, Photolithographic-stereolithographic-tandem fabrication of 4D smart scaffolds for improved stem cell cardiomyogenic differentiation, Biofabrication 10 (2018), 035007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Miao S, Cui H, Nowicki M, Xia L, Almeida J, Zhou X, Zhu W, Lee S, Sarkar K, Zhang Z, Zhang LG, Stereolithographic 4D bioprinting of multi-responsive architectures for neural engineering, Adv. Biosyst (2018) 10.1002/adbi.201800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Peng W, Datta P, Ayan B, Ozbolat V, Sosnoski D, Ozbolat IT, 3D bioprinting for drug discovery and development in pharmaceutics, Acta Biomater. 57 (2017) 26–46. [DOI] [PubMed] [Google Scholar]
- [40].Pati F, Gantelius J, Svahn HA, 3D bioprinting of tissue/organ models, Angew. Chem. Int. Ed. Eng 55 (2016) 4650–4665. [DOI] [PubMed] [Google Scholar]
- [41].Vanderburgh J, Sterling JA, Guelcher SA, 3D printing of tissue engineered constructs for in vitro modeling of disease progression and drug screening, Ann. Biomed. Eng 45 (2017) 164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Michael H, Wojciech P, Histology: A Text and Atlas: With Correlated Cell and Molecular Biology, Wolters Kluwer/Lippincott Williams & Wilkins Health, 2011. [Google Scholar]
- [43].Betts JG, Anatomy & Physiology, Rice University, 2013. [Google Scholar]
- [44].Widmaier EP, Raff H, Strang KT, Vander's Human Physiology, 12 ed., Mc-Graw-Hill Education, Boston, NY, USA, 2010. [Google Scholar]
- [45].Dilley RJ, Morrison WA, Vascularisation to improve translational potential of tissue engineering systems for cardiac repair, Int. J. Biochem. Cell Biol 56 (2014) 38–46. [DOI] [PubMed] [Google Scholar]
- [46].Coulombe KL, Bajpai VK, Andreadis ST, Murry CE, Heart regeneration with engineered myocardial tissue, Annu. Rev. Biomed. Eng 16 (2014) 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Adler CP, Friedburg H, Myocardial DNA content, ploidy level and cell number in geriatric hearts: post-mortem examinations of human myocardium in old age, J. Mol. Cell. Cardiol 18 (1986) 39–53. [DOI] [PubMed] [Google Scholar]
- [48].Rodeheffer RJ, Gerstenblith G, Becker LC, Fleg JL, Weisfeldt ML, Lakatta EG, Exercise cardiac-output is maintained with advancing age in healthy-human subjects - cardiac dilatation and increased stroke volume compensate for a diminished heart-rate, Circulation 69 (1984) 203–213. [DOI] [PubMed] [Google Scholar]
- [49].Laflamme MA, Murry CE, Heart regeneration, Nature 473 (2011) 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Streeter DD Jr., Spotnitz HM, Patel DP, Ross J Jr., Sonnenblick EH, Fiber orientation in the canine left ventricle during diastole and systole, Circ. Res 24 (1969) 339–347. [DOI] [PubMed] [Google Scholar]
- [51].Akintewe OO, Roberts EG, Rim NG, Ferguson MAH, Wong JY, Design approaches to myocardial and vascular tissue engineering, Annu. Rev. Biomed. Eng 19 (2017) 389–414. [DOI] [PubMed] [Google Scholar]
- [52].Korolj A, Wang EY, Civitarese RA, Radisic M, Biophysical stimulation for in vitro engineering of functional cardiac tissues, Clin. Sci. (Lond.) 131 (2017) 1393–1404. [DOI] [PubMed] [Google Scholar]
- [53].Chien KR, Domian IJ, Parker KK, Cardiogenesis and the complex biology of regenerative cardiovascular medicine, Science 322 (2008) 1494–1497. [DOI] [PubMed] [Google Scholar]
- [54].Pepine CJ, New concepts in the pathophysiology of acute myocardial infarction, Am. J. Cardiol 64 (1989) 2B–8B. [DOI] [PubMed] [Google Scholar]
- [55].Karam JP, Muscari C, Montero-Menei CN, Combining adult stem cells and polymeric devices for tissue engineering in infarcted myocardium, Biomaterials 33 (2012) 5683–5695. [DOI] [PubMed] [Google Scholar]
- [56].Cohen S, Leor J, Rebuilding broken hearts. Biologists and engineers working together in the fledgling field of tissue engineering are within reach of one of their greatest goals: constructing a living human heart patch, Sci. Am 291 (2004) 44–51. [PubMed] [Google Scholar]
- [57].Cui H, Liu Y, Cheng Y, Zhang Z, Zhang P, Chen X, Wei Y, In vitro study of electroactive tetraaniline-containing thermosensitive hydrogels for cardiac tissue engineering, Biomacromolecules 15 (2014) 1115–1123. [DOI] [PubMed] [Google Scholar]
