Abstract
A respiratory infection caused by antibiotic-resistant bacteria can be life-threatening. In recent years, there has been tremendous effort put towards therapeutic application of bacteriophages (phages) as an alternative or supplementary treatment option over conventional antibiotics. Phages are natural parasitic viruses of bacteria that can kill the bacterial host, including those that are resistant to antibiotics. Inhaled phage therapy involves the development of stable phage formulations suitable for aerosol delivery followed by preclinical and clinical studies for assessment of efficacy, pharmacokinetics and safety. We presented an overview of recent advances in phage formulation for aerosol delivery and their efficacy in acute and chronic rodent lung infection models. We have reviewed and presented on the prospects of inhaled phage therapy as a complementary treatment option with current antibiotics and as a preventative means. Inhaled phage therapy has the potential to transform the prevention and treatment of bacterial respiratory infections, including those caused by antibiotic-resistant bacteria.
Keywords: Bacteriophages, Phages, antibiotic resistant bacteria, formulation, biopharmaceutics, aerosol, respiratory infection
Graphical Abstract
1. Introduction
Bacteriohage (phage) therapy is regaining attention as a potential treatment option for bacterial infections, including those caused by multidrug-resistant (MDR) bacteria. Phage therapy utilizes obligatory lytic phages to kill its host, bacteria. During the lytic cycle of infection, a phage self-replicates by injecting its genetic material into the target bacterium taking over the host’s cellular machinery to synthesize new phages. The phage progeny are released from the host via cell lysis and the cycle restarts. The initial phage infection of bacteria is termed primary infection and the infection of bacteria by the progeny released from lysed cells is termed secondary infection [1]. Passive therapy has been defined as the use of a much higher number of phages in relation to the number of bacterial cells present during a primary infection. In contrast, if the number of phages is lower, and hence the therapy is likely to depend on self-replication and secondary infection of phage, the term active phage therapy is used. In the treatment of bacterial lung infections with bacteriophages, even with a high concentration of phages delivered to the lungs through inhalation, in situ phage replication (secondary infection) might be essential. Through replication, phages may gain access to more secluded and difficult to access infection sites with heavy bacterial load in the deeper lung [2]. Some of the key advantages of phage therapy over conventional antibiotic treatment are that phages are (i) naturally occurring antibacterials with low inherent toxicity, (ii) effective against MDR bacterial infections, (iii) self-replicating agents, (iv) able to co-evolve with bacteria, (v) highly specific nature avoids disturbance of non-targeted bacteria, and (vi) can penetrate biofilms, a problematic state of bacteria in, for example, cystic fibrosis (CF) patients [3, 4].The, species-specific nature of phages is of particular interest as a targeted treatment can limit the unintended adverse impacts on the patient’s microbiome which is commonly observed in antibiotic treatment [5]. However, despite the potential benefits, the use of phages for the treatment of pulmonary bacterial infections has been relatively underexplored.
Inhaled phage therapy was reported in the 1960s and its use was continuously improved in the Eastern European countries, particularly in Georgia, Russia and Poland [6]. Earlier studies on pulmonary phage treatments in humans have recently been reviewed by Abedon [2]. In addition to many successful reports, there have been records of treatment failure most likely due to lack of knowledge of phage diversity, specificity and quality control in the earlier studies [7]. With the recent renewed interest in phage therapy, significant efforts have been poured into understanding its antibacterial activity in preclinical and clinical studies. A recent review by Malik et al. [8] discussed acute and chronic bacterial infections and phage formulations with a focus on phage encapsulation for improved delivery systems. Several studies reported isolation of phages from the environment and patients which were then amplified and purified for research use [6, 9, 10]. Furthermore, inhalation technology such as nebulizers has allowed topical delivery of phages to the site of infection [11]. Liquid and dry powder phage formulations for nebulization and inhalation have been extensively studied, along with numerous in vitro and in vivo studies to determine safety and efficacy of the phage therapy.
In this review, we discuss recent advances on pulmonary phage therapy for treatment of infections caused by bacteria including Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumonia, Burkholderia cepacia complex (BCC), and Mycobacterium tuberculosis. In this article, we will first cover phage formulation and delivery methods, then preclinical and clinical evaluations, followed by phage therapy against biofilms, adjunctive therapy with antibiotics and its prophylactic use. Possible safety concerns and challenges faced will be covered in the last section.
2. Phage Formulation and delivery
Only limited knowledge is available on processing phages into well-defined pharmaceutical formulations, and their long-term stability and impact on in vivo phage efficacy. Formulating phages into inhalable forms for pulmonary infections is a dual challenge requiring both aerosol performance and phage biochemical stability. Progress has been partly hindered by poor understanding of the mechanism responsible for stabilisation of phages in both liquid and solid formulations. As phages for therapy are generally composed of a protein capsid enclosing a single molecule of genetic material, current phage formulation strategies are largely adapted from the knowledge obtained in the development of protein-based pharmaceuticals. Recent reviews on the influence of external factors, including temperature, pH, ionic strength, interfacial adsorption and mechanical stresses on the stability of phages during processing and storage have confirmed the rationality of this approach [6, 12, 13].
2.1. Liquid suspension formulations
2.1.1. Free phage formulations
Most phage research for respiratory infections in animal models has been focused on the delivery of liquid formulations using intra-nasal instillation [14–16] and nebulization [11, 17–23]. This is mainly because the preparation of liquid phage formulations is relatively simple with little formulation development required for phage stability. Challenges remain as the most commonly used phage stabilizers, including phosphate (PBS, pH 7) and Tris are not yet approved for inhalation. Liquid phage formulations are easily aerosolized into fine droplets using most commercially available nebulizers [11]. In general, products containing multiple phages with different infection mechanisms against the target pathogen are recommended to achieve better therapy outcomes by reducing the likelihood of phage resistant cells [24]. The stability of phage upon formulation processes is phage-dependent [25, 26] with some being more robust and some being more vulnerable. Hence, minimal processing steps would be favorable to maintain high survival rates of all phages in the formulation. Therefore, it is not surprising that commercial phage preparations such as those in the Republic of Georgia for therapeutic use [27, 28] and Food and Drug Administration (FDA) approved products for food protection [29–31] are marketed as liquid suspensions. Usually, these phage products are recommended to store under refrigeration (2–8 °C) with a shelf life of 1–2 years [27, 28].
Despite their popularity, only limited data is available for stabilizing phages in liquid phage suspensions. While crude phage lysates were used in early studies [6], the removal of bacterial endotoxin for nebulized products is recommended and it would likely be a quality requirement for their approval [19], Details of phage propagation and purification procedures are described elsewhere [6, 10]. This section will focus on the storage stability of liquid phage formulations. Purified phages are commonly stored in buffer systems, such as PBS [19], salt-magnesium (SMB, pH 7.5) [11, 14, 15, 23, 32] and Tris (pH 7.5) [32], due to poor stability of phages in pure water. Presence of Mg2+ ion (1-10 mM), gelatin (800 μg/ml) or ficoll (3 – 6%) can help stabilize phages [32]. Short-term stability of a 3-phage mix in PBS was demonstrated [19], showing no statistically significant titer loss (< 0.5 log10) over a storage period of 6 months at both 4 °C and room temperature. Although the choice of buffer systems for phage storage in different phage studies is usually decided by custom rather than systematic investigation, the titer drops need to be minimized (< 1 log10 during the shelf life may be desired) for viable commercial products.
2.1.2. Liposome-encapsulated formulations
Encapsulation of phages into liposomes has been recently proposed to prolong the survival rate and the biodistribution of phages [33–36]. Colom et al. [33] encapsulated a cocktail of three phages (UAB_Phi20, UAB_Phi78, or UAB_Ph87) targeting Salmonella enterica into cationic liposomes containing 3.2% w/v trehalose. Liposome formulation was produced using a conventional thin film hydration followed by extrusion. The mean liposome size was 308 nm with an encapsulation efficiency of 47-49%. No titer loss was noted in this liposome formulation after storage at 4 °C for a period of 3 months. Using the same liposome generation method, Singla et al. [35, 36] encapsulated a lytic podovirus phage against K. pneumoniae into liposomes. Addition of Tween 80 and charge inducers, dicetyl phosphate (negative) and stearylamine (positive), reduced the liposome size from ~1000 nm to < 200 nm, depending on the actual compositions of the formulations. High encapsulation efficiency of 92% was achieved with the positive charge inducer. Liposome-phage formulations were structurally and biologically stable for nine weeks at 4 °C and room temperature (<0.1 log10 loss at 4 °C and 0.4 log10 loss at room temperature). However, the size of liposome increased from 120 nm to 320 nm with a 1.2 log10 phage titer loss after storage at 37 °C for nine weeks. In another study, anti-tuberculosis phages were packed into giant unilamellar liposomes (≥ 5 μm) using polyvinyl alcohol gel assisted formation followed by extrusion and inversed emulsion methods [34]. These formulations were suggested to be delivered by an inhalation route due to large size of the liposomes; however, realistically, it is difficult to deliver 5 μm liposomes by inhalation as the aerosol droplets have to be > 5 μm, unless the patient uses an extremely slow inhalations [37]. Inhaled liposomal formulations can localize the medication in the lungs and improve the therapeutic outcome with reduced systemic adverse effects [38–40]. The studies performed to date have focused on short-term stability. Long-term stability studies are essential for determining the true potential of liposome-encapsulated phages. The relevance of accelerated stability testing on long-term stability of a phage formulation stored at 4°C or room temperature must, too, be determined.
2.1.3. Delivery
Nebulization has been the primary method for phage aerosolization and delivery into the respiratory tract. Commercial nebulizers utilize various modes of aerosol generation mechanism including vibrating mesh, compressed air jet, ultrasound and colliding liquid jets. The potential for inhaled phage therapy using nebulizers has been investigated in several in vitro and in vivo studies. Golshahi et al. [11] was the first group to conduct comprehensive analysis of phage nebulization using two commercial nebulizers, Pari LC star and eFlow nebulizers. Readers are directed to an article by Hoe et al. [6] for a review of this study.
In a more recent work, Sahota et al. [18] used AeroEclipse jet nebulizer and Omron mesh nebulizer to aerosolize two anti-pseudomonal phages, PELP20 and PELI40. A 5 mL phage lysate (3 × 109 PFU/mL, 1.5 × 1010 PFU/mL) was nebulized for 5 min into a cascade impactor, followed by biological assay to determine the phage titer. The amount of viable phages that could reach the lower respiratory tract (< 4.7 μm) with AeroEclipse nebulizer was 15% (2.3 × 109 PFU) and 2% (1.6 × 108 PFU) for PELP20 and PELI40, respectively. Using Omron nebulizer, only 1.3% (1.9 × 108 PFU) of PELP20 could potentially reach the lower airways. Carrigy et al. [41] used a vibrating mesh nebulizer, jet nebulizer, and soft mist inhaler to nebulize an anti-tuberculosis phage D29. Jet nebulizer caused a greater titre reduction of 3.7 log10, whereas vibrating mesh nebulizer and soft mist inhaler resulted in 0.4 log10 and 0.6 log10 titre reduction, respectively. Vibrating mesh nebulizer could deliver the highest phage titre in a given time (108 PFU/min), followed by soft mist inhaler (106 PFU/actuation) and jet nebulizer (104 PFU/min). Astudillo et al. [42] assessed the structural stability of anti-pseudomonas phage PEV44 before and after nebulization using air-jet, vibrating mesh and Omron static-mesh nebulizers. Jet nebulizer caused the most severe structural damages with 83% of phage population found “broken” (i.e. head detached from the tail). Mesh type nebulizer caused damages to approximately half of the phage population. These studies showed that viable phage delivery depends on the mechanical stress of nebulization, which seems to be phage-dependent, and delivery efficiency of the nebulizers.
