Abstract
3D bioprinting is emerging as a promising technology for fabricating complex tissue constructs with tailored biological components and mechanical properties. Recent advances have enabled scientists to precisely position materials and cells to build functional tissue models for in vitro drug screening and disease modeling. This review presents state-of-the-art 3D bioprinting techniques and discusses the choice of cell source and biomaterials for building functional tissue models that can be used for personalized drug screening and disease modeling. In particular, we focus on 3D-bioprinted liver models, cardiac tissues, vascularized constructs, and cancer models for their promising applications in medical research, drug discovery, toxicology, and other pre-clinical studies.
Keywords: 3D printing, tissue model, drug screening, disease model, in vitro culture, tissue engineering, biomaterials
Graphical Abstract
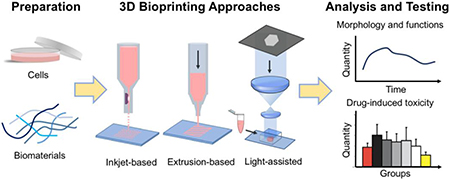
Schematic diagram showing the use of 3D bioprinting to build in vitro constructs that can be used for drug testing and disease modeling.
1. Introduction
Three dimensional (3D) bioprinting, an extension of 3D printing, is based on additive manufacturing technology and provides controlled fabrication of 3D structures in all X, Y, and Z directions [1–3]. The complex structures to be formed can be designed using a computer-aided design (CAD) software or scanned from medical images including magnetic resonance imaging (MRI) or computed tomography (CT) scans [4,5]. 3D bioprinting has emerged as a promising technology for fabricating complex tissue constructs with tailored biological components and mechanical properties [1]. By utilizing this transformative technology, bioinks, including hydrogels, cells, and growth factors, can be precisely positioned to create 3D in vitro culture environments [6,7]. In this way, native tissue architecture, cellular composition and vasculature can be recapitulated in vitro to create biomimetic tissue models, which can be used for studying disease mechanisms, screening drugs and other clinical applications [8,9]. With further involvement of induced pluripotent stem cell (iPSC)-derived cells, personalized tissue models in healthy and disease states can be built to customize the drug screening and treatment process.
Here we will review the development of in vitro tissue models using 3D bioprinting approaches. We first look at the state-of-the-art 3D bioprinting platforms, the commonly used cell source and biomaterial choice for building functional tissue models that can be used for personalized drug screening and disease modeling. Then we focus on 3D-bioprinted liver constructs, cardiac tissues, vascularized structures, and cancer models for their wide applications in medical research, drug discovery, toxicology, and other pre-clinical studies. Finally, we will discuss the limitations of current technologies and the direction for future work.
2. Current 3D bioprinting approaches to build in vitro tissue models
3D bioprinting has the advantage of reconstructing complex structures from CT or MRI images and producing accurate structures from predetermined digital designs such as CAD models. In order to build functional tissue models, the combination of 3D bioprinting technology with appropriate choice of cells and biomaterials is essential [1,10,11]. The fabrication capability of the 3D printer and the requirement on materials are highly dependent on the type of printer [12,13]. The choice of cell source also leads to various application potentials of the tissue model [14,15]. In the following sections, we discuss these in more detail.
2.1. Current 3D bioprinting technology
The primary types of 3D bioprinting technologies include inkjet-based, extrusion-based, and light-assisted printing. Each of the 3D printing approaches has the capability to both print scaffolds for cell seeding and encapsulate cells directly within scaffolds to build tissue constructs. However, these platforms differ in various aspects including their printing mechanisms, resolution, time, and material choice. Based on recent publications in the past three years, we found that extrusion-based printing is the most used technique [16–72] followed by light-assisted [73–96] and inkjet-based printing approaches [45,97–107]. Below we evaluate and compare these platforms more thoroughly.
2.1.1. Inkjet-based bioprinting
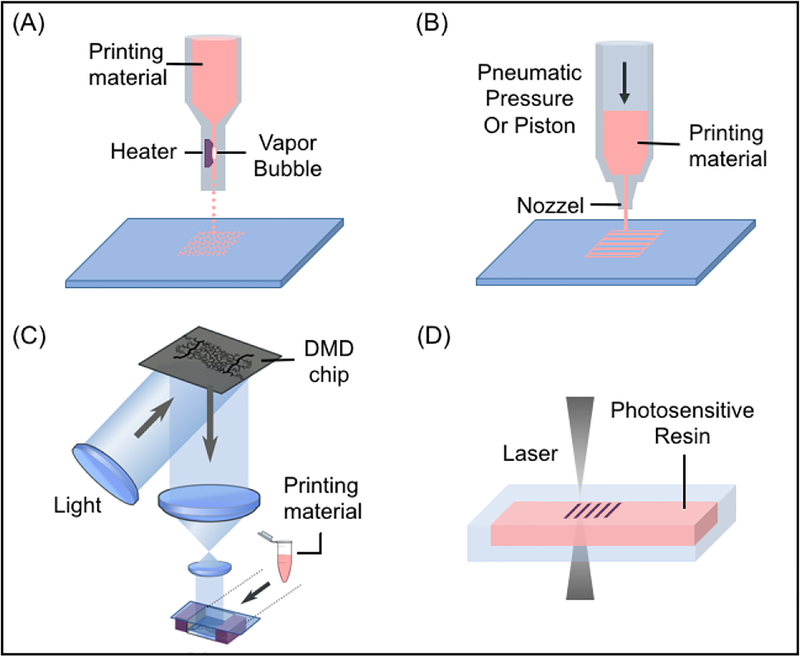
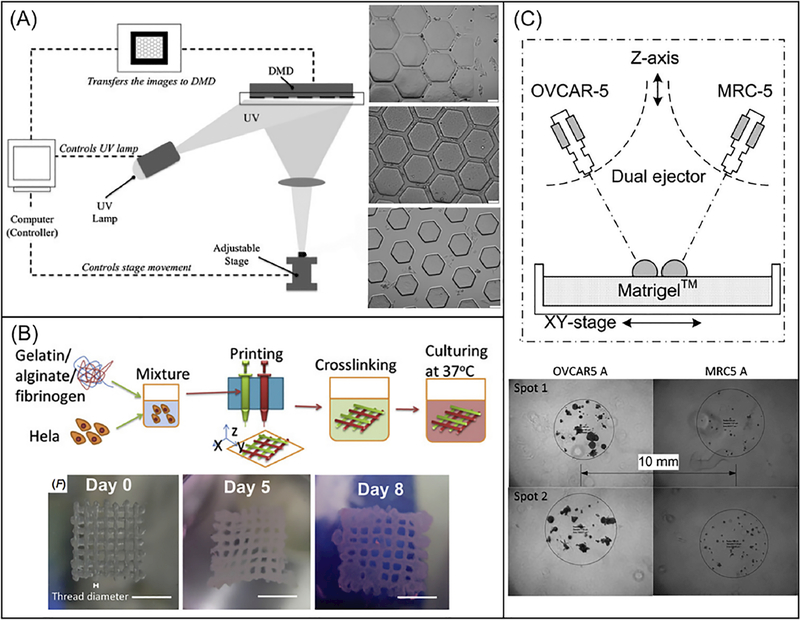
Inkjet-based bioprinting systems are modified from conventional desktop inkjet printers to dispense precise picoliter droplets of bioink (material solution or cell-material mixture) on printing stage (Fig. 1A) [108,109]. There are multiple approaches to inkjet printing, including thermal, piezoelectric, and electromagnetic [110]. Among these types, the thermal approach is more commonly used because of the relatively high cell viability after printing, user-friendly design, and lower cost in general. During thermal inkjet printing, localized heating increases the temperature to 300°C for several microseconds and inflates an air bubble to push droplets out from the nozzle head [110]. In the piezoelectric method, droplets are produced by the pulse pressure generated from a piezoelectric actuator [111]. The electromagnetic approach is based on electromagnetic principles including Lorentz force and permanent magnet-based configurations [112]. Electromagnetic printing generates relatively larger droplets than thermal or piezoelectric approaches [113].
Fig. 1.
Schematic diagrams showing the printing approaches: (A) inkjet-based bioprinting systems, (B) extrusion-based bioprinting systems, (C) DLP-based bioprinting and (D) TPP-based bioprinting platforms.
For current inkjet-based bioprinters, a printing speed in the range of hundreds of millimeters per second and a printing resolution as high as 20 μm has been reported [10,114]. Resolution of the printed constructs relies on the nozzle diameter as well as the properties of the bioink. Smaller diameter nozzle heads generally render higher printing resolution but also increases the potential for clogging, thus the variety of materials that can be printed with inkjet-based method is limited. Generally, only materials with relatively low viscosity or water-based materials are suitable in order to minimize the chance of clogging. This requirement in turn limits the size and structural integrity of the constructs produced by this printing technology. While inkjet-based method is flexible and inexpensive, the limitations on materials, frequent nozzle clogging, slow printing speed due to point-by-point deposition, and potential damage to cells from shear or thermal stress are issues waiting to be resolved before the expansion of its applications to building more complex tissue models.
2.1.2. Extrusion-based bioprinting
Extrusion-based bioprinting systems deposit continuous filaments compared to the individual droplets of inkjet-based bioprinters (Fig. 1B). This technology uses a set of automated motors to control the stage or the printer nozzle and a dispensing system to deposit bioink at a precise time and location that is digitally controlled by a computer. Multiple approaches can be used to drive the dispensing system, including pressure-based control, mechanical control, or solenoid control [1]. In this case, acellular or cell-laden bioinks can be printed onto a receiving substrate in a layer-by-layer fashion.
For microscale nozzle printing, a more versatile selection of bioinks are compatible with this technology. These include cell spheroid suspension, decellularized extracellular matrix (dECM) solutions, and hydrogels with a wider range of viscosity such as poly(ethylene glycol) (PEG)-based hydrogels, gelatin, hyaluronic acid (HA), and alginate [17,115–117]. Printing of more viscous hydrogels can provide a stronger mechanical support in the final structure. Notably, the flexibility of using more biocompatible inks during extrusion-based printing also make it more suitable for building a variety of tissue models. In addition to the wider choice of printing materials, extrusion-based printing is also advantageous in terms of printing and deposition speed as well as upscaling potential. However, extrusion-based bioprinting has the lowest reported printing speed among the three types of printing approaches, in the range of 10 to 50 μm/s [1,10]. Additionally, the resolution of the printed constructs is generally compromised to allow for 3D structures with a larger footprint. The reported minimal printed feature resolution can be 5μm but is generally over 100 μm [1,116,118]. Extrusion-based printing also suffers from shear induced cell death, which is similar to inkjet printing technology [1,116,118]. Nevertheless, tissue models that lack microscale features such as bone, cartilage and organoids, can still be robustly built using extrusion-based bioprinting [116,118,119]. Furthermore, some biomaterials as well as tissues can be readily fabricated by modeling with customized molds that are prepared by extrusion-based 3D printing technology [120,121].
2.1.3. Light-assisted bioprinting
Light-assisted bioprinting methods have gained popularity in recent decades for their high cell viability post-printing as well as superior printing speed and resolution. Light-assisted bioprinting systems have many variations, where each type has the potential to modulate different parameters of the printed constructs, including mechanical properties, chemical compositions, cell and material distributions. Two types of light-assisted bioprinting are mainly applied in tissue engineering and discussed in detail below - digital light processing (DLP)-based bioprinting and the two-photon polymerization (TPP)-based bioprinting.
2.1.3.1. DLP-based bioprinting
DLP-based bioprinting platforms utilize a digital micro-mirror device (DMD) chip, a motorized translational stage, and a motorized printing head that are all controllable by computer (Fig. 1C) [122,123]. The DMD chip consists of around two million micro-mirrors, which allows for precise light projection patterning as each micro-mirror can be turned on or off independently throughout the printing process. The illumination of UV light or other light source projects onto the prepolymer solution only when the micro-mirror is in its arbitrary “on” state [10]. Two bioprinting systems, dynamic optical projection stereolithography (DOPsL) and microscale continuous printing (μCOP), emerged recently as DLP-based bioprinting platforms with DOPsL highlighting the dynamic printing while μCOP highlighting the continuous printing.
The resolution of DLP-based printers is usually at the microscale level, depending on the focal size of the light beam from each of the micro-mirrors. With DLP-based bioprinting, there is no artificial interface between dots as with inkjet printing or between lines as with extrusion-based printing. This is because an entire plane of pattern is projected onto the prepolymer solution all at once, and the stage moves while the printing patterns continuously refresh. Absence of the artificial interfaces results in better mechanical integrity of the printed structure. DLP-based printers fabricate the entire volume of a structure in a few seconds such that the printing speed is based on a volumetric scale of a few cubic millimeter per second, which is much faster than the printing speed of other conventional approaches [10]. The flexible pattern input, rapid printing speed, and high printing resolution allow researchers to build complex structures with high precision, including microwells [124], microfluidic mixing chambers [125], complex tissue structure [126–130], fractal geometries [131], and constructs with tunable Poisson ratios [132,133]. Materials that are compatible with this printing technique include various photopolymerizable polymers, such as gelatin methacrylate (GelMA), poly(ethylene glycol) diacrylate (PEGDA), and glycidyl methacrylate hyaluronic acid (GMHA). While the material selection is confined within photopolymerizable materials, the limitation can be mitigated with the expanding library of photocurable materials.
DLP-based bioprinting has great potential to build complex tissue structures with microscale resolution. With the capability of modulating scaffold mechanical property, DLP-based bioprinting can also be used to print tissue disease models. The versatility of this printing technique can be demonstrated by the large amount of complex tissue constructs fabricated using this method, including but not limited to vasculature network [127], aligned cardiac scaffolds, and liver microarchitecture [126].
2.1.3.2. TPP-based bioprinting
TPP-based bioprinting is a type of laser-based direct-writing technique developed from stereolithography, which generates structures by repeatedly and selectively photopolymerizable photo-sensitive monomers with a rastering laser (Fig. 1D) [134]. The printing mechanism of TPP is based on the two-photon absorption phenomenon, where the probability of two photon absorption by a molecule is associated with the square of light intensity [135]. This confines the dimension of the printing voxel to below 1 μm3 [136]. The feature resolution produced by TPP can be achieved around 100 nm [137], making it ideal for printing nanoscale and microscale features. Meanwhile, the tradeoff of such high resolution is limited in construct size and printing speed. The printing speed of TTP-based printers lies in the range of 200–1,600 mm/s, as reported for laser assisted printers [1,10], which is faster than extrusion-based printers and comparable to inkjet printers. A number of polymers have been successfully printed with this method, including type I collagen [138], bovine serum albumin [139], laminin [140], streptavidin [141] and PEG-based hydrogels [142].
Unlike inkjet- or extrusion-based methods that can employ various polymerization mechanisms during printing, laser-based printing primarily utilizes free-radical polymerization, which limits its selection of materials. However, laser-based bioprinting methods still provide numerous advantages such as good cell viability, high feature resolution and fast printing speed, which make them promising tools for creating disease models and drug testing platforms [10].
The applications of light-assisted printing approaches to develop in vitro tissue models has increased dramatically in recent years. Different light-assisted printing platforms can address the challenges in building tissue models with various requirements. For example, TPP-based printing provides superior feature resolution for building structure with single cell resolution, and the DLP-based printing is capable of rapid printing of complex architecture in liver tissues [126,143]. Nevertheless, some additional limitations of light-assisted printing approach need to be kept in mind. Most light-assisted printing platforms cannot provide the flexibility to selectively deposit bioink, as compared to other two types of printing approaches, so washing steps will be needed when there is a change of either material or cell type [1]. In addition, light-assisted printing platforms mostly use a printing reservoir or chamber to contain the bioink for light to selectively polymerize [1]. This can often lead to wasted unpolymerized bioink inside the printing reservoir, which can be an issue when cells or materials are in limited quantity. Thus, the appropriate choice for a particular printing approach is always application oriented and needs to be based on both the printing capability and the potential limitations.
2.2. Cell source and preparation
Most tissues are not acellular therefore incorporation of cells is essential to create functional tissue constructs. To build 3D printed in vitro tissue models, there are mainly two ways of cell incorporation: cell seeding onto already printed scaffolds and cell encapsulation during the printing process. For the seeding method, cells can be seeded directly or mixed together with a carrier matrix like collagen. The latter approach has been more popular for cell types that are sensitive, less proliferative, and dependent on cell matrix interactions [1,144,145]. In the case of cell encapsulation, a cell suspension solution is premixed with the biomaterial solution and allowed to solidify through various methods depending on the printing approach. Overall, the choice of cellularization approach depends on a variety of factors such as the intended purpose of study, printing method employed, and the cell type used.
