Abstract
Background
HIV is a neuropathogenic virus that may result in detrimental neurodevelopmental (ND) outcomes early in life. This is the first study to evaluate the effect of HIV-1 subtype on neurodevelopment of Ugandan pre-school children.
Methods
Neurodevelopment of 87 HIV-1 infected and 221 HIV exposed uninfected (HEU) Ugandan children aged 1.8 to 4.9 years was assessed using four scales of the Mullen Scales of Early Learning (MSEL), two scales of the Color Object Association Test (COAT), and one score of the Early Childhood Vigilance Test (ECVT). HIV-1 subtype was defined by phylogenetic analyses. General linear models were used to relate test scores to HIV-1 subtype (A versus D) while adjusting for relevant covariates. The scores were benchmarked against HEU group to facilitate the interpretation.
Results
Seventy-one percent of children infected with subtype A vs. 60% of children with subtype D were currently on antiretroviral therapy (ART) (p=0.49). Children with HIV-1 subtype A infection were older when compared to subtype D (3.29 vs. 2.76 years, respectively, p=0.03), but similar regarding sex, socioeconomic status, weight-for-age z-score, CD4+ and CD8+ (% and total), viral load. No statistically significant differences by HIV-1 subtype were observed in the MSEL, COAT, and ECVT. Differences ≥0.33 of the standard deviation were observed for the MSEL Composite Score, Receptive Language (MSEL), and Total Memory (COAT).
Conclusions
In contrast to previously reported differences in ND outcomes of school-aged children by HIV-1 subtype, ND scores among pre-school children were similar for subtypes A and D, with few potential differences on language production and memory outcomes that favored subtype A. Further investigation with larger sample sizes and longitudinal follow-up is needed.
Keywords: HIV subtype, children, neurodevelopment
HIV's entry into the central nervous system (CNS) occurs early in the course of infection[1]. Being neuropathogenic, HIV can cross the blood brain barrier inside target cells, can infect target cells within this system, and may have a severe negative effect on neurodevelopment[2]. HIV has been shown to have a detrimental effect on cognition and motor functions of perinatally infected children [3], even among children who have a CD4 cell count > 350 cells/μl [4]. In conjunction with environmental factors, poverty, and nutrition, HIV has emerged as an additional potential cause of neurodevelopmental (ND) delay among children in countries that have high disease prevalence[5-8]. The virus has been shown to have a direct effect on overall ND, including motor deficits, visual and learning disorders among children with and without antiretroviral therapy (ART) [3, 9]. ART scale-up in low-income countries contributes to neurodevelopmental improvement in infected children; however, social and environmental factors also have an important role on a child's ND [8, 10]. The association between HIV infection and ND in resource-constrained settings is complex and intertwined with multiple proximal and distal factors, including nutrition, access to early ART, and the child's learning environment [11]. Thus, it is fundamental to identify neurocognitive deficits early in life and ensure access to interventions that aim to enhance neurocognition.
HIV-1 is divided into three groups: M (major), O (outlier) and N (new, non-M, and non-O) [12]. Group M is subdivided into nine different subtypes (A-D, F-H, J, K), and multiple recombinants exist with different geographic distributions [13]. In East Africa, for example, subtypes A, C, and D are the most prevalent [14], and, specifically in Uganda, HIV subtypes A and D are the most common [14]. HIV subtypes are classified phylogenetically on the basis of differences in their envelope nucleotide sequences [15].
To the best of our knowledge, only a handful of studies have reported on the neurocognitive performance of Ugandan adults and children based on HIV subtype, immunological, and ART status [16-18]. Among school aged Ugandan HIV- infected children who had not yet received ART, HIV subtype A infection was associated with children having poorer neurocognitive performance when compared to subtype D [16]. However, Bangirana et al., reported no differences in neurocognitive outcomes when comparing Ugandan children on ART infected by HIV subtype A or D [19].
Given that findings reported in the literature have been inconsistent and mainly focused on school-age children, we aimed to evaluate differences in neurocognitive outcomes among young Ugandan HIV-infected children on the basis of HIV subtype and to compare their outcomes to those of HIV exposed uninfected (HEU) children.
