Abstract