In general, there are mainly three sources of cells commonly used for building 3D printed tissue models: primary cells, cell lines, and stem cell-derived cells. Primary cells are cells directed isolated from human or animal tissues. If these cells are properly harvested from the targeted tissue at the desired health stage, they are great candidates to recapitulate the specific tissue functions at the specific point or stage [146]. However, the availability of human primary cells is low and sometimes very rare like in the case of primary cardiomyocytes. In addition, there are always batch-to-batch or donor-to-donor variations. The tissue constructs that use primary cells are also not patient specific, making it less preferable for personalized platforms. Cell lines are cells that can be subcultured repeatedly in vitro and have acquired homogenous genotypic and phenotypic characteristics. Cell lines are cheaper and easily accessible, and likely have standard culture procedure. For these reasons, cell lines are good choices when used as the starting or testing cells for building new tissue models. They are also widely used in cancer models when focusing on specific cancer cell behavior. However, these cells are mostly modified so their structures and functional performance may differ from the targeted cells. Like primary cells, they cannot be applied to personalized platforms. Stem cell-derived cell type is the third kind of cells commonly used in 3D printed tissue constructs. When primary cells are less available and cell lines are not ideal, stem cell-derived cells are often good choices to consider. These include mesenchymal stem cells (MSC), embryonic stem cells (ESC) and iPSC-derived cells. In particular, human iPSC-derived cells are gaining increasing popularity for their potential to recapitulate individual differences. They are widely applied in many kinds of in vitro tissue models. These cells however still have limitations in that they are often not functionally mature enough and the also there can be inconsistency between differentiation batches [147,148].
Recently co-culture platforms are gaining increasing attention due to the better support they provide on cell survival and tissue function [126,127,143,149]. Including multiple types of cells in the printing process therefore is becoming a more common strategy. Most of the co-culture platforms are printed by extrusion-based and light-assisted bioprinters [126,127,143,149]. Due to the addition of multiple cell types, these platforms make it possible to study interactions between different cell types and provide paracrine support from non-parenchymal cell types. Such benefits are particularly significant when studying cancer behaviors and modeling organs whose function rely on the contributions from multiple cell types.
Regardless of the type of cell source chosen, there can always be high variations between cells used for different batches of 3D printing. Such variations come from a variety of sources including but not limited to user handling techniques, culture medium and chemicals [150], variations in culture environment [150], aging and mutations of cells [151–153], and differentiation inconsistency [151]. Therefore, implementing certain assays or methods to characterize cells before printing is essential to achieve consistency and reproducibility between experiments [154,155]. The specific assay and methods chosen are usually highly cell type dependent, but general characterizations on cell viability, purity and phenotype can be applied to all cell types [126,155,156]. Such characterizations can also be used following printing to study the impacts on cells due to the cell preparation technique, printing process, and 3D culture method.
2.3. Biomaterial choice
To design tissue scaffolds with the desired physical and chemical properties in 3D bioprinting, proper biomaterial selection is an important consideration. More specifically, biomaterials can be divided into two main categories: naturally-derived (e.g. collagen [30], gelatin [158], fibrin [158], hyaluronic acid [158], silk proteins [158], chitosan [159], alginate [117], dECM [160]) and synthetic (e.g. poly(lactic acid) (PLA) [159], poly(lactic-glycolic) acid (PLGA) [161], PEGDA [125]). Naturally-derived materials are attractive because the complexity of their biophysical and biochemical constituents closely recapitulate the native extracellular matrix (ECM). In turn, these intrinsic properties have been demonstrated to strongly support cell adhesion, proliferation, differentiation, migration, and biocompatibility [162]. However, natural biomaterials are often mechanically weak with higher potential for variation between batches. Synthetic materials are highly defined and can be easily reproduced to control for a wide range of properties including degradation rate, cell adhesive moieties, mechanical strength, and structure [162]. For instance, synthetic polymer backbones can be decorated with cell recognition peptides sequences such as RGD and YIGSR to improve cell adhesion onto the substrates [163]. This flexibility enables the user to adopt a bottom-up approach to engineer a microenvironment mimicking the chemical and physical elements of the ECM found in vivo. Despite these advantages, it remains difficult to fully recapitulate components of the native tissue ECM artificially and the potential for poor tissue integration as well as the production of cytotoxic degradable byproducts may pose concerns with respect to long term biocompatibility [164].
To circumvent these challenges, composite hydrogels incorporating both natural and synthetic biomaterials have been employed by combining the advantages of both to better emulate the characteristics of natural tissues. More specifically, synthetic materials can be used to impart mechanical strength while naturally-derived materials contribute ECM components into the hydrogel matrix to improve cell viability and functionality. For example, Hutson et al. developed PEG-GelMA hydrogels which can be copolymerized to exhibit tunable stiffness and degradation profiles with improved fibroblast binding and viability compared to PEG only hydrogels [165]. Interpenetrating hydrogel networks composed of polyvinyl alcohol (PVA)-gelatin and a PEG porogen have also been used by Miao et al. to vary modulus (i.e. 10 kPa to 100 kPa) by modifying the concentration and molecular weight of PVA as well as the gelatin content for cartilage regeneration [166]. In alternative approaches, the ability to deposit multimaterial in 3D printing has also been employed by depositing synthetic materials such as polydimethylsiloxane (PDMS), and poly(caprolactone) (PCL) to fabricate a supportive framework into which natural materials including collagen, gelatin, fibrin, and dECM may be subsequently placed in between [160,167,168]. Furthermore, nanoparticles could be incorporated into the hydrogels to create functional structures. For instance, Gou et al. 3D-printed a hydrogel nanocomposite based liver-mimetic device that can effectively cleanse the blood [123].
As we move towards the production of biomimetic tissues there is an increasing need to develop novel biomaterials that possess complex biophysical and biochemical cues to promote tissue-specific function and maturation. While most naturally-derived bioinks utilize gelatin, collagen, and hyaluronic acid these materials only represent single components of the ECM and lack other important constituents such as growth factors, proteoglycans, glycosaminoglycans, laminin, fibronectin, and elastin [169]. As a result, the use of dECM derived from tissues and organs has gained interest for applications in tissue engineering and regenerative medicine. The process of decellularization aims to remove all cellular components using a combination of mechanical, chemical, and enzymatic treatments to yield a collagenous matrix material while retaining constituents of the native ECM. Studies have also demonstrated that ECM derived from different tissues are compositionally distinct and cells respond to these matrices in a tissue-specific manner that is important in maintaining phenotype and functionality [169–171]. With regards to 3D bioprinting, seminal work by Pati et al. showed the development of decellularized tissue bioinks from pepsin solubilized cardiac, adipose, and cartilage tissues to fabricate cell-laden constructs with the use of a nozzle-based printer. These dECM printed constructs enhanced functionality of encapsulated rat myoblasts, human adipose-derived stem cells, and human inferior turbinate-tissue derived mesenchymal stem cells for each of the cardiac, adipose, and cartilage printed constructs, respectively, in comparison to collagen bioink controls [160]. This study demonstrates the versatility of dECM in 3D bioprinting to fabricate tissue constructs to better recapitulate the microenvironment of in vivo tissues and organs.
In the context of 3D bioprinting, a range of mechanisms have been employed to print both naturally-derived and synthetic hydrogel precursors. Regardless of the bioink selected, the biomaterials must be able to quickly form a hydrogel network during the printing process either through chemical or physical crosslinking mechanisms. For example, in light-assisted 3D bioprinting systems crosslinking is achieved through free-radical polymerization of photopolymerizable bioinks [172], whereas in nozzle-based printing modalities other methods including thermal gelation [173], ionic crosslinking [117], and via pH sensitivity [174] have been used. In addition to the crosslinking mechanism employed, the properties of the bioink must be considered and carefully selected for optimizing printing parameters in different 3D printing systems. In extrusion-based 3D-printing, a major consideration for users is solution viscosity. Lower viscosity materials may implement aforementioned crosslinking methods near the extruder tip, whereas higher viscosities allow the printing of self-supporting structures that maintain their shape until crosslinking occurs. In this case, adjustments must be made to the nozzle gauge and printing speed to accommodate material viscosity or using materials with shear thinning properties [175]. Other factors including platform temperature and humidity along with shear stress through the extruder are also important considerations, especially during the direct printing of cells with extrusion-based methods [176]. In light-assisted 3D bioprinting, both fluid and viscous materials are compatible, thus opening users to materials with a larger range of mechanical properties. However, these printing systems are limited to photocurable bioinks which requires synthetic and natural biomaterials to be functionalized with photocrosslinkable groups such as PEGDA, GelMA, and GMHA. In addition, the opacity of the chosen biomaterial is also an important consideration since this will impact the light penetration depth and subsequently effect the resolution and quality of the final structure [177].
The decision on the choice of biomaterials depends on a variety of factors, including but not limited to the type of printing approach, tissue of interest, and the biological process to model. To build in vitro tissue models for disease modeling and personalized drug screening, future research is needed to develop materials with high tunability on the mechanical, chemical, and biological properties to recapitulate the protein composition as well as the native tissue environment at the targeted health stage.
3. Drug screening and disease modeling applications in various organs
In the following sections, the applications of 3D bioprinting technologies to build liver, cardiac, vascularized, and cancer models are discussed. More specifically, the use of different 3D bioprinting approaches as well as the performance of current 3D printed tissue constructs in terms of their tissue-specific functions, drug metabolizing potentials, and drug dose responses are reviewed.
Liver models
Liver associated diseases are major contributors of morbidity and mortality in the United States [178]. These abnormalities can lead to the formation of excessive fibrous tissue, result in the reduction of both liver-specific and systematic functions, and transition to non-reversible end-stage liver failure that can only rely on liver transplantation [179]. In addition, as the liver serves a vital role in xenobiotic metabolism and detoxification, the investigation of hepatotoxicity is an essential component of any preclinical drug study [180]. Conventional animal models are often costly and unreliable in translation to human studies due to variations in hepatocellular functions of different species [181–183]. Moreover, both liver disease progression and drug response vary between individuals. Failure in hepatotoxicity prediction often leads to post-market withdrawal of a drug. Therefore, effective in vitro human liver models that base on personalized cell type are considered as a highly promising approach to better understand the disease mechanism, serve as a drug screening platform, and potentially treat disease in a regenerative medicine approach.
Over the past decades, liver tissue engineering has made significant progress towards the establishment of in vitro liver models for both fundamental pathophysiological studies and drug screening [184–187]. The sources of cells used for these in vitro liver models include primary hepatocytes, hepatic cell lines isolated from tumors or liver slices, and stem cell-derived hepatic cells [182,183,187]. Monolayer culture, organoid culture and co-culture platforms have been established using culture plates [188,189], commercially available wells [190], microfluidic perfusable chip [191,192], dielectrophoresis micropatterning [193] and physical mask-based additive photopatterning methods [182]. However, the liver specific functions of hepatocytes cultured in such platforms declined over weeks of in vitro culture [187–189,194,195]. Therefore, liver constructs that better mimic native environment and help maintain in vitro liver functions is in great demand. 3D bioprinting technology, with its potential to pattern cells and biomaterials in a precise manner, provides a great tool to achieve novel and biomimetic in vitro liver models with increasing structural complexity.
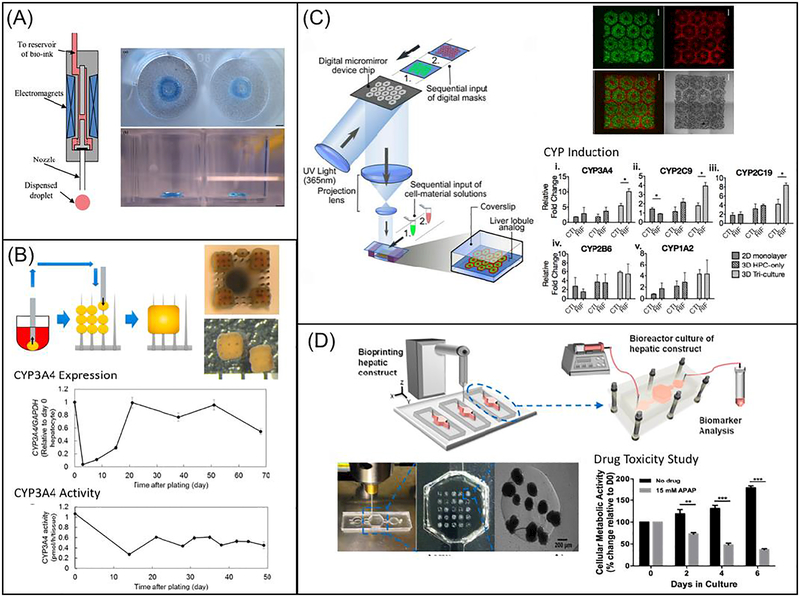
Different 3D bioprinting approaches have been utilized to create liver tissue constructs. Faulkner-Jones et al. reported the use of inkjet-based bioprinter to encapsulate human iPSC and ESC-derived hepatocyte like cells (HLCs) in alginate hydrogels to create 3D ring structures (Fig. 2A) [196]. Alginate was chosen based on its good biocompatibility, low immunogenicity, low toxicity and hydrophilic nature [196]. The cell laden alginate droplets were exposed to calcium chloride solution followed by barium chloride before incubating in culture medium [196]. The viability and albumin secreting function of HLCs were well maintained following this valve-based bioprinting. Kang et al. used extrusion-based bioprinting to generate a 3D hepatic structure [197]. Here, a five-layer alginate scaffold containing mouse induced hepatocyte-like cells, each measuring 25 by 25 millimeters, was constructed. During in vitro culture, the expression of albumin, ASGR1 and HNF4a gradually increased [197]. The construct was also transplanted in vivo with increased proliferation and higher albumin expression observed [197]. This work demonstrated the use of 3D bioprinted liver scaffold as an effective option for liver therapy. Kizawa et al. also demonstrated a scaffold-free 3D bioprinting technology to build liver tissue that could stably maintain bile acid secretion as well as drug, glucose, and lipid metabolism for weeks (Fig. 2B) [198]. This was achieved by connecting spheroids of human primary hepatocytes using the 3D printer. Their work provided insight on the long term culture of 3D bioprinted liver construct in vitro. In particular, the group studied the expression and activity of CYP3A4 enzymes and showed that both were maintained for around a period of 2 months. In order to mimic the complex microarchitecture of liver, Ma et al. reported the use of DLP-based bioprinting technology to build biomimetic liver tissue at microscale resolution (Fig. 2C) [126]. The 3D bioprinted liver construct consisted of a hexagonal array of human iPSC-derived hepatic cells with supporting cells (Fig. 2C). Hepatic cells cultured in this 3D bioprinted tri-culture model demonstrated better liver specific function and drug metabolism potential following CYP induction than those cultured in conventional 2D monolayer and 3D single culture platforms [126]. Directly printing into a microfluidic chamber to build liver-on-a-chip platform has also been demonstrated by Bhise and colleagues (Fig. 2D) [199]. Droplets of HepG2 spheroid-GelMA mixture were printed on a glass slide within the cell culture chamber of a bioreactor, followed by immediate UV crosslinking [199]. The engineered hepatic construct remained functional during the 30-day culture period and showed a drug response similar to published data [199].
Fig. 2.
3D bioprinting of liver tissue models: (A) Schematic diagram showing the inkjet-based printing setup (left) and bright field image showing the printed construct stained in blue (right). (Reprinted from: [196]) (B) Schematic diagram showing the process of building the construct from spheroids, with the top and side views of the construct on the top right. Plots showing expression (middle) and activity (bottom) of CYP3A4 over time (Reprinted from: [198]) (C) Schematic diagram showing the DLP-based bioprinting system, with the fluorescence and bright field images of 3D printed liver construct on the top right. Bar charts showing CYP enzyme induction on lower right. Scale bars are 500 μm. (Reprinted from:[126]) (D) Schematic diagram showing the direct 3D printing within microfluidic chip, with images showing the microfluidic setup and printed structure on lower left. Bar chart showing drug toxicity study on bottom right (Reprinted from:[199])
The applications of 3D printing technology to build in vitro liver models as shown in the above examples have demonstrated great benefits in providing long term culture with well-maintained liver-specific functions and drug metabolism potential. Nevertheless, maintaining functional liver cell functions for longer than thirty days and achieving drug response profiles comparable to native liver still remain as great challenges in the field.