Turgeon et al. [43] compared the effect of aerosolization on the viability of different types of phages using three nebulization methods: atomizer, 6-jet collision nebulizer and Aeroneb nebulizer. Five structurally distinct lytic phages were used, including Leviviridae faimly MS2, Cystoviridae family Φ6, Microviridae family ΦX174, Corticoviridae family PM2 and Tectiviridae family PR772. The results confirmed that phage stability upon nebulization is phage-dependent with phages MS2 and ΦX174 being most robust. The behaviour of phages in aerosol was found to be diverse. More recently, the same group looked at the effect of relative humidity (RH) and temperature on aerosolized phages [44]. Phages MS2, Φ6, ΦX174 and PR772 at a titer of 109 to 1010 PFU/mL were aerosolized using a 6-jet collision nebulizer into a RH and temperature controlled chamber. The aerosolized phages were left for 14 h, followed by sample analysis. The mass median aerodynamic diameter of the aerosolized virus particles was 1.12 ± 0.05 μm. Phage MS2 remained viable at a range of RH between 20-80%. Phage ΦX174 was highly stable at 80% RH, but unstable at lower RH of 20%. Phage PR772 was unstable at 50% and 80% RH, and resulted in complete inactivation at 20% RH. Phage Φ6 could withstand a low RH of 20% even after 14 h, but lost bioactivity at 80% RH. Phage MS2 was the most resistant over the range of RH studied. This study highlighted the necessity to control temperature and RH for phage nebulization studies in order to understand their sensitivity and robustness at different environmental conditions. Use of robust phages capable of withstanding different environmental conditions, such as high RH, would ensure biological stability during clinical treatment using jet nebulizers..
Cooper et al. [19] assessed nebulization efficiency of a phage cocktail consisting of anti-pseudomonal phages GL-1, GL-12.5 and LP-M10. Ten millilitres of the phage suspension was nebulized using a Porta-neb nebulizer connected to an Andersen cascade impactor. No statistically significant loss of titer was observed after nebulization. Phage particles recovered in the impactor showed that the majority would have been deposited in the throat and upper airways (75%) and the remaining particles in the secondary bronchi and alveoli. Phage stability post-nebulization can be phage-dependent, but this study demonstrated the feasibility of nebulizing a phage cocktail with minimal loss of infectivity. While the total PFU recovered suggested that there were minimal losses, it is not possible to determine if the survival or distributiom of the individual phages was equal.
2.2. Phage solid formulations
Following the development pathway of protein-based pharmaceuticals, phages are processed to dry powder formulations for ease of transport and storage, as well as extended storage stability. Freeze drying has been demonstrated to be an excellent technique in stabilizing phages in the solid state for long-term storage [45–50]. A recent review summarized the protective effects of pharmaceutically acceptable excipients on the stability of phages [13]. Depending on the concentration, sugars (sucrose [49, 50], lactose [47, 50] and trehalose [49]) provide excellent protection for phages during freeze drying and storage. Of these sugars, only lactose is approved for inhalation delivery and hence, the rest will require safety testing for regulatory approval. Inhalation of small molecules as dry powders may cause irritation and bronchoconstrictions in non-cystic fibrosis patients and caution should be taken [51]. Nonetheless, tobramycin, colistin methanesulfonate and mannitol are used for treatment of chronic pulmonary infections in cystic fibrosis patients. Merabishvili et al. [49] showed that freeze drying a Myoviridae phage ISP against S. aureus with 0.5 M sucrose or trehalose resulted in 1 log10 titer loss during processing and a further loss of 1 log10 after 37 month storage at 4 °C. Significant titer loss (> 4 log10) was observed in formulations containing lower sugar concentration of 0.1 M after 37 months [49]. In addition to sucrose, the influence of inorganic salts on phage stability during freeze-drying and storage was reported by Dini et al. [50]. A Podoviridae coliphage CA933P was freeze-dried with different amounts of sucrose (0.1, 0.3 and 0.5 M) and salt-magnesium (SM) buffer. Higher sucrose concentrations were detrimental to phage stability during freeze-drying [50], which is opposite to the findings on the IPS phage reported by Merabishvili et al. [49]. No further titer loss of the sucrose stabilized phage was observed for at least 120 days. Although the mechanisms of stabilization of phage in solid state are still unclear, the high transition temperatures (Tg) of these sugars (60 °C for sucrose, 108 °C for lactose and 115 °C for trehalose) and/or their capability as a water substitute are believed to play important roles. These freeze dried phage formulations can be either reconstituted for nebulization or milled to produce powders for dry powder inhalers (DPI) administration [47, 48]. However, potential degradation of the phage by milling has to be considered due to the mechanical stress of the process.
Compared with nebulizers, DPI are simple to use and do not require regular cleaning and disinfection. Furthermore, DPI are more portable than nebulizers and do not require electricity for operation, which is particularly useful for patients in developing countries. Spray dried phage particles contain sugars as an internal excipient, but do not require the use of lactose as an external carrier. The feasibility of spray drying has been demonstrated for producing inhalable phage powders with moderate titer loss (< 1 log10) [25, 26, 52, 53]. Spray drying is a widely used technique that produces fine drug particles for pulmonary delivery as a single-step method and is less expensive than freeze drying. Due to the susceptibility of phage towards thermal stresses, low drying temperatures (~ 40 °C outlet temperature) are often used. The production process and stability of phages in powder form appeat to be phage-specific or at least depends on the type of phage [25] [26] [54]. Phage powders were most stable when stored at 4 °C and 0% RH. Both high temperature (25 °C) and/or RH (54%) caused significant inactivation of phages, but the humidity seems to be a more important factor for phage inactivation due to the crystallization of trehalose upon storage. The difference in degradation rate of phages would potentially aggravate the challenge of formulating phage mixes in dry powder formulations.
Recently, we have also produced stable phage powders with reasonable aerosol performance by co-spray drying a Pseudomonas podovirus phage PEV2 with multi-component excipient systems consisting of trehalose, mannitol and leucine [53, 55]. A titer loss of 1.3 log10 was noted for powders containing a high portion of trehalose (40%, 60% and 80%). In a more recent study, we have demonstrated that sugar and leucine are sufficient for biological and physicochemical stabilization of PEV phages [52]. The storage stability of these spray dried powder systems was also assessed [53, 56] at various RH conditions (0, 22 and 60% RH) at 4 °C. Results showed that phage activity was retained with no further titer loss for at least 12 months at 0% and 22% RH. For powders stored at 60% RH, no titer loss was measured after 1 month storage for all formulations, but no viable phages were detected in these powders after 3 and 12 months. In another study, we assessed the stability of spray dried powders containing phages PEV2 and PEV40 at 20 °C under vacuum [57]. A formulation containing 30% leucine maintained PEV2 viability whereas those containing 40% leucine resulted in titre reduction of 0.8 log10 over 12 months. PEV40 phage powders resulted in 0.5 log10 titre loss over 12 month regardless of the proportion of leucine present in the formulation. Since moisture protective packaging is usually used for spray dried pharmaceuticals, exposure to high humidity will be short term when the powders are unpacked prior to usage. In this context, 1 month stability at 60% RH could be considered as adequate. Stability of phages in inhalable powders at room temperature eliminates the necessity for cold-chain infrastructure. This can decrease the associated costs, allowing the products to be more readily used.
2.3. Propellant-based phage formulations
Due to the affordability and simple operation, pressurized metered dose inhalers (pMDIs) have long been favored by patients and healthcare professionals for asthma and chronic obstructive pulmonary disease management. However, there was only one report on the feasibility of formulating phage into pMDIs [58]. Hoe et al. [58] formulated aqueous FKZ/D3 and KS4-M phages (myoviridae) suspensions in a reverse emulsion with Tyloxapol surfactant (100 mg/mL) and filled into hydrofluoroalkane 134a pMDI canisters with a 50 μL metering valve. Phages were successfully actuated from the pMDIs with a titer loss of < 1 log10. Although the long term stability of these formulations was not assessed, this study showed the tolerability of some myoviridae phages to the shear stresses associated with flash atomization in pMDIs.
3. In vivo evaluation of phage therapy
3.1. Efficacy
In vivo safety and efficacy studies are an important step in developing a commercial phage product. Over the past decade, several groups have attempted to address these issues by administering phages via intra-nasal route to treat pulmonary bacterial infections in rodents [14–16, 20, 59–62]. These studies used acute lung infection models to demonstrate the potential for therapeutic application. Studies described by Abedon [2] will not be discussed here. Mice are often rendered neutropenic with neutrophil cells depleted to observe the antibacterial activities of phage-alone, unaided by the host’s immune response. Under neutropenia, the animal becomes highly susceptible to bacterial infection, which resembles respiratory infections in immunocompromised patients. Semler et al. [20] administer phages KS12 and KS4-M, at a theoretical multiplicity of infection (MOI) of 11 and 150 respectively, active against a Burkholderia cepacia strain strain using a nose-only inhalation device in a neutropenic mice model. Phage concentration delivered to the lung tissue was back-calculated to determine the MOI post-inhalation. Bacterial burden in the lungs was significantly reduced 2 days post-treatment. In a separate study, treatment with phage KS5 showed a significant bacterial killing at an MOI of 32, but was ineffective when an MOI of 2 was used. Low phage concentration may have led to simply an insufficient dose-response effect of a passive therapy or a long pre-proliferation period with a high risk of phage clearance from the infection site. Initial studies looking at the frequency of resistance and phage adsorption rates would have helped better understand the MOI effect.
Pabary et al. [60] assessed the efficacy of a phage cocktail using an acute P. aeruginosa lung infection mouse model. Both bacterial challenge and phage were administered intra-nasally. BALB/c mice were infected with PAO1 at 2.5 × 107 CFU and received phage treatment (2.4 × 107 PFU in 20μl) by nasal gavage either simultaneously, 24 h post-infection, or 48 h pre-infection. Infective burden was determined at various times in bronchoalveolar lavage fluid samples. A complete clearance of bacteria was observed in all mice having received simultaneous administration. Bacterial clearance was found in 86% and 71% of mice that received delayed and prophylactic treatments, respectively. Phage treatment prevented systemic spread of bacteria regardless of administration time.
