Abstract
The Biomarker Qualification Program was established at the Center for Drug Evaluation and Research (CDER), Food and Drug Administration (FDA) to expedite the integration of promising biomarkers across multiple drug development programs. The first set of biomarkers qualified in 2008 consisted of seven nonclinical safety biomarkers for the detection of acute drug-induced nephrotoxicity in rats, and included urinary Kidney Injury Molecule-1 (KIM-1). This manuscript discusses the use of KIM-1 in drug development and research before and after CDER’s qualification of KIM-1. Use was determined by analyzing relevant documents identified by keyword searches using three databases: 1) an FDA internal database, Document Archiving, Reporting, and Regulatory Tracking System (DARRTS) 2) ClinicalTrials.gov, and 3) PubMed. Results indicate increased use of KIM-1 as a biomarker for detection of kidney injury in drug development programs reviewed by CDER, as well as in research following qualification.
Keywords: biomarker qualification (BQ), context of use (COU), drug development, kidney injury, Kidney Injury Molecule (KIM-1)
Introduction
A biomarker is a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions. [1] Biomarkers have multiple uses in drug development; these include identifying subjects more likely to experience an outcome or response to a treatment administered in a clinical trial, monitoring potential drug toxicities during a trial, and determining whether a treatment administered in a clinical trial is achieving the intended outcome or response.
Historically, biomarkers have gained acceptance in drug development after they were used by the scientific and medical communities over time. To improve the efficiency of the acceptance process, the Center for Drug Evaluation and Research (CDER) currently administers two additional pathways that evaluate the acceptability of biomarkers for use in drug development. One pathway is the evaluation of a biomarker for use with a specific candidate drug in an Investigational New Drug (IND) Application or New Drug/Biologics License Application (NDA/BLA). Broad acceptance of the biomarker in drug development can be challenging with this approach because the supporting biomarker data are retained within the specific candidate drug/biologic submission and are often not publicly available for others to use. The second pathway is the Biomarker Qualification Program, which qualifies a biomarker for a specific context of use (COU) to be utilized across multiple drug development programs. CDER established the Biomarker Qualification Program in 2007 as part of the Food and Drug Administration’s (FDA’s) Critical Path Initiative to make drug development tools publicly available to expedite drug development and regulatory review. [2–4]
Qualification of a biomarker is defined as a conclusion, that within the stated COU, the biomarker can be relied upon to have a specific interpretation and application in medical product development and regulatory review. [3] Once a biomarker is qualified, the biomarker can be used under the COU for which it obtained qualification during the development of any candidate drug without the need for CDER to reconsider or reconfirm the suitability of the qualified biomarker for that specific COU. To date, thirteen biomarkers have been qualified, including ten safety biomarkers for use in nonclinical safety assessment studies.
Pioneering collaborative efforts between industry and academia in the Predictive Safety Testing Consortium (PSTC) led to the submission of nonclinical safety biomarker data to CDER, FDA for qualification of the biomarkers through the Biomarker Qualification Pilot Process. The PSTC submission was the first submission to be reviewed at CDER, FDA jointly with European Medicines Agency (EMEA) for biomarker qualification and resulted in the successful qualification of seven urinary safety biomarkers for use in nonclinical studies: Kidney Injury Molecule (KIM-1), urine albumin, total protein, β2-Microglobulin, Cystatin C, Clusterin and Trefoil Factor-3. [5–8] These biomarkers were qualified for the non-invasive detection of nephrotoxicity, to be used in addition to traditional biomarkers and histopathology in nonclinical safety assessment studies. [9]
This research effort was aimed at evaluating the use of qualified biomarkers in drug development and research. We selected KIM-1, one of the seven qualified nonclinical safety biomarkers described above, for our pilot study. The initial reports about the utility of KIM-1 as a biomarker of the kidney injury response to drug toxicity appeared in 2004–2006 [10,11]. Additional data provided by PSTC led to the qualification of urinary KIM-1 for the detection of drug-induced acute kidney tubular alterations in Good Laboratory Practice rat studies used to support clinical trials. Reviews and data from qualification were made public to support the use of KIM-1 in drug development and to support the continued development of KIM-1 for additional contexts of use in the future. The qualification letter for KIM-1 discussed gaining supportive data for clinical use: “While considerable human data exist for some of these novel biomarkers, they are not currently qualified for routine monitoring of drug-induced nephrotoxicity in the clinical setting. In cases where additional evaluation of drug effect on the kidney is deemed useful, the sponsor and FDA’s clinical review division will decide on a case by case basis how best to implement the use of these biomarkers in a clinical development program. Human qualification studies, including studies evaluating the pattern of elevation and the degree and timeframe of reversibility of elevation of these markers after human exposure to known nephrotoxicants such as aminoglycosides, may be helpful in moving these markers into clinical use.”