3.1. Heart and muscle models
Cardiovascular diseases are the foremost cause of death in the United States [200]. Roughly one billion U.S. dollars are spent researching and developing a new drug, only to fail clinical trials at rates as high as 80% for cardiovascular drugs [201]. Furthermore, some drugs may disproportionately benefit or harm certain genotypes, ethnicities, sexes, and ages, while clinical trials are not necessarily representative of possible users of the drug [202]. Cardiotoxicity is often evaluated in cell cultures missing the native 3D extracellular microenvironment, inducing non-physiological alignment and therefore compromising intercellular communication, profoundly confounding results. Consequently, cardiotoxicity is the primary reason for the retraction of pharmaceuticals from the market [200]. Hence there is significant need for predictive preclinical models, which are currently reliant on animal models lacking translational relevance to humans, as well as a biomimetic platform for personalized drug screening.
As most users of pharmaceuticals are adult humans, primary human adult cardiomyocytes are an ideal cell source, however, being terminally differentiated they cannot expand in culture and acquiring more of these cells would necessitate the routine collection of biopsies of heart tissue from patients. Alternative cell sources of interest are human ESC-derived cardiomyocytes and human iPSC-derived cardiomyocytes (iPSC-CMs) [203]. With the potential to represent individual differences, human iPSC-CMs are more preferred in studying disease mechanisms and for screening potential therapeutic drugs. Biomaterials are widely used as the scaffold for developing cardiac tissues, and so must mimic biochemical and mechanical properties of the native extracellular matrix. Commonly employed natural biomaterials for cardiac tissue engineering include extracellular matrix proteins such as fibrin [204], collagen [205], gelatin [206], and decellularized cardiac matrix [160,207]. Synthetic options include polyacrylamide hydrogels, which allow for independent control of both mechanical and biochemical properties [208]. These versatile hydrogels have elastic moduli that can be tuned to mimic the elasticity of both healthy [209] and infarcted myocardium [210] (10–15 kPa and >50 kPa, respectively) and can attach to the user’s choice of extracellular matrix proteins [211].
Most work toward developing these disease modeling and drug discovery/screening platforms has focused on recreating micro-tissues of the left ventricular myocardium, the site of most cardiac pathologies and the primary pumping chamber of the heart. These micro-tissues are generally created by seeding cells atop or encapsulating cells within a scaffold that mimics the extracellular matrix by supporting and directing tissue growth [212]. Cardiomyocytes in heart interact with microscale features within a 3D multilayered construct. 3D bioprinting is thus a promising fabrication tool as it offers unprecedented control of 3D architecture, able to create arbitrarily complex geometries in fine detail with high precision and accuracy, and is superior to traditional methods of creating 3D scaffolds (e.g. electrospinning, freeze-drying, gas-foaming, and particle or porogen leaching) which only allow for control of bulk properties [1,10]. Precisely patterned microtopological and biochemical cues can promote the growth of confluent, aligned, as well as structurally and electrically anisotropic tissue to match the structure and function of native myocardium [213,214]. 3D bioprinting also allows for the direct patterning of cells [1], avoiding the inevitable and potentially confounding effect of cell aggregation due to relatively uncontrolled cell distribution in the traditional approach of seeding cells into a prefabricated scaffold. Furthermore, 3D printing can accommodate an ever-growing library of biomaterials with tunable mechanical and biochemical properties and allows for significantly simplified fabrication, rapid iteration, and increased dimensionality compared to traditional patterning techniques such as microcontact printing/micromolding.
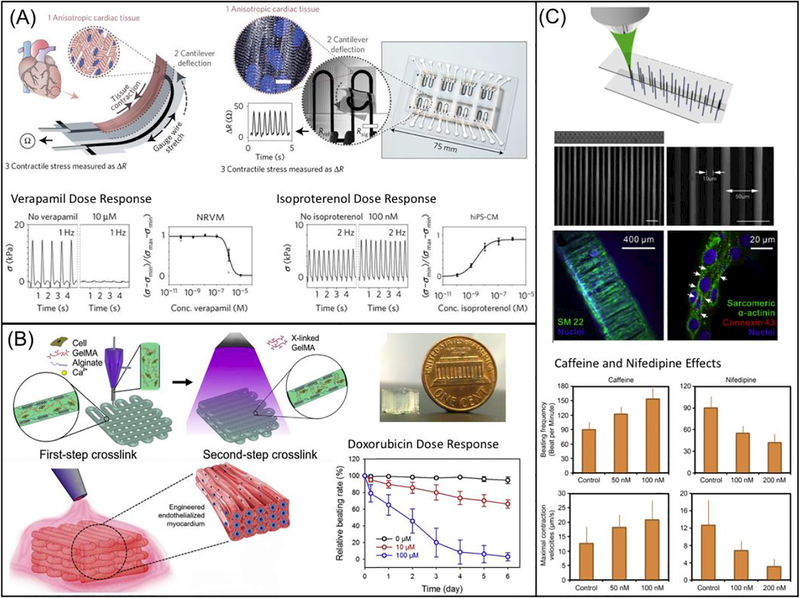
Lind et al. designed microarchitectures that guide the self-assembly of laminar rat-derived cardiac tissues, embedding noninvasive contractile stress sensors (Fig. 3A) [215]. The system was fully fabricated via direct ink writing multimaterial 3D printing of six functional bioinks based on highly conductance, piezoresistive, and biocompatible soft materials. This microphysiological device acquires data that is delivered electronically as opposed to optically, reducing labor, eliminating the need for dedicated microscopy setups, and allowing measurements to be taken in an incubator. The engineered micro-tissue exhibited inotropic responses to L-type calcium channel blocker, verapamil, and β-adrenergic agonist isoproterenol, comparable to data from engineered 3D neonatal rat ventricular myocardial tissues and isolated postnatal whole rat hearts, demonstrating the model’s potential as a drug screening platform [215]. Zhang et al. also reported the use of a composite alginate/GelMA bioink mixed with endothelial cells to directly print a hydrogel scaffold through a combination of extrusion and photocuring process (Fig. 3B) [216]. The endothelial cells gradually migrated toward the microfiber peripheries, forming a controlled anisotropic, confluent layer of endothelium, which was then seeded with rat-derived cardiomyocytes [216]. An aligned, spontaneously and synchronously contracting tissue was thus generated and embedded in a microfluidic perfusion bioreactor to create a myocardium-on-a-chip for evaluating cardiotoxicity. The endothelialized micro-tissue demonstrated dose-dependent reduction in beating rate to the common anti-cancer drug doxorubicin comparable to prior studies [216]. A similar fabrication method and cardiotoxicity test was performed on endothelialized human iPSC-derived micro-tissue, with results that corresponded well to those observed in the rat-derived micro-tissue, suggesting translational potential for personalized drug screening [216].
Fig. 3.
3D bioprinting of cardiac tissue models: (A) Schematic diagram showing the design of a multimaterial, patterned, piezoresistive stress sensor that aligns cardiomyocytes in a thick tissue and sense cardiac force output by changes in resistivity during contraction. Scale bar are 10 μm. Plots showing verapamil and isoproterenol dose response are on the bottom. (Reprinted from: [215]) (B) Schematic diagram showing the extrusion-based 3D printing system that generate multimaterial prints of cardiomyocytes and endothelial cells in naturally-based alginate and GelMA scaffold. The image of printed construct is shown on the top right and the doxorubicin dose response is shown on the lower right. (Reprinted from: [216]) (C) Schematic diagram showing TPP-based printing of micron-scale filaments, which was seeded with healthy and Long-QT iPSC-CMs. Fluorescence images in the middle showing the construct in bulk and cell alignment in various conditions. Bar charts on the bottom showing effects of caffeine and nifedipine on beating frequency and maximal contraction (Reprinted from: [217]).
TPP has also been utilized to fabricate filamentous scaffolds of synthetic [217] and natural polymers with micron-scale resolution [143]. Zhen et. al exposed a photoresist and produced 5 and 10 μm diameter filaments (Fig. 3C). When iPSC-CMs were seeded onto the scaffold they aligned along the vertical filaments and their contraction velocity was analyzed. Both healthy and diseased (long QT syndrome) cardiomyocytes were observed, and their dose responses to various drugs including caffeine, nifedipine (calcium channel blocker), E4031 (potassium channel blocker), and propranolol (beta-blocker) were recorded [217]. Gao et al. also used TTP to produce 15 μm wide times 100 μm tall lines of GelMA hydrogels. The direct write was repeated producing a layer-by-layer scaffold. iPSC-CMs were seeded with endothelial and smooth muscle cells at a 2:1:1 ratio on fibronectin and collagen supported by the polymerized scaffold and were analyzed for calcium transients and conduction velocity across the entire scaffold. The samples were implanted on a myocardial infarction mouse model, with 11% cell engraftment and significantly improved ejection fraction and fractional shortening at 4 weeks, demonstrating the potential of 3D-printed scaffolds to recover cardiac function [143].
The platforms that have been developed so far typically only involved one cell type. Integrating other cell types and structures remains a challenge but would further improve the physiological relevance of these models. Another challenge that remains is corresponding data generated by a microscale, organ-on-a-chip platform to a human-scale response. As 3D printing technology advances in terms of resolution, speed, flexibility, and scalability, so does our ability to control tissue architecture and print cardiomyocytes with high viability.
3.2. Vascularized tissue models
One of the main roadblocks to engineering functional tissue models is the lack of functional vasculature, which plays a key role in transporting nutrients and oxygen to the cells along with removing waste from the cells in the engineered tissues [10,218,219]. Without proximity (100–200 μm) to the vascular network, living tissues can become necrotic and lose their function in a very short time [220,221]. To address this challenge, multiple approaches have been advanced to induce effective vascularization in engineered tissue constructs, such as the incorporation of pro-angiogenic growth factors or endothelial cells [222–227], endothelial cell-laden hydrogel fiber assembly [228], PDMS molding for spatially defined endothelial cords [220], and 3D stamping for complex branched vessel structures [229]. While different degrees of vascularization have been achieved, there remain some major limitations to these approaches. For example, the induction of angiogenesis with pro-angiogenic growth factors is a slow process, which cannot provide functional vascular network immediately after grafting [230,231]. The PDMS molding method is limited to simple geometric endothelial cords which cannot be perfused in vitro [220]. The 3D stamping method can provide perfusable branched vessel structures with micro/nano pores to facilitate the diffusion of nutrients and oxygen, however, the entire process consists of multiple molding and aligning processes, which can be very labor intensive [229]. With the proven flexibility and capability to create highly complex biological constructs from various biomaterials, 3D bioprinting stands out as one of the most promising solutions to incorporate perfusable and functional vasculature network in the engineered tissues [1,16,17,168,221,232].
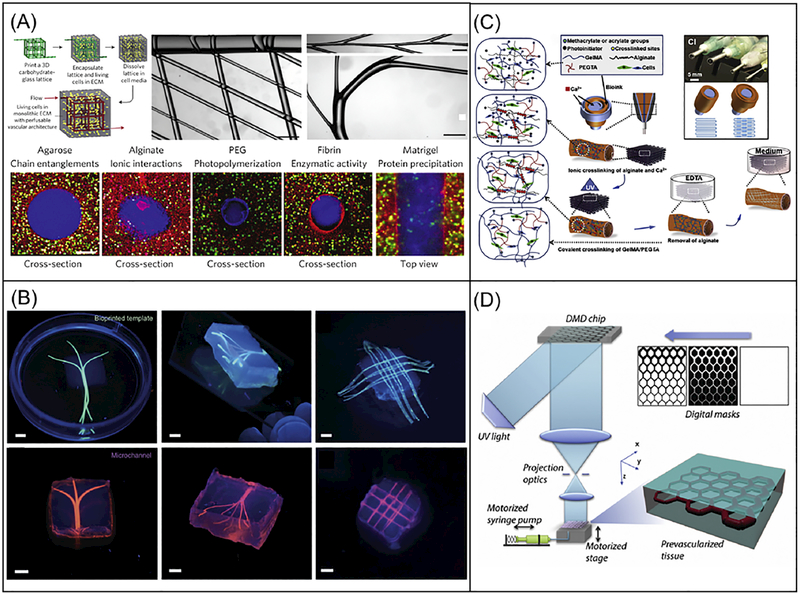
To create the perfusable hollow vascular channels, the most common strategy is to 3D print solid interconnected networks with sacrificial materials followed by the cast molding of a second material [219,221,232]. After the sacrificial template is removed, an interconnected hollow vessel structure is left for endothelialization and perfusion of blood or cell culture media. Extrusion-based bioprinters have emerged as the most popular tool to perform this sacrificial bioprinting strategy. Various materials have been developed to serve as the sacrificial ink used by extrusion-based bioprinters. Miller and colleagues used carbohydrate glass to print a rigid 3D filament network and used it as the template to cast mold a variety of cell-laden ECMs, which can be crosslinked as a bulk to encapsulate the carbohydrate filament network [219]. The carbohydrate filament network was then dissolved, leaving a perfusable vascular architecture (Fig. 4A). The extrusion-based bioprinter used in this work exhibited the capability to print multiscale structures with various designs and feature sizes (Fig. 4A). It was also demonstrated that this vascular network can be readily perfused and lined with endothelial cells to support the viability and function of the hepatocytes encapsulated around it (Fig. 4A). Similarly, Kolesky et al. used Pluronic F127 as the sacrificial ink and demonstrated the 3D printing of vascularized, heterogeneous cell-laden tissues with a multi-nozzle extrusion-based bioprinter [232]. It was further demonstrated that this same method can be used to print thick vascularized tissues (> 1cm in thickness), which can be perfused in vitro for long time periods [168]. To further explore the use of naturally-derived hydrogel materials for improved biocompatibility, Bertassoni and colleagues employed alginate as the sacrificial ink, which can be physically pulled out of the cast molded tissue construct and provide the microchannel networks for perfusion (Fig. 4B) [221].
Fig. 4.
3D bioprinting of vascularized tissue models. (A) Schematic of a sacrificial bioprinting method using an extrusion-based bioprinter and carbohydrate glass as the sacrificial template, with images showing multiscale structures on top middle and right. Scale bars are 1mm. Bottom cross-sectional fluorescence images showing lumen structures from a variety of cell-laden ECM materials. Scale bars are 200 μm. (Reprinted from: [219]). (B) Fluorescence images showing the bioprinted alginate templates (green) enclosed in GelMA hydrogels and the respective microchannels perfused with a fluorescent microbead suspension (pink) after removal of the alginate templates. Scale bars are 3mm. (Reprinted from: [221]). (C) Schematic of the direct vasculature printing with a multilayered coaxial extrusion-based bioprinter and a blend bioink with two independent crosslinking mechanisms. (Reprinted from: [17]). (D) Schematic of the DLP-based bioprinting system for the rapid printing of prevascularized 3D tissues with direct encapsulation of endothelial and supportive cells in a continuous fashion. (Reprinted from: [127]).
The sacrificial bioprinting method usually involves multiple steps that can be very time-consuming: 1) printing the sacrificial template, 2) cast molding cell-laden ECMs to encapsulate the sacrificial template, 3) dissolving the sacrificial template to provide hollow interconnected vascular network, and 4) perfusing endothelial cells to line the hollow vascular network. To improve the efficiency of the biofabrication process, efforts have been made to print functional vascularized tissues directly with endothelial cells in one single step. Jia and colleagues developed a multilayered coaxial extrusion-based bioprinter and a blend bioink with two independent crosslinking mechanisms [17]. As shown in Fig. 4C, the blend bioink, consisting of GelMA, sodium alginate, 4-arm poly(ethylene glycol)-tetra-acrylate (PEGTA), and endothelial cells is delivered through the sheath layer of the coaxial nozzle and ionically crosslinked by the Ca2+ delivered through both the core channel of the coaxial nozzle and the ambient spray of CaCl2 solution, thus providing temporary structural support. After the bioprinting process, the photopolymerizable GelMA and PEGTA were covalently crosslinked by UV, providing permanent fixation of the microchannel structures. The alginate component can then be dissolved for improved cell spreading and proliferation in the printed tissue construct. While this work demonstrated the direct printing with endothelial cells into the wall of the vessels, it still involves the dissolving process of the supportive alginate component. Also, the serial writing process of the extrusion-based bioprinting has limited fabrication speed and compromises the mechanical integrity of the printed scaffolds due to the interfaces between the extruded lines. To address these challenges, Zhu et al. employed a DLP-based bioprinter for the rapid printing of prevascularized 3D tissues with the direct encapsulation of endothelial and supportive cells (Fig. 4D) [127]. With this system, heterogeneous 3D tissues were printed with precisely controlled material and cell distributions in one single step without sacrificial material dissolving or cell perfusion. The regionally controlled biomaterial interfaces induced the endothelial cells to form lumen like structures in vitro, which also survived and anastomosed with the host circulation in vivo. Moreover, the prevascularized tissue constructs were printed in a continuous fashion which offers better mechanical integrity.