- [58].Der Sarkissian S, Levesque T, Noiseux N, Optimizing stem cells for cardiac repair: current status and new frontiers in regenerative cardiology, World J. Stem Cells 9 (2017) 9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Young PP, Schafer R, Cell-based therapies for cardiac disease: a cellular therapist's perspective, Transfusion 55 (2015) 441–451, (quiz 440). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Feric NT, Radisic M, Strategies and challenges to myocardial replacement therapy, Stem Cells Transl. Med. 5 (2016) 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Rimann M, Graf-Hausner U, Synthetic 3D multicellular systems for drug development, Curr. Opin. Biotechnol. 23 (2012) 803–809. [DOI] [PubMed] [Google Scholar]
- [62].Astashkina A, Grainger DW, Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments, Adv. Drug Deliv. Rev 69–70 (2014) 1–18. [DOI] [PubMed] [Google Scholar]
- [63].Morimoto Y, Hsiao AY, Takeuchi S, Point-, line-, and plane-shaped cellular constructs for 3D tissue assembly, Adv. Drug Deliv. Rev 95 (2015) 29–39. [DOI] [PubMed] [Google Scholar]
- [64].Schroer AK, Shotwell MS, Sidorov VY, Wikswo JP, Merryman WD, I-Wire Heart-on-a-Chip II: biomechanical analysis of contractile, three-dimensional cardiomyocyte tissue constructs, Acta Biomater. 48 (2017) 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nuxoll E, BioMEMS in drug delivery, Adv. Drug Deliv. Rev 65 (2013) 1611–1625. [DOI] [PubMed] [Google Scholar]
- [66].Tsui JH, Lee W, Pun SH, Kim J, Kim DH, Microfluidics-assisted in vitro drug screening and carrier production, Adv. Drug Deliv. Rev 65 (2013) 1575–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Vladisavljevic GT, Khalid N, Neves MA, Kuroiwa T, Nakajima M, Uemura K, Ichikawa S, Kobayashi I, Industrial lab-on-a-chip: design, applications and scale-up for drug discovery and delivery, Adv. Drug Deliv. Rev 65 (2013) 1626–1663. [DOI] [PubMed] [Google Scholar]
- [68].Zheng XT, Yu L, Li P, Dong H, Wang Y, Liu Y, Li CM, On-chip investigation of cell-drug interactions, Adv. Drug Deliv. Rev 65 (2013) 1556–1574. [DOI] [PubMed] [Google Scholar]
- [69].Esch EW, Bahinski A, Huh D, Organs-on-chips at the frontiers of drug discovery, Nat. Rev. Drug Discov 14 (2015) 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Huh D, Hamilton GA, Ingber DE, From 3D cell culture to organs-on-chips, Trends Cell Biol. 21 (2011) 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Farooqi KM, Sengupta PP, Echocardiography and three-dimensional printing: sound ideas to touch a heart, J. Am. Soc. Echocardiogr 28 (2015) 398–403. [DOI] [PubMed] [Google Scholar]
- [72].Foley TA, El Sabbagh A, Anavekar NS, Williamson EE, Matsumoto JM, 3D-printing: applications in cardiovascular imaging, Curr. Radiol. Rep 5 (2017, 43). [Google Scholar]
- [73].Kuk M, Mitsouras D, Dill KE, Rybicki FJ, Dwivedi G, 3D printing from cardiac computed tomography for procedural planning, Curr. Cardiovasc. Imaging 10 (2017, 21). [Google Scholar]
- [74].Farooqi KM, Saeed O, Zaidi A, Sanz J, Nielsen JC, Hsu DT, Jorde UP, 3D printing to guide ventricular assist device placement in adults with congenital heart disease and heart failure, JACC Heart Fail. 4 (2016) 301–311. [DOI] [PubMed] [Google Scholar]
- [75].Lee VK, Dai G, Printing of three-dimensional tissue analogs for regenerative medicine, Ann. Biomed. Eng. 45 (2017) 115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Park JH, Jang J, Lee JS, Cho DW, Three-dimensional printing of tissue/organ analogues containing living cells, Ann. Biomed. Eng 45 (2017) 180–194. [DOI] [PubMed] [Google Scholar]
- [77].Skardal A, Atala A, Biomaterials for integration with 3-D bioprinting, Ann. Biomed. Eng. 43 (2015) 730–746. [DOI] [PubMed] [Google Scholar]
- [78].Serpooshan V, Mahmoudi M, Hu DA, Hu JB, Wu SM, Bioengineering cardiac constructs using 3D printing, J. 3D Print. Med 1 (2017) 123–139. [Google Scholar]
- [79].Rubart M, Field LJ, Cardiac regeneration: repopulating the heart, Annu. Rev. Physiol 68 (2006) 29–49. [DOI] [PubMed] [Google Scholar]
- [80].Weinberger F, Mannhardt I, Eschenhagen T, Engineering cardiac muscle tissue: a maturating field of research, Circ. Res 120 (2017) 1487–1500. [DOI] [PubMed] [Google Scholar]
- [81].Gorabi AM, Tafti SHA, Soleimani M, Panahi Y, Sahebkar A, Cells, scaffolds and their interactions in myocardial tissue regeneration, J. Cell. Biochem 118 (2017) 2454–2462. [DOI] [PubMed] [Google Scholar]
- [82].Kochegarov A, Lemanski LF, New trends in heart regeneration: a review, J. Stem Cells Regen. Med 12 (2016) 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Segers VF, Lee RT, Stem-cell therapy for cardiac disease, Nature 451 (2008) 937–942. [DOI] [PubMed] [Google Scholar]