Fothergill et al. [63] developed a novel chronic lung infection mouse model using P. aeruginosa with persistent infection for 28 days. A clinical P. aeruginosa strain LESB65 at a mid-log phase (2 × 106 CFU) was administered intra-nasally to female BALB/cOlaHsd mice. The inoculated bacteria colonized in the upper respiratory tract, including nasopharynx and paranasal sinuses, allowing adaptation and migration into the lungs. The type of mouse strain [64] and the bacterial isolate used [65] were important in establishing the infection model of interest. With the right combination, the authors could establish chronic respiratory tract infections. Using this model, Waters et al. [66] tested the efficacy of phage PELP20 by administering intra-nasally two doses at 2 × 107 PFU at 24 h and 36 h post-infection, followed by bacterial count in the lung tissues at 48 h. In a separate experiment, the two doses were given at 48 h and 60 h post-infection with bacteria enumeration at 72 h. A complete clearance of bacteria in the lung was observed in both treatment groups. Phage administration at 6 and 6.5 days post-infection resulted in a complete clearance of bacteria in 70% of mice and a significant reduction in the other 30% of mice.
Overall, inhaled phage therapy has shown to be highly efficacious for combating acute and chronic infections in the lung [20, 60, 63, 66]. Intra-nasal administration has been the preferred route for pulmonary delivery of phages in animals due to the convenience and ease of administration. When planning and evaluating animal studies, it is imperative to be mindful of substantial loss of deliverables to the gastrointestinal tract due to mucociliary clearance [11]. A phage dose should be adjusted to consider this loss in order for it to truly reflect the administered dose. However, for clinical evaluation, nebulizers and dry powder inhalers would be used through oral inhalation. Hence, the use of other delivery systems including intra-tracheal instillation or inhalation chambers would better mimic clinical treatment. Chronic pulmonary infections in cystic fibrosis (CF) patients are often polymicrobial, and no animal infection model is available yet, that can reflect such complex interactions between the microbial organisms. Development of in vivo and/or ex vivo models would be beneficial for testing phage mixes against bacteria in a poly-microbiome environment. Further preclinical studies may be necessary to draw conclusions on the recommended dose, dosing frequency, and their suitability for human use. A murine thigh infection model can predict the efficacy of antibiotic against bacterial infection, which can help elucidate optimal drug dosing regimen in humans [67]. Such studies could help with modelling the pharmacokinetic/pharmacodynamics for inhaled phage therapy. Pharmacokinetic/pharmacodynamics studies should be carefully designed not to unnecessary slow down the product development process, but to expedite our understanding of safety and efficacy of inhaled phage therapeutics.
3.2. Pharmacokinetics
Liu et al. [23] assessed the pharmacokinetics of a mycobacteriophage (D29) in healthy mice. They used endotracheal administration with a Penn-Century Micro-Sprayer® aerosolizer attached to a high pressure syringe to deliver 5 × 108 PFU of the phage to the animal. Approximately 10% of the aerosolized phages (4 × 107 PFU) reached the lung and were eliminated at 72 h. Low concentrations of phage were found in other organs, including spleen, kidney and brain, which were eliminated at 72 h. Phage detection in brain tissues suggests the ability to translocate to olfactory bulbs through the nerves or pass through the blood-brain barrier [68]. However, there is a possibility of cross contamination of phages during the sample processing. When phage was delivered via intraperitoneal route, only 105 PFU reached the lung with phages eliminated after 12 h. Concentration of phage in spleen and kidney were initially high (105 PFU) but the number rapidly reduced over 12 hours.
The pharmacokinetics of phages differs from conventional pharmaceuticals. Phages are self-replicating with the capability to eradicate bacteria even with a relatively low initial concentration. Phage kinetics is non-linear and several factors play an important role in determining the outcome of the treatment, including the presence of target pathogen, relative number density of bacteria and phage, and the optimal time of phage administration (See review by Payne and Jansen [1]). The number of target bacteria may need to be above a proliferation threshold for phages to multiply. Below the threshold, the chances of a phage getting in contact with bacterial cells are reduced, and phages may be cleared from the infection site. This suggests that the timing of phage administration may also be critical. In clinical settings, the bacterial load in a patient’s lung should be sufficiently high to reach the proliferation threshold of bacteria. Hence, the key aspects to be studied are the minimum phage concentration required and administration times for a successful treatment.
4. Combination Therapy with Antibiotics
Emergence of phage-resistant bacteria [69] has led to repurposing of combination therapy using phages and antibiotics. Theoretically, the combined effects of the two antibacterial agents can be additive, synergistic or antagonistic. Over the past few years, studies have repeatedly shown synergistic interaction between antibiotics and phages in vitro, which can potentially improve the clinical outcome in the treatment of pulmonary bacterial infections [70].
4.1. Burkholderia cepacia
Phage-antibiotic synergy was assessed in vitro and in vivo using BCC phages KS12 and KS14 and six antibiotics representing four different drug classes [71]. In the presence of a β-lactam antibiotic meropenem, KS12 and KS14 showed a significant increase in plaque size. The increase in plaque size suggests enhanced bactericidal effect of phages with antibiotics. By using antibiotics at sub-inhibitory concentration, phages may have increased access to receptors on bacterial cells that have become elongated [71]. Phage KS12 and meropenem combination treatment promoted survival of B. cenocepacia K56-2-infected Galleria mellonella larvae over controls treated with phage or meropenem alone. Survival rate of larvae was 78% upon combined treatment, whereas monotherapy resulted in 20-30% survival rate.
4.2. Klebsiella pneumonia
Verma et al. [72] assessed the efficacy of phage KPO1K2 in combination with ciprofloxacin on young (12 h old) K. pneumonia biofilms. Monotherapy using ciprofloxacin or phages reduced the bacterial load in the biofilm by 1.76 log10 or > 4 log10, respectively, 3 h post-treatment. Phage-antibiotic combination therapy could not further reduce bacterial load compared with phage-alone treatment. However, combination therapy significantly reduced the emergence of phage- or antibiotic-resistant mutants in both planktonic cells and biofilm.
4.3. Pseudomonas aeruginosa
Torres-Barceló et al. [73] used phage LUZ7 with streptomycin at a sub-lethal concentration against P. aeruginosa. The phage-antibiotic combination substantially decreased bacterial load compared to monotherapy, and minimized the development of mutants that are resistance to antibiotic or phage. Coulter et al. [74] reported the emergence of antibiotic- and phage- resistant cells was reduced by 60% and 99%, respectively, when 48 h-old P. aeruginosa biofilm was treated with combination of phage PB-1 and tobramycin combination. Similar results were found by Chaudhry et al. [75] where phage plus ceftazidime, ciprofloxacin or tobramycin reduced the number of antibiotic-resistant P. aeruginosa PA14 in biofilms. Moreover, Knezevic et al. [76] tested the antibacterial activity of phage s-1 and sub-inhibitory concentration of ceftriaxone. Phage and antibiotic synergy was observed for all targeted strains with 1.3 – 2.6 log10 reductions in bacterial count. Nouraldin et al. [77] assessed the efficacy of phage and antibiotic combination against 15 clinical isolates on both planktonic and biofilm states. Amikacin and meropenem showed synergistic effect with phages against planktonic cells, whereas amikacin with phages was effective against biofilms.
4.4. Staphylococcus aureus
Kirby et al. [78] used a continuous culture system to model population dynamics S. aureus when treated with gentamicin and anti-staphylococcal phage. A computer model generated from the experimental system showed that the presence of gentamicin induced aggregation of bacterials cells, forming biofilm. Conversely, bacterial cells with increased capacity to aggregate are more susceptible to bactericidal activities of Sa5 phages.
4.5. Streptococcus pneumonia
Vouillamoz et al. [79] tested the efficacy of combined application of phage lysin CpI-1 and antibiotics to treat S. pneumonia infection in vivo. Combination treatment significantly increased the percentage survival of mice with pneumococcal bacteremia by 80% at day 7 post-treatment compared with daptomycin or CpI-1 monotherapy each with 35% and 0% survival, respectively.
Overall, there is strong evidence demonstrating synergistic antimicrobial effect of phage and antibiotics, but the underlying molecular mechanisms are unclear. A recent study by Chan et al. [80] showed a possible reason for such synergy. When drug-resistant bacteria are exposed to phages, the bacteria will start to develop phage-resistance as a protective mechanism. During this process, the drug-resistant mechanism (loss of efflux pump) is compromised. By losing sensitivity to phages, bacteria regained sensitivity to antibiotics. There are many different classes of antibiotics and phages that could potentially demonstrate synergistic interactions. Further research is needed to develop antibiotic-phage therapy that could better the clnical needs of patients. As in vitro studies far outnumber in vivo studies, more preclinical evaluations are necessary for assessing the efficacy and benefits of combined therapy over monotherapy.
5. Phage therapy for prophylactic treatment
Pulmonary phage therapy for prophylactic treatment is an underexplored area with only a few preclinical and clinical studies. According to Abedon [2], there exist only a handful of studies from the last century with focus on prophylactic pulmonary phage delivery. Some of these studies are hard to find as they are not indexed in databases such as Pubmed, Medline or Scifinder and most of them are not available in English. The following section only considers English literature that can be found in the mentioned databases.
Debarbieux and colleagues [16] investigated the suitability of phage PAK-P1 for prophylactic use in an animal lung model against P. aeruginosa. Using a bioluminescent strain, a real-time view of lung infection progression was recorded to provide visual and quantitative representation of the infection. Eight BALB/C male mice were randomly allocated to either a group receiving a phage-treatment or a control group receiving only PBS. The mice received either PBS or 108 PFU of PAK-P1 phages 24 h prior to bacteria inoculation at 107 CFU. At 2 h post-bacterial challenge, the photon emission in the control group was approximately 5 times higher than the phage-treated group (3.6 × 105 vs. 0.7 × 105 photon/s). At 6 h, the difference was even more pronounced (Fig. 1) with the phage treated group showing 33 times lower emission compared with control (0.2 × 105 vs. 6.6 × 105 photon/s). A 100% survival was observed in phage-treated mice after 16 days, whereas 0% survival was recorded for control group within 2 days, indicating that phages have prophylactic effect against P. aeruginosa lung infection.
Figure 1.
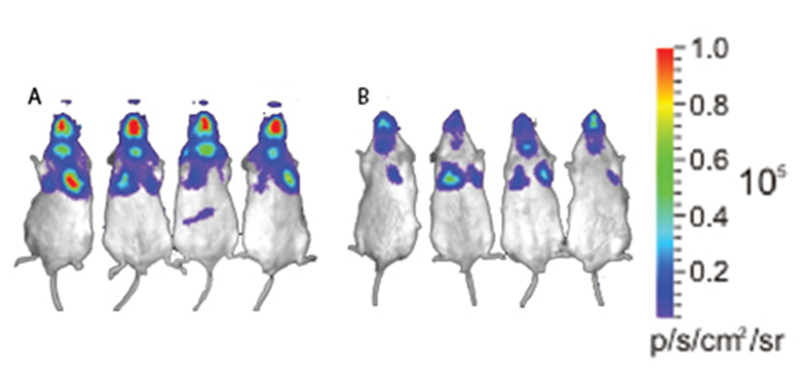
Effect of phage treatment on lethal infection in mice. Mice were treated with PBS (left) or phage solution (right). All mice were infected with bioluminescent Pseudomonas aeruginosa prior to treatment. PAK-P1 phage were given at a phage-to-bacterium ratio of 10:1. Red colour indicates ‘high’ bacterial count and purple colour indicates ‘low’ bacterial count. Figure is adapted from [16].