As shown in the aforementioned examples, 3D bioprinting has proven to be a valuable tool to address the challenges in vascular tissue engineering with the versatility and flexibility to fabricate various designs with a wide range of biomaterials as well as the precision to control microscale features. Nonetheless, much work must be done to engineer a fully functional vascularized tissue in vitro that can mimic the native blood vessels. For example, most of the current work is limited in printing tissues at millimeter or centimeter scales [168]. Printing organ-scale vasculature network remains a big challenge, possibly due to the lack of a material and a biofabrication platform that can provide a mechanically strong vascular structure to support such a large tissue and in the meantime retain the biocompatibility to support cell viability and function [168,229]. Such mechanical strength is also desired for grafting the tissue in vivo and connecting with the host circulation. Also, methods to arrange the endothelial cells and other supportive cells like the native blood vessel structure and induce the formation of capillary network to the desired tissue regions for optimal material exchange between the circulation and parenchymal cells remain to be an important task for the tissue engineering field [219,233]. To address these challenges, multidisciplinary collaborations involving bioengineers, materials scientists, and biologists are greatly needed to advance the current 3D bioprinting strategies and combine it with novel biomaterials to achieve fully functional vasculatures.
3.3. Cancer models
Conventional two-dimensional cancer models cultured in vitro have provided many essential insights into cancer and led to some key therapeutic successes [234]. However, the monolayer models cannot replicate the native features of 3D tumor tissues [235]. For example, tumor-stroma interactions have been increasingly recognized as one factor that influences the treatment responses of tumors to various drugs. These impacts on tumor drug response include stroma-induced drug resistance and stroma-induced synthetic lethality [236]. Drug development for cancer has been stagnant for decades, with over 95% drugs failed during clinical trials [237], indicating the urgent need of predictive preclinical models. The advancement in 3D bioprinting techniques has given rise to a few in vitro cancer models that better replicate the tumor microenvironment (TME), which are critical to tumor proliferation, metastasis, and responses to drugs. The following section focuses on several aspects of tumor progression, including cancer cell migration, proliferation and functionality, tumor-stroma interactions, and the 3D printed models built to study these behaviors.
TME is highly complex and heterogeneous, and its features, including mechanical stimulation, biochemical gradients, geometric cues, tissue architectures, and cell-cell/matrix interactions [238], affect the metastatic events through numerous interactions with the cancer cells. Metastatic progression has been verified to have led to 90% of death from cancer [239], as well as known to be linked with a significant decrease in 5-year survival rates – an important indicator of cancer prognosis. Thus, one focus of 3D bioprinting has been cancer metastasis to elucidate the highly varied mechanisms of cancer metastasis. Huang and colleagues utilized DLP-based bioprinting to generate biomimetic chips with incorporated vasculatures to study effects of geometric cues on migration speed of tumor cells (HeLa cell) and normal cells (10T1/2) [240]. PEGDA was used to construct the structure because of its tunable mechanical properties and biocompatibility. The embedded vasculatures possessed three different chamber width (25, 45, and 120 μm) to mimic blood vessels of different sizes in vivo, as shown in (Fig. 5A). The results demonstrated that HeLa cells migrated at increased speeds in narrower channels, while the fibroblast migration speed was not affected by the channel widths. This work introduced a method to model different responses of cancerous cells and noncancerous cells to different geometric cues, which could potentially be used as a tool to screen anti-migratory molecules.
Fig. 5.
(A). Schematic diagram of a DLP-based system that uses a programmable DMD to selectively illuminate UV light onto photosensitive monomer solution. Bright-field images on the right showing HeLa cells-seeded PEGDA scaffolds with channels of 25-μm width (top), 45-μm width (middle), and 120-μm width (bottom). Scale bars are 100 μm. (Reprinted from: [240]). (B). Schematic process of an extrusion-based printing of gelatin/alginate/fibrinogen constructs with Hela cells to model cervical tumor. Bright field images on the bottom showing 3D printed Hela cell constructs on day 0, day 5 and day 8. Scale bar are 5 mm. (Reprinted from: [241]). (C). Schematic of an ejection printing platform composed of an automated stage and two nanoliter ejectors to dispense cancer cells (OVCAR-5) and fibroblasts (MRC-5). Bright-field image on the bottom showing 3D printed constructs with OVCAR-5 and MRC-5 cells. (Reprinted from: [149]).
TME features affect not only the migration events, but also the cancer cell proliferation and tumor characteristics. One group reported constructing a 10 × 10 × 2 mm3 grid cervical tumor model with Hela cells in a hydrogel mixture of gelatin, alginate, and fibrinogen (Fig. 5B) [241]. The construct was fabricated by extrusion-printing, utilizing different methods to crosslink the hydrogel components, as shown in Fig. 5B. By better replicating the heterogeneity and mimicking the native microenvironment, the 3D printed tumor model exhibited higher proliferation rate and higher simulated tumor characteristics including matrix metalloproteinase protein (MMP) expression and chemoresistance against the anticancer treatment paclitaxel than the 2D control model. More biomimetic cell-cell interactions and cell-matrix interactions within the 3D models may be the origin of the differences in cell behavior and functionalities.
To study tumor-stroma interactions, Xu and colleagues used droplet printing technique to pattern human ovarian cancer cells and fibroblasts onto a Matrigel substrate (Fig. 5C) [149]. The printing system consisted of micron-resolution XYZ stages and nanoscale dispensing valves, and 150 μm diameter nozzles were used to eject droplets. The printed OVCAR-5 with co-culture of the fibroblasts proliferated and formed 3D acinar structures that resembled the ovarian cancer micronodules (Fig. 5C). The results demonstrated that patterning cancer cells with normal stromal cells could enable generation of more physiologically relevant tumor models for better understanding of the cancer mechanisms. 3D-printing technology could also be employed to built special tumor models for evaluating novel formulations in vivo. For instance, Yang et al. used 3D printing technology to build a subcutaneous glioblastoma xenograft that mimics the resection tumor cavity [242]. This tumor model was then used to evaluate the efficiency of a customized drug-releasable implant in preventing glioblastoma recurrence after surgery [242]
Current 3D printed cancer models are still limited in the types of cells and the design of the model to truly represent TME in vitro. Future work on using patient specific cells and primary cancer cells from patients will provide more insights on the patient-specific and disease stage-specific cell behavior and cell-cell interactions. Further work is needed to develop biomaterials and printing approaches to build scaffolds that can mimic the dynamic biochemical and mechanical environments.
4. Challenges and future outlook
3D bioprinting technology presents the capability of precisely positioning biomaterials and living cells to reconstruct complex structures that can be used for disease modeling and drug screening. In each of the fields of liver, heart, vascular structure and cancer, researchers have used this technology to build tissue models with organ-specific functions, drug testing applications, and transplantation potentials. Despite the recent achievements in this field, challenges still remain on the printing platform, cells, and materials used to build tissue models with difficulties to fully recapitulates the cellular organization and structural complexity comparable to native tissues.
Technological challenges with regard to 3D printing platforms include the need for increased resolution, printing speed, biocompatibility and scaling-up. Currently only light-assisted bioprinters can achieve microscale resolution, which also depends on the type of material used and the cell concentration in the printing mixture. Higher printing resolution is still in great demand to produce complex single cell structures like capillary networks and blastocyst cavity. Higher printing speed also remains an essential challenge for printing organ level structure. The viability of cells in printing solution decreases as printing time increases, particularly for metabolically active cell types like liver and muscle cells. The biocompatibility of 3D printing platforms has been reported to be satisfactory in the aspects of cell viability, but the impacts on the gene expression and functional aspects are largely understudied. Depending on the type of bioprinter used, there are various mechanical and optical disturbances to cells involved. Further studies on the mechanical and optical impacts from the bioprinting process will provide more insights into the biocompatibility of 3D printing process. Lastly, there are still challenges to the scale up of bioprinted tissue constructs. Current reported applications were largely based on a small sample sizes. In order to consistently generate large amount of tissue models for clinical and commercial applications, future work is needed to standardize the printers, cells, materials as well as the printing process.
There are also great limitations on the window of materials used for 3D bioprinting. Due to the requirements on biomaterials to possess specific qualities, the common types of materials used for 3D bioprinting are reduced to only a few [243]. Efforts have been made to develop multimaterial bioinks for extrusion-based printing approaches [243]. More recently, decellularized ECM has also been studied to create printable biomaterials for extrusion-based and light-assisted bioprinting platforms [167]. Unlike highly purified forms of ECM components commonly used, such as gelatin and collagen bioinks, dECM bioinks containing the heterogeneous constituents of the native ECM provides an avenue for researchers to fabricate tissue-specific cell-laden constructs with tissue-matched microenvironments. This approach becomes more important as we strive to develop 3D printed biomimetic tissues since the native ECM plays a critical role in modulating biological activities including cell proliferation, maturation, migration, and differentiation [169]. Together, these efforts will lead to the eventual goal of developing 3D-printable and cell-compatible materials with tunability on the mechanical, chemical and biological properties to recapitulate the protein composition as well as the native tissue environment of the specific patient at the targeted health stage.
To apply 3D printed tissue models to personalized drug screening and disease modeling, patient specific cell sources, including human iPSC-derived cells and primary diseased cells from patients, will be the main focus. Current studies already demonstrated the use of iPSC-derived cells to build various tissue types [130,196]. However, the maturation of differentiated cells to reach the functional level of adult cells still remains a huge challenge in the field. Primary cells directly harvested from patients are very scarce and not widely applied in current work. Research to advance human iPSC differentiation consistency and maturation protocol is widely demanded. Future applications of using primary diseased cells from patients to build co-culture or tri-culture platforms will also provide more insights on the development of personalized disease modeling.
While choosing the appropriate combination of bioprinting technology, materials and cells is essential in developing complex tissues, establishing the physiologically relevant microenvironment with appropriate physical, chemical and biological features remains as the ultimate goal [1]. The review of the four specific application areas outlined the efforts of researchers to create such microenvironment for the corresponding tissue types [126,199,216,217]. In particular, physical features including ECM alignment [215–217], tissue microarchitecture [126,240], and ECM stiffness [126,215] have been considered and mimicked by various groups working on bioprinted cardiac, liver and vascularized tissues. Chemical features including complex ECM components [160,241] and growth factors [244] have also been incorporated across various tissue and cancer systems. Integrating biological features such as cellular composition [126,216], cytokine interactions from co-culture systems [149] and vasculature [126,127] are also widely adopted. Despite the current attempts to achieve one or a few features of the tissue type, great challenges remain in simultaneously incorporating all physiologically relevant features to recapitulate a particular microenvironment. Future advancements in printing technology, material development and cell sourcing will facilitate this process of establishing physiologically relevant physical, chemical and biological features in a particular microenvironment.
Bioprinting technology provides the possibility to develop in vitro tissue models with physiological relevant cell composition, material properties, complex micro-structures and proper vascularization, but this is only the front end of the development. Further innovations on post-printing culture platforms such as bioreactors and the incorporation of microfluidic devices will be needed to assist functional maturation and maintenance especially for large vascularized tissue constructs. Along with these developments, technological advancement in imaging systems and analyzing tools will also be in high demand to analyze large tissue constructs. By applying these dynamic systems, the eventual goal of generating a human-on-a-chip can be realized to create a fully integrated platform studying the interdependent effects of multiple miniaturized organs [245]. As such, each organ system can be connected via microfluidics networks and used to revolutionize future drug testing by incorporating biosensors to monitor real-time downstream effects on metabolism, pH, and blood flow on various organs in parallel [245]. Overall, advancements in both research and technology in the fields of medicine, engineering, and biology will be needed to solve these challenges to fully realize the potential of 3D bioprinting in developing sophisticated in vitro disease models and precision medicine.
5. Acknowledgements
The work was supported in part by grants from the California Institute for Regenerative Medicine (RT3-07899), National Institutes of Health (R01EB021857, R21HD090662) and National Science Foundation (CMMI- 1547005 and CMMI-1644967).
Abbreviations
- CAD
computer-aided design
- CT
computed tomography
- dECM
decellularized extracellular matrix
- DLP
digital light processing
- DMD
digital micro-mirror device
- DOPsL
dynamic optical projection stereolithography
- ECM
native extracellular matrix
- ESC
embryonic stem cells
- GelMA
gelatin methacrylate
- GMHA
glycidyl methacrylate hyaluronic acid
- HA
hyaluronic acid
- HLCs
hepatocyte like cells
- iPSC
induced pluripotent stem cell
- iPSC-CMs
iPSC-derived cardiomyocytes
- MMP
matrix metalloproteinase protein
- MRI
magnetic resonance imaging
- MSC
mesenchymal stem cells
- PCL
poly(caprolactone)
- PDMS
polydimethylsiloxane
- PEG
poly(ethylene glycol)
- PEGDA
poly(ethylene glycol) diacrylate
- PEGTA
poly(ethylene glycol)-tetra-acrylate
- PLA
poly(lactic acid)
- PLGA
poly(lactic-glycolic) acid
- PVA
polyvinyl alcohol
- TME
tumor microenvironment
- TPP
two-photon polymerization
- 3D
Three dimensional
- μCOP
microscale continuous printing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
All authors declare no competing interests.