- [84].Duval K, Grover H, Han LH, Mou Y, Pegoraro AF, Fredberg J, Chen Z, Modeling physiological events in 2D vs. 3D Cell culture, Physiology (Bethesda) 32 (2017) 266–277. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- [85].Singh A, Singh A, Sen D, Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6years (2010–2015), Stem Cell Res Ther 7 (2016) 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zheng SX, Weng YL, Zhou CQ, Wen ZZ, Huang H, Wu W, Wang JF, Wang T, Comparison of cardiac stem cells and mesenchymal stem cells transplantation on the cardiac electrophysiology in rats with myocardial infarction, Stem Cell Rev. 9 (2013) 339–349. [DOI] [PubMed] [Google Scholar]
- [87].Taylor DA, Parikh RB, Sampaio LC, Bioengineering hearts: simple yet complex, Curr. Stem Cell Rep 3 (2017) 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chong JJH, Cell Therapy for Left Ventricular Dysfunction: An Overview for Cardiac Clinicians, Heart, Lung and Circulation, 21, 2012532–542. [DOI] [PubMed] [Google Scholar]
- [89].Garbern JC, Lee RT, Cardiac stem cell therapy and the promise of heart regeneration, Cell Stem Cell 12 (2013) 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P, Adult cardiac stem cells are multipotent and support myocardial regeneration, Cell 114 (2003) 763–776. [DOI] [PubMed] [Google Scholar]
- [91].Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P, Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure, Proc. Natl. Acad. Sci. U. S. A 102 (2005) 8692–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kharaziha M, Memic A, Akbari M, Brafman DA, Nikkhah M, Nano-enabled approaches for stem cell-based cardiac tissue engineering, Adv. Healthc. Mater 5 (2016) 1533–1553. [DOI] [PubMed] [Google Scholar]
- [93].Sun X, Altalhi W, Nunes SS, Vascularization strategies of engineered tissues and their application in cardiac regeneration, Adv. Drug Deliv. Rev 96 (2016) 183–194. [DOI] [PubMed] [Google Scholar]
- [94].He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ, Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization, Circ. Res 93 (2003) 32–39. [DOI] [PubMed] [Google Scholar]
- [95].Mummery C, Ward-Van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R, Tertoolen L, Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells, Circulation 107 (2003) 2733–2740. [DOI] [PubMed] [Google Scholar]
- [96].Moccia F, Diofano F, Rebuzzini P, Zuccolo E, Embryonic stem cells for cardiac regeneration, in: Madonna R (Ed.), Stem Cells and Cardiac Regeneration, Springer International Publishing, Cham, 2016, pp. 9–29. [Google Scholar]
- [97].Li Y, Li L, Chen ZN, Gao G, Yao R, Sun W, Engineering-derived approaches for iPSC preparation, expansion, differentiation and applications, Biofabrication 9 (2017), 032001. [DOI] [PubMed] [Google Scholar]
- [98].Gao L, Kupfer ME, Jung JP, Yang L, Zhang P, Da Sie Y, Tran Q, Ajeti V, Freeman BT, Fast VG, Campagnola PJ, Ogle BM, Zhang J, Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold, Circ. Res 120 (2017) 1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Madonna R, Ferdinandy P, Schulz R, Induced pluripotent stem cells for cardiac regeneration, in: Madonna R (Ed.), Stem Cells and Cardiac Regeneration, Springer International Publishing, Cham, 2016, pp. 31–43. [Google Scholar]
- [100].Emmert MY, Wolint P, Wickboldt N, Gemayel G, Weber B, Brokopp CE, Boni A, Falk V, Bosman A, Jaconi ME, Hoerstrup SP, Human stem cell-based three-dimensional microtissues for advanced cardiac cell therapies, Biomaterials 34 (2013) 6339–6354. [DOI] [PubMed] [Google Scholar]
- [101].Liu HB, Zhang J, Xin SY, Liu C, Wang CY, Zhao D, Zhang ZR, Mechanosensitive properties in the endothelium and their roles in the regulation of endothelial function, J. Cardiovasc. Pharmacol 61 (2013) 461–470. [DOI] [PubMed] [Google Scholar]
- [102].Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT, Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration, Circulation 110 (2004) 962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Condorelli G, Borello U, De Angelis L, Latronico M, Sirabella D, Coletta M, Galli R, Balconi G, Follenzi A, Frati G, Cusella De Angelis MG, Gioglio L, Amuchastegui S, Adorini L, Naldini L, Vescovi A, Dejana E, Cossu G, Cardiomyocytes induce endothelial cells to trans-differentiate into cardiac muscle: implications for myocardium regeneration, Proc. Natl. Acad. Sci. U. S. A 98 (2001) 10733–10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Badorff C, Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes, Circulation 107 (2003) 1024–1032. [DOI] [PubMed] [Google Scholar]