It was hypothesized that publicly posting the qualification of a biomarker along with the supportive data would encourage increased use of the biomarker in drug development under the COU that the biomarker initially obtained qualification under; and would also lead to increased exploratory use of the biomarker in drug development and research that may support other new potential contexts of use. The aim of this study was to evaluate these hypotheses by assessing the use of KIM-1 in drug development programs and research before and after its qualification on April 14, 2008.
Results
DARRTS Search Results
The Document Archiving, Reporting & Regulatory Tracking System (DARRTS) search yielded 361 FDA-generated documents associated with 154 regulatory applications that specifically discussed or referenced the use of urinary KIM-1 as a biomarker to detect kidney injury, including drug-induced nephrotoxicity or as a pharmacodynamic/response biomarker to assess response to medical products.
These regulatory applications comprised of 143 INDs, 10 NDAs and 1 BLA and included referenced use of KIM-1 in nonclinical (including rats and other animal species) as well as in clinical trial settings. Further analyses were limited to documents associated with INDs that referenced KIM-1 use.
From January 1, 2005 until the qualification of KIM-1 on April 13, 2008, documents associated with four INDs referenced KIM-1. After qualification (April 14, 2008 – December 31, 2016), documents associated with 139 INDs referred to KIM-1. A sharp increase in the number of INDs with FDA-generated documents that discussed the use of KIM-1 was observed in the year following qualification, and this number remained relatively constant over subsequent years (Figure 1).
Figure 1.
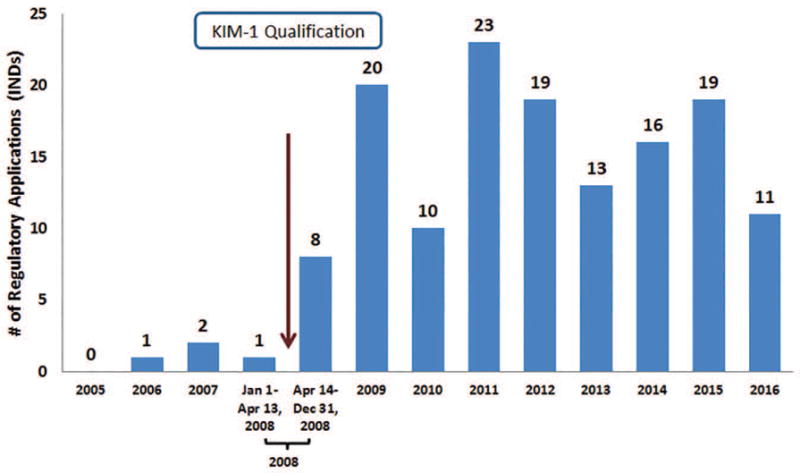
Number of regulatory applications (INDs) with FDA- generated documents in the DARRTS database that referenced KIM-1 as a drug development tool from 2005–2016.
Reference to KIM-1 use in INDs associated with different review divisions in CDER’s Office of New Drugs was evaluated. Following KIM-1 qualification, results show an increase in references to KIM-1 in regulatory documents across review divisions in CDER. Prior to KIM-1 qualification, regulatory documents from only three review divisions discussed the use of KIM-1. After qualification, references to use of KIM-1 expanded to include 16 review divisions (Figure 2).
Figure 2.