References
- [1].Murphy SV, Atala A, 3D bioprinting of tissues and organs., Nat. Biotechnol 32 (2014) 773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- [2].Stanton MM, Samitier J, Sánchez S, Bioprinting of 3D hydrogels, Lab Chip. 15 (2015) 3111–3115. doi: 10.1039/C5LC90069G. [DOI] [PubMed] [Google Scholar]
- [3].Irvine S, Venkatraman S, Bioprinting and differentiation of stem cells, Molecules. 21 (2016) 1188. doi: 10.3390/molecules21091188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Collins SF, Bioprinting is changing regenerative medicine forever, Stem Cells Dev 23 (2014) 79–82. doi: 10.1089/scd.2014.0322. [DOI] [PubMed] [Google Scholar]
- [5].Giannopoulos AA, Mitsouras D, Yoo S-J, Liu PP, Chatzizisis YS, Rybicki FJ, Applications of 3D printing in cardiovascular diseases, Nat. Rev. Cardiol 13 (2016) 701–718. doi: 10.1038/nrcardio.2016.170. [DOI] [PubMed] [Google Scholar]
- [6].Jammalamadaka U, Tappa K, Recent advances in biomaterials for 3D printing and tissue engineering, J. Funct. Biomater 9 (2018) 22. doi: 10.3390/jfb9010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Skardal A, Atala A, Biomaterials for integration with 3-D bioprinting, Ann. Biomed. Eng 43 (2015) 730–746. doi: 10.1007/s10439-014-1207-1. [DOI] [PubMed] [Google Scholar]
- [8].Markstedt K, Mantas A, Tournier I, Martínez Ávila H, Hägg D, Gatenholm P, 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications, Biomacromolecules. 16 (2015) 1489–1496. doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- [9].Cui H, Zhu W, Holmes B, Zhang LG, Biologically inspired smart release system based on 3D bioprinted perfused scaffold for vascularized tissue regeneration, Adv. Sci 3 (2016) 1600058. doi: 10.1002/advs.201600058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhu W, Ma X, Gou M, Mei D, Zhang K, Chen S, 3D printing of functional biomaterials for tissue engineering, Curr. Opin. Biotechnol 40 (2016) 103–112. doi: 10.1016/j.copbio.2016.03.014. [DOI] [PubMed] [Google Scholar]
- [11].Lee JM, Yeong WY, Design and printing strategies in 3D bioprinting of cell-hydrogels: A review, Adv. Healthc. Mater 5 (2016) 2856–2865. doi: 10.1002/adhm.201600435. [DOI] [PubMed] [Google Scholar]
- [12].Huang J, Fu H, Li C, Dai J, Zhang Z, Recent advances in cell-laden 3D bioprinting: materials, technologies and applications, J. 3D Print. Med 1 (2017) 245–268. doi: 10.2217/3dp-2017-0010. [DOI] [Google Scholar]
- [13].Ji S, Guvendiren M, Recent advances in bioink design for 3D bioprinting of tissues and organs., Front. Bioeng. Biotechnol 5 (2017) 23. doi: 10.3389/fbioe.2017.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Leberfinger AN, Ravnic DJ, Dhawan A, Ozbolat IT, Concise review: Bioprinting of stem cells for transplantable tissue fabrication, Stem Cells Transl. Med 6 (2017) 1940–1948. doi: 10.1002/sctm.17-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Peng W, Datta P, Ayan B, Ozbolat V, Sosnoski D, Ozbolat IT, 3D bioprinting for drug discovery and development in pharmaceutics, Acta Biomater 57 (2017) 26–46. doi: 10.1016/j.actbio.2017.05.025. [DOI] [PubMed] [Google Scholar]
- [16].Gao Q, He Y, Fu J, Liu A, Ma L, Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery, Biomaterials. 61 (2015) 203–215. doi: 10.1016/j.biomaterials.2015.05.031. [DOI] [PubMed] [Google Scholar]
- [17].Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, Pi Q, Byambaa B, Dokmeci MR, Shin SR, Khademhosseini A, Direct 3D bioprinting of perfusable vascular constructs using a blend bioink, Biomaterials. 106 (2016) 58–68. doi: 10.1016/j.biomaterials.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kang LH, Armstrong PA, Lee LJ, Duan B, Kang KH, Butcher JT, Optimizing photo-encapsulation viability of heart valve cell types in 3D printable composite hydrogels, Ann. Biomed. Eng 45 (2017) 360–377. doi: 10.1007/s10439-016-1619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shanjani Y, Pan CC, Elomaa L, Yang Y, A novel bioprinting method and system for forming hybrid tissue engineering constructs, Biofabrication. 7 (2015) 45008. doi: 10.1088/1758-5090/7/4/045008. [DOI] [PubMed] [Google Scholar]
- [20].Schütz K, Placht A-M, Paul B, Brüggemeier S, Gelinsky M, Lode A, Three-dimensional plotting of a cell-laden alginate/methylcellulose blend: towards biofabrication of tissue engineering constructs with clinically relevant dimensions, J. Tissue Eng. Regen. Med 11 (2017) 1574–1587. doi: 10.1002/term.2058. [DOI] [PubMed] [Google Scholar]
- [21].Wang LL, Highley CB, Yeh Y-C, Galarraga JH, Uman S, Burdick JA, Three-dimensional extrusion bioprinting of single- and double-network hydrogels containing dynamic covalent crosslinks, J. Biomed. Mater. Res. Part A. 106 (2018) 865–875. doi: 10.1002/jbm.a.36323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ouyang L, Yao R, Mao S, Chen X, Na J, Sun W, Three-dimensional bioprinting of embryonic stem cells directs highly uniform embryoid body formation, Biofabrication. 7 (2015) 44101. doi: 10.1088/1758-5090/7/4/044101. [DOI] [PubMed] [Google Scholar]
- [23].Tabriz AG, Hermida MA, Leslie NR, Shu W, Three-dimensional bioprinting of complex cell laden alginate hydrogel structures, Biofabrication. 7 (2015) 45012. doi: 10.1088/1758-5090/7/4/045012. [DOI] [PubMed] [Google Scholar]
- [24].Kim BS, Jang J, Chae S, Gao G, Kong J-S, Ahn M, Cho D-W, Three-dimensional bioprinting of cell-laden constructs with polycaprolactone protective layers for using various thermoplastic polymers, Biofabrication. 8 (2016) 35013. doi: 10.1088/1758-5090/8/3/035013. [DOI] [PubMed] [Google Scholar]
- [25].Bertlein S, Brown G, Lim KS, Jungst T, Boeck T, Blunk T, Tessmar J, Hooper GJ, Woodfield TBF, Groll J, Thiol-ene clickable gelatin: A platform bioink for multiple 3D biofabrication technologies, Adv. Mater 29 (2017) 1703404. doi: 10.1002/adma.201703404. [DOI] [PubMed] [Google Scholar]
- [26].AnilKumar S, Allen SC, Tasnim N, Akter T, Park S, Kumar A, Chattopadhyay M, Ito Y, Suggs LJ, Joddar B, The applicability of furfuryl-gelatin as a novel bioink for tissue engineering applications, J. Biomed. Mater. Res. Part B Appl. Biomater (2018). doi: 10.1002/jbm.b.34123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jin Y, Liu C, Chai W, Compaan A, Huang Y, Self-supporting nanoclay as internal scaffold material for direct printing of soft hydrogel composite structures in air, ACS Appl. Mater. Interfaces. 9 (2017) 17456–17465. doi: 10.1021/acsami.7b03613. [DOI] [PubMed] [Google Scholar]
- [28].Liu W, Zhang YS, Heinrich MA, De Ferrari F, Jang HL, Bakht SM, Alvarez MM, Yang J, Li Y-C, Trujillo-de Santiago G, Miri AK, Zhu K, Khoshakhlagh P, Prakash G, Cheng H, Guan X, Zhong Z, Ju J, Zhu GH, Jin X, Shin SR, Dokmeci MR, Khademhosseini A, Rapid continuous multimaterial extrusion bioprinting., Adv. Mater 29 (2017). doi: 10.1002/adma.201604630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dubbin K, Tabet A, Heilshorn SC, Quantitative criteria to benchmark new and existing bio-inks for cell compatibility, Biofabrication. 9 (2017) 44102. doi: 10.1088/1758-5090/aa869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Paxton N, Smolan W, Böck T, Melchels F, Groll J, Jungst T, Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability, Biofabrication. 9 (2017) 44107. doi: 10.1088/1758-5090/aa8dd8. [DOI] [PubMed] [Google Scholar]
- [31].Shafiee A, McCune M, Forgacs G, Kosztin I, Post-deposition bioink self-assembly: a quantitative study, Biofabrication. 7 (2015) 45005. doi: 10.1088/1758-5090/7/4/045005. [DOI] [PubMed] [Google Scholar]
- [32].Koo Y, Kim G, New strategy for enhancing in situ cell viability of cell-printing process via piezoelectric transducer-assisted three-dimensional printing, Biofabrication. 8 (2016) 25010. doi: 10.1088/1758-5090/8/2/025010. [DOI] [PubMed] [Google Scholar]
- [33].Chimene D, Peak CW, Gentry JL, Carrow JK, Cross LM, Mondragon E, Cardoso GB, Kaunas R, Gaharwar AK, Nanoengineered ionic–covalent entanglement (NICE) bioinks for 3D bioprinting, ACS Appl. Mater. Interfaces. 10 (2018) 9957–9968. doi: 10.1021/acsami.7b19808. [DOI] [PubMed] [Google Scholar]
- [34].Colosi C, Shin SR, Manoharan V, Massa S, Costantini M, Barbetta A, Dokmeci MR, Dentini M, Khademhosseini A, Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink, Adv. Mater 28 (2016) 677–684. doi: 10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Di Giuseppe M, Law N, Webb B, Macrae RA, Liew LJ, Sercombe TB, Dilley RJ, Doyle BJ, Mechanical behaviour of alginate-gelatin hydrogels for 3D bioprinting, J. Mech. Behav. Biomed. Mater 79 (2018) 150–157. doi: 10.1016/j.jmbbm.2017.12.018. [DOI] [PubMed] [Google Scholar]
- [36].Lee H, Yoo JJ, Kang H-W, Cho D-W, Investigation of thermal degradation with extrusion-based dispensing modules for 3D bioprinting technology, Biofabrication. 8 (2016) 15011. doi: 10.1088/1758-5090/8/1/015011. [DOI] [PubMed] [Google Scholar]
- [37].Tan YJ, Tan X, Yeong WY, Tor SB, Hybrid microscaffold-based 3D bioprinting of multi-cellular constructs with high compressive strength: A new biofabrication strategy, Sci. Rep 6 (2016) 39140. doi: 10.1038/srep39140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xu C, Lee W, Dai G, Hong Y, Highly elastic biodegradable single-network hydrogel for cell printing, ACS Appl. Mater. Interfaces. 10 (2018) 9969–9979. doi: 10.1021/acsami.8b01294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Duchi S, Onofrillo C, O’Connell CD, Blanchard R, Augustine C, Quigley AF, Kapsa RMI, Pivonka P, Wallace G, Di Bella C, Choong PFM, Handheld co-axial bioprinting: Application to in situ surgical cartilage repair, Sci. Rep 7 (2017) 5837. doi: 10.1038/s41598-017-05699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jin Y, Compaan A, Bhattacharjee T, Huang Y, Granular gel support-enabled extrusion of three-dimensional alginate and cellular structures, Biofabrication. 8 (2016) 25016. doi: 10.1088/1758-5090/8/2/025016. [DOI] [PubMed] [Google Scholar]
- [41].Gettler BC, Zakhari JS, Gandhi PS, Williams SK, Formation of adipose stromal vascular fraction cell-laden spheroids using a three-dimensional bioprinter and superhydrophobic surfaces, Tissue Eng. Part C. Methods. 23 (2017) 516–524. doi: 10.1089/ten.TEC.2017.0056. [DOI] [PubMed] [Google Scholar]
- [42].Liu W, Heinrich MA, Zhou Y, Akpek A, Hu N, Liu X, Guan X, Zhong Z, Jin X, Khademhosseini A, Zhang YS, Extrusion bioprinting of shear-thinning gelatin methacryloyl bioinks, Adv. Heal. Mater 6 (2017) 1601451. doi: 10.1002/adhm.201601451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rocca M, Fragasso A, Liu W, Heinrich MA, Zhang YS, Embedded multimaterial extrusion bioprinting, SLAS Technol. Transl. Life Sci. Innov 23 (2018) 154–163. doi: 10.1177/2472630317742071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ouyang L, Yao R, Zhao Y, Sun W, Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells, Biofabrication. 8 (2016) 35020. doi: 10.1088/1758-5090/8/3/035020. [DOI] [PubMed] [Google Scholar]
- [45].Kim BS, Lee J-S, Gao G, Cho D-W, Direct 3D cell-printing of human skin with functional transwell system, Biofabrication. 9 (2017) 25034. doi: 10.1088/1758-5090/aa71c8. [DOI] [PubMed] [Google Scholar]
- [46].Sakai S, Ohi H, Hotta T, Kamei H, Taya M, Differentiation potential of human adipose stem cells bioprinted with hyaluronic acid/gelatin-based bioink through microextrusion and visible light-initiated crosslinking, Biopolymers. 109 (2018) e23080. doi: 10.1002/bip.23080. [DOI] [PubMed] [Google Scholar]
- [47].Ersumo N, Witherel CE, Spiller KL, Differences in time-dependent mechanical properties between extruded and molded hydrogels, Biofabrication. 8 (2016) 35012. doi: 10.1088/1758-5090/8/3/035012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].O’Connell CD, Di Bella C, Thompson F, Augustine C, Beirne S, Cornock R, Richards CJ, Chung J, Gambhir S, Yue Z, Bourke J, Zhang B, Taylor A, Quigley A, Kapsa R, Choong P, Wallace GG, Development of the biopen: a handheld device for surgical printing of adipose stem cells at a chondral wound site, Biofabrication. 8 (2016) 15019. doi: 10.1088/1758-5090/8/1/015019. [DOI] [PubMed] [Google Scholar]
- [49].McElheny C, Hayes D, Devireddy R, Design and fabrication of a low-cost three-dimensional bioprinter, J. Med. Device 11 (2017) 41001. doi: 10.1115/1.4037259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Diamantides N, Wang L, Pruiksma T, Siemiatkoski J, Dugopolski C, Shortkroff S, Kennedy S, Bonassar LJ, Correlating rheological properties and printability of collagen bioinks: the effects of riboflavin photocrosslinking and pH, Biofabrication. 9 (2017) 34102. doi: 10.1088/1758-5090/aa780f. [DOI] [PubMed] [Google Scholar]
- [51].Liu W, Zhong Z, Hu N, Zhou Y, Maggio L, Miri AK, Fragasso A, Jin X, Khademhosseini A, Zhang YS, Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments, Biofabrication. 10 (2018) 24102. doi: 10.1088/1758-5090/aa9d44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dai X, Liu L, Ouyang J, Li X, Zhang X, Lan Q, Xu T, Coaxial 3D bioprinting of self-assembled multicellular heterogeneous tumor fibers, Sci. Rep 7 (2017) 1457. doi: 10.1038/s41598-017-01581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Apelgren P, Amoroso M, Lindahl A, Brantsing C, Rotter N, Gatenholm P, Kölby L, Chondrocytes and stem cells in 3D-bioprinted structures create human cartilage in vivo, PLoS One. 12 (2017) e0189428. doi: 10.1371/journal.pone.0189428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ho L, Hsu S, Cell reprogramming by 3D bioprinting of human fibroblasts in polyurethane hydrogel for fabrication of neural-like constructs, Acta Biomater 70 (2018) 57–70. doi: 10.1016/j.actbio.2018.01.044. [DOI] [PubMed] [Google Scholar]
- [55].Mistry P, Aied A, Alexander M, Shakesheff K, Bennett A, Yang J, Bioprinting using mechanically robust core-shell cell-laden hydrogel strands, Macromol. Biosci 17 (2017) 1600472. doi: 10.1002/mabi.201600472. [DOI] [PubMed] [Google Scholar]
- [56].Wu Z, Su X, Xu Y, Kong B, Sun W, Mi S, Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation, Sci. Rep 6 (2016) 24474. doi: 10.1038/srep24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Byambaa B, Annabi N, Yue K, Trujillo-de Santiago G, Alvarez MM, Jia W, Kazemzadeh-Narbat M, Shin SR, Tamayol A, Khademhosseini A, Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue, Adv. Heal. Mater 6 (2017) 1700015. doi: 10.1002/adhm.201700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tijore A, Behr J-M, Irvine SA, Baisane V, Venkatraman S, Bioprinted gelatin hydrogel platform promotes smooth muscle cell contractile phenotype maintenance, Biomed. Microdevices. 20 (2018) 32. doi: 10.1007/s10544-018-0274-8. [DOI] [PubMed] [Google Scholar]
- [59].Shao H, Yang X, He Y, Fu J, Liu L, Ma L, Zhang L, Yang G, Gao C, Gou Z, Bioactive glass-reinforced bioceramic ink writing scaffolds: sintering, microstructure and mechanical behavior, Biofabrication. 7 (2015) 35010. doi: 10.1088/1758-5090/7/3/035010. [DOI] [PubMed] [Google Scholar]