- [105].Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM, Isolation of putative progenitor endothelial cells for angiogenesis, Science 275 (1997) 964–967. [DOI] [PubMed] [Google Scholar]
- [106].Elnakish MT, Hassan F, Dakhlallah D, Marsh CB, Alhaider IA, Khan M, Mesenchymal stem cells for cardiac regeneration: translation to bedside reality, Stem Cells Int. 2012 (2012) 646038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Sil AK, Maeda S, Sano Y, Roop DR, Karin M, IkappaB kinase-alpha acts in the epidermis to control skeletal and craniofacial morphogenesis, Nature 428 (2004) 660–664. [DOI] [PubMed] [Google Scholar]
- [108].Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC, Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium, Nature 428 (2004) 668–673. [DOI] [PubMed] [Google Scholar]
- [109].Brown RD, Ambler SK, Mitchell MD, Long CS, The cardiac fibroblast: therapeutic target in myocardial remodeling and failure, Annu. Rev. Pharmacol. Toxicol 45 (2005) 657–687. [DOI] [PubMed] [Google Scholar]
- [110].Xin M, Olson EN, Bassel-Duby R, Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair, Nat. Rev. Mol. Cell Biol 14 (2013) 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Souders CA, Bowers SL, Baudino TA, Cardiac fibroblast: the renaissance cell, Circ. Res. 105 (2009) 1164–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Doppler SA, Deutsch MA, Lange R, Krane M, Cardiac regeneration: current therapies-future concepts, J. Thorac. Dis 5 (2013) 683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Nam YJ, Song K, Luo X, Daniel E, Lambeth K, West K, Hill JA, Dimaio JM, Baker LA, Bassel-Duby R, Olson EN, Reprogramming of human fibroblasts toward a cardiac fate, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 5588–5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Laframboise WA, Scalise D, Stoodley P, Graner SR, Guthrie RD, Magovern JA, Becich MJ, Cardiac fibroblasts influence cardiomyocyte phenotype in vitro, Am. J. Phys. Cell Phys 292 (2007) C1799–C1808. [DOI] [PubMed] [Google Scholar]
- [115].Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D, In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes, Nature 485 (2012) 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Porter KE, Turner NA, Cardiac fibroblasts: at the heart of myocardial remodeling, Pharmacol. Ther 123 (2009) 255–278. [DOI] [PubMed] [Google Scholar]
- [117].Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlstrom H, Lendahl U, Frisen J, Generalized potential of adult neural stem cells, Science 288 (2000) 1660–1663. [DOI] [PubMed] [Google Scholar]
- [118].Pollack GH, Cardiac pacemaking: an obligatory role of catecholamines?, Science 196 (1977) 731–738. [DOI] [PubMed] [Google Scholar]
- [119].Wallner M, Duran JM, Mohsin S, Troupes CD, Vanhoutte D, Borghetti G, Vagnozzi RJ, Gross P, Yu D, Trappanese DM, Kubo H, Toib A, Sharp TE 3rd, Harper SC, Volkert MA, Starosta T, Feldsott EA, Berretta RM, Wang T, Barbe MF, Molkentin JD, Houser SR, Acute catecholamine exposure causes reversible myocyte injury without cardiac regeneration, Circ. Res 119 (2016) 865–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Cipitria A, Salmeron-Sanchez M, Mechanotransduction and growth factor signalling to engineer cellular microenvironments, Adv. Healthc. Mater 6 (2017, 1700052). [DOI] [PubMed] [Google Scholar]
- [121].Parker KK, Ingber DE, Extracellular matrix, mechanotransduction and structural hierarchies in heart tissue engineering, Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci 362 (2007) 1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Jung JP, Squirrell JM, Lyons GE, Eliceiri KW, Ogle BM, Imaging cardiac extracellular matrices: a blueprint for regeneration, Trends Biotechnol. 30 (2012) 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Daley WP, Peters SB, Larsen M, Extracellular matrix dynamics in development and regenerative medicine, J. Cell Sci 121 (2008) 255–264. [DOI] [PubMed] [Google Scholar]
- [124].Donderwinkel I, van Hest JCM, Cameron NR, Bio-inks for 3D bioprinting: recent advances and future prospects, Polym. Chem 8 (2017) 4451–4471. [Google Scholar]
- [125].Jose RR, Rodriguez MJ, Dixon TA, Omenetto F, Kaplan DL, Evolution of bioinks and additive manufacturing technologies for 3D bioprinting, ACS Biomater. Sci. Eng 2 (2016) 1662–1678. [DOI] [PubMed] [Google Scholar]