Methods
Participants
This is a secondary data analysis of baseline data from 87 HIV infected children and 221 HEU controls who participated in a randomized controlled trial of a parenting intervention called Mediational Intervention for Sensitizing Caregivers (MISC). During enrollment, all children underwent physical examination by trained staff, which included auditory and visual testing. HIV infected children aged 2 to 5 years with no history of non-HIV illness or injury that could have caused a CNS insult, including HIV encephalopathy, were recruited from The AIDS Support Organization (TASO) treatment center in Tororo, along with TASO satellite clinics from the surrounding area of Busia. After consent, 120 HIV-infected children were enrolled and evaluated between March 2012 and April 2013. HIV status was confirmed by blood test upon enrollment into the MISC trial. Dried blood spots (DBS) were collected for all participants and stored for analysis. Out of the 120 HIV infected children who were enrolled into MISC, sufficient viral material to conduct subtype assignment was available for 87 children (72.5%). No statistically significant differences in socioeconomic or biological characteristics were observed between HIV infected children with subtype (n=87) assignment and those without (n=33). HEU children were recruited from rosters of a completed malaria chemoprevention study [20] (n=175) or by referral from a trial of prevention of mother to child transmission of HIV (n=46) at the Infectious Disease Research Collaboration at Tororo District Hospital, Uganda [21].
Study procedures and ethical considerations
Child neurocognitive measures were administered in the project office by trained testers in one of three local languages (Japhadola, Ateso, or Luganda) between March and September of 2012. Study testers were trained on site by the PI and co-author (MJB). On site test supervision was provided by the following authors: HRE, IF, RO, and MJB. Quality assurance and re-training was provided by data managers on-site and authors: IF, MJB, and HRE and by following standardized operating procedures developed for each test.
The Institutional Review Boards of Michigan State University, the School of Medicine Research Ethics Committee at Makerere University, and the Ugandan National Council for Science and Technology approved this study. The trial was registered on ClinicalTrials.gov (NCT01640561).
Measures
Child demographics included age, sex, and parent report of current ART (yes/no). Weight-for-age z-scores were computed using the macro created by the World Health Organization [22].
An economic wealth index was constructed from a checklist of eight material possessions (shoes, radio, mattress, blanket, bicycle, motorcycle, cows, and goats) and six housing quality items (type of roof, availability of water supply, type of fuel used, frequency of meat in diet, and food security). Respondents reported presence/absence of each asset at their homes. Top 20th, middle 60th, and bottom 20th percentiles were defined based on factor scores derived from principal component analysis of the 14 items.
The MSEL is a comprehensive test measuring specific developmental domains. Items are combined into four cognitive scales (Visual Reception, Fine Motor, Receptive Language and Expressive Language) that can be summarized to yield an overall score (MSEL Composite score) as well as domain-specific sub-scores. The Composite score serves as a general measure of fluid intelligence reflective of general cognitive ability [23]. Age-adjusted T-scores were obtained from standardized samples from the U.S., and lower scores indicate diminished cognitive abilities. The Mullen test has been extensively used by our group with young children in rural Uganda showing high sensitivity [24-26].
The COAT was used to assess visual memory independent of language competency [27]. The principal outcomes are immediate recall and total recall Lower scores indicate poorer performance. The COAT has been previously used as an experimental measure of memory for Ugandan children [25]..
The ECVT is a measure of vigilance used in preschool children to evaluate sustained attention [28, 29]. Children are required to monitor a colorful computer screen for the sudden appearance of active and engaging cartoon. The principal outcome is the proportion of time looking at the animation as scored from a video recorded from a webcam mounted on the computer.
Home Observation for the Measurement of the Environment (HOME) [30] was used to measure quality of child-caregiver interactions in the home environment. This measure has been validated previously in Uganda [31]. For this analysis we use the Infant-Toddler version, which includes 45 yes/no items. A total HOME score was generated by summing the number of ‘yes’ responses to each item, with higher scores indicating higher quality.
HIV subtype assignment for either A or D was done using a modification of a previously published method [32]. PCR-based assay with sequencing of a 324 bp of gp41 ENV gene. Subtype was defined by phylogenetic analyses with subtype references. HIV subtype was classified as being subtype A or D on the basis of ENV sequencing. For all children, baseline viral load testing, CD4 and CD8 cell counts were completed using a 5 ml blood draw at each visit, with samples evaluated by the Joint Clinical Research Center Mbale District Hospital. HIV subtype assignment was performed at Johns Hopkins Medical Institutions, Baltimore, Maryland.