- [60].Bhuthalingam R, Lim PQ, Irvine SA, Venkatraman SS, Automated robotic dispensing technique for surface guidance and bioprinting of cell, J. Vis. Exp (2016). doi: 10.3791/54604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ribeiro A, Blokzijl MM, Levato R, Visser CW, Castilho M, Hennink WE, Vermonden T, Malda J, Assessing bioink shape fidelity to aid material development in 3D bioprinting, Biofabrication. 10 (2017) 14102. doi: 10.1088/1758-5090/aa90e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Müller M, Öztürk E, Arlov Ø, Gatenholm P, Zenobi-Wong M, Alginate sulfate–nanocellulose bioinks for cartilage bioprinting applications, Ann. Biomed. Eng 45 (2017) 210–223. doi: 10.1007/s10439-016-1704-5. [DOI] [PubMed] [Google Scholar]
- [63].Reid JA, Mollica PA, Johnson GD, Ogle RC, Bruno RD, Sachs PC, Accessible bioprinting: adaptation of a low-cost 3D-printer for precise cell placement and stem cell differentiation, Biofabrication. 8 (2016) 25017. doi: 10.1088/1758-5090/8/2/025017. [DOI] [PubMed] [Google Scholar]
- [64].Akkineni AR, Ahlfeld T, Lode A, Gelinsky M, A versatile method for combining different biopolymers in a core/shell fashion by 3D plotting to achieve mechanically robust constructs, Biofabrication. 8 (2016) 45001. doi: 10.1088/1758-5090/8/4/045001. [DOI] [PubMed] [Google Scholar]
- [65].Kesti M, Müller M, Becher J, Schnabelrauch M, D’Este M, Eglin D, Zenobi-Wong M, A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation, Acta Biomater 11 (2015) 162–172. doi: 10.1016/j.actbio.2014.09.033. [DOI] [PubMed] [Google Scholar]
- [66].Skardal A, Devarasetty M, Kang HW, Mead I, Bishop C, Shupe T, Lee SJ, Jackson J, Yoo J, Soker S, Atala A, A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs, Acta Biomater 25 (2015) 24–34. doi: 10.1016/j.actbio.2015.07.030. [DOI] [PubMed] [Google Scholar]
- [67].Cochis A, Bonetti L, Sorrentino R, Contessi Negrini N, Grassi F, Leigheb M, Rimondini L, Farè S, 3D printing of thermo-responsive methylcellulose hydrogels for cell-sheet engineering, Mater 11 (2018) 579. doi: 10.3390/ma11040579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Rodriguez MJ, Dixon TA, Cohen E, Huang W, Omenetto FG, Kaplan DL, 3D freeform printing of silk fibroin, Acta Biomater 71 (2018) 379–387. doi: 10.1016/j.actbio.2018.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yin J, Yan M, Wang Y, Fu J, Suo H, 3D bioprinting of low-concentration cell-laden gelatin methacrylate (GelMA) bioinks with a two-step cross-linking strategy, ACS Appl. Mater. Interfaces 10 (2018) 6849–6857. doi: 10.1021/acsami.7b16059. [DOI] [PubMed] [Google Scholar]
- [70].McBeth C, Lauer J, Ottersbach M, Campbell J, Sharon A, Sauer-Budge AF, 3D bioprinting of GelMA scaffolds triggers mineral deposition by primary human osteoblasts, Biofabrication. 9 (2017) 15009. doi: 10.1088/1758-5090/aa53bd. [DOI] [PubMed] [Google Scholar]
- [71].Gu Q, Tomaskovic-Crook E, Wallace GG, Crook JM, 3D bioprinting human induced pluripotent stem cell constructs for in situ cell proliferation and successive multilineage differentiation, Adv. Heal. Mater 6 (2017) 1700175. doi: 10.1002/adhm.201700175. [DOI] [PubMed] [Google Scholar]
- [72].Di Bella C, Duchi S, O’Connell CD, Blanchard R, Augustine C, Yue Z, Thompson F, Richards C, Beirne S, Onofrillo C, Bauquier SH, Ryan SD, Pivonka P, Wallace GG, Choong PF, In situ handheld three-dimensional bioprinting for cartilage regeneration, J. Tissue Eng. Regen. Med 12 (2018) 611–621. doi: 10.1002/term.2476. [DOI] [PubMed] [Google Scholar]
- [73].Miao S, Zhu W, Castro NJ, Nowicki M, Zhou X, Cui H, Fisher JP, Zhang LG, 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate, Sci. Rep 6 (2016) 27226. doi: 10.1038/srep27226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wang Z, Jin X, Tian Z, Menard F, Holzman JF, Kim K, A novel, well-resolved direct laser bioprinting system for rapid cell encapsulation and microwell fabrication, Adv. Heal. Mater (2018) 1701249. doi: 10.1002/adhm.201701249. [DOI] [PubMed] [Google Scholar]
- [75].Wang Z, Abdulla R, Parker B, Samanipour R, Ghosh S, Kim K, A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks, Biofabrication. 7 (2015) 45009. doi: 10.1088/1758-5090/7/4/045009. [DOI] [PubMed] [Google Scholar]
- [76].Stichler S, Böck T, Paxton N, Bertlein S, Levato R, Schill V, Smolan W, Malda J, Teßmar J, Blunk T, Groll J, Double printing of hyaluronic acid/poly(glycidol) hybrid hydrogels with poly(ε-caprolactone) for MSC chondrogenesis, Biofabrication. 9 (2017) 44108. doi: 10.1088/1758-5090/aa8cb7. [DOI] [PubMed] [Google Scholar]
- [77].Dinh N-D, Luo R, Tankeh M, Christine A, Lin WN, Shih W-C, Goh J. Cho-Hong, Chen C-H, Effective light directed sssembly of building blocks with microscale control, Small. 13 (2017). doi: 10.1002/smll.201700684. [DOI] [PubMed] [Google Scholar]
- [78].Zhang Z, Xu C, Xiong R, Chrisey DB, Huang Y, Effects of living cells on the bioink printability during laser printing, Biomicrofluidics. 11 (2017) 34120. doi: 10.1063/1.4985652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Xiong R, Zhang Z, Chai W, Huang Y, Chrisey DB, Freeform drop-on-demand laser printing of 3D alginate and cellular constructs, Biofabrication. 7 (2015) 45011. doi: 10.1088/1758-5090/7/4/045011. [DOI] [PubMed] [Google Scholar]
- [80].Keriquel V, Oliveira H, Rémy M, Ziane S, Delmond S, Rousseau B, Rey S, Catros S, Amédée J, Guillemot F, Fricain J-C, In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications, Sci. Rep 7 (2017) 1778. doi: 10.1038/s41598-017-01914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Koch L, Deiwick A, Franke A, Schwanke K, Haverich A, Zweigerdt R, Chichkov BN, Laser bioprinting of human induced pluripotent stem cells – the effect of printing and biomaterials on cell survival, pluripotency, and differentiation, Biofabrication. (2018). doi: 10.1088/1758-5090/aab981. [DOI] [PubMed] [Google Scholar]
- [82].Vinson BT, Phamduy TB, Shipman J, Riggs B, Strong AL, Sklare SC, Murfee WL, Burow ME, Bunnell BA, Huang Y, Chrisey DB, Laser direct-write based fabrication of a spatially-defined, biomimetic construct as a potential model for breast cancer cell invasion into adipose tissue, Biofabrication. 9 (2017) 25013. doi: 10.1088/1758-5090/aa6bad. [DOI] [PubMed] [Google Scholar]
- [83].Burks HE, Phamduy TB, Azimi MS, Saksena J, Burow ME, Collins-Burow BM, Chrisey DB, Murfee WL, Laser direct-write onto live tissues: A novel model for studying cancer cell migration, J. Cell. Physiol 231 (2016) 2333–2338. doi: 10.1002/jcp.25363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Morris VB, Nimbalkar S, Younesi M, McClellan P, Akkus O, Mechanical properties, cytocompatibility and manufacturability of chitosan: PEGDA hybrid-gel scaffolds by stereolithography, Ann. Biomed. Eng 45 (2017) 286–296. doi: 10.1007/s10439-016-1643-1. [DOI] [PubMed] [Google Scholar]
- [85].Cadau S, Rival D, Andre-Frei V, Chavan MM, Fayol D, Salducci M, Brisson B, Guillemot F, New bioprinted skin, cosmetic in vitro model, J. Cosmet. Sci 68 (2017) 85–90. [PubMed] [Google Scholar]
- [86].Gi HJ, Han D, Park J-K, Optoelectrofluidic printing system for fabricating hydrogel sheets with on-demand patterned cells and microparticles, Biofabrication. 9 (2017) 15011. doi: 10.1088/1758-5090/aa564c. [DOI] [PubMed] [Google Scholar]
- [87].Bourget J-M, Kérourédan O, Medina M, Rémy M, Thébaud NB, Bareille R, Chassande O, Amédée J, Catros S, Devillard R, Patterning of endothelial cells and mesenchymal stem cells by laser-assisted bioprinting to study cell migration, Biomed Res. Int 2016 (2016) 1–7. doi: 10.1155/2016/3569843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zhang Z, Chai W, Xiong R, Zhou L, Huang Y, Printing-induced cell injury evaluation during laser printing of 3T3 mouse fibroblasts, Biofabrication. 9 (2017) 25038. doi: 10.1088/1758-5090/aa6ed9. [DOI] [PubMed] [Google Scholar]
- [89].Yang W, Yu H, Wei F, Li G, Wang Y, Liu L, Selective pattern of cancer cell accumulation and growth using UV modulating printing of hydrogels, Biomed. Microdevices. 17 (2015) 104. doi: 10.1007/s10544-015-0013-3. [DOI] [PubMed] [Google Scholar]
- [90].Kawecki F, Clafshenkel WP, Auger FA, Bourget J-M, Fradette J, Devillard R, Self-assembled human osseous cell sheets as living biopapers for the laser-assisted bioprinting of human endothelial cells, Biofabrication. (2018). doi: 10.1088/1758-5090/aabd5b. [DOI] [PubMed] [Google Scholar]
- [91].Xiong R, Zhang Z, Chai W, Chrisey DB, Huang Y, Study of gelatin as an effective energy absorbing layer for laser bioprinting, Biofabrication. 9 (2017) 24103. doi: 10.1088/1758-5090/aa74f2. [DOI] [PubMed] [Google Scholar]
- [92].Jang J, Kim TG, Kim BS, Kim S-W, Kwon S-M, Cho D-W, Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking, Acta Biomater 33 (2016) 88–95. doi: 10.1016/j.actbio.2016.01.013. [DOI] [PubMed] [Google Scholar]
- [93].Stichler S, Jungst T, Schamel M, Zilkowski I, Kuhlmann M, Böck T, Blunk T, Teßmar J, Groll J, Thiol-ene clickable poly(glycidol) hydrogels for biofabrication, Ann. Biomed. Eng 45 (2017) 273–285. doi: 10.1007/s10439-016-1633-3. [DOI] [PubMed] [Google Scholar]
- [94].Zhang Z, Xiong R, Mei R, Huang Y, Chrisey DB, Time-resolved imaging study of jetting dynamics during laser printing of viscoelastic alginate solutions, Langmuir. 31 (2015) 6447–6456. doi: 10.1021/acs.langmuir.5b00919. [DOI] [PubMed] [Google Scholar]
- [95].Wang Z, Tian Z, Jin X, Holzman JF, Menard F, Kim K, Visible light-based stereolithography bioprinting of cell-adhesive gelatin hydrogels, in: 2017 39th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., IEEE, 2017: pp. 1599–1602. doi: 10.1109/EMBC.2017.8037144. [DOI] [PubMed] [Google Scholar]
- [96].Sakai S, Kamei H, Mori T, Hotta T, Ohi H, Nakahata M, Taya M, Visible light-induced hydrogelation of an alginate derivative and application to stereolithographic bioprinting using a visible light projector and acid red, Biomacromolecules. 19 (2018) 672–679. doi: 10.1021/acs.biomac.7b01827. [DOI] [PubMed] [Google Scholar]
- [97].Gao G, Schilling AF, Hubbell K, Yonezawa T, Truong D, Hong Y, Dai G, Cui X, Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA, Biotechnol. Lett 37 (2015) 2349–2355. doi: 10.1007/s10529-015-1921-2. [DOI] [PubMed] [Google Scholar]
- [98].Sakai S, Ueda K, Gantumur E, Taya M, Nakamura M, Drop-on-drop multimaterial 3D bioprinting realized by peroxidase-mediated cross-linking, Macromol. Rapid Commun 39 (2018) 1700534. doi: 10.1002/marc.201700534. [DOI] [PubMed] [Google Scholar]
- [99].Benning L, Gutzweiler L, Tröndle K, Riba J, Zengerle R, Koltay P, Zimmermann S, Stark GB, Finkenzeller G, Assessment of hydrogels for bioprinting of endothelial cells, J. Biomed. Mater. Res. Part A. 106 (2018) 935–947. doi: 10.1002/jbm.a.36291. [DOI] [PubMed] [Google Scholar]
- [100].Gao G, Yonezawa T, Hubbell K, Dai G, Cui X, Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging, Biotechnol. J 10 (2015) 1568–1577. doi: 10.1002/biot.201400635. [DOI] [PubMed] [Google Scholar]
- [101].Hart LR, Li S, Sturgess C, Wildman R, Jones JR, Hayes W, 3D printing of biocompatible supramolecular polymers and their composites, ACS Appl. Mater. Interfaces 8 (2016) 3115–3122. doi: 10.1021/acsami.5b10471. [DOI] [PubMed] [Google Scholar]
- [102].Christensen K, Xu C, Chai W, Zhang Z, Fu J, Huang Y, Freeform inkjet printing of cellular structures with bifurcations, Biotechnol. Bioeng 112 (2015) 1047–1055. doi: 10.1002/bit.25501. [DOI] [PubMed] [Google Scholar]
- [103].Samson AAS, Lee J, Song JM, Paper-based inkjet bioprinting to detect fluorescence resonance energy transfer for the assessment of anti-inflammatory activity, Sci. Rep 8 (2018) 591. doi: 10.1038/s41598-017-18995-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Gao G, Hubbell K, Schilling AF, Dai G, Cui X, Bioprinting cartilage tissue from mesenchymal stem cells and PEG hydrogel, in: Methods Mol. Biol, 2017: pp. 391–398. doi: 10.1007/978-1-4939-7021-6_28. [DOI] [PubMed] [Google Scholar]
- [105].Rimann M, Bono E, Annaheim H, Bleisch M, Graf-Hausner U, Standardized 3D bioprinting of soft tissue models with human primary cells, J. Lab. Autom 21 (2016) 496–509. doi: 10.1177/2211068214567146. [DOI] [PubMed] [Google Scholar]
- [106].Tse CCW, Smith PJ, Inkjet printing for biomedical applications, Methods Mol Biol 1771 (2018) 107–117. doi: 10.1007/978-1-4939-7792-5_9. [DOI] [PubMed] [Google Scholar]
- [107].Hendriks J, Willem Visser C, Henke S, Leijten J, Saris DBF, Sun C, Lohse D, Karperien M, Optimizing cell viability in droplet-based cell deposition, Sci. Rep 5 (2015) 11304. doi: 10.1038/srep11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hölzl K, Lin S, Tytgat L, Van Vlierberghe S, Gu L, Ovsianikov A, Bioink properties before, during and after 3D bioprinting, Biofabrication. 8 (2016) 32002. doi: 10.1088/1758-5090/8/3/032002. [DOI] [PubMed] [Google Scholar]
- [109].Pereira RF, Bártolo PJ, 3D bioprinting of photocrosslinkable hydrogel constructs, J. Appl. Polym. Sci 132 (2015) 42458. doi: 10.1002/app.42458. [DOI] [Google Scholar]
- [110].Cui X, Boland T, D’Lima DD, Lotz MK, Thermal inkjet printing in tissue engineering and regenerative medicine., Recent Pat. Drug Deliv. Formul 6 (2012) 149–155. doi: 10.2174/187221112800672949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Li J, Chen M, Fan X, Zhou H, Recent advances in bioprinting techniques: approaches, applications and future prospects, J. Transl. Med 14 (2016) 271. doi: 10.1186/s12967-016-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Andò B, Marletta V, An all-inkjet printed bending actuator with embedded sensing feature and an electromagnetic driving mechanism, Actuators. 5 (2016) 21. doi: 10.3390/act5030021. [DOI] [Google Scholar]
- [113].Kollamaram G, Hopkins SC, Glowacki BA, Croker DM, Walker GM, Inkjet printing of paracetamol and indomethacin using electromagnetic technology: Rheological compatibility and polymorphic selectivity, Eur. J. Pharm. Sci 115 (2018) 248–257. doi: 10.1016/j.ejps.2018.01.036. [DOI] [PubMed] [Google Scholar]
- [114].Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A, A 3D bioprinting system to produce human-scale tissue constructs with structural integrity, Nat. Biotechnol 34 (2016) 312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- [115].Graham AD, Olof SN, Burke MJ, Armstrong JPK, Mikhailova EA, Nicholson JG, Box SJ, Szele FG, Perriman AW, Bayley H, High-resolution patterned cellular constructs by droplet-based 3D printing, Sci. Rep 7 (2017) 7004. doi: 10.1038/s41598-017-06358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ozbolat IT, Hospodiuk M, Current advances and future perspectives in extrusion-based bioprinting, Biomaterials. 76 (2016) 321–343. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- [117].Axpe E, Oyen ML, Applications of alginate-based bioinks in 3D bioprinting, Int. J. Mol. Sci 17 (2016) E1976. doi: 10.3390/ijms17121976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].You F, Eames BF, Chen X, Application of extrusion-based hydrogel bioprinting for cartilage tissue engineering, Int. J. Mol. Sci 18 (2017) 1597. doi: 10.3390/ijms18071597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Hung BP, Naved BA, Nyberg EL, Dias M, Holmes CA, Elisseeff JH, Dorafshar AH, Grayson WL, Three-dimensional printing of bone extracellular matrix for craniofacial regeneration., ACS Biomater. Sci. Eng 2 (2016) 1806–1816. doi: 10.1021/acsbiomaterials.6b00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Tao J, Hu Y, Wang S, Zhang J, Liu X, Gou Z, Cheng H, Liu Q, Zhang Q, You S, Gou M, A 3D-engineered porous conduit for peripheral nerve repair, Sci. Rep 7 (2017) 46038. doi: 10.1038/srep46038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Hu Y, Wu Y, Gou Z, Tao J, Zhang J, Liu Q, Kang T, Jiang S, Huang S, He J, Chen S, Du Y, Gou M, 3D-engineering of cellularized conduits for peripheral nerve regeneration, Sci. Rep 6 (2016) 32184. doi: 10.1038/srep32184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Hribar KC, Soman P, Warner J, Chung P, Chen S, Light-assisted direct-write of 3D functional biomaterials, Lab Chip. 14 (2014) 268–275. doi: 10.1039/C3LC50634G. [DOI] [PubMed] [Google Scholar]