- [126].Segers VF, Lee RT, Protein therapeutics for cardiac regeneration after myocardial infarction, J. Cardiovasc. Transl. Res 3 (2010) 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Ventrelli L, Ricotti L, Menciassi A, Mazzolai B, Mattoli V, Nanoscaffolds for guided cardiac repair: the new therapeutic challenge of regenerative medicine, J. Nanomater 2013 (2013) 108485. [Google Scholar]
- [128].Martinelli V, Cellot G, Toma FM, Long CS, Caldwell JH, Zentilin L, Giacca M, Turco A, Prato M, Ballerini L, Mestroni L, Carbon nanotubes promote growth and spontaneous electrical activity in cultured cardiac myocytes, Nano Lett. 12 (2012) 1831–1838. [DOI] [PubMed] [Google Scholar]
- [129].Cui H, Shao J, Wang Y, Zhang P, Chen X, Wei Y, PLA- PEG-PLA and its electroactive tetraaniline copolymer as multi-interactive injectable hydrogels for tissue engineering, Biomacromolecules 14 (2013) 1904–1912. [DOI] [PubMed] [Google Scholar]
- [130].Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT, Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells, Circulation 111 (2005) 442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Tokunaga M, Liu ML, Nagai T, Iwanaga K, Matsuura K, Takahashi T, Kanda M, Kondo N, Wang P, Naito AT, Komuro I, Implantation of cardiac progenitor cells using self-assembling peptide improves cardiac function after myocardial infarction, J. Mol. Cell. Cardiol 49 (2010) 972–983. [DOI] [PubMed] [Google Scholar]
- [132].Kharaziha M, Shin SR, Nikkhah M, Topkaya SN, Masoumi N, Annabi N, Dokmeci MR, Khademhosseini A, Tough and flexible CNT-polymeric hybrid scaffolds for engineering cardiac constructs, Biomaterials 35 (2014) 7346–7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Cui H, Cui L, Zhang P, Huang Y, Wei Y, Chen X, In situ electroactive and antioxidant supramolecular hydrogel based on cyclodextrin/copolymer inclusion for tissue engineering repair, Macromol. Biosci 14 (2014) 440–450. [DOI] [PubMed] [Google Scholar]
- [134].Navaei A, Saini H, Christenson W, Sullivan RT, Ros R, Nikkhah M, Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs, Acta Biomater. 41 (2016) 133–146. [DOI] [PubMed] [Google Scholar]
- [135].Zhou J, Chen J, Sun H, Qiu X, Mou Y, Liu Z, Zhao Y, Li X, Han Y, Duan C, Tang R, Wang C, Zhong W, Liu J, Luo Y, Mengqiu Xing M, Wang C, Engineering the heart: evaluation of conductive nanomaterials for improving implant integration and cardiac function, Sci. Rep 4 (2014) 3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Song JJ, Ott HC, Organ engineering based on decellularized matrix scaffolds, Trends Mol. Med 17 (2011) 424–432. [DOI] [PubMed] [Google Scholar]
- [137].Robinson KA, Li J, Mathison M, Redkar A, Cui J, Chronos NA, Matheny RG, Badylak SF, Extracellular matrix scaffold for cardiac repair, Circulation 112 (2005) I135–I143. [DOI] [PubMed] [Google Scholar]
- [138].Moroni F, Mirabella T, Decellularized matrices for cardiovascular tissue engineering, Am. J. Stem Cells 3 (2014) 1–20. [PMC free article] [PubMed] [Google Scholar]
- [139].Engelmayr GC Jr, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE, Accordion-like honeycombs for tissue engineering of cardiac anisotropy, Nat. Mater 7 (2008) 1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Taylor EN, Hoffman MP, Barefield DY, Aninwene GE 2nd, Abrishamchi AD, Lynch TLT, Govindan S, Osinska H, Robbins J, Sadayappan S, Gilbert RJ, Alterations in multi-scale cardiac architecture in association with phosphorylation of myosin binding protein-C, J. Am. Heart Assoc 5 (2016) e002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Rohmer D, Sitek A, Gullberg GT, Reconstruction and visualization of fiber and laminar structure in the normal human heart from ex vivo diffusion tensor magnetic resonance imaging (DTMRI) data, Investig. Radiol 42 (2007) 777–789. [DOI] [PubMed] [Google Scholar]
- [142].Bian W, Badie N, Himel HDT, Bursac N, Robust T-tubulation and maturation of cardiomyocytes using tissue-engineered epicardial mimetics, Biomaterials 35 (2014) 3819–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Kim DH, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, Suh KY, Tung L, Levchenko A, Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Fleischer S, Shapira A, Feiner R, Dvir T, Modular assembly of thick multifunctional cardiac patches, Proc. Natl. Acad. Sci. U. S. A 114 (2017) 1898–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Montgomery M, Zhang B, Radisic M, Cardiac tissue vascularization: from angiogenesis to microfluidic blood vessels, J. Cardiovasc. Pharmacol. Ther 19 (2014) 382–393. [DOI] [PubMed] [Google Scholar]