Statistical Analysis
Distribution of demographics and socio-economic characteristics, as well as immunological and neurodevelopmental scores were summarized according to HIV status and subtype classification. Two sets of general linear models (GLM) were built. First, unadjusted models related the COAT, MSEL, and ECVT (one at a time) to a 3-level variable reflecting membership in the uninfected reference group, HIV subtype A or HIV D subtype group. Second, age, sex, weight-for-age, socio-economic status, and HOME score were included in our first adjusted model. The p-values for the differences among three groups (subtype A, subtype D, and HEU) were reported, as well as the p-values for the tests comparing HIV subtypes A versus D. Third, ART status and viral load were added to the adjusted model to estimate the effects of HIV subtype on neurodevelopment over and above these factors. Given that key variables, such as viral load and ART status are only relevant among those infected, the adjusted models did not include the HEU group. The least square (LS) means by HIV subtype were output from the adjusted models, and differences between them were tested. All statistical tests were two-sided.
The available sample sizes for the HIV subtypes allowed to detect mean differences of at least 0.75 of the standard deviation with power of 0.80 or greater in two-sided tests conducted at 0.05 level of significance. Differences of smaller magnitude may not have been detected as statistically significant, therefore the p-values in tables are accompanied by the estimates of the effect sizes. The effect sizes for the comparisons by subtype are expressed as Cohen's d, difference between means in the standard deviation units in the unadjusted analyses, and differences between the LS means divided by the square root of the mean squared error in the adjusted analyses. Analyses were performed in SAS 9.4 and STATA 12.
Results
Group A children were significantly older than group D (3.29 vs. 2.76 years, p=<.01) (Table 1), with no other pairwise group differences. Three groups also differed with respect to WAZ (p=.04), with HEU children having lower WAZ (-1.20) than subtype A group (-0.80), p=.02. Mean WAZ for group D (-0.72) was not different from HEU (p=.12) due to the sample size of group D. The proportion of boys differed across three groups (p<.01), with subtype D having the lowest point estimate for the percentage of boys (22%), however test of pairwise differences in proportions with HEU and A groups resulted in p-values of 0.09 and 0.08, respectively.
Table 1. Demographic characteristics and immunological parameters of 87 children living with HIV and 221 uninfected controls in Tororo, Uganda.
| HIV-1 Subtype | HEU group | ||||
|---|---|---|---|---|---|
| Characteristics | A N=69 (79.3%) | D N=18 (20.7%) | P-value for subtype comparison | Uninfected reference group N=221 | P-value for comparison of A, D, and reference groups |
| Age (yrs), Mean (SD) | 3.29 (0.93) | 2.76 (0.74) | <.01 | 2.87 (0.42) | <0.01 |
| Sex, N (%) | 0.08 | <0.01 | |||
| Boys | 31 (45) | 4 (22) | 125 (57) | ||
| Girls | 38 (55) | 14 (78) | 96 (43) | ||
| Wealth Group | 0.99 | 0.72 | |||
| Lowest 20% | 12 (17) | 3 (17) | 46 (21) | ||
| Middle 60% | 46 (67) | 12 (67) | 124 (58) | ||
| Upper 20% | 11 (16) | 3 (16) | 45 (21) | ||
| WAZ, Mean (SD) | -0.80 (1.65) | -0.72 (1.40) | 0.84 | -1.20 (1.12) | 0.04 |
| CD4+ %, Mean (SD) | 45.63 (13.81) | 43.47 (14.40) | 0.57 | N/A | |
| CD8+ %, Mean (SD) | 47.57 (13.88) | 45.00 (15.66) | 0.51 | N/A | |
| CD4+ lymphocyte count (copies/ml), Mean (SD) | 1439.6 (801.5) | 1362.9 (744.5) | 0.72 | N/A | |
| CD8+ lymphocyte count (copies/ml), Mean (SD) | 1544.3 (947.7) | 1270.5 (641.5) | 0.26 | N/A | |
| Log plasma HIV RNA level (copies/ml), Mean (SD) | 8.58 (2.90) | 10.01 (2.92) | 0.09 | N/A | |
| Currently on ART, N (%) | 0.49 | ||||
| No | 21 (30) | 7 (39) | N/A | ||
| Yes | 48 (70) | 11 (61) | N/A |
HEU: HIV Exposed Uninfected
Statistically significant differences in the adjusted means among three groups were observed in ECVT scores in the unadjusted model (HEU mean=0.69 vs. group A mean=0.62 vs. group D mean=0.55, p<0.01) (Table 2). Effect sizes of subtype A vs. D were near ½ of the standard deviation for COAT Total Memory (d=0.47, difference of 4.45 points) and ECVT scores (d=0.53, difference of 0.07). Figure 1 shows the unadjusted mean differences for the five MSEL domains and the Composite score. In the adjusted analysis that include HEU effect sizes over 1/3 of the standard deviation were observed for Gross Motor (d=0.34, difference of 1.05 points) Visual Reception (d=0.39, difference of 1.68 points) and the Composite score (d=0.38, difference of 5.3 points) of the MSEL (Table 2).