Morello et al. [15] demonstrated that prophylactic treatment of phage P3-CHA can rescue mice infected with P. aeruginosa CHA strain. Bacterial inoculation at 3 × 106 CFU via intra-nasal route resulted in 0 % survival within two days of infection. Intra-nasal delivery of P3-CHA phage at 3 × 108 PFU four days prior to bacterial infection led to 100% survival. In another group, the mice were given an equivalent dose of heat-treated (80 °C) phages. All mice died in this group within 2 days, indicating that active phages are responsible for treatment and survival of lung infected mice. Histopathological examination showed that the lung damage in the P3-CHA treated mice was less severe than in the control group. The lesion severity score was significantly lower (p=0.012) for mice treated prophylactically with phages (7 out of 25) compared to the mice that were not treated with phages (16 out of 25). Additionally, bacterial count in the lung was significantly lower in the mice with treated with phages compared to the control group (p=0.008).
Singla et al. [35] examined the prophylactic effect of liposome-entrapped lytic phage KPO1K2 for the prevention of K. pneumonia induced lobar pneumonia in BALB/c mice. Liposome-entrapped phages were given intraperitoneally at an MOI of 0.01 at 6, 24, 48 and 72 h before bacterial challenge (104 CFU). Phage-liposome formulation provided protection when administered at 6, 24 and 48 h pre-inoculation. In a separate study, non-encapsulated phage was administered at an MOI of 1 at 3, 6 and 24 h before the bacterial challenge. The mice were protected from infection against K. pneumonia induced lobar pneumonia when injected at 3 and 6 h pre-inoculation. Treatment with liposome-entrapped phages increased the retention time in the host, and significantly reduced the levels of pro-inflammatory mediators and increased anti-inflammatory mediators as compared to non-encapsulated phages. Similar experiments were done by Chhibber et al. [61]. An anti-Klebsiella phage at a MOI of 200 was intraperitoneally delivered at 3, 6 and 24 h prior to bacterial challenge. The group that received treatment at 3 h proved most effective with complete clearance of bacteria in the lung after 5 days (Fig. 2). Administration at 6 h also provided protection against K. pneumonia with complete clearance after 7 days. Prophylactic treatment at 24 h prior to inoculation failed to clear the bacteria in the lung, probably due to a rapid phage elimination; after 12 h phage delivery, the concentration of phages fell below the optimum effective dose, which was not enough to fully protect mice from bacterial infection.
Figure 2.
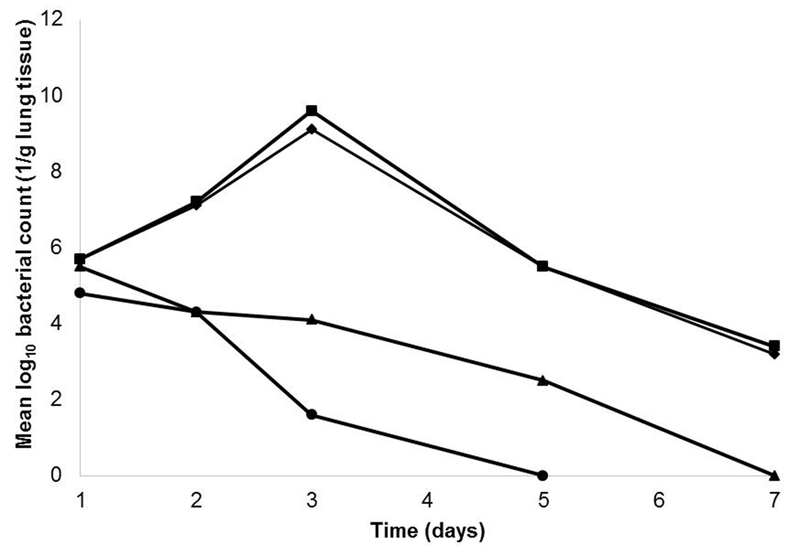
Bacterial counts in lung tissue homogenate over time. Mice were treated with phage preparation at 3, 6 and 24 h prior to bacterial challenge. The control group did not receive phage solution prior to infection. Figure is adapted from [61].
Aleshkin et al. [81] prepared a phage cocktail to cover a wide range of bacterial infections. The cocktail consisted of 8 phages that can kill A. baumannii, K. pneumonia, P. aeruginosa and S. aureus (2 phages per species). Toxicity profile of phage cocktail KPV15 was tested by intraperitoneally injecting a dose of 108 PFU to mice. No acute or chronic intoxication was reported, which was determined by no influence on the physiological condition, weight and morphology of the animals’ organs. In a separate study, KPV15 at 108 PFU was injected prior to bacterial challenge with K. pneumonia. A 100% survival rate was observed for mice receiving phage treatment at 12 or 24 h prior to inoculation, demonstrating a prophylactic effect. Authors also reported the use of KPV15 to treat 13 critically ill patients infected with a multidrug-resistant K. pneumonia. Patients received 5 mL of KPV15 (> 108 PFU/mL) through an endotracheal tube. Eight out of 13 patients reported elimination of K. pneumonia post-phage treatment. Sadly, the authors did not comment on the standard care of treatment used, control group or adverse events, which makes the assessement of phage activity difficult. Nonetheless, the authors suggested that the phage cocktail could be a useful tool in healthcare-associated infections.
These studies and others [82–86] have shed light on phages as a possible prophylactic agents. Phages can not only prevent bacterial growth, but also protect the host organism from the bacterial invasion for days. The kinetics of inhaled phages could be further studied both in healthy and infected animal models in order to extend our knowledge and evaluate the elimination rate of active phages from the infection site in the lungs as well as from systemic circulation. This will help understand dose regimens for potential prophylactic use of inhaled phages. Efforts to develop phages as prophylactic treatment could potentially benefit healthcare providers, immunocompromised patients and family members of patients suffering from lung infections.
6. Clinical trial evaluation
Extensive review and summary of earlier clinical studies of inhaled phage therapy are described somewhere else [2]. Most case studies have been conducted at the George Eliava Institute of Bacteriophage, Microbiology and Virology in Georgia and the Institute of Immunology and Experimental Therapy in Poland. The Eliava Institute has extensive experience with the selection and isolation of phages for therapeutic use. Monophage preparations and phage cocktails have been developed to combat a variety of bacterial pathogens. A well-known example is the pyophage preparation [17, 25] containing five different phages that specifically target S. aureus, S. pyogenes, P. mirabilis, P. vulgaris, P. aeruginosa, and E. coli. and a staphylococcal phage preparation (Sb-1 phage). In a case study from 2011, Sb-1 and pyophage were used to treat a 7-year old CF patient [87]. The initial bacterial concentration in the patient’s sputum was 1 × 107 CFU/mL for S. aureus, and 8 × 106 CFU/mL for P. aeruginosa. Conventional antibiotics failed to treat the patient’s lungs with chronic colonization of P. aeruginosa and S. aureus. Initially, pyophage was applied nine times via nebulization at a 4-6 weeks interval between treatments, which led to a dramatic reduction of P. aeruginosa. The pyophage preparation alone, however, had no effect on S. aureus. The addition of Sb-1 to the pyophage cocktail (administered five times in total with a nebulizer) led to a drastic decrease in the amount of S. aureus in the lungs. One month after termination of inhaled phage therapy, the concentration of P. aeruginosa and S. aureus levels remained low at around 10-100 CFU/mL and 103-105 CFU/mL, respectively. Institute of Immunology and Experimental Therapy has been focusing on the therapeutic application of phages for several decades providing significant contributions to our current knowledge of phage therapy. Slopek et al. [98] evaluated efficacy of phages in 550 patients affected by suppurative bacterial infections. The treatments took place between 1981 and 1986 at 10 different hospitals. Of 550 patients, 93 cases had respiratory infections with lung abscess, bronchitis, and pneumonia induced by S. pyogenes, K. pneumoniae, P. aeruginosa, E. coli and Salmonella. Phage suspension (10 mL) was administered orally and locally using inhalation delivery. Desired therapeutic effect was observed in 83 cases while 8 cases showed a transient improvement. No therapeutic effect was observed in 2 cases. Detailed information, including types of nebulizer used, treatment time, phage stability during nebulization, sample purity (endotoxin level) and formulation content are unreported in many case studies. Although the majority of the treatments were not conducted using clinical standards acceptable for drug approval in the Western world, they have demonstrated therapeutic potential for phages and deepened our understanding on how phages can be applied.
To gain regulatory approval for market access, any new therapeutic products must usually go through a long and comprehensive process involving preclinical and clinical trials. In the US, it takes an average of 12 years for the approval of new drug from preclinical testing and the costs run into millions of dollars [88]. The length, size and complexity of human clinical trials are the primary reason for the high costs [88]. Lack of robust clinical trials is not surprising as phage therapy is relatively new in Western countries and proper drug development takes significant resources and time. The number of phage therapy clinical trials on ClinicalTrials.gov is very limited and those with focus on respiratory diseases are even rarer. Only one trial was identified (ClinicalTrials.gov identifier number NCT01818206) and the aim was to assess the efficacy of a cocktail of 10 phages against P. aeruginosa in sputum samples from CF patients. The phage cocktail used significantly decreased P. aeruginosa levels with a concomitant increase in phage concentration in 46% of the sputum samples [89]. Patient’s age, gender, colonization period of bacteria, previous treatment with antibiotics and lung function (forced expiratory volume) did not correlate with the efficacy of phage therapy. The capability of phages to invade their host in sputum demonstrated that phage therapy can be used to treat chronic lung infections. The biopharmaceutical company AmpliPhi Biosciences has developed a phage cocktail, AB-PA01, that can be used to treat P. aeruginosa lung infections, which are common in individuals with CF. The cocktail has shown promising preclinical results and the company is planning for clinical trials in humans. Large scale double-blind inhaled phage therapy trials are mandatory to address safety and efficacy in humans.
7. Challenges of phage therapy
Urgent need for novel antibacterial agents for treatment of MDR bacterial infection has pushed forward phage research with more resources being invested into this field. Nonetheless, a number of issues are yet to be addressed before inhaled phage therapy can be accepted as a treatment option in Western countries.