- [146].Parsa H, Ronaldson K, Vunjak-Novakovic G, Bioengineering methods for myocardial regeneration, Adv. Drug Deliv. Rev 96 (2016) 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Dimarakis L, Levicar N, Nihoyannopoulos P, Gordon MY, Habib NA, In vitro stem cell differentiation into cardiomyocytes, J. Cardiothoracic-Renal Res 1 (2006) 115–121. [Google Scholar]
- [148].Scuderi GJ, Butcher J, Naturally engineered maturation of cardiomyocytes, Front. Cell. Dev. Biol 5 (2017) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Eschenhagen T, Eder A, Vollert I, Hansen A, Physiological aspects of cardiac tissue engineering, Am. J. Physiol. Heart Circ. Physiol 303 (2012) H133–H143. [DOI] [PubMed] [Google Scholar]
- [150].Hirschi KK, Li S, Roy K, Induced pluripotent stem cells for regenerative medicine, Annu. Rev. Biomed. Eng 16 (2014) 277–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Tandon V, Zhang B, Radisic M, Murthy SK, Generation of tissue constructs for cardiovascular regenerative medicine: from cell procurement to scaffold design, Biotechnol. Adv 31 (2013) 722–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Hansen A, Eder A, Bonstrup M, Flato M, Mewe M, Schaaf S, Aksehirlioglu B, Schwoerer AP, Uebeler J, Eschenhagen T, Development of a drug screening platform based on engineered heart tissue, Circ. Res 107 (2010) 35–44. [DOI] [PubMed] [Google Scholar]
- [153].Schaaf S, Shibamiya A, Mewe M, Eder A, Stohr A, Hirt MN, Rau T, Zimmermann WH, Conradi L, Eschenhagen T, Hansen A, Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology, PLoS One 6 (2011), e26397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Davies SW, Wedzicha JA, Hypoxia and the heart, Br. Heart J 69 (1993) 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Giordano FJ, Oxygen, oxidative stress, hypoxia, and heart failure, J. Clin. Invest 115 (2005) 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Takimoto E, Kass DA, Role of oxidative stress in cardiac hypertrophy and remodeling, Hypertension 49 (2007) 241–248. [DOI] [PubMed] [Google Scholar]
- [157].Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA, The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response, Cell 157 (2014) 565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Boopathy AV, Pendergrass KD, Che PL, Yoon YS, Davis ME, Oxidative stress-induced Notch1 signaling promotes cardiogenic gene expression in mesenchymal stem cells, Stem Cell Res Ther 4 (2013) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Yutzey KE, Switched at birth, Nature 509 (2014) 572–573. [DOI] [PubMed] [Google Scholar]
- [160].Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T, Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts, Nat. Med 12 (2006) 452–458. [DOI] [PubMed] [Google Scholar]
- [161].Mofrad AZ, Mashayekhan S, Bastani D, Simulation of the effects of oxygen carriers and scaffold geometry on oxygen distribution and cell growth in a channeled scaffold for engineering myocardium, Math. Biosci. 294 (2017) 160–171. [DOI] [PubMed] [Google Scholar]
- [162].Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G, Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds, Tissue Eng. 12 (2006) 2077–2091. [DOI] [PubMed] [Google Scholar]
- [163].Fung YC, Biomechanical Aspects of Growth and Tissue Engineering, Biomechanics: Motion, Flow, Stress, and Growth, Springer New York, New York, NY, 1990499–546. [Google Scholar]
- [164].Shachar M, Cohen S, Cardiac tissue engineering, ex-vivo: design principles in biomaterials and bioreactors, Heart Fail. Rev 8 (2003) 271–276. [DOI] [PubMed] [Google Scholar]
- [165].Radisic M, Marsano A, Maidhof R, Wang Y, Vunjak-Novakovic G, Cardiac tissue engineering using perfusion bioreactor systems, Nat. Protoc 3 (2008) 719–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Carrier RL, Rupnick M, Langer R, Schoen FJ, Freed LE, Vunjak-Novakovic G, Perfusion improves tissue architecture of engineered cardiac muscle, Tissue Eng. 8 (2002) 175–188. [DOI] [PubMed] [Google Scholar]
- [167].Radisic M, Yang L, Boublik J, Cohen RJ, Langer R, Freed LE, Vunjak-Novakovic G, Medium perfusion enables engineering of compact and contractile cardiac tissue, Am. J. Physiol. Heart Circ. Physiol 286 (2004) H507–H516. [DOI] [PubMed] [Google Scholar]
- [168].Chen HC, Hu YC, Bioreactors for tissue engineering, Biotechnol. Lett. 28 (2006) 1415–1423. [DOI] [PubMed] [Google Scholar]
- [169].Portner R, Nagel-Heyer S, Goepfert C, Adamietz P, Meenen NM, Bioreactor design for tissue engineering, J. Biosci. Bioeng 100 (2005) 235–245. [DOI] [PubMed] [Google Scholar]