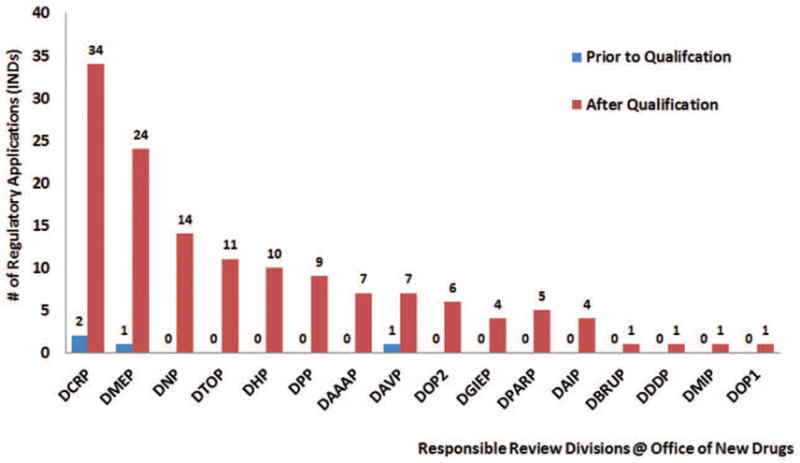
Number of regulatory applications (INDs) with FDA communications in the DARRTS database that referenced KIM-1 as a drug development tool per review division in the Office of New Drugs, CDER from 2005 to 2016.
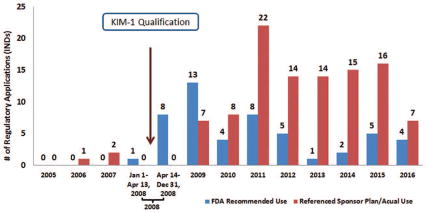
Evaluation of the FDA-generated documents in the DARRTS database indicated that FDA reviewers increasingly recommended the use of KIM-1 to sponsors following its qualification in 2008. Prior to qualification, only one IND was identified in which use of KIM-1 was recommended by FDA reviewers. Immediately following qualification (i.e., April 14, 2008– December 31, 2008), use of KIM-1 was recommended by FDA reviewers in communications with sponsors in 8 distinct INDs. The number of recommendations decreased over the subsequent years but remained significantly above pre-qualification levels. In contrast, references to the planned or actual use of KIM-1, by sponsors, increased more gradually following qualification of KIM-1 (2009) but remained elevated after this time frame (from 2010–2016) (Figure 3).
Figure 3.
Number of regulatory applications (INDs) with FDA-generated documents in the DARRTS database that referenced FDA reviewer recommendations for the use of KIM-1 vs sponsor’s planned or actual use of KIM-1 as a drug development tool.
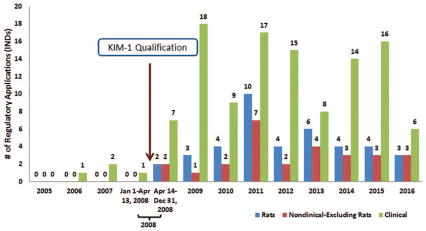
As previously discussed, urinary KIM-1 is qualified for the non-invasive detection of nephrotoxicity, to be used in addition to traditional markers and histopathology in rat safety assessment studies. [9] No application or documents associated with the applications, referred to use of KIM-1 in nonclinical studies prior to qualification. After qualification, references to the use of KIM-1 in rat studies were found in documents associated with 40 INDs. References to use of KIM-1 in nonclinical settings other than rats was found in the documents associated 27 INDs (Figure 4). These uses included evaluation of urinary KIM-1 in other nonclinical species such as mice, monkeys, and dogs for detection of drug-induced nephrotoxicity. Prior to qualification, there were four regulatory applications with communications which referenced the use of KIM-1 in the clinical setting. Following qualification 110 regulatory applications referred to the use of KIM-1 in the clinical setting (Figure 4).
Figure 4.
Regulatory applications (INDs) with FDA- generated documents in the DARRTS database that referred to KIM-1 as a drug development tool, grouped by study type: rats, nonclinical (excluding rats), and clinical. Please note that a single regulatory document may contain multiple uses of KIM-1 (for example, use of KIM-1 in the nonclinical as well as clinical studies).
ClinicalTrials.gov Search Results
The ClinicalTrials.gov database [12] was searched for KIM-1 to identify studies using KIM-1 that may not have been submitted to FDA in a regulatory application, since the database contains information on observational studies conducted within and outside the US in addition to interventional studies and expanded access programs.