Similar to antibiotics, bacteria can develop resistance to a particular phage as a defence mechanism. Potential phage resistance mechanisms have been documented by Labrie et al. [69]. Bacteria can acquire resistance by blocking the cell receptors to inhibit phage adsorption, preventing phage DNA/RNA entry or degrading them once they enter, or through other abortive infection systems. In response to bacterial anti-viral mechanisms, phages evolve continuously to circumvent and retain anti-bacterial abilities. The use of phage mixes has been recommended as an strategy to reduce the risk of bacterial phage resistance development [90]. Phage mixes formulation involves more than just mixing of two or more phages in a suspension. Optimal performance results when individual phages complement one another, i.e. each phage targeting different surface receptors. By targeting multiple receptors simultaneously, the bacterium is pressured to develop mutations to several genes, and the chances for simultaneous mutations are low. The development of phages in mixes, however, brings other challenges such as stability testing of each phage in the mixture to determine suitability of formulation, and increased complexity of pharmacology which may require extensive preclinical investigation. A growing number of groups and companies are exploring genetically engineered phages to expand their host range, minimize phage-resistance and promote biofilm degradation [91]. Engineered phages have the potential to improve the therapeutic potential, yet they will need to demonstrate their superiority over naturally occurring phages and undergo rigorous testing to ensure their safety prior to regulatory approval.
Phage therapeutic manufacture for lung delivery comprises of production, purification and formulation for inhalation. Currently, the cost associated with phage production and purification would be more expensive than antibiotics. However, it is expected to become more affordable as the technology advances [92] and the volume of production increases due to demand, saving healthcare costs and providing access to those in need [93].
Phage products are already being marketed for clinical use in some Eastern European countries [94]. In some European countries, the use of phage therapy for individual patients who have run out of options is covered by the Physician Practice Act and World Medical Association Declaration of Helsinki [95, 96]; thus therapy is perceived as an experimental treatment under the supervision of medical ethical committees. Magisterial phage preparation is another approach explored to address perceived regulatory challenges in Europe [97]. Under Belgian legislation, magisterial preparation produced under Good Manufacturing Practice (GMP) is approved by the Pharmacopeia Commission, and the hospital microbiological lab can be licensed as a supplier of the materials. The question remains on what should be the standard requirement for a GMP phage preparation and how such system could be financially viable. However, it is unlikely that compassionate use of phages will replace robust and controlled clinical trials acceptable by regulatory agencies like the US Food and Drug Administration and the European Medicines Agency. Once the necessary safety and efficacy data has been generated, the regulatory agencies would need to endorse new drug development paths to allow for the unique characteristic of the technology to be exploited in the most efficient manner. For example, due to the risk of phage-resistance, phage preparations will need to be reformulated overtime. In some cases, tailor-made phage or phage mixes should be considered to optimize the treatment outcomes. Personalized phage therapy provides the advantage of eliminating ineffective phages and adding the highly effective phages. Such tailored phage treatments are more effective than standardized phage mixes [98]. However, personalized medicine is inevitably time consuming and costly for the patients. Hence, such personalised phage therapy would necessitate rapid identification of the pathogen and cost consideration. In a reported phage therapy case, 34 people from 15 different institutions were involved in providing personalized phage mixes against Acinetobacter baumannii [99]. The patient was successfully treated, but this form of treatment may not be viable in the long run due to time and costs associated. Reformulation and personalized phage mixes need to happen in an efficienct manner without the need for too extensive preclinical and clinical studies.
The importance of additional clinical studies has been discussed in section 7, but substantial financial investment pushes the need for intellectual property protection on the phage product for the investors. Since phages are naturally occurring microorganisms classified as the most abundant living beings, there is no considerable benefits in patenting individual phages, and private companies may be hesitant to invest [100]. Currently, phage isolated from the environment is patentable, but with limited intellectual property protection [101]. Industries interested in market approval of phage therapeutics should explore Orphan Drug Act for approval and intellectual property protection. Genetically engineered phages or phage formulation technologies for inhalation delivery may offer avenue for intellectual property protection to draw in much-needed funding.
As phages vary in their robustness, each phage product, liquid or solid formulations, may require different biochemical stabilizers and storage conditions (see section 2). The majority of the clinical studies reported to date have used nebulizers to deliver liquid phage formulations probably due to ease of formulation production. Dry powders are considered as the next generation respiratory drug delivery system as it provides long storage stability without requiring refrigeration [102]. Phage stability in dry powder formulations can vary, which requires optimization of production process for each individual phage (e.g. spray drying conditions, excipient and formulation parameters). This poses a challenge for formulating dry powder containing phage cocktail; moreover, substitution of phage due to emergence of phage-resistance will require a continous development effort. To determine the shelf-life of a phage product, individual phage mixed in a cocktail should be quantitatively examined for stability assessment. Unfortunately, quantitative analysis to differentiate each phage in a cocktail mixture using plaque assay is challenging. Furthermore, it is difficult to distinguish subtle titer reduction from phage destabilization due to variability in plaque assay [103]. For this same reason, precise representation of viable phages in fine particle fraction (particles < 5 μm in aerosol cloud) is ambiguous; fine particle fraction is the gold-standard for determining aerosol performance of inhaled therapeutics. The correlation between real-time and accelerated stability testing of phage products has not been established, which means phage product may need to undergo long-term stability assessments , which can delay the initiation of trials and registration. Undoubtedly, a company could launch the product with a shorter expiry date and then gradually develop evidence for longer term stability.
Phage lysates produced in Gram-negative and Gram-positive bacteria often contain endotoxins, including lipopolysaccharide (LPS) and lipoteichoic acid, respectively. Endotoxins can be removed from phage preparations using cesium chloride density gradient centrifugation [104], affinity techniques [105], water immiscible solvents such as 1-octanol or 1-butanol [106] and commercially available Endo-trap kit (Hyglos, Germany) [19]. For the production of therapeutic products only those purification systems that avoid the use of dangerous chemicals should be considered. Liu et al. [23] assessed the concentration of leukocyte, neutrophils, lymphocytes and TNF-α in bronchoalveolar fluid after endotracheal delivery of mycobacteriophage D29. Twentyfour hours post-phage administration, no significant differences were detected compared with the control group. Only a slight increase in lymphocyte number was detected after 72 h, indicating a minor inflammatory response. The presence of endotoxin and other host cell protein might have induced the inflammatory response, but the purity of the phage preparation was not discussed in the study. Lung epithelial cells remained intact as reflected by consistent level of IL-1 and LDH after 24 h and 72 h. Debarbieux et al. [16] delivered intra-nasally to lung infected mice an anti-Pseudomonal phage amplified and purified in PBS solution in-house. The levels of inflammatory markers, IL-6 and TNF-α, were low in the lungs and were comparable to those receiving PBS solution only, suggesting that phages alone do not stimulate inflammatory response. Carmody et al. [14] also tested the pro-inflammatory potential of the in-house phage preparation. A group of mice were mock-infected with PBS using tracheotomy followed by intra-nasal or intraperitoneal BcepIL02 phage administration at 24 h post-infection. These studies repeatedly demonstrated that lab- purified phage preparations do not induce an inflammatory response. Phage purification is an essential step for removing any bacterial debris, endotoxins and unwanted materials such as the growth media. During this process, phages may be inactivated with a subsequent titer reduction [107, 108]. For powder formulations, phage titer may be further reduced during production, but can be maintained in the presence of bulking- and phage-stabilizing excipients such as lactose and trehalose [52, 53]. One of the concerns of phage therapy is that it can release endotoxins following rapid bacteriolysis of Gram-negative and Gram-positive bacteria. This could theoretically trigger a pro-inflammatory response and ultimately, systemic organ failure known as the Jarisch-Herxheimer effect. Recent studies both humans [109] and animals [110] resulted in no evidence of such reaction. Case studies in humans are in agreement with preclinical studies; no adverse events have been reported so far.
Our understanding of the optimal phage dose, dose frequency, administration timing and optimal modalities (single phage, phage cocktail, combination therapy to conventional antibiotics) for a variety of clinical indications remains limited. Preclinical studies indicate that phage treatment at a high MOI (i.e. high dose) provides optimal protection against acute respiratory infections [15, 16, 20, 60]. Successful therapy is achieved when phage is administered shortly after bacterial challenge [16, 59]. However, these were acute models of study and in the clinical setting, patients suffer from chronic infections, often polymicrobial, where it is difficult to gauge the concentration of bacteria in the lungs. Hence, there may be a tendency to administer the highest dose to avoid clearance of phages before the proliferation threshold can be met. The number of in vivo studies on pharmacokinetics of inhaled phages in infected and non-infected models has been too small to draw conclusions on dose frequency. The relevance of these studies for human lung infections also remains uncertain. Given the long-term report of inherent safety of phages in humans, it may be more relevant to study these parameters directly in Phase I/II clinical trials. The effect of phage administration, including multiple dosing and the development of antiphage antibodies (potentially rendering therapy failure) also needs to be further elucidated. Intravenous administration of phages can activate both the adaptive and innate immune system [111] causing rapid titer reduction in the blood [112]. Pre-existing inflammation caused by bacterial infection may speed up phagocytic clearance of phages [113]. On the other hand, the innate immune system can induce antibacterial activities, clearing MDR and/or phage-resistant bacteria from the site of infection [114, 115]. Phages generate neutralizing antibodies in serum, but it is unclear whether such antiphage antibodies are produced in the lungs. Łusiak-Szelachowska et al. [116] demonstrated that the level of antiphage antibodies in serum is not associated with the efficacy of phage therapy. The effect of antiphage antibodies after repeated inhaled phage therapy is an area yet to be explored. In silico modelling for clinical phage therapy application is challenging due to complex interaction between the phage, bacteria and the immune system.
8. Conclusion
Despite the renewed attention inhaled phage therapy has received in the last 20 years, its use still lacks rigorous in vivo studies and some of the fundamental questions remain to be answered. There have been reports on the use of inhaled phage therapy for compassionate use in European countries, but the detailed clinical protocols and data are often unreported. General consensus for inhaled phage therapy is that it is safe in humans with no reported adverse events [117–120]. The US FDA expressed their positive view on phage therapy and the need for pragmatic regulatory guidelines for this new antibacterial agent [100]. Robust double-blinded randomized clinical trials, pharmacokinetic/pharmacodynamics and further research on stability of phage and phage mixes in liquid and dry powder formulations are essential to meet the current regulatory models. It would benefit to add phage therapy on top of the current standard treatment especially for patients with life-threatening infections. For example, Orphan Drug Act could be utilized to lower the financial burdens and stringency associated with clinical trials. Furthermore, smaller clinical trial sizes and shorter clinical trial times could accelerate approval pathway. The current regulations do not allow full explotation of the beneits of phage therapy. However, the regulatory agency is actively working together to find out the best regulatory path for phage therapy. Despite all these challenges, inhaled phage therapy holds remarkable potential to help treat respiratory infections, particularly those caused by MDR bacteria.