Table 2. Unadjusted and adjusted least square means, and 95% confidence intervals for the MSEL, COAT and ECVT scores of 87 children living with HIV and 221 uninfected controls in Tororo, Uganda.
| Adjusted Means by HIV-1 subtype | Adjusted mean for reference group | ||||
|---|---|---|---|---|---|
| Domains | A LS Mean (95% CI) | D LS Mean (95% CI) | A v. D P-value (effect size) | HEU** LS Mean (95% CI) | P-value for comparison of A, D, and reference groups |
| ECVT | |||||
| Proportion of time looking | 0.59 (0.54, 0.64) | 0.56 (0.49, 0.65) | 0.58 (0.18) | 0.70 (0.67, 0.72) | <0.01 |
| COAT | |||||
| Immediate Memory | 2.75 (0.24, 5.26) | 4.25 (-0.34, 8.83) | 0.56 (0.16) | 2.11 (0.69, 3.53) | 0.64 |
| Total Memory | 6.76 (3.49, 10.02) | 5.07 (-0.91, 11.05) | 0.62 (0.14) | 5.97 (4.12, 7.82) | 0.86 |
| MSEL | |||||
| Gross Motor | 25.34 (24.55, 26.14) | 26.39 (24.93, 27.85) | 0.21 (0.34) | 26.99 (26.54, 27.44) | <0.01 |
| Fine Motor | 33.51 (30.80, 36.22) | 29.44 (24.48, 34.40) | 0.15 (0.18) | 34.05 (32.52, 35.59) | 0.21 |
| Visual Reception | 32.12 (29.63, 34.61) | 30.44 (25.89, 34.99) | 0.52 (0.39) | 29.09 (27.68, 30.50) | 0.10 |
| Receptive Language | 37.90 (35.47, 40.34) | 35.21 (30.76, 39.66) | 0.29 (0.29) | 37.97 (36.59, 39.34) | 0.49 |
| Language | 33.90 (31.13, 36.68) | 32.05 (26.98, 37.13) | 0.52 (0.17) | 35.44 (33.87, 37.01) | 0.33 |
| Composite MSEL | 72.31 (68.70, 75.93) | 67.01 (60.40, 73.63) | 0.16 (0.38) | 70.74 (68.69, 72.79) | 0.36 |
Figure 1. Boxplot of Mullen Scales of Early Learning Scores by HIV-1 subtype.
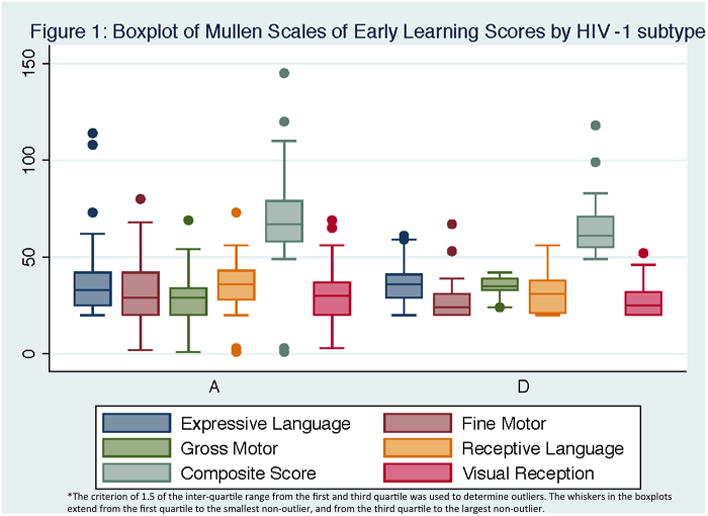
In adjusted analyses, children with HIV subtype A had higher LS mean (LSM) scores in the MSEL Composite (LSM=72.97 vs. LSM=66.16, d=0.39), Receptive Language (LSM=38.17 vs. LSM=33.76, d=0.41), and Total Memory of the COAT (LSM=9.30 vs. LSM=5.40, d=0.42) when compared to children with HIV subtype D (Table 3).