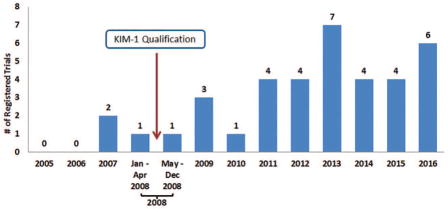
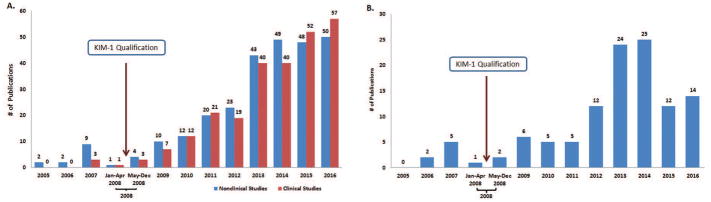
Thirty-seven registered clinical studies were identified in ClinicalTrials.gov that reported use of urinary KIM-1 as a kidney injury biomarker (from 2005 to 2016). Registered studies were categorized by trial start date; the results are shown in Figure 5. Use of KIM-1 to detect kidney injury was reported in 3 clinical studies that started between January 2005 and April 2008; following KIM-1 qualification, use increased with 34 studies referencing the use at the end of 2016. Possible overlap between the DARRTS and ClinicalTrials.gov results was examined. No overlap was observed.
Figure 5.
Number of clinical trials with summary information at ClinicalTrials.gov website that referenced the use of KIM-1 for detecting kidney injury, including drug-induced nephrotoxicity
PubMed Search Results
The PubMed [13] search using the keyword KIM-1 (or its synonyms) yielded 708 publications from January 1, 2005, to December 31, 2016. These documents cover a broad use of KIM-1 and are not limited to the COU for which KIM-1 was qualified. Fifty-five publications were excluded from further analyses since they either referred to uses not related to the biomarker “KIM-1” (including names, part of an address, or reference to an unrelated entity, such as a cell line) or referred to the use of KIM-1 as a therapeutic target. Also, publications in languages other than English were excluded.
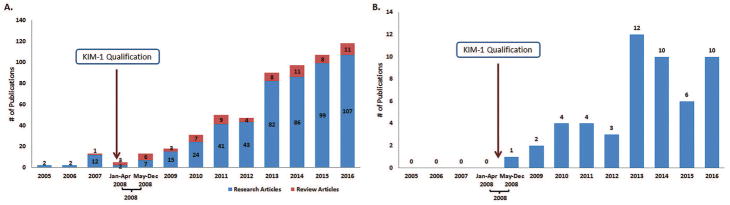
Five hundred and ninety-three publications that reported the use of KIM-1 as a biomarker for detecting kidney injury including drug-induced nephrotoxicity were retained for the next step. Of these, 22 were published before qualification and 571 were published after qualification (Figure 6A). The number of research articles as well as review articles that referenced the use of KIM-1 as a biomarker for kidney injury, including drug-induced nephrotoxicity, increased following qualification. Higher rates of increase were observed for the research articles that referenced KIM-1 use compared to the review articles. (Figure 6A).
Figure 6.
(A) Number of publications in PubMed that reference the use of KIM-1 for detection of kidney injury, including drug-induced nephrotoxicity from 2005–2016; (B) Publications in PubMed that reference the qualification of KIM-1 by FDA from 2005–2016.
The publications were screened to identify the research articles and review articles that referred to KIM-1 qualification by FDA. Of the 593 publications that reported use of KIM-1 as a biomarker of kidney injury or nephrotoxicity, 52 referenced the qualification of KIM-1 (Figure 6B).
The PubMed search results showed that use of KIM-1 as a biomarker for kidney injury including drug-induced nephrotoxicity increased in nonclinical as well as clinical studies. In the studies identified, the use of KIM-1 in nonclinical as well as in clinical studies was comparable, with 52% in nonclinical studies and 48% in clinical studies. (Figure 7A).
Figure 7.
(A) Publication references in PubMed of the use of KIM-1 for detecting kidney injury, including drug-induced nephrotoxicity, grouped by the study type: nonclinical and clinical. Note: Six publications had information on KIM-1 use in nonclinical as well as in clinical studies. (B) Publication references in PubMed of the use of urinary KIM-1 protein for detecting kidney injury, including drug-induced nephrotoxicity, in rats.