Acknowledgements
H-K Chan is supported by a research grant from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R33AI121627. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Abbreviations
- MDR
multidrug resistant
- BCC
Burkholderia cepacia complex
- CF
cystic fibrosis
- RH
relative humidity
- DPI
dry powder inhaler
- pMDIs inhalers
pressurized metered dose
- FDA
Food and Drug Administration
- MOI
multiplicity of infection
- LPS
lipopolysaccharide
- PFU
plaque forming units
References
- [1].Payne RJ, Jansen VA, Pharmacokinetic principles of bacteriophage therapy, Clin Pharmacokinet, 42 (2003) 315–325. [DOI] [PubMed] [Google Scholar]
- [2].Abedon ST, Phage therapy of pulmonary infections, Bacteriophage, 5 (2015) e1020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Loc-Carrillo C, Abedon ST, Pros and cons of phage therapy, Bacteriophage, 1 (2011) 111–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wittebole X, De Roock S, Opal SM, A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens, Virulence, 5 (2014) 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK, Engstrand L, Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome, PLoS One, 5 (2010) e9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hoe S, Semler DD, Goudie AD, Lynch KH, Matinkhoo S, Finlay WH, Dennis JJ, Vehring R, Respirable bacteriophages for the treatment of bacterial lung infections, Journal of aerosol medicine and pulmonary drug delivery, 26 (2013) 317–335. [DOI] [PubMed] [Google Scholar]
- [7].Tsonos J, Vandenheuvel D, Briers Y, De Greve H, Hernalsteens JP, Lavigne R, Hurdles in bacteriophage therapy: deconstructing the parameters, Veterinary microbiology, 171 (2014) 460–469. [DOI] [PubMed] [Google Scholar]
- [8].Malik DJ, Sokolov IJ, Vinner GK, Mancuso F, Cinquerrui S, Vladisavljevic GT, Clokie MRJ, Garton NJ, Stapley AGF, Kirpichnikova A, Formulation, stabilisation and encapsulation of bacteriophage for phage therapy, Advances in Colloid and Interface Science, 249 (2017) 100–133. [DOI] [PubMed] [Google Scholar]
- [9].Merabishvili M, Pirnay JP, Verbeken G, Chanishvili N, Tediashvili M, Lashkhi N, Glonti T, Krylov V, Mast J, Van Parys L, Lavigne R, Volckaert G, Mattheus W, Verween G, De Corte P, Rose T, Jennes S, Zizi M, De Vos D, Vaneechoutte M, Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials, PLoS One, 4 (2009) e4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Carlson K, Appendix. Working with bacteriophages: common techniques and methodological approaches, in: Kutter E, Sulakvelidze A (Eds.) Bacteriophages: biology and applications, CRC Press, Boca Raton, Fla: 2005, pp. 437–494. [Google Scholar]
- [11].Golshahi L, Seed KD, Dennis JJ, Finlay WH, Toward modern inhalational bacteriophage therapy: nebulization of bacteriophages of Burkholderia cepacia complex, Journal of aerosol medicine and pulmonary drug delivery, 21 (2008) 351–360. [DOI] [PubMed] [Google Scholar]
- [12].Jonczyk E, Klak M, Miedzybrodzki R, Gorski A, The influence of external factors on bacteriophages--review, Folia microbiologica, 56 (2011) 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bodier-Montagutelli E, Morello E, L’Hostis G, Guillon A, Dalloneau E, Respaud R, Pallaoro N, Blois H, Vecellio L, Gabard J, Heuze-Vourc’h N, Inhaled phage therapy: a promising and challenging approach to treat bacterial respiratory infections, Expert opinion on drug delivery, (2016) 1–14. [DOI] [PubMed] [Google Scholar]
- [14].Carmody LA, Gill JJ, Summer EJ, Sajjan US, Gonzalez CF, Young RF, LiPuma JJ, Efficacy of Bacteriophage Therapy in a Model of Burkholderia cenocepacia Pulmonary Infection, Journal of Infectious Diseases, 201 (2010) 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Morello E, Saussereau E, Maura D, Huerre M, Touqui L, Debarbieux L, Pulmonary Bacteriophage Therapy on Pseudomonas aeruginosa Cystic Fibrosis Strains: First Steps Towards Treatment and Prevention, PLoS One, 6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Debarbieux L, Leduc D, Maura D, Morello E, Criscuolo A, Grossi O, Balloy V, Touqui L, Bacteriophages Can Treat and Prevent Pseudomonas aeruginosa Lung Infections, Journal of Infectious Diseases, 201 (2010) 1096–1104. [DOI] [PubMed] [Google Scholar]
- [17].Kutateladze M, Adamia R, Phage therapy experience at the Eliava Institute, Medecine et maladies infectieuses, 38 (2008) 426–430. [DOI] [PubMed] [Google Scholar]
- [18].Sahota JS, Smith CM, Radhakrishnan P, Winstanley C, Goderdzishvili M, Chanishvili N, Kadioglu A, O’Callaghan C, Clokie MR, Bacteriophage Delivery by Nebulization and Efficacy Against Phenotypically Diverse Pseudomonas aeruginosa from Cystic Fibrosis Patients, Journal of aerosol medicine and pulmonary drug delivery, 28 (2015) 353–360. [DOI] [PubMed] [Google Scholar]
- [19].Cooper CJ, Denyer SP, Maillard JY, Stability and purity of a bacteriophage cocktail preparation for nebulizer delivery, Letters in applied microbiology, 58 (2014) 118–122. [DOI] [PubMed] [Google Scholar]
- [20].Semler DD, Goudie AD, Finlay WH, Dennis JJ, Aerosol Phage Therapy Efficacy in Burkholderia cepacia Complex Respiratory Infections, Antimicrobial Agents and Chemotherapy, 58 (2014) 4005–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cao Z, Zhang J, Niu YD, Cui N, Ma Y, Cao F, Jin L, Li Z, Xu Y, Isolation and characterization of a “phiKMV-like” bacteriophage and its therapeutic effect on mink hemorrhagic pneumonia, PLoS One, 10 (2015) e0116571. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [22].Liu K, Wen Z, Li N, Yang W, Wang J, Hu L, Dong X, Lu J, Li J, Impact of relative humidity and collection media on mycobacteriophage D29 aerosol, Applied and environmental microbiology, 78 (2012) 1466–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu KY, Yang WH, Dong XK, Cong LM, Li N, Li Y, Wen ZB, Yin Z, Lan ZJ, Li WP, Li JS, Inhalation Study of Mycobacteriophage D29 Aerosol for Mice by Endotracheal Route and Nose-Only Exposure, Journal of aerosol medicine and pulmonary drug delivery, 29 (2016) 393–405. [DOI] [PubMed] [Google Scholar]
- [24].Gill JJ, Hyman P, Phage choice, isolation, and preparation for phage therapy, Current pharmaceutical biotechnology, 11 (2010) 2–14. [DOI] [PubMed] [Google Scholar]
- [25].Matinkhoo S, Lynch KH, Dennis JJ, Finlay WH, Vehring R, Spray-Dried Respirable Powders Containing Bacteriophages for the Treatment of Pulmonary Infections, Journal of Pharmaceutical Sciences, 100 (2011) 5197–5205. [DOI] [PubMed] [Google Scholar]
- [26].Vandenheuvel D, Singh A, Vandersteegen K, Klumpp J, Lavigne R, Van den Mooter G, Feasibility of spray drying bacteriophages into respirable powders to combat pulmonary bacterial infections, European Journal of Pharmaceutics and Biopharmaceutics, 84 (2013) 578–582. [DOI] [PubMed] [Google Scholar]
- [27].JSC Biochimpharm, Products.
- [28].Eliava Biopreparation, Products.
- [29].Intralytix Inc, Product.
- [30].Phage Guard.
- [31].Sarhan WA, Azzazy HM, Phage approved in food, why not as a therapeutic?, Expert review of anti-infective therapy, 13 (2015) 91–101. [DOI] [PubMed] [Google Scholar]
- [32].Tovkach FI, Zhuminska GI, Kushkina AI, Long-term preservation of unstable bacteriophages of enterobacteria, Mikrobiolohichnyi zhurnal (Kiev, Ukraine : 1993), 74 (2012) 60–66. [PubMed] [Google Scholar]
- [33].Colom J, Cano-Sarabia M, Otero J, Cortes P, Maspoch D, Llagostera M, Liposome-Encapsulated Bacteriophages for Enhanced Oral Phage Therapy against Salmonella spp, Applied and environmental microbiology, 81 (2015) 4841–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nieth A, Verseux C, Barnert S, Suss R, Romer W, A first step toward liposome-mediated intracellular bacteriophage therapy, Expert opinion on drug delivery, 12 (2015) 1411–1424. [DOI] [PubMed] [Google Scholar]
- [35].Singla S, Harjai K, Katare OP, Chhibber S, Bacteriophage-loaded nanostructured lipid carrier: improved pharmacokinetics mediates effective resolution of Klebsiella pneumoniae-induced lobar pneumonia, The Journal of infectious diseases, 212 (2015) 325–334. [DOI] [PubMed] [Google Scholar]
- [36].Singla S, Harjai K, Raza K, Wadhwa S, Katare OP, Chhibber S, Phospholipid vesicles encapsulated bacteriophage: A novel approach to enhance phage biodistribution, Journal of virological methods, 236 (2016) 68–76. [DOI] [PubMed] [Google Scholar]
- [37].Zeman KL, Wu J, Bennett WD, Targeting aerosolized drugs to the conducting airways using very large particles and extremely slow inhalations, Journal of aerosol medicine and pulmonary drug delivery, 23 (2010) 363–369. [DOI] [PubMed] [Google Scholar]
- [38].Cipolla D, Wu HY, Chan HK, Gonda I, NOVEL INHALED LIPOSOMAL CIPROFLOXACIN FORMULATIONS FOR PERSONALIZED THERAPY, Journal of aerosol medicine and pulmonary drug delivery, 28 (2015) A13–A13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cipolla D, Wu HY, Gonda I, Chan HK, Aerosol Performance and Long-Term Stability of Surfactant-Associated Liposomal Ciprofloxacin Formulations with Modified Encapsulation and Release Properties, Aaps Pharmscitech, 15 (2014) 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cipolla D, Wu HY, Gonda I, Chan HK, Aerosol Performance and Stability of Liposomes Containing Ciprofloxacin Nanocrystals, Journal of aerosol medicine and pulmonary drug delivery, 28 (2015) 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Carrigy NB, Chang RY, Leung SSY, Harrison M, Petrova Z, Pope WH, Hatfull GF, Britton WJ, Chan HK, Sauvageau D, Finlay WH, Vehring R, Anti-Tuberculosis Bacteriophage D29 Delivery with a Vibrating Mesh Nebulizer, Jet Nebulizer, and Soft Mist Inhaler, Pharm Res, 34 (2017) 2084–2096. [DOI] [PubMed] [Google Scholar]
- [42].Astudillo A, Leung SSY, Kutter E, Morales S, Chan H-K, Nebulization effects on structural stability of bacteriophage PEV 44, European Journal of Pharmaceutics and Biopharmaceutics, 125 (2018) 124–130. [DOI] [PubMed] [Google Scholar]