Table 3. Adjusted least square means, and 95% confidence intervals for the MSEL, COAT and ECVT scores of 87 children living with HIV living in Tororo, Uganda.
| Adjusted Means by HIV-1 subtype | |||
|---|---|---|---|
| Domains | A LS Mean (95% CI) | D LS Mean (95% CI) | P-value (effect size) |
| ECVT | |||
| Proportion of time looking | 0.62 (0.58, 0.65) | 0.60 (0.53, 0.68) | 0.75 (0.08) |
| COAT | |||
| Immediate Memory | 3.84 (2.83, 4.84) | 3.75 (1.86, 5.63) | 0.93 (0.03) |
| Total Memory | 9.30 (6.49, 12.12) | 5.40 (0.12, 10.69) | 0.16 (0.42) |
| MSEL | |||
| Gross Motor | 26.31 (25.18, 27.46) | 27.18 (25.22, 29.13) | 0.42 (0.23) |
| Fine Motor | 33.26 (29.28, 37.24) | 29.36 (21.88, 36.83) | 0.32 (0.30) |
| Visual Reception | 32.10 (29.06, 35.15) | 30.72 (25.00, 36.44) | 0.65 (0.14) |
| Receptive Language | 38.17 (34.89, 41.44) | 33.76 (27.61, 39.91) | 0.18 (0.41) |
| Expressive Language | 35.74 (32.22, 39.27) | 32.87 (26.25, 39.49) | 0.41 (0.28) |
| Composite MSEL | 72.97 (67.65, 78.29) | 66.16 (56.18, 76.15) | 0.20 (0.39) |
Adjusted for age, sex, HOME score, socio-economic status, weight-for-age z-score, HAART status and viral load
MSEL: Mullen Scales of Early Learning, COAT: Color Object Association Test; ECVT: Early Childhood Vigilance Test
Bold type denotes statistically significant (P-value<0.05)
Discussion
This work represents the first study evaluating ND in pre-school children by HIV subtype in sub-Saharan Africa using a homogeneous sample of children in terms of socio-demographic, immunological, and treatment status. Results presented here suggest that even though the statistical significance for the differences in infant neurodevelopment outcomes for subtypes A versus D was not reached, some of the outcomes, such as language production and memory, may be better among those with subtype A based on the standardized effect sizes. After adjusting for potential confounding factors, other outcomes were similar for the two subtype groups and the control group of HEU children.
Research on the effects of HIV subtype on ND in children is limited and supports the hypothesis that HIV subtype A has a greater impact on cognitive ability and visual attention than subtype D [16]. On the other hand, studies describing the impact of HIV on adult ND suggest that subtype D has a greater negative impact, however, results are difficult to extrapolate to children living with HIV [17, 18, 33-35]. In our results, adjusted models showed higher scores with differences near 0.4 of the standard deviation among infants infected with subtype A when compared to subtype D in Receptive Language and Composite scores of the MSEL, and for the Total Memory score of the COAT. Findings presented in this analysis can be viewed as hypothesis-generating. The hypothesis that general cognitive ability and Receptive Language might be affected early on among children infected with HIV subtype D will need future testing with larger samples. Our findings and this hypothesis are consistent with what has been reported among adults infected with HIV subtype D in Uganda [18].
However, our findings regarding infant neurodevelopment differ from those reported in a previous study by our group in Uganda among a sample of ART naive children 6-12 years of age, where poorer neurocognitive performance in children infected with HIV subtype A was reported [16]. That study used a different set of neurocognitive tests that were appropriate for school-age children (Kaufman Assessment Battery for Children Second Edition, KABC-II; Bruininks-Oseretsky Test for Motor Proficiency second edition, BOT-2; and the Test of Variables of Attention, TOVA), and viral loads among children infected with HIV subtype A were higher than in those infected with subtype D [16]. In the present study, while older age of the subtype A group compared to subtype D can explain some of the differences in the unadjusted means, the means adjusted for age and other potential confounders still favored subtype A. Also, a similar proportion of children infected with subtype A and D were currently on ART. This could result in less neuropathogenic effects among these children, highlighting the importance of providing treatment to all children living with HIV [19, 36]. However, caution is warranted when interpreting the effects of HIV subtype on children's ND scores given the small sample size and the lack of statistical significance observed when comparing subtype groups. Nonetheless, these studies add to the knowledge on the effect of HIV subtypes on specific areas of the developing brain at different developmental stages.