- [123].Gou M, Qu X, Zhu W, Xiang M, Yang J, Zhang K, Wei Y, Chen S, Bio-inspired detoxification using 3D-printed hydrogel nanocomposites., Nat. Commun 5 (2014) 3774. doi: 10.1038/ncomms4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Hribar KC, Finlay D, Ma X, Qu X, Ondeck MG, Chung PH, Zanella F, Engler AJ, Sheikh F, Vuori K, Chen S, Nonlinear 3D projection printing of concave hydrogel microstructures for long-term multicellular spheroid and embryoid body culture, Lab Chip. 15 (2015) 2412–2418. doi: 10.1039/C5LC00159E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Liu J, Hwang HH, Wang P, Whang G, Chen S, Direct 3D-printing of cell-laden constructs in microfluidic architectures., Lab Chip. 16 (2016) 1430–1438. doi: 10.1039/c6lc00144k. [DOI] [PubMed] [Google Scholar]
- [126].Ma X, Qu X, Zhu W, Li Y-S, Yuan S, Zhang H, Liu J, Wang P, Lai CSE, Zanella F, Feng G-S, Sheikh F, Chien S, Chen S, Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting, Proc. Natl. Acad. Sci 113 (2016) 2206–2211. doi: 10.1073/pnas.1524510113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Zhu W, Qu X, Zhu J, Ma X, Patel S, Liu J, Wang P, Lai CSE, Gou M, Xu Y, Zhang K, Chen S, Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture, Biomaterials. 124 (2017) 106–115. doi: 10.1016/j.biomaterials.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Grogan SP, Chung PH, Soman P, Chen P, Lotz MK, Chen S, D’Lima DD, Digital micromirror device projection printing system for meniscus tissue engineering, Acta Biomater 9 (2013) 7218–7226. doi: 10.1016/j.actbio.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Soman P, Tobe BTD, Lee JW, Winquist AAM, Singec I, Vecchio KS, Snyder EY, Chen S, Three-dimensional scaffolding to investigate neuronal derivatives of human embryonic stem cells, Biomed. Microdevices 14 (2012) 829–838. doi: 10.1007/s10544-012-9662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Cha C, Soman P, Zhu W, Nikkhah M, Camci-Unal G, Chen S, Khademhosseini A, Structural reinforcement of cell-laden hydrogels with microfabricated three dimensional scaffolds, Biomater. Sci 2 (2014) 703–709. doi: 10.1039/C3BM60210A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Warner J, Soman P, Zhu W, Tom M, Chen S, Design and 3D printing of hydrogel scaffolds with fractal geometries, ACS Biomater. Sci. Eng 2 (2016) 1763–1770. doi: 10.1021/acsbiomaterials.6b00140. [DOI] [PubMed] [Google Scholar]
- [132].Lee JW, Soman P, Park JH, Chen S, Cho DW, A tubular biomaterial construct exhibiting a negative poisson’s ratio, PLoS One. 11 (2016) 1–14. doi: 10.1371/journal.pone.0155681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Soman P, Fozdar DY, Lee JW, Phadke A, Varghese S, Chen S, A three-dimensional polymer scaffolding material exhibiting a zero Poisson’s ratio, Soft Matter. 8 (2012) 4946. doi: 10.1039/c2sm07354d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Cumpston BH, Ananthavel SP, Barlow S, Dyer DL, Ehrlich JE, Erskine LL, Heikal AA, Kuebler SM, Lee I-YS, McCord-Maughon D, Qin J, Röckel H, Rumi M, Wu X-L, Marder SR, Perry JW, Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication, Nature. 398 (1999) 51–54. doi: 10.1038/17989. [DOI] [Google Scholar]
- [135].Zipfel WR, Williams RM, Webb WW, Nonlinear magic: multiphoton microscopy in the biosciences, Nat. Biotechnol 21 (2003) 1369–1377. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
- [136].Farsari M, Chichkov BN, Materials processing: Two-photon fabrication, Nat. Photonics 3 (2009) 450–452. doi: 10.1038/nphoton.2009.131. [DOI] [Google Scholar]
- [137].Truby RL, Lewis JA, Printing soft matter in three dimensions, Nature. 540 (2016) 371–378. doi: 10.1038/nature21003. [DOI] [PubMed] [Google Scholar]
- [138].Bell A, Kofron M, Nistor V, Multiphoton crosslinking for biocompatible 3D printing of type I collagen, Biofabrication. 7 (2015) 35007. doi: 10.1088/1758-5090/7/3/035007. [DOI] [PubMed] [Google Scholar]
- [139].Engelhardt S, Hoch E, Borchers K, Meyer W, Krüger H, Tovar GEM, Gillner A, Fabrication of 2D protein microstructures and 3D polymer–protein hybrid microstructures by two-photon polymerization, Biofabrication. 3 (2011) 25003. doi: 10.1088/1758-5082/3/2/025003. [DOI] [PubMed] [Google Scholar]
- [140].Su PJ, Tran QA, Fong JJ, Eliceiri KW, Ogle BM, Campagnola PJ, Mesenchymal stem cell interactions with 3D ECM modules fabricated via multiphoton excited photochemistry, Biomacromolecules. 13 (2012) 2917–2925. doi: 10.1021/bm300949k. [DOI] [PubMed] [Google Scholar]
- [141].Wylie RG, Ahsan S, Aizawa Y, Maxwell KL, Morshead CM, Shoichet MS, Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels, Nat. Mater 10 (2011) 799–806. doi: 10.1038/nmat3101. [DOI] [PubMed] [Google Scholar]
- [142].Zhang W, Soman P, Meggs K, Qu X, Chen S, Tuning the poisson’s ratio of biomaterials for investigating cellular response, Adv. Funct. Mater 23 (2013) 3226–3232. doi: 10.1002/adfm.201202666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Gao L, Kupfer ME, Jung JP, Yang L, Zhang P, Da Sie Y, Tran Q, Ajeti V, Freeman BT, Fast VG, Campagnola PJ, Ogle BM, Zhang J, Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold, Circ. Res 120 (2017) 1318–1325. doi: 10.1161/CIRCRESAHA.116.310277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Hastings CL, Roche ET, Ruiz-Hernandez E, Schenke-Layland K, Walsh CJ, Duffy GP, Drug and cell delivery for cardiac regeneration, Adv. Drug Deliv. Rev 84 (2015) 85–106. doi: 10.1016/j.addr.2014.08.006. [DOI] [PubMed] [Google Scholar]
- [145].Steele AN, MacArthur JW, Woo YJ, Stem cell therapy: healing or hype? why stem cell delivery doesn’t work., Circ. Res 120 (2017) 1868–1870. doi: 10.1161/CIRCRESAHA.117.310584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Caddeo S, Boffito M, Sartori S, Tissue engineering approaches in the design of healthy and pathological in vitro tissue models., Front. Bioeng. Biotechnol 5 (2017) 40. doi: 10.3389/fbioe.2017.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Medvedev SP, Shevchenko AI, Zakian SM, Induced pluripotent stem cells: problems and advantages when applying them in regenerative medicine., Acta Naturae. 2 (2010) 18–28. [PMC free article] [PubMed] [Google Scholar]
- [148].Li Y-C, Zhu K, Young T-H, Induced pluripotent stem cells, form in vitro tissue engineering to in vivo allogeneic transplantation., J. Thorac. Dis 9 (2017) 455–459. doi: 10.21037/jtd.2017.02.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Xu F, Celli J, Rizvi I, Moon S, Hasan T, Demirci U, A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform, Biotechnol. J 6 (2011) 204–212. doi: 10.1002/biot.201000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Yao T, Asayama Y, Animal-cell culture media: History, characteristics, and current issues, Reprod. Med. Biol 16 (2017) 99–117. doi: 10.1002/rmb2.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Morrison G, Liu C, Wing C, Delaney SM, Zhang W, Dolan ME, Evaluation of inter-batch differences in stem-cell derived neurons, Stem Cell Res 16 (2016) 140–148. doi: 10.1016/j.scr.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Kinarivala N, Shah K, Abbruscato TJ, Trippier PC, Passage variation of PC12 cells results in inconsistent susceptibility to externally induced apoptosis, ACS Chem. Neurosci 8 (2017) 82–88. doi: 10.1021/acschemneuro.6b00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Liu P, Kaplan A, Yuan B, Hanna JH, Lupski JR, Reiner O, Passage number is a major contributor to genomic structural variations in mouse iPSCs, Stem Cells. 32 (2014) 2657–2667. doi: 10.1002/stem.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Carmen J, Burger SR, McCaman M, Rowley JA, Developing assays to address identity, potency, purity and safety: cell characterization in cell therapy process development, Regen. Med 7 (2012) 85–100. doi: 10.2217/rme.11.105. [DOI] [PubMed] [Google Scholar]
- [155].Wang L, Xu M, Luo L, Zhou Y, Si P, Iterative feedback bio-printing-derived cell-laden hydrogel scaffolds with optimal geometrical fidelity and cellular controllability, Sci. Rep 8 (2018) 2802. doi: 10.1038/s41598-018-21274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Fang Y, Eglen RM, Three-dimensional cell cultures in drug discovery and development., SLAS Discov. Adv. Life Sci. R D 22 (2017) 456–472. doi: 10.1177/1087057117696795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, Kates SL, Awad HA, 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration, Biomaterials. 35 (2014) 4026–4034. doi: 10.1016/j.biomaterials.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Gomes S, Leonor IB, Mano JF, Reis RL, Kaplan DL, Natural and genetically engineered proteins for tissue engineering., Prog. Polym. Sci 37 (2012) 1–17. doi: 10.1016/j.progpolymsci.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Almeida CR, Serra T, Oliveira MI, Planell JA, Barbosa MA, Navarro M, Impact of 3-D printed PLA- and chitosan-based scaffolds on human monocyte/macrophage responses: Unraveling the effect of 3-D structures on inflammation, Acta Biomater 10 (2014) 613–622. doi: 10.1016/j.actbio.2013.10.035. [DOI] [PubMed] [Google Scholar]
- [160].Pati F, Jang J, Ha D-H, Won Kim S, Rhie J-W, Shim J-H, Kim D-H, Cho D-W, Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink, Nat. Commun 5 (2014) 1–11. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Kim JD, Choi JS, Kim BS, Chan Choi Y, Cho YW, Piezoelectric inkjet printing of polymers: Stem cell patterning on polymer substrates, Polymer (Guildf). 51 (2010) 2147–2154. doi: 10.1016/j.polymer.2010.03.038. [DOI] [Google Scholar]
- [162].Keane TJ, Badylak SF, Biomaterials for tissue engineering applications, Semin. Pediatr. Surg 23 (2014) 112–118. doi: 10.1053/j.sempedsurg.2014.06.010. [DOI] [PubMed] [Google Scholar]
- [163].Zhu J, Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering, Biomaterials. 31 (2010) 4639–4656. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Lu L, Peter SJ, Lyman MD, Lai HL, Leite SM, Tamada JA, Uyama S, Vacanti JP, Langer R, Mikos AG, In vitro and in vivo degradation of porous poly(DL-lactic-co-glycolic acid) foams., Biomaterials. 21 (2000) 1837–1845. [DOI] [PubMed] [Google Scholar]
- [165].Hutson CB, Nichol JW, Aubin H, Bae H, Yamanlar S, Al-Haque S, Koshy ST, Khademhosseini A, Synthesis and characterization of tunable poly(ethylene glycol): gelatin methacrylate composite hydrogels, Tissue Eng 17 (2011) 1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Miao T, Miller EJ, McKenzie C, Oldinski RA, Physically crosslinked polyvinyl alcohol and gelatin interpenetrating polymer network theta-gels for cartilage regeneration, J. Mater. Chem. B 3 (2015) 9242–9249. doi: 10.1039/C5TB00989H. [DOI] [PubMed] [Google Scholar]
- [167].Lee H, Han W, Kim H, Ha D-H, Jang J, Kim BS, Cho D-W, Development of liver decellularized extracellular matrix bioink for three-dimensional cell printing-based liver tissue engineering, Biomacromolecules. 18 (2017) 1229–1237. doi: 10.1021/acs.biomac.6b01908. [DOI] [PubMed] [Google Scholar]
- [168].Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA, Three-dimensional bioprinting of thick vascularized tissues., Proc. Natl. Acad. Sci. U. S. A 113 (2016) 3179–3184. doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Crapo PM, Gilbert TW, Badylak SF, An overview of tissue and whole organ decellularization processes, Biomaterials. 32 (2011) 3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Russo V, Yu C, Belliveau P, Hamilton A, Flynn LE, Comparison of human adipose-derived stem cells isolated from subcutaneous, omental, and intrathoracic adipose tissue depots for regenerative applications., Stem Cells Transl. Med 3 (2014) 206–217. doi: 10.5966/sctm.2013-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Beachley VZ, Wolf MT, Sadtler K, Manda SS, Jacobs H, Blatchley MR, Bader JS, Pandey A, Pardoll D, Elisseeff JH, Tissue matrix arrays for high-throughput screening and systems analysis of cell function, Nat. Methods. 12 (2015) 1197–1204. doi: 10.1038/nmeth.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA, 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs, Adv Mater 26 (2014) 3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- [173].Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, Woodruff TK, Shah RN, A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice, Nat. Commun 8 (2017) 15261. doi: 10.1038/ncomms15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Yoon H, Lee J-S, Yim H, Kim G, Chun W, Development of cell-laden 3D scaffolds for efficient engineered skin substitutes by collagen gelation, RSC Adv 6 (2016) 21439–21447. doi: 10.1039/C5RA19532B. [DOI] [Google Scholar]
- [175].Highley CB, Rodell CB, Burdick JA, Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels, Adv. Mater 27 (2015) 5075–5079. doi: 10.1002/adma.201501234. [DOI] [PubMed] [Google Scholar]
- [176].Smith CM, Christian JJ, Warren WL, Williams SK, Characterizing environmental factors that impact the viability of tissue-engineered constructs fabricated by a direct-write bioassembly tool, Tissue Eng 13 (2007) 373–383. doi: 10.1089/ten.2006.0101. [DOI] [PubMed] [Google Scholar]
- [177].Zhu W, Li J, Leong YJ, Rozen I, Qu X, Dong R, Wu Z, Gao W, Chung PH, Wang J, Chen S, 3D-printed artificial microfish, Adv. Mater 27 (2015) 4411–4417. doi: 10.1002/adma.201501372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, Volk ML, The epidemiology of cirrhosis in the United States: a population-based study., J. Clin. Gastroenterol 49 (2015) 690–696. doi: 10.1097/MCG.0000000000000208. [DOI] [PubMed] [Google Scholar]
- [179].Bataller R, Brenner D, Liver fibrosis, J. Clin. Invest 115 (2005) 209–218. doi: 10.1172/JCI200524282.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Kaplowitz N, Idiosyncratic drug hepatotoxicity, Nat. Rev. Drug Discov 4 (2005) 489–499. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- [181].Tsang VL, Chen A. a, Cho LM, Jadin KD, Sah RL, DeLong S, West JL, Bhatia SN, Liu Tsang V, Chen A. a, Cho LM, Jadin KD, Sah RL, DeLong S, West JL, Bhatia SN, Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels., FASEB J 21 (2007) 790–801. doi: 10.1096/fj.06-7117com. [DOI] [PubMed] [Google Scholar]
- [182].Khetani SR, Bhatia SN, Microscale culture of human liver cells for drug development., Nat. Biotechnol 26 (2008) 120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- [183].Hewitt NJ, Gómez Lechón MJ, Houston JB, Hallifax D, Brown HS, Maurel P, Kenna JG, Gustavsson L, Lohmann C, Skonberg C, Guillouzo A, Tuschl G, Li AP, LeCluyse E, Groothuis GMM, Hengstler JG, Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies, Drug Metab. Rev 39 (2007) 159–234. doi: 10.1080/03602530601093489. [DOI] [PubMed] [Google Scholar]