Next, the analysis focused on research articles that described urinary KIM-1 protein as a biomarker for kidney injury including drug-induced nephrotoxicity in rats. Specifically, research articles that described the use of KIM-1 from other sample types (e.g., kidney biopsy or plasma), forms (e.g., mRNA) and use in other species were removed. The number of research articles that referred to the use of urinary KIM-1 in rats as a biomarker for kidney injury including drug-induced nephrotoxicity increased from 8 (in the two years and four months before KIM-1 qualification) to 105 (in the period following KIM-1 qualification to 2016). Publications per year increased from 2012–2016, with the number of publications peaking in 2014 (Figure 7B).
Discussion
The potential impact of biomarker qualification on the use of KIM-1 in drug development and research was evaluated by identifying references to its use, before and after qualification in three databases: the DARRTS database housing FDA-generated documents associated with regulatory submissions, ClinicalTrials.gov, and PubMed.
Review of the DARRTS database revealed an increased number of references to the use of KIM-1 in drug development programs following qualification. This increase can be attributed at least in part, to FDA reviewers’ recommendations to use KIM-1 in settings where there was a priori concern that a drug might cause kidney injury, as well as increased in the reference to planned or actual use of KIM-1 by sponsors in drug development. The keyword search in DARRTS (details in the methods section) is limited to FDA-generated documents, and thus, sponsor’s use of KIM-1 was determined indirectly through identification of FDA documents that cited KIM-1 use by the sponsors. This number may be an underestimation, as it is solely based on language referred to in FDA-generated documents such as reviews and meeting minutes, and not on sponsor protocols. Additionally, reference to KIM-1 expanded across multiple therapeutic areas following qualification (from three to 16 review divisions from 2008 to 2016). In the INDs that were identified, KIM-1 was used in nonclinical studies with rats (N=40) and other nonclinical species (N=27), including mice, monkeys and dogs, as well as in clinical studies (N=114). These findings show evidence of an increase in the use of KIM-1 in clinical studies, consistent with FDA recommendations for the use of urinary KIM-1 and other qualified urinary biomarkers in exploratory clinical studies to evaluate their ability to monitor for potential nephrotoxicity in humans, and to possibly mitigate risk to subjects. [9]
ClinicalTrials.gov was used as an additional resource for evaluating the use of urinary KIM-1 in clinical trials. Analyses indicate an increase in the use of KIM-1 in clinical studies following qualification of KIM-1 for the detection of drug-induced nephrotoxicity in rats. The number of clinical trials that referred to KIM-1 use for detection of kidney injury including drug-induced nephrotoxicity in this database (N=37) may be an underestimation since ClinicalTrials.gov provides summary information about study protocols and may not include the exploratory use of KIM-1 in the trial summaries. Interestingly, no overlap was observed between the studies in the DARRTS database and the ClinicalTrials.gov database. This could be explained by the limited summary information on the Clinical Trials.gov website as well as the inability to search trial protocols using the DARRTS database. Clinicaltrials.gov may include observational or exploratory studies as well as studies conducted exclusively outside the US, which may not be conducted under an IND and thus found in the DARRTS database. In addition, not all clinical studies conducted in the United States are required by law to be registered at ClinicalTrials.gov. [12]
The number of publications associated with the use of KIM-1 for detection of kidney injury including drug-induced nephrotoxicity increased more gradually within the first four years after KIM-1 qualification in the PubMed search results. Also, several publications (N=52) referred to the qualification of KIM-1 by CDER suggesting awareness of KIM-1’s qualification in the scientific community. It should be noted that terms such as approval or acceptance or validation by FDA were used in lieu of qualification in these publications. The slower increase in publications referencing use of KIM-1 may reflect in part lack of commercially available assays for KIM-1, in rats and humans, since such assays were not commercially available until 2009–2010. [14] Also, this delay may represent the time needed for conducting studies, completing the analyses, publishing the results and capturing the published information by PubMed. A limitation of this database is that the publications are not necessarily drug-development centric and often, it is difficult to distinguish drug-induced nephrotoxicity from non-drug-induced kidney injury based on the published data.
The PubMed and ClinicalTrials.gov searches referenced the use of KIM-1 as a biomarker of kidney injury including drug-induced nephrotoxicity. Studies in these databases include interventions that were either procedural (e.g., surgery) or drugs or devices (e.g., tourniquet system). In contrast, the DARRTS search results were limited to the use of KIM-1 in drug development programs.