One mechanism that has been hypothesized for HIV subtype A's higher degree of encephalopathogenicity relative to subtype D is on the basis of chemokine receptor tropisim (CCR5 vs. CXCR4) [37]. CCR5 may facilitate infection of macrophages, HIV's main target cells for transport across the blood brain barrier into the CNS, resulting in infection of microglia and other target cells in the CNS [38]. However, the association between -1HIV and neuropathogenesis is likely to be more complex than the association between HIV subtype and chemokine receptors, involving other factors such as age, socio-economic and ART status, time of ART initiation, type of ART being received, ART adherence, viral load, and nutritional status.
Results presented here should be viewed in light of certain limitations. First, our results are based on the associations using cross-sectional data, hindering our capacity to establish causality. Children included in this study were evaluated at a young age prior to development of cognitive skills that can potentially be measured later in life. Second, standard interpretation of T-scores for the MSEL proposes that any scores at or below 7th percentile or -1.5 to -2 standard deviations below the average indicate significant delays. However, without specific norms for this Ugandan population, it is difficult to establish appropriate cut-off scores to gauge clinical significance of any differences. Therefore this study relied upon the standardized effect size to address the magnitude of the differences. Also, sample size was too small to analyze the interaction between ART status and HIV subtype in relation to the neurocognitive outcomes. Finally, only 18 children with HIV subtype D were included in the analysis, which may result in wide confidence intervals and thus, should be interpreted with caution.
Conclusions
ND in young children (2- 5 years old) infected with HIV subtype A compared to subtype D was similar, with some differences favoring subtype A found in language development, overall cognition, and memory. The estimates of the effect sizes provided in this report can be used to formally power future prospective studies, including longitudinal studies that would test the association between HIV subtype and neurodevelopment and capture the complex pathological interaction between HIV and CNS, while accounting for other important factors.
Acknowledgments
Dr. Ruiseñor-Escudero conceptualized and designed the study, drafted the initial manuscript and approved the final manuscript as submitted.
Dr. Sikorskii conducted data analysis and critically reviewed and approved the final manuscript.
Drs. Familiar Lopez and Boivin critically reviewed all sections of the manuscript providing substantial input.
Drs. Persaud and Ziemniack did HIV subtype characterization and provided input to the manuscript.
Drs. Nakasujja and Opoka coordinated and supervised data collection and reviewed the manuscript before final submission.
All authors approved the final manuscript as submitted and agree to accountable for all aspects of the work.
This research was supported by NIH grant R01 HD070723 (PIs: Boivin, Bass). Thanks to all the work of the testers, trainers and staff working in Tororo, Uganda.
Financial Disclosure: National Institutes of Health (NIH) grant# RO1 HD070723. HIV subtyping was supported by the Department of Psychiatry at Michigan State University.
Funding sources: All phases of this study were supported by the National Institutes of Health (NIH) grant# RO1 HD070723. HIV subtyping was supported by the Department of Psychiatry at Michigan State University.
Footnotes
Conflict of Interest: The authors have no potential conflict of interest.