- [170].Martin I, Wendt D, Heberer M, The role of bioreactors in tissue engineering, Trends Biotechnol. 22 (2004) 80–86. [DOI] [PubMed] [Google Scholar]
- [171].Kleber AG, Rudy Y, Basic mechanisms of cardiac impulse propagation and associated arrhythmias, Physiol. Rev. 84 (2004) 431–488. [DOI] [PubMed] [Google Scholar]
- [172].Nerbonne JM, Kass RS, Molecular physiology of cardiac repolarization, Physiol. Rev. 85 (2005) 1205–1253. [DOI] [PubMed] [Google Scholar]
- [173].Cheung DYC, Duan B, Butcher JT, Chapter 21 - bioprinting of cardiac tissues A2 - Atala, Anthony, in: Yoo JJ (Ed.), Essentials of 3D Biofabrication and Translation, Academic Press, Boston, 2015, pp. 351–370. [Google Scholar]
- [174].Papadaki M, Bursac N, Langer R, Merok J, Vunjak-Novakovic G, Freed LE, Tissue engineering of functional cardiac muscle: molecular, structural, and electrophysiological studies, Am. J. Physiol. Heart Circ. Physiol 280 (2001) H168–H178. [DOI] [PubMed] [Google Scholar]
- [175].Bursac N, Papadaki M, Cohen RJ, Schoen FJ, Eisenberg SR, Carrier R, Vunjak-Novakovic G, Freed LE, Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies, Am. J. Phys. 277 (1999) H433–H444. [DOI] [PubMed] [Google Scholar]
- [176].Balint R, Cassidy NJ, Cartmell SH, Electrical stimulation: a novel tool for tissue engineering, Tissue Eng. Part B 19 (2013) 48–57. [DOI] [PubMed] [Google Scholar]
- [177].Tandon N, Cannizzaro C, Chao PH, Maidhof R, Marsano A, Au HT, Radisic M, Vunjak-Novakovic G, Electrical stimulation systems for cardiac tissue engineering, Nat. Protoc 4 (2009) 155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Tandon N, Marsano A, Maidhof R, Wan L, Park H, Vunjak-Novakovic G, Optimization of electrical stimulation parameters for cardiac tissue engineering, J. Tissue Eng. Regen. Med 5 (2011) e115–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Zhu W, Ye T, Lee SJ, Cui H, Miao S, Zhou X, Shuai D, Zhang LG, Enhanced neural stem cell functions in conductive annealed carbon nanofibrous scaffolds with electrical stimulation, Nanomed-nanotechnol. (2017, 10.1016/j.nano.2017.03.018). [DOI] [PubMed] [Google Scholar]
- [180].Cui H, Liu Y, Deng M, Pang X, Zhang P, Wang X, Chen X, Wei Y, Synthesis of biodegradable and electroactive tetraaniline grafted poly(ester amide) copolymers for bone tissue engineering, Biomacromolecules 13 (2012) 2881–2889. [DOI] [PubMed] [Google Scholar]
- [181].Yang B, Yao F, Hao T, Fang W, Ye L, Zhang Y, Wang Y, Li J, Wang C, Development of electrically conductive double-network hydrogels via one-step facile strategy for cardiac tissue engineering, Adv. Healthc. Mater 5 (2016) 474–488. [DOI] [PubMed] [Google Scholar]
- [182].Lim HL, Chuang JC, Tran T, Aung A, Arya G, Varghese S, Dynamic electromechanical hydrogel matrices for stem cell culture, Adv. Funct. Mater 21 (2011) 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Cui H, Wang Y, Cui L, Zhang P, Wang X, Wei Y, Chen X, In vitro studies on regulation of osteogenic activities by electrical stimulus on biodegradable electroactive polyelectrolyte multilayers, Biomacromolecules 15 (2014) 3146–3157. [DOI] [PubMed] [Google Scholar]
- [184].Wang Y, Cui H, Wu Z, Wu N, Wang Z, Chen X, Wei Y, Zhang P, Modulation of osteogenesis in Mc3t3-E1 cells by different frequency electrical stimulation, PLoS One 11 (2016), e0154924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Liu Y, Cui H, Zhuang X, Wei Y, Chen X, Electrospinning of aniline pentamer-graft-gelatin/PLLA nanofibers for bone tissue engineering, Acta Biomater. 10 (2014) 5074–5080. [DOI] [PubMed] [Google Scholar]
- [186].Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Baharvand H, Kiani S, Al-Deyab SS, Ramakrishna S, Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering, J. Tissue Eng. Regen. Med 5 (2011) e17–e35. [DOI] [PubMed] [Google Scholar]
- [187].Gaebel R, Ma N, Liu J, Guan J, Koch L, Klopsch C, Gruene M, Toelk A, Wang W, Mark P, Wang F, Chichkov B, Li W, Steinhoff G, Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration, Biomaterials 32 (2011) 9218–9230. [DOI] [PubMed] [Google Scholar]
- [188].Gaetani R, Doevendans PA, Metz CH, Alblas J, Messina E, Giacomello A, Sluijter JP, Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells, Biomaterials 33 (2012) 1782–1790. [DOI] [PubMed] [Google Scholar]
- [189].Gaetani R, Feyen DA, Verhage V, Slaats R, Messina E, Christman KL, Giacomello A, Doevendans PA, Sluijter JP, Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction, Biomaterials 61 (2015) 339–348. [DOI] [PubMed] [Google Scholar]