The use of KIM-1 in studies for contexts other than the qualified COU provides additional data that can help understand the usefulness of this biomarker for other purposes. For example, our results show that KIM-1 data has been utilized by sponsors for contexts other than the qualified COU in early stages of drug development. It has been reported that the qualified nonclinical renal safety biomarkers have been utilized for multiple purposes, including candidate molecule prioritization, early safety reads on efficacy studies, candidate selection, and go/no goes decisions at the pre-IND stage. [15] It has also been reported that the qualified nephrotoxicity biomarkers “have decreased animal use and the number of interim time points to be assessed, with fewer necropsy and pathology endpoints [15]”. Some published data also support a potential role for urinary KIM-1 for the detection of kidney injury including drug-induced nephrotoxicity for use in clinical studies. [16, 17] More data on the performance of the nonclinical drug-induced nephrotoxicity biomarkers, including KIM-1, in clinical studies are needed to establish their usefulness and possibly lead to qualification in humans.
Collectively, the analyses suggest that KIM-1 use in research and drug development increased after the qualification of the biomarker by CDER’s Biomarker Qualification Program. In addition to qualification, the increase in KIM-1 use in drug development may be attributable to other factors, including, commercial assay availability, increased KIM-1 use by the scientific community and discussions with FDA review staff.
Conclusion
Our study demonstrates that qualification of urinary KIM-1 for the detection of acute drug-induced nephrotoxicity assessment in rats was followed by increased reference to its use in drug development programs and increased use in research by the larger scientific community. The increased use of KIM-1 was not limited to use in rat studies and was observed in other nonclinical scenarios as well as in clinical studies in drug development and research. These results support the hypothesis that public posting of the qualified biomarkers along with the supportive data may encourage an increase in the use of the biomarkers in drug development, even though other factors including assay availability may have contributed to the results. Research on the uptake of additional qualified biomarkers in drug development and research is likely to help in understanding the contribution of these factors better. These nonclinical biomarkers hold considerable promise if they can be established as translational biomarkers to be used both in nonclinical and clinical studies, to predict toxicity across species and may speed innovation for many different types of therapeutics.
Methods
The use of KIM-1 in drug development and research was evaluated by analyzing documents identified by searching three databases: 1) An internal FDA database, DARRTS, 2) ClinicalTrials.gov, and 3) PubMed. The database searches were limited to documents generated/published from January 1, 2005 to December 31, 2016. Keyword searches were conducted in these databases using all known synonyms of KIM-1(i.e., Kidney Injury Molecule-1, Hepatitis A virus cellular receptor 1, HAVCR-1, T cell immunoglobulin domain and mucin domain protein 1, TIM-1, and TIMD1). A full text review of the documents identified through the keyword search was performed, and documents containing references not related to the biomarker “KIM-1” (including names, part of an address, or reference to an unrelated entity, such as a cell line) were removed. In addition, documents that described or referred to the use of KIM-1 as a therapeutic target were also excluded.
DARRTS search
DARRTS is an information technology platform for managing, tracking, and reporting on drug applications. A Communication Content Search was conducted in DARRTS using the dates and keywords for KIM-1 described above. The Communication Content Search is a text search of all FDA-generated documents (also referred to as FDA communications) associated with individual drug applications that are recorded in DARRTS: reviews, correspondences, and application forms. Documents identified by the search that referenced the use of urinary KIM-1 were selected. Next, documents that did not refer to KIM-1 as a drug development tool were removed from the results. Further analysis was limited to FDA-generated documents associated with individual INDs since INDs are likely to be the fastest to incorporate KIM-1 and are closest to the actual study using KIM-1. The results were grouped by the year, and results for 2008 were further divided into two groups: 1) January 1 –April 13 and 2) April 14 –December 31.
To evaluate references to KIM-1 use in FDA-generated documents for detection of kidney injury in drug development, the references to KIM-1 were grouped by their associated INDs and each IND was sorted by the year of the earliest communication date. To evaluate references to the use of KIM-1 in applications across review divisions in CDER, applications were then sorted by the responsible review division in CDER’s Office of New Drugs. To evaluate the contribution of FDA reviewers and sponsors, the documents associated with regulatory applications were organized by recommendations for KIM-1 use by FDA reviewers and references to its planned use or actual use by sponsors in review documentation. To evaluate reference to the use of KIM-1 as a drug development tool in different species, the documents associated with regulatory applications were organized into one of the following categories determined by the study application of KIM-1: rat studies, nonclinical studies (excluding rats), and clinical studies.