- [43].Turgeon N, Toulouse MJ, Martel B, Moineau S, Duchaine C, Comparison of five bacteriophages as models for viral aerosol studies, Applied and environmental microbiology, 80 (2014) 4242–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Verreault D, Marcoux-Voiselle M, Turgeon N, Moineau S, Duchaine C, Resistance of Aerosolized Bacterial Viruses to Relative Humidity and Temperature, Applied and environmental microbiology, 81 (2015) 7305–7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ackermann HW, 5500 Phages examined in the electron microscope, Archives of Virology, 152 (2007) 227–243. [DOI] [PubMed] [Google Scholar]
- [46].Puapermpoonsiri U, Ford SJ, van der Walle CF, Stabilization of bacteriophage during freeze drying, International journal of pharmaceutics, 389 (2010) 168–175. [DOI] [PubMed] [Google Scholar]
- [47].Golshahi L, Lynch KH, Dennis JJ, Finlay WH, In vitro lung delivery of bacteriophages KS4-M and Theta KZ using dry powder inhalers for treatment of Burkholderia cepacia complex and Pseudomonas aeruginosa infections in cystic fibrosis, Journal of Applied Microbiology, 110 (2011) 106–117. [DOI] [PubMed] [Google Scholar]
- [48].Alfadhel M, Puapermpoonsiri U, Ford SJ, McInnes FJ, van der Walle CF, Lyophilized inserts for nasal administration harboring bacteriophage selective for Staphylococcus aureus: in vitro evaluation, International journal of pharmaceutics, 416 (2011) 280–287. [DOI] [PubMed] [Google Scholar]
- [49].Merabishvili M, Vervaet C, Pirnay J-P, De Vos D, Verbeken G, Mast J, Chanishvili N, Vaneechoutte M, Stability of Staphylococcus aureus Phage ISP after Freeze-Drying (Lyophilization), PLoS One, 8 (2013) e68797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dini C, de Urraza PJ, Effect of buffer systems and disaccharides concentration on Podoviridae coliphage stability during freeze drying and storage, Cryobiology, 66 (2013) 339–342. [DOI] [PubMed] [Google Scholar]
- [51].Murray MP, Govan JR, Doherty CJ, Simpson AJ, Wilkinson TS, Chalmers JD, Greening AP, Haslett C, Hill AT, A randomized controlled trial of nebulized gentamicin in non-cystic fibrosis bronchiectasis, American journal of respiratory and critical care medicine, 183 (2011) 491–499. [DOI] [PubMed] [Google Scholar]
- [52].Chang RY, Wong J, Mathai A, Morales S, Kutter E, Britton W, Li J, Chan HK, Production of highly stable spray dried phage formulations for treatment of Pseudomonas aeruginosa lung infection, Eur J Pharm Biopharm, 121 (2017) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Leung SSY, Parumasivam T, Gao FG, Carrigy NB, Vehring R, Finlay WH, Morales S, Britton WJ, Kutter E, Chan HK, Production of inhalation phage powders using spray freeze drying and spray drying techniques for treatment of respiratory infections, Pharm Res, 33 (2016) 1486–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vandenheuvel D, Meeus J, Lavigne R, Van den Mooter G, Instability of bacteriophages in spray-dried trehalose powders is caused by crystallization of the matrix, International journal of pharmaceutics, 472 (2014) 202–205. [DOI] [PubMed] [Google Scholar]
- [55].Leung S.S.Y. WJ, Guerra HV, Samnick K, Prud’ homme RK, Chan H-K, Porous mannitol carrier for pulmonary delivery of cyclosporine A nanoparticles, International Journal of Pharmaceutics, 2015. [DOI] [PubMed] [Google Scholar]
- [56].Leung SSY, Parumasivam T, Gao FG, Carter EA, Carrigy NB, Vehring R, Finlay WH, Morales S, Britton WJ, Kutter E, Chan HK, Effects of storage conditions on the stability of spray dried, inhalable bacteriophage powders, International journal of pharmaceutics, 521 (2017) 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Leung SSY, Parumasivam T, Nguyen A, Gengenbach T, Carter EA, Carrigy NB, Wang H, Vehring R, Finlay WH, Morales S, Britton WJ, Kutter E, Chan HK, Effect of storage temperature on the stability of spray dried bacteriophage powders, Eur J Pharm Biopharm, 127 (2018) 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hoe S, Boraey MA, Ivey JW, Finlay WH, Vehring R, Manufacturing and device options for the delivery of biotherapeutics, Journal of aerosol medicine and pulmonary drug delivery, 27 (2014) 315–328. [DOI] [PubMed] [Google Scholar]
- [59].Alemayehu D, Casey PG, McAuliffe O, Guinane CM, Martin JG, Shanahan F, Coffey A, Ross RP, Hill C, Bacteriophages phiMR299-2 and phiNH-4 can eliminate Pseudomonas aeruginosa in the murine lung and on cystic fibrosis lung airway cells, mBio, 3 (2012) e00029–00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pabary R, Singh C, Morales S, Bush A, Alshafi K, Bilton D, Alton EW, Smithyman A, Davies JC, Antipseudomonal Bacteriophage Reduces Infective Burden and Inflammatory Response in Murine Lung, Antimicrob Agents Chemother, 60 (2016) 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chhibber S, Kaur S, Kumari S, Therapeutic potential of bacteriophage in treating Klebsiella pneumoniae B5055-mediated lobar pneumonia in mice, Journal of medical microbiology, 57 (2008) 1508–1513. [DOI] [PubMed] [Google Scholar]
- [62].Henry M, Lavigne R, Debarbieux L, Predicting in vivo efficacy of therapeutic bacteriophages used to treat pulmonary infections, Antimicrob Agents Chemother, 57 (2013) 5961–5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fothergill JL, Neill DR, Loman N, Winstanley C, Kadioglu A, Pseudomonas aeruginosa adaptation in the nasopharyngeal reservoir leads to migration and persistence in the lungs, Nature communications, 5 (2014) 4780. [DOI] [PubMed] [Google Scholar]
- [64].Bragonzi A, Murine models of acute and chronic lung infection with cystic fibrosis pathogens, International journal of medical microbiology : IJMM, 300 (2010) 584–593. [DOI] [PubMed] [Google Scholar]
- [65].Carter ME, Fothergill JL, Walshaw MJ, Rajakumar K, Kadioglu A, Winstanley C, A subtype of a Pseudomonas aeruginosa cystic fibrosis epidemic strain exhibits enhanced virulence in a murine model of acute respiratory infection, The Journal of infectious diseases, 202 (2010) 935–942. [DOI] [PubMed] [Google Scholar]
- [66].Waters EM, Neill DR, Kaman B, Sahota JS, Clokie MR, Winstanley C, Kadioglu A, Phage therapy is highly effective against chronic lung infections with Pseudomonas aeruginosa, Thorax, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Dudhani RV, Turnidge JD, Nation RL, Li J, fAUC/MIC is the most predictive pharmacokinetic/pharmacodynamic index of colistin against Acinetobacter baumannii in murine thigh and lung infection models, The Journal of antimicrobial chemotherapy, 65 (2010) 1984–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Badhan RK, Kaur M, Lungare S, Obuobi S, Improving brain drug targeting through exploitation of the nose-to-brain route: a physiological and pharmacokinetic perspective, Current drug delivery, 11 (2014) 458–471. [DOI] [PubMed] [Google Scholar]
- [69].Labrie SJ, Samson JE, Moineau S, Bacteriophage resistance mechanisms, Nature reviews. Microbiology, 8 (2010) 317–327. [DOI] [PubMed] [Google Scholar]
- [70].Torres-Barcelo C, Hochberg ME, Evolutionary Rationale for Phages as Complements of Antibiotics, Trends in microbiology, 24 (2016) 249–256. [DOI] [PubMed] [Google Scholar]
- [71].Kamal F, Dennis JJ, Burkholderia cepacia complex Phage-Antibiotic Synergy (PAS): antibiotics stimulate lytic phage activity, Applied and environmental microbiology, 81 (2015) 1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Verma V, Harjai K, Chhibber S, Restricting ciprofloxacin-induced resistant variant formation in biofilm of Klebsiella pneumoniae B5055 by complementary bacteriophage treatment, The Journal of antimicrobial chemotherapy, 64 (2009) 1212–1218. [DOI] [PubMed] [Google Scholar]
- [73].Torres-Barcelo C, Arias-Sanchez FI, Vasse M, Ramsayer J, Kaltz O, Hochberg ME, A window of opportunity to control the bacterial pathogen Pseudomonas aeruginosa combining antibiotics and phages, PLoS One, 9 (2014) e106628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Coulter LB, McLean RJ, Rohde RE, Aron GM, Effect of bacteriophage infection in combination with tobramycin on the emergence of resistance in Escherichia coli and Pseudomonas aeruginosa biofilms, Viruses, 6 (2014) 3778–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chaudhry WN, Concepcion-Acevedo J, Park T, Andleeb S, Bull JJ, Levin BR, Synergy and Order Effects of Antibiotics and Phages in Killing Pseudomonas aeruginosa Biofilms, PLoS One, 12 (2017) e0168615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Knezevic P, Curcin S, Aleksic V, Petrusic M, Vlaski L, Phage-antibiotic synergism: a possible approach to combatting Pseudomonas aeruginosa, Research in microbiology, 164 (2013) 55–60. [DOI] [PubMed] [Google Scholar]
- [77].Nouraldin AAM, Baddour MM, Harfoush RAH, Essa SAM, Bacteriophage-antibiotic synergism to control planktonic and biofilm producing clinical isolates of Pseudomonas aeruginosa, Alexandria Journal of Medicine, 52 (2016) 99–105. [Google Scholar]
- [78].Kirby AE, Synergistic action of gentamicin and bacteriophage in a continuous culture population of Staphylococcus aureus, PLoS One, 7 (2012) e51017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Vouillamoz J, Entenza JM, Giddey M, Fischetti VA, Moreillon P, Resch G, Bactericidal synergism between daptomycin and the phage lysin Cpl-1 in a mouse model of pneumococcal bacteraemia, International journal of antimicrobial agents, 42 (2013) 416–421. [DOI] [PubMed] [Google Scholar]
- [80].Chan BK, Sistrom M, Wertz JE, Kortright KE, Narayan D, Turner PE, Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa, Scientific reports, 6 (2016) 26717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Aleshkin AV, Ershova ON, Volozhantsev NV, Svetoch EA, Popova AV, Rubalskii EO, Borzilov AI, Aleshkin VA, Afanas’ev SS, Karaulov AV, Galimzyanov KM, Rubalsky OV, Bochkareva SS, Phagebiotics in treatment and prophylaxis of healthcare-associated infections, Bacteriophage, 6 (2016) e1251379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zimecki M, Artym J, Kocieba M, Weber-Dabrowska B, Borysowski J, Gorski A, Effects of prophylactic administration of bacteriophages to immunosuppressed mice infected with Staphylococcus aureus, BMC microbiology, 9 (2009) 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zimecki M, Artym J, Kocieba M, Weber-Dabrowska B, Borysowski J, Gorski A, Prophylactic effect of bacteriophages on mice subjected to chemotherapy-induced immunosuppression and bone marrow transplant upon infection with Staphylococcus aureus, Medical microbiology and immunology, 199 (2010) 71–79. [DOI] [PubMed] [Google Scholar]