- [184].Guguen-Guillouzo C, Corlu A, Guillouzo A, Stem cell-derived hepatocytes and their use in toxicology, Toxicology. 270 (2010) 3–9. doi: 10.1016/j.tox.2009.09.019. [DOI] [PubMed] [Google Scholar]
- [185].Yoon No D, Lee K-H, Lee J, Lee S-H, 3D liver models on a microplatform: well-defined culture, engineering of liver tissue and liver-on-a-chip, Lab Chip. 15 (2015) 3822–3837. doi: 10.1039/C5LC00611B. [DOI] [PubMed] [Google Scholar]
- [186].Bhatia SN, Underhill GH, Zaret KS, Fox IJ, Cell and tissue engineering for liver disease, Sci. Transl. Med 6 (2014) 245sr2. doi: 10.1126/scitranslmed.3005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Sivaraman LG, Leach A, Townsend JK, Iida S, Hogan T, Stolz BJ, Fry DB, Samson R, Tannenbaum LD, Griffith SR, A microscale in vitro physiological model of the liver: predictive screens for drug metabolism and enzyme induction, Curr. Drug Metab 6 (2005) 569–591. doi: 10.2174/138920009787048400. [DOI] [PubMed] [Google Scholar]
- [188].Du Y, Han R, Wen F, Ng San San S, Xia L, Wohland T, Leo HL, Yu H, Synthetic sandwich culture of 3D hepatocyte monolayer, Biomaterials. 29 (2008) 290–301. doi: 10.1016/j.biomaterials.2007.09.016. [DOI] [PubMed] [Google Scholar]
- [189].Takayama K, Kawabata K, Nagamoto Y, Kishimoto K, Tashiro K, Sakurai F, Tachibana M, Kanda K, Hayakawa T, Furue MK, Mizuguchi H, 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing, Biomaterials. 34 (2013) 1781–1789. doi: 10.1016/j.biomaterials.2012.11.029. [DOI] [PubMed] [Google Scholar]
- [190].Gaskell H, Sharma P, Colley HE, Murdoch C, Williams DP, Webb SD, Characterization of a functional C3A liver spheroid model., Toxicol. Res. (Camb). 5 (2016) 1053–1065. doi: 10.1039/c6tx00101g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Domansky K, Inman W, Serdy J, Dash A, Lim MHM, Griffith LG, Perfused multiwell plate for 3D liver tissue engineering., Lab Chip. 10 (2010) 51–58. doi: 10.1039/b913221j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Toh Y-C, Lim TC, Tai D, Xiao G, van Noort D, Yu H, A microfluidic 3D hepatocyte chip for drug toxicity testing, Lab Chip. 9 (2009) 2026–2035. doi: 10.1039/b900912d. [DOI] [PubMed] [Google Scholar]
- [193].Ho C-T, Lin R-Z, Chen R-J, Chin C-K, Gong S-E, Chang H-Y, Peng H-L, Hsu L, Yew T-R, Chang S-F, Liu C-H, Liver-cell patterning lab c hip: mimicking the morphology of liver lobule tissue., Lab Chip. 13 (2013) 3578–3587. doi: 10.1039/C3LC50402F. [DOI] [PubMed] [Google Scholar]
- [194].Berger DR, Ware BR, Davidson MD, Allsup SR, Khetani SR, Enhancing the functional maturity of iPSC-derived human hepatocytes via controlled presentation of cell-cell interactions in vitro., Hepatology. (2014) 1–44. doi: 10.1002/hep.27621. [DOI] [PubMed] [Google Scholar]
- [195].Kim K, Ohashi K, Utoh R, Kano K, Okano T, Preserved liver-specific functions of hepatocytes in 3D co-culture with endothelial cell sheets, Biomaterials. 33 (2012) 1406–1413. doi: 10.1016/j.biomaterials.2011.10.084. [DOI] [PubMed] [Google Scholar]
- [196].Faulkner-Jones A, Fyfe C, Cornelissen D-J, Gardner J, King J, Courtney Aidan, Shu W, Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D, Biofabrication. 7 (2015) 44102. doi: 10.1088/1758-5090/7/4/044102. [DOI] [PubMed] [Google Scholar]
- [197].Kang K, Kim Y, Lee SB, Kim JS, Park S, Kim W, Yang H-M, Kim S-J, Jeong J, Choi D, Three-dimensional bio-printing of hepatic structures with direct-converted hepatocyte-like cells, Tissue Eng. Part A. 24 (2018) 576–583. doi: 10.1089/ten.TEA.2017.0161. [DOI] [PubMed] [Google Scholar]
- [198].Kizawa H, Nagao E, Shimamura M, Zhang G, Torii H, Scaffold-free 3D bio-printed human liver tissue stably maintains metabolic functions useful for drug discovery, Biochem. Biophys. Reports 10 (2017) 186–191. doi: 10.1016/j.bbrep.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Bhise NS, Manoharan V, Massa S, Tamayol A, Ghaderi M, Miscuglio M, Lang Q, Shrike Zhang Y, Shin SR, Calzone G, Annabi N, Shupe TD, Bishop CE, Atala A, Dokmeci MR, Khademhosseini A, A liver-on-a-chip platform with bioprinted hepatic spheroids, Biofabrication. 8 (2016) 14101. doi: 10.1088/1758-5090/8/1/014101. [DOI] [PubMed] [Google Scholar]
- [200].Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee, Heart disease and stroke statistics−−2015 update: a report from the American Heart Association., Circulation. 131 (2015) e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- [201].Ciociola AA, Cohen LB, Kulkarni P, the F.-R.M.C. of the A.C. of Gastroenterology, How drugs are developed and approved by the FDA: current process and future directions, Am J Gastroenterol 109 (2014) 620–623. 10.1038/ajg.2013.407. [DOI] [PubMed] [Google Scholar]
- [202].Ribas J, Sadeghi H, Manbachi A, Leijten J, Brinegar K, Zhang YS, Ferreira L, Khademhosseini A, Cardiovascular organ-on-a-chip platforms for drug discovery and development, Appl. Vitr. Toxicol 2 (2016) 82–96. doi: 10.1089/aivt.2016.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Lundy SD, Gantz JA, Pagan CM, Filice D, Laflamme MA, Pluripotent stem cell derived cardiomyocytes for cardiac repair, Curr. Treat. Options Cardiovasc. Med 16 (2014) 319. doi: 10.1007/s11936-014-0319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Ye KY, Sullivan KE, Black LD, Encapsulation of cardiomyocytes in a fibrin hydrogel for cardiac tissue engineering, J. Vis. Exp (2011) 3251. doi: 10.3791/3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Legant WR, Pathak A, Yang MT, Deshpande VS, McMeeking RM, Chen CS, Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues, Proc. Natl. Acad. Sci 106 (2009) 10097–10102. doi: 10.1073/pnas.0900174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].McCain ML, Agarwal A, Nesmith HW, Nesmith AP, Parker KK, Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues, Biomaterials. 35 (2014) 5462–5471. doi: 10.1016/j.biomaterials.2014.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL, Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering., Biomaterials. 30 (2009) 5409–5416. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Gribova V, Crouzier T, Picart C, A material’s point of view on recent developments of polymeric biomaterials: control of mechanical and biochemical properties, J. Mater. Chem 21 (2011) 14354–14366. doi: 10.1039/C1JM11372K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Ribeiro AJS, Ang Y-S, Fu J-D, Rivas RN, Mohamed TMA, Higgs GC, Srivastava D, Pruitt BL, Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness, Proc. Natl. Acad. Sci 112 (2015) 12705–12710. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang H-Y, Speicher DW, Sanger JW, Sanger JM, Discher DE, Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating., J. Cell Sci 121 (2008) 3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Chaudhuri R, Ramachandran M, Moharil P, Harumalani M, Jaiswal AK, Biomaterials and cells for cardiac tissue engineering: Current choices, Mater. Sci. Eng. C 79 (2017) 950–957. doi: 10.1016/j.msec.2017.05.121. [DOI] [PubMed] [Google Scholar]
- [212].Hirt MN, Hansen A, Eschenhagen T, Cardiac tissue engineering: state of the art., Circ. Res 114 (2014) 354–367. doi: 10.1161/CIRCRESAHA.114.300522. [DOI] [PubMed] [Google Scholar]
- [213].Rao C, Prodromakis T, Kolker L, Chaudhry UAR, Trantidou T, Sridhar A, Weekes C, Camelliti P, Harding SE, Darzi A, Yacoub MH, Athanasiou T, Terracciano CM, The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells, Biomaterials. 34 (2013) 2399–2411. doi: 10.1016/j.biomaterials.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Salick MR, Napiwocki BN, Sha J, Knight GT, Chindhy SA, Kamp TJ, Ashton RS, Crone WC, Micropattern width dependent sarcomere development in human ESC-derived cardiomyocytes, Biomaterials. 35 (2014) 4454–4464. doi: 10.1016/j.biomaterials.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M, Park S, Kotikian A, Nesmith AP, Campbell PH, Vlassak JJ, Lewis JA, Parker KK, Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing., Nat. Mater 16 (2016) 303–308. doi: 10.1038/nmat4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, Aleman J, Colosi C, Busignani F, Dell’Erba V, Bishop C, Shupe T, Demarchi D, Moretti M, Rasponi M, Dokmeci MR, Atala A, Khademhosseini A, Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip, Biomaterials. 110 (2016) 45–59. doi: 10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Ma Z, Koo S, Finnegan MA, Loskill P, Huebsch N, Marks NC, Conklin BR, Grigoropoulos CP, Healy KE, Three-dimensional filamentous human diseased cardiac tissue model, Biomaterials. 35 (2014) 1367–1377. doi: 10.1016/j.biomaterials.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Novosel EC, Kleinhans C, Kluger PJ, Vascularization is the key challenge in tissue engineering, Adv. Drug Deliv. Rev 63 (2011) 300–311. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- [219].Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen D-HT, Cohen DM, Toro E, Chen A. a, Galie P. a, Yu X, Chaturvedi R, Bhatia SN, Chen CS, Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues., Nat. Mater 11 (2012) 768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Baranski JD, Chaturvedi RR, Stevens KR, Eyckmans J, Carvalho B, Solorzano RD, Yang MT, Miller JS, Bhatia SN, Chen CS, Geometric control of vascular networks to enhance engineered tissue integration and function, Proc. Natl. Acad. Sci 110 (2013) 7586–7591. doi: 10.1073/pnas.1217796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, Barabaschi G, Demarchi D, Dokmeci MR, Yang Y, Khademhosseini A, Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs., Lab Chip. 14 (2014) 2202–2211. doi: 10.1039/c4lc00030g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Lee KY, Peters MC, Anderson KW, Mooney DJ, Controlled growth factor release from synthetic extracellular matrices, Nature. 408 (2000) 998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- [223].Fischbach C, Mooney DJ, Polymers for pro- and anti-angiogenic therapy, Biomaterials. 28 (2007) 2069–2076. doi: 10.1016/j.biomaterials.2006.12.029. [DOI] [PubMed] [Google Scholar]
- [224].Richardson TP, Peters MC, Ennett a B., Mooney DJ, Polymeric system for dual growth factor delivery., Nat. Biotechnol 19 (2001) 1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- [225].Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk C. a, Mulligan RC, D’Amore P. a, Langer R, Engineering vascularized skeletal muscle tissue., Nat. Biotechnol 23 (2005) 879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- [226].Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Huber I, Habib M, Gepstein L, Levenberg S, Tissue engineering of vascularized cardiac muscle from human embryonic stem cells, Circ. Res 100 (2007) 263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- [227].Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK, Tissue engineering: creation of long-lasting blood vessels., Nature. 428 (2004) 138–139. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- [228].Leong MF, Toh JKC, Du C, Narayanan K, Lu HF, Lim TC, Wan A.C. a, Ying JY, Patterned prevascularised tissue constructs by assembly of polyelectrolyte hydrogel fibres., Nat. Commun 4 (2013) 2353. doi: 10.1038/ncomms3353. [DOI] [PubMed] [Google Scholar]
- [229].Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, Wells LA, Massé S, Kim J, Reis L, Momen A, Nunes SS, Wheeler AR, Nanthakumar K, Keller G, Sefton MV, Radisic M, Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis, Nat. Mater 1 (2016) 1–42. doi: 10.1038/nmat4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Chen X, Aledia AS, Ghajar CM, Griffith CK, Putnam AJ, Hughes CCW, George SC, Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature., Tissue Eng. Part A. 15 (2009) 1363–1371. doi: 10.1089/ten.tea.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Sahota PS, Burn JL, Brown NJ, MacNeil S, Approaches to improve angiogenesis in tissue-engineered skin, Wound Repair Regen 12 (2004) 635–642. doi: 10.1111/j.1067-1927.2004.12608.x. [DOI] [PubMed] [Google Scholar]
- [232].Kolesky DB, Truby RL, Gladman a. S., Busbee T. a., Homan K. a., Lewis J. a., 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs, Adv. Mater 26 (2014) 3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- [233].Galie P. a, Nguyen D-HT, Choi CK, Cohen DM, Janmey P. a, Chen CS, Fluid shear stress threshold regulates angiogenic sprouting., Proc. Natl. Acad. Sci. U. S. A 111 (2014) 7968–7973. doi: 10.1073/pnas.1310842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].McMillin DW, Negri JM, Mitsiades CS, The role of tumour–stromal interactions in modifying drug response: challenges and opportunities, Nat. Rev. Drug Discov 12 (2013) 217–228. doi: 10.1038/nrd3870. [DOI] [PubMed] [Google Scholar]
- [235].Leonard F, Godin B, 3D in vitro model for breast cancer research using magnetic levitation and bioprinting method., Methods Mol. Biol 1406 (2016) 239–251. doi: 10.1007/978-1-4939-3444-7_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S, Mitsiades N, Schlossman RL, Munshi NC, Kung AL, Griffin JD, Richardson PG, Anderson KC, Mitsiades CS, Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity, Nat. Med 16 (2010) 483–489. doi: 10.1038/nm.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Unger C, Kramer N, Walzl A, Scherzer M, Hengstschläger M, Dolznig H, Modeling human carcinomas: Physiologically relevant 3D models to improve anti-cancer drug development, Adv. Drug Deliv. Rev 79 (2014) 50–67. doi: 10.1016/j.addr.2014.10.015. [DOI] [PubMed] [Google Scholar]
- [238].Albritton JL, Miller JS, 3D bioprinting: improving in vitro models of metastasis with heterogeneous tumor microenvironments, Dis. Model. Mech 10 (2017) 3–14. doi: 10.1242/dmm.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Chaffer CL, Weinberg R. a, A perspective on cancer cell metastasis., Science (80-.). 331 (2011) 1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- [240].Huang TQ, Qu X, Liu J, Chen S, 3D printing of biomimetic microstructures for cancer cell migration., Biomed. Microdevices. 16 (2014) 127–132. doi: 10.1007/s10544-013-9812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Zhao Y, Yao R, Ouyang L, Ding H, Zhang T, Zhang K, Cheng S, Sun W, Three-dimensional printing of Hela cells for cervical tumor model in vitro., Biofabrication. 6 (2014) 35001. doi: 10.1088/1758-5082/6/3/035001. [DOI] [PubMed] [Google Scholar]
- [242].Yang Y, Du T, Zhang J, Kang T, Luo L, Tao J, Gou Z, Chen S, Du Y, He J, Jiang S, Mao Q, Gou M, A 3D-Engineered Conformal Implant Releases DNA Nanocomplexs for Eradicating the Postsurgery Residual Glioblastoma, Adv. Sci 4 (2017). doi: 10.1002/advs.201600491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Rutz AL, Hyland KE, Jakus AE, Burghardt WR, Shah RN, A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels, Adv. Mater 27 (2015) 1607–1614. doi: 10.1002/adma.201405076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Cui X, Breitenkamp K, Lotz M, D’Lima D, Synergistic action of fibroblast growth factor-2 and transforming growth factor-beta1 enhances bioprinted human neocartilage formation, Biotechnol. Bioeng 109 (2012) 2357–2368. doi: 10.1002/bit.24488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Eisenstein M, Artificial organs: Honey, I shrunk the lungs, Nature. 519 (2015) S16--S18. doi: 10.1038/519S16a. [DOI] [PubMed] [Google Scholar]