ClinicalTrials.gov search
ClinicalTrials.gov is a registry and results database with information on publicly and privately funded clinical studies on a wide range of diseases and conditions. [12]
An advanced search was conducted in ClinicalTrials.gov using the dates and keywords for KIM-1 described above. The advanced search is a text search of summary information about the clinical study protocol that includes the disease or condition; intervention; title, description, and design of the study; eligibility criteria; study locations and contact information; and sometimes includes the study outcomes. Results identified by the search that referenced use of urinary KIM-1 were selected. Next, the documents that did not reference the use of KIM-1 for detection of kidney injury, including drug-induced nephrotoxicity, were removed. The results were grouped by the year the trial started, and trials starting in 2008 were further divided into two groups determined by the range of months the trial started: 1) January–April and 2) May–December.
PubMed search
PubMed is an online search engine that accesses a MEDLINE database containing over 27 million citations for biomedical literature compiled by the United States National Library of Medicine. [18] A search was conducted in PubMed using the dates and keywords for KIM-1 described above. Results identified by the search were removed if the citation did not provide access to the full article written in English, or did not reference the use of KIM-1 for detection of kidney injury, including drug-induced nephrotoxicity. The search results included the use of KIM-1 from multiple sources such as urine, plasma/serum or kidney tissue as well as two forms, protein and mRNA.
The remaining articles were divided into research articles and review articles and were grouped by the year published. The results were grouped by the year, and results for 2008 were further divided into two groups: 1) January–April and 2) May–December.
Articles were reviewed and references to the qualification were counted. References to KIM-1 in research articles were sorted into one of the following categories determined by the study application of KIM-1: nonclinical studies, and clinical studies. Lastly, the use of urinary KIM-1 protein as a biomarker of kidney injury, including drug-induced nephrotoxicity in rats in research articles grouped by the year.
Study Highlights.
What is the current knowledge on the topic?
A: It has so far not been evaluated if use of KIM-1, or any other biomarker qualified by CDER, FDA, for drug development or research, has changed after qualification.
What question did this study address?
A: The goal of this study was to evaluate the use of KIM-1 in drug development and research before and after the qualification of KIM-1.
What does this study add to our knowledge?
A. Analysis of search results from three databases showed an increase in the use of KIM-1 in drug development and research. These results support CDER’s hypothesis that public posting of the qualified biomarkers along with the supportive data may encourage an increase in the use of the biomarkers in drug development.
How might this change clinical pharmacology or translational science?
A. KIM-1 and other qualified nonclinical safety biomarkers hold considerable promise if they can be established as translational biomarkers to be used both in nonclinical and clinical studies, to predict toxicity across species and may speed innovation for many different types of therapeutics.
Acknowledgments
Funding
Dr. Joachim Ix was supported by a grant from the National Institute of Kidney and Diabetes and Digestive Diseases (NIDDK) K24DK110427.
This project was supported in part by an appointment to the Research Participation Program at CDER administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration.
Thanks to Dr. ShaAvhrée Buckman-Garner, Dr. Norman L Stockbridge, Dr. Elizabeth Hausner, Dr. Carolina Panico, Dr. Robert O’Neill, Mark Geanacopoulos, Dr. Mitra Ahadpour and Dr. Juan Ruiz for review and comments on the manuscript. Thanks to Dr. Ling Lan for her statistical input and expertise.
Abbreviations
- BLA
Biologics License Application
- CDER
Center for Drug Evaluation and Research
- COU
Context of Use
- DAAAP
Division of Anesthesia, Analgesia, and Addiction Products
- DAIP
Division of Anti-Infective Products
- DARRTS
Document Archiving, Reporting, and Regulatory Tracking System
- DAVP
Division of Anti-Viral Products
- DBRUP
Division of Bone, Reproductive and Urologic Products
- DCRP
Division of Cardiovascular and Renal Products
- DDDP
Division of Dermatology and Dental Products
- DGIEP
Division of Gastroenterology and Inborn Errors Products
- DHP
Division of Hematology Products
- DMEP
Division of Metabolic and Endocrine Products
- DMIP
Division of Medical Imaging Products
- DNP
Division of Neurology Products
- DOP
Division of Oncology Products
- DPARP
Division of Pulmonary, Allergy and Rheumatology Products
- DPP
Division of Psychiatry Products
- DSPTP
Division of Special Pathogen and Transplant Products
- DTOP
Division of Transplant and Ophthalmology Products
- FDA
Food and Drug Administration
- IND
Investigational New Drug Application
- KIM-1
Kidney Injury Molecule-1
- NDA
New Drug Application
- OND
Office of New Drugs
Footnotes
Conflict of Interest/Disclosure
The authors declared no competing interests for this work.