Clinical Trial Registration: clinicaltrials.gov Identifier NCT01640561
References
- 1.Kaul M, Zheng J, Okamoto S, Gendelman H, Lipton S. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death & Differentiation. 2005;12:878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 2.Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Current HIV/AIDS Reports. 2011;8(1):54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nozyce M, Hittelman J, Muenz L, Durako SJ, Fischer ML, Willoughby A. Effect of perinatally acquired human immunodeficiency virus infection on neurodevelopment in children during the first two years of life. Pediatrics. 1994;94(6 Pt 1):883–891. [PubMed] [Google Scholar]
- 4.Ruel TD, Boivin MJ, Boal HE, Bangirana P, Charlebois E, Havlir DV, et al. Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clinical Infectious Diseases. 2012:cir1037. doi: 10.1093/cid/cir1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, et al. Child development: risk factors for adverse outcomes in developing countries. The lancet. 2007;369(9556):145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 6.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B, et al. Developmental potential in the first 5 years for children in developing countries. The lancet. 2007;369(9555):60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engle PL, Black MM, Behrman JR, De Mello MC, Gertler PJ, Kapiriri L, et al. Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. The lancet. 2007;369(9557):229–242. doi: 10.1016/S0140-6736(07)60112-3. [DOI] [PubMed] [Google Scholar]
- 8.Sherr L, Croome N, Castaneda KP, Bradshaw K, Romero RH. Developmental challenges in HIV infected children—An updated systematic review. Children and Youth Services Review. 2014;45:74–89. [Google Scholar]
- 9.Blanchette N, Smith ML, King S, Fernandes-Penney A, Read S. Cognitive development in school-age children with vertically transmitted HIV infection. Developmental neuropsychology. 2002;21(3):223–241. doi: 10.1207/S15326942DN2103_1. [DOI] [PubMed] [Google Scholar]
- 10.Le Doaré K, Bland R, Newell ML. Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Pediatrics. 2012:2012–0405. doi: 10.1542/peds.2012-0405. peds. [DOI] [PubMed] [Google Scholar]
- 11.Boivin MJ, Ruiseñor-Escudero H, Familiar-Lopez I. CNS Impact of Perinatal HIV Infection and Early Treatment: the Need for Behavioral Rehabilitative Interventions Along with Medical Treatment and Care. Current HIV/AIDS Reports. 2016;13(6):318–327. doi: 10.1007/s11904-016-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liner KJ, Hall CD, Robertson KR. Impact of human immunodeficiency virus (HIV) subtypes on HIV-associated neurological disease. Journal of neurovirology. 2007;13(4):291–304. doi: 10.1080/13550280701422383. [DOI] [PubMed] [Google Scholar]
- 13.LANL. HIV circulating recombinant forms (CRFs) 2014 [Google Scholar]
- 14.Hemelaar J, Gouws E, Ghys PD, Osmanov S. Global trends in molecular epidemiology of HIV-1 during 2000-2007. AIDS (London, England) 2011;25(5):679–689. doi: 10.1097/QAD.0b013e328342ff93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spira S, Wainberg MA, Loemba H, Turner D, Brenner BG. Impact of clade diversity on HIV-1 virulence, antiretroviral drug sensitivity and drug resistance. Journal of Antimicrobial Chemotherapy. 2003;51(2):229–240. doi: 10.1093/jac/dkg079. [DOI] [PubMed] [Google Scholar]
- 16.Boivin MJ, Ruel TD, Boal HE, Bangirana P, Cao H, Eller LA, et al. HIV-subtype A is associated with poorer neuropsychological performance compared with subtype D in antiretroviral therapy-naive Ugandan children. AIDS (London, England) 2010;24(8):1163–1170. doi: 10.1097/qad.0b013e3283389dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacktor N, Nakasujja N, Redd AD, Manucci J, Laeyendecker O, Wendel SK, et al. HIV subtype is not associated with dementia among individuals with moderate and advanced immunosuppression in Kampala, Uganda. Metabolic brain disease. 2014 doi: 10.1007/s11011-014-9498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacktor N, Nakasujja N, Skolasky RL, Rezapour M, Robertson K, Musisi S, et al. HIV subtype D is associated with dementia, compared with subtype A, in immunosuppressed individuals at risk of cognitive impairment in Kampala, Uganda. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;49(5):780–786. doi: 10.1086/605284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bangirana P, Ruel TD, Boivin MJ, Pillai SK, Giron LB, Sikorskii A, et al. Absence of neurocognitive disadvantage associated with paediatric HIV subtype A infection in children on antiretroviral therapy. Journal of the International AIDS Society. 2017;20(2) doi: 10.1002/jia2.25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamya MR, Kapisi J, Bigira V, Clark TD, Kinara S, Mwangwa F, et al. Efficacy and safety of three regimens for the prevention of malaria in young HIV-exposed Ugandan children: a randomized controlled trial. AIDS (London, England) 2014;28(18):2701–2709. doi: 10.1097/QAD.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marquez C, Okiring J, Chamie G, Ruel TD, Achan J, Kakuru A, et al. Increased morbidity in early childhood among HIV-exposed uninfected children in Uganda is associated with breastfeeding duration. Journal of tropical pediatrics. 2014;60(6):434–441. doi: 10.1093/tropej/fmu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Child growth standards. Organization WH; 2015. [Google Scholar]