- [84].Matsuzaki S, Yasuda M, Nishikawa H, Kuroda M, Ujihara T, Shuin T, Shen Y, Jin Z, Fujimoto S, Nasimuzzaman MD, Wakiguchi H, Sugihara S, Sugiura T, Koda S, Muraoka A, Imai S, Experimental protection of mice against lethal Staphylococcus aureus infection by novel bacteriophage phi MR11, The Journal of infectious diseases, 187 (2003) 613–624. [DOI] [PubMed] [Google Scholar]
- [85].Capparelli R, Parlato M, Borriello G, Salvatore P, Iannelli D, Experimental phage therapy against Staphylococcus aureus in mice, Antimicrob Agents Chemother, 51 (2007) 2765–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wills QF, Kerrigan C, Soothill JS, Experimental Bacteriophage Protection against Staphylococcus aureus Abscesses in a Rabbit Model, Antimicrobial Agents and Chemotherapy, 49 (2005) 1220–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kvachadze L, Balarjishvili N, Meskhi T, Tevdoradze E, Skhirtladze N, Pataridze T, Adamia R, Topuria T, Kutter E, Rohde C, Evaluation of lytic activity of staphylococcal bacteriophage Sb‐1 against freshly isolated clinical pathogens, Microbial biotechnology, 4 (2011) 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Van Norman GA, Drugs, Devices, and the FDA: Part 1: An Overview of Approval Processes for Drugs, JACC: Basic to Translational Science, 1 (2016) 170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Saussereau E, Vachier I, Chiron R, Godbert B, Sermet I, Dufour N, Pirnay JP, De Vos D, Carrié F, Molinari N, Debarbieux L, Effectiveness of bacteriophages in the sputum of cystic fibrosis patients, Clinical Microbiology and Infection, 20 (2014) O983–O990. [DOI] [PubMed] [Google Scholar]
- [90].Chan BK, Abedon ST, Loc-Carrillo C, Phage cocktails and the future of phage therapy, Future microbiology, 8 (2013) 769–783. [DOI] [PubMed] [Google Scholar]
- [91].Barbu M, DelRosario Y, Hubby B, Engineered bacteriophage therapeutics against multidrug-resistant pathogens, The FASEB Journal, 31 (2017) 101–104. [Google Scholar]
- [92].Kramberger P, Honour RC, Herman RE, Smrekar F, Peterka M, Purification of the Staphylococcus aureus bacteriophages VDX-10 on methacrylate monoliths, Journal of virological methods, 166 (2010) 60–64. [DOI] [PubMed] [Google Scholar]
- [93].Miedzybrodzki R, Fortuna W, Weber-Dabrowska B, Gorski A, Phage therapy of staphylococcal infections (including MRSA) may be less expensive than antibiotic treatment, Postepy higieny i medycyny doswiadczalnej (Online), 61 (2007) 461–465. [PubMed] [Google Scholar]
- [94].Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM, Phage treatment of human infections, Bacteriophage, 1 (2011) 66–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Verbeken G, Pirnay JP, Lavigne R, Jennes S, De Vos D, Casteels M, Huys I, Call for a dedicated European legal framework for bacteriophage therapy, Archivum immunologiae et therapiae experimentalis, 62 (2014) 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Pelfrene E, Willebrand E, Cavaleiro Sanches A, Sebris Z, Cavaleri M, Bacteriophage therapy: a regulatory perspective, Journal of Antimicrobial Chemotherapy, 71 (2016) 2071–2074. [DOI] [PubMed] [Google Scholar]
- [97].Verbeken G, Pirnay J-P, De Vos D, Jennes S, Zizi M, Lavigne R, Casteels M, Huys I, Optimizing the European Regulatory Framework for Sustainable Bacteriophage Therapy in Human Medicine, Arch Immunol Ther Exp 60 (2012) 161–172. [DOI] [PubMed] [Google Scholar]
- [98].Zhukov-Verezhnikov NN, Peremitina LD, Berillo EA, Komissarov VP, Bardymov VM, Therapeutic effect of bacteriophage preparations in the complex treatment of suppurative surgical diseases, Sovetskaia meditsina, (1978) 64–66. [PubMed] [Google Scholar]
- [99].Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, Barr JJ, Reed SL, Rohwer F, Benler S, Segall AM, Taplitz R, Smith DM, Kerr K, Kumaraswamy M, Nizet V, Lin L, McCauley MD, Strathdee SA, Benson CA, Pope RK, Leroux BM, Picel AC, Mateczun AJ, Cilwa KE, Regeimbal JM, Estrella LA, Wolfe DM, Henry MS, Quinones J, Salka S, Bishop-Lilly KA, Young R, Hamilton T, Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection, Antimicrobial Agents and Chemotherapy, 61 (2017) e00954–00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Brussow H, What is needed for phage therapy to become a reality in Western medicine?, Virology, 434 (2012) 138–142. [DOI] [PubMed] [Google Scholar]
- [101].Pirnay J-P, Verbeken G, Rose T, Jennes S, Zizi M, Huys I, Lavigne R, Merabishvili M, Vaneechoutte M, Buckling A, De Vos D, Introducing yesterday’s phage therapy in today’s medicine, Future Virology, 7 (2012) 379–390. [Google Scholar]
- [102].Chew NY, Chan HK, The role of particle properties in pharmaceutical powder inhalation formulations, Journal of aerosol medicine : the official journal of the International Society for Aerosols in Medicine, 15 (2002) 325–330. [DOI] [PubMed] [Google Scholar]
- [103].Bodier-Montagutelli E, Morello E, L’Hostis G, Guillon A, Dalloneau E, Respaud R, Pallaoro N, Blois H, Vecellio L, Gabard J, Heuze-Vourc’h N, Inhaled phage therapy: a promising and challenging approach to treat bacterial respiratory infections, Expert opinion on drug delivery, 14 (2017) 959–972. [DOI] [PubMed] [Google Scholar]
- [104].Van Belleghem JD, Merabishvili M, Vergauwen B, Lavigne R, Vaneechoutte M, A comparative study of different strategies for removal of endotoxins from bacteriophage preparations, Journal of Microbiological Methods, 132 (2017) 153–159. [DOI] [PubMed] [Google Scholar]
- [105].Boratynski J, Syper D, Weber-Dabrowska B, Lusiak-Szelachowska M, Pozniak G, Gorski A, Preparation of endotoxin-free bacteriophages, Cellular & molecular biology letters, 9 (2004) 253–259. [PubMed] [Google Scholar]
- [106].Szermer-Olearnik B, Boratyński J, Removal of Endotoxins from Bacteriophage Preparations by Extraction with Organic Solvents, PLoS ONE, 10 (2015) e0122672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Adriaenssens EM, Lehman SM, Vandersteegen K, Vandenheuvel D, Philippe DL, Cornelissen A, Clokie MRJ, García AJ, De Proft M, Maes M, Lavigne R, CIM® monolithic anion-exchange chromatography as a useful alternative to CsCl gradient purification of bacteriophage particles, Virology, 434 (2012) 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Bourdin G, Schmitt B, Marvin Guy L, Germond J-E, Zuber S, Michot L, Reuteler G, Brüssow H, Amplification and Purification of T4-Like Escherichia coli Phages for Phage Therapy: from Laboratory to Pilot Scale, Applied and environmental microbiology, 80 (2014) 1469–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sarker SA, Sultana S, Reuteler G, Moine D, Descombes P, Charton F, Bourdin G, McCallin S, Ngom-Bru C, Neville T, Akter M, Huq S, Qadri F, Talukdar K, Kassam M, Delley M, Loiseau C, Deng Y, El Aidy S, Berger B, Brussow H, Oral Phage Therapy of Acute Bacterial Diarrhea With Two Coliphage Preparations: A Randomized Trial in Children From Bangladesh, EBioMedicine., 4 (2016) 124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Soothill J, Hawkins C, Änggår E, Harper D, Therapeutic use of bacteriophages, The Lancet Infectious Diseases, 4 (2004) 544–545. [DOI] [PubMed] [Google Scholar]
- [111].Nilsson AS, Phage therapy--constraints and possibilities, Ups J Med Sci, 119 (2014) 192–198. doi: 110.3109/03009734.03002014.03902878. Epub 03002014 Mar 03009730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Sokoloff AV, Bock I, Zhang G, Sebestyen MG, Wolff JA, The interactions of peptides with the innate immune system studied with use of T7 phage peptide display, Mol Ther, 2 (2000) 131–139. [DOI] [PubMed] [Google Scholar]
- [113].Hodyra-Stefaniak K, Miernikiewicz P, Drapala J, Drab M, Jonczyk-Matysiak E, Lecion D, Kazmierczak Z, Beta W, Majewska J, Harhala M, Bubak B, Klopot A, Gorski A, Dabrowska K, Mammalian Host-Versus-Phage immune response determines phage fate in vivo, Scientific reports, 5 (2015) 14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Leung CYJ, Weitz JS, Modeling the synergistic elimination of bacteria by phage and the innate immune system, J Theor Biol, 429:241–252. (2017) 10.1016/j.jtbi.2017.1006.1037. Epub 2017 Jun 1028. [DOI] [PubMed] [Google Scholar]
- [115].Roach DR, Leung CY, Henry M, Morello E, Singh D, Di Santo JP, Weitz JS, Debarbieux L, Synergy between the Host Immune System and Bacteriophage Is Essential for Successful Phage Therapy against an Acute Respiratory Pathogen, Cell Host & Microbe, 22 38–47. e34. [DOI] [PubMed] [Google Scholar]
- [116].Lusiak-Szelachowska M, Zaczek M, Weber-Dabrowska B, Miedzybrodzki R, Letkiewicz S, Fortuna W, Rogoz P, Szufnarowski K, Jonczyk-Matysiak E, Olchawa E, Walaszek KM, Gorski A, Antiphage activity of sera during phage therapy in relation to its outcome, Future Microbiol., 12:109–117. (2017) 10.2217/fmb-2016-0156. Epub 2016 Sep 2219. [DOI] [PubMed] [Google Scholar]
- [117].Bruttin A, Brussow H, Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy, Antimicrob Agents Chemother, 49 (2005) 2874–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].McCallin S, Alam Sarker S, Barretto C, Sultana S, Berger B, Huq S, Krause L, Bibiloni R, Schmitt B, Reuteler G, Brussow H, Safety analysis of a Russian phage cocktail: from metagenomic analysis to oral application in healthy human subjects, Virology, 443 (2013) 187–196. [DOI] [PubMed] [Google Scholar]
- [119].Rhoads DD, Wolcott RD, Kuskowski MA, Wolcott BM, Ward LS, Sulakvelidze A, Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial, Journal of wound care, 18 (2009) 237–238, 240–233. [DOI] [PubMed] [Google Scholar]
- [120].Sarker SA, McCallin S, Barretto C, Berger B, Pittet AC, Sultana S, Krause L, Huq S, Bibiloni R, Bruttin A, Reuteler G, Brussow H, Oral T4-like phage cocktail application to healthy adult volunteers from Bangladesh, Virology, 434 (2012) 222–232. [DOI] [PubMed] [Google Scholar]