Disclaimer:
This article reflects the view of the authors and should not be construed to represent FDA’s views and policies.
Author Contributions
R.C., S.S., A.T., J.H.I., K.H., L.M., and S.A. wrote the manuscript; S.A., R.C., and S.S. designed the research; R.C. performed the research; R.C., S.S., A.T., and S.A. analyzed the data.
References
- 1.FDA-NIH Biomarker working Group. [Accessed 9 April 2018];BEST (Biomarker, EndpointS, and other Tools) Resource. 2016 < https://www.ncbi.nlm.nih.gov/books/NBK326791/>. [PubMed]
- 2.Food and Drug Administration. [Accessed 9 April 2018];Challenge and Opportunity on the Critical Path to New Medical Products. 2004 < http://www.who.int/intellectualproperty/documents/en/FDAproposals.pdf>.
- 3.Food and Drug Administration. [Accessed 9 April 2018];Guidance for Industry and FDA Staff: Qaulification Process for Drug Development Tools. 2014 < https://www.fda.gov/downloads/drugs/guidances/ucm230597.pdf>.
- 4.Food and Drug Administration. [Accessed 9 April 2018];Biomarker Qualification Program. < https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/BiomarkerQualificationProgram/default.htm>.
- 5.Food and Drug Administration. [Accessed 9 April 2018];List of Qualified Biomarkers. < https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/BiomarkerQualificationProgram/ucm535383.htm>.
- 6.Goodsaid F, Papaluca M. Evolution of biomarker qualification at the health authorities. Nat Biotechnol. 2010;28:441–3. doi: 10.1038/nbt0510-441. [DOI] [PubMed] [Google Scholar]
- 7.Dieterle F, et al. Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol. 2010;28:463–9. doi: 10.1038/nbt.1622. [DOI] [PubMed] [Google Scholar]
- 8.Dieterle F, et al. Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol. 2010;28:455–62. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- 9.Food and Drug Administration. [Accessed 9 April 2018];Biomarker qualification letter RE: Review Submission of the Qualification of Seven Biomarkers of Drug-Induced Nephrotoxicity in Rats. 2008 < http://wayback.archive-it.org/7993/20170723111045/https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/UCM285031.pdf>.
- 10.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–63. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 11.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–29. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 12.U.S. National Library of Medicine. [Accessed 9 April 2018];ClinicalTtrials.gov Website. < https://clinicaltrials.gov/>.
- 13.US National Library of Medicine NIH. [Accessed 9 April 2018];PubMed. < https://www.ncbi.nlm.nih.gov/pubmed/>.
- 14.Chaturvedi S, Farmer T, Kapke GF. Assay validation for KIM-1: human urinary renal dysfunction biomarker. Int J Biol Sci. 2009;5:128–34. doi: 10.7150/ijbs.5.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dennis Eslie H, Walker Elizabeth G, Baker Amanda F, Miller Richard T. Opportunities and Challenges of Safety Biomarker Qualification: Perspectives from the Predictive Safety Testing Consortium. Drug Development Research. 2013;74:112–26. [Google Scholar]
- 16.van Meer L, Moerland M, Cohen AF, Burggraaf J. Urinary kidney biomarkers for early detection of nephrotoxicity in clinical drug development. Br J Clin Pharmacol. 2014;77:947–57. doi: 10.1111/bcp.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tekce BK, Uyeturk U, Tekce H, Uyeturk U, Aktas G, Akkaya A. Does the kidney injury molecule-1 predict cisplatin-induced kidney injury in early stage? Ann Clin Biochem. 2015;52:88–94. doi: 10.1177/0004563214528312. [DOI] [PubMed] [Google Scholar]
- 18.(US), N.C.f.B.I. [Accessed 9 April 2018];PubMed Help. 2005 < https://www.ncbi.nlm.nih.gov/books/NBK3827/>.