- 23.Mullen E. Mullen scales of early learning. 21. Circle Pines, MN: American Guidance Service Inc; 1995. [Google Scholar]
- 24.Busman RA, Page CF, Oka E, Giordani B, Boivin MJ. Factors contributing to the psychosocial adjustment of Ugandan preschool children with HIV/AIDS. In: Boivin MJ, Giordani B, editors. Neuropsychology of children in Africa: Perspectives on risk and resilience. New York: Springer; 2013. [Google Scholar]
- 25.Boivin MJ, Bangirana P, Nakasujja N, Page CF, Shohet C, Givon D, et al. A year-long caregiver training program improves cognition in preschool Ugandan children with human immunodeficiency virus. The Journal of pediatrics. 2013;163(5):1409–1416e1405. doi: 10.1016/j.jpeds.2013.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koura GK, Boivin MJ, Davidson LL, Ouédraogo S, Zoumenou R, Alao MJ, et al. Usefulness of child development assessments for low-resource settings in francophone Africa. Journal of developmental and behavioral pediatrics: JDBP. 2013;34(7) doi: 10.1097/DBP.0b013e31829d211c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan CM, Johnson AL, Hughes SJ, Shapiro EG. The Color Object Association Test (COAT): the development of a new measure of declarative memory for 18-to 36-month-old toddlers. Child Neuropsychology. 2007;14(1):21–41. doi: 10.1080/09297040601100430. [DOI] [PubMed] [Google Scholar]
- 28.Goldman DZ, Shapiro EG, Nelson CA. Measurement of vigilance in 2-year-old children. Developmental neuropsychology. 2004;25(3):227–250. doi: 10.1207/s15326942dn2503_1. [DOI] [PubMed] [Google Scholar]
- 29.Zelinsky D, Hughes S, Rumsey R, Jordan C, Shapiro E. The early childhood vigilance task: A new technique for the measurement of sustained attention in very young children. Journal of the International Neuropsychological Society. 1996;2(23):7. [Google Scholar]
- 30.Caldwell B, Bradley R. Home Observation for Measurement of the Environment Administration Manual. Tempe, Arizona: Family and Human Dynamics Research Institute, Arizona State University; 2003. [Google Scholar]
- 31.Bangirana P, John CC, Idro R, Opoka RO, Byarugaba J, Jurek AM, et al. Socioeconomic predictors of cognition in Ugandan children: implications for community interventions. PLoS One. 2009;4(11):e7898. doi: 10.1371/journal.pone.0007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redd AD, Mullis CE, Serwadda D, Kong X, Martens C, Ricklefs SM, et al. The rates of HIV superinfection and primary HIV incidence in a general population in Rakai, Uganda. Journal of Infectious Diseases. 2012:jis325. doi: 10.1093/infdis/jis325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaleebu P, French N, Mahe C, Yirrell D, Watera C, Lyagoba F, et al. Effect of human immunodeficiency virus (HIV) type 1 envelope subtypes A and D on disease progression in a large cohort of HIV-1-positive persons in Uganda. J Infect Dis. 2002;185(9):1244–1250. doi: 10.1086/340130. [DOI] [PubMed] [Google Scholar]
- 34.Baeten JM, Chohan B, Lavreys L, Chohan V, McClelland RS, Certain L, et al. HIV-1 subtype D infection is associated with faster disease progression than subtype A in spite of similar plasma HIV-1 loads. J Infect Dis. 2007;195(8):1177–1180. doi: 10.1086/512682. [DOI] [PubMed] [Google Scholar]
- 35.Kiwanuka N, Laeyendecker O, Robb M, Kigozi G, Arroyo M, McCutchan F, et al. Effect of human immunodeficiency virus Type 1 (HIV-1) subtype on disease progression in persons from Rakai, Uganda, with incident HIV-1 infection. The Journal of infectious diseases. 2008;197(5):707–713. doi: 10.1086/527416. [DOI] [PubMed] [Google Scholar]
- 36.Organization WH. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015 [PubMed] [Google Scholar]
- 37.Kaleebu P, Nankya IL, Yirrell DL, Shafer LA, Kyosiimire-Lugemwa J, Lule DB, et al. Relation between chemokine receptor use, disease stage, and HIV-1 subtypes A and D: results from a rural Ugandan cohort. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2007;45(1):28–33. doi: 10.1097/QAI.0b013e3180385aa0. [DOI] [PubMed] [Google Scholar]
- 38.Gray L, Roche M, Churchill MJ, Sterjovski J, Ellett A, Poumbourios P, et al. Tissue-specific sequence alterations in the human immunodeficiency virus type 1 envelope favoring CCR5 usage contribute to persistence of dual-tropic virus in the brain. Journal of virology. 2009;83(11):5430–5441. doi: 10.1128/JVI.02648-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