- [190].Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, Kim DH, Cho DW, Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink, Nat. Commun 5 (2014) 3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Jang J, Park HJ, Kim SW, Kim H, Park JY, Na SJ, Kim HJ, Park MN, Choi SH, Park SH, Kim SW, Kwon SM, Kim PJ, Cho DW, 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair, Biomaterials 112 (2017) 264–274. [DOI] [PubMed] [Google Scholar]
- [192].Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, Aleman J, Colosi C, Busignani F, Dell'Erba V, Bishop C, Shupe T, Demarchi D, Moretti M, Rasponi M, Dokmeci MR, Atala A, Khademhosseini A, Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip, Biomaterials 110 (2016) 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Tijore A, Irvine SA, Sarig U, Mhaisalkar P, Baisane V, Venkatraman SS, Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel, Biofabrication( 10, 2018, 025003). [DOI] [PubMed] [Google Scholar]
- [194].Ong CS, Fukunishi T, Zhang H, Huang CY, Nashed A, Blazeski A, Disilvestre D, Vricella L, Conte J, Tung L, Tomaselli GF, Hibino N, Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes, Sci. Rep 7 (2017) 4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue HJ, Ramadan MH, Hudson AR, Feinberg AW, Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels, Sci. Adv 1 (2015), e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Wang Z, Lee SJ, Cheng HJ, Yoo JJ, Atala A, 3D bioprinted functional and contractile cardiac tissue constructs, Acta Biomater. 70 (2018) 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Izadifar M, Chapman D, Babyn P, Chen X, Kelly ME, UV-assisted 3D bioprinting of nanoreinforced Hybrid cardiac patch for myocardial tissue engineering, Tissue Eng. Part C 24 (2018) 74–88. [DOI] [PubMed] [Google Scholar]
- [198].Hockaday LA, Kang KH, Colangelo NW, Cheung PY, Duan B, Malone E, Wu J, Girardi LN, Bonassar LJ, Lipson H, Chu CC, Butcher JT, Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds, Biofabrication 4 (2012), 035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Duan B, Hockaday LA, Kang KH, Butcher JT, 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels, J. Biomed. Mater. Res. A 101 (2013) 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Duan B, Kapetanovic E, Hockaday LA, Butcher JT, Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells, Acta Biomater. 10 (2014) 1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Visconti RP, Kasyanov V, Gentile C, Zhang J, Markwald RR, Mironov V, Towards organ printing: engineering an intra-organ branched vascular tree, Expert. Opin. Biol. Ther. 10 (2010) 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Rouwkema J, Khademhosseini A, Vascularization and angiogenesis in tissue engineering: beyond creating static networks, Trends Biotechnol. 34 (2016) 733–745. [DOI] [PubMed] [Google Scholar]
- [203].Griffith CK, Miller C, Sainson RC, Calvert JW, Jeon NL, Hughes CC, George SC, Diffusion limits of an in vitro thick prevascularized tissue, Tissue Eng. 11 (2005) 257–266. [DOI] [PubMed] [Google Scholar]
- [204].Phan DTT, Wang X, Craver BM, Sobrino A, Zhao D, Chen JC, Lee LYN, George SC, Lee AP, Hughes CCW, A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications, Lab Chip 17 (2017) 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Mosadegh B, Xiong G, Dunham S, Min JK, Current progress in 3D printing for cardiovascular tissue engineering, Biomed. Mater 10 (2015), 034002. [DOI] [PubMed] [Google Scholar]
- [206].Cui X, Gao G, Qiu Y, Accelerated Myotube formation using bioprinting technology for biosensor applications, Biotechnol. Lett 35 (2013) 315–321. [DOI] [PubMed] [Google Scholar]
- [207].Zhang YS, Aleman J, Shin SR, Kilic T, Kim D, Mousavi Shaegh SA, Massa S, Riahi R, Chae S, Hu N, Avci H, Zhang W, Silvestri A, Sanati Nezhad A, Manbohi A, De Ferrari F, Polini A, Calzone G, Shaikh N, Alerasool P, Budina E, Kang J, Bhise N, Ribas J, Pourmand A, Skardal A, Shupe T, Bishop CE, Dokmeci MR, Atala A, Khademhosseini A, Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors, Proc. Natl. Acad. Sci. U. S. A 114 (2017) E2293–E2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M, Park SJ, Kotikian A, Nesmith AP, Campbell PH, Vlassak JJ, Lewis JA, Parker KK, Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing, Nat. Mater 16 (2017) 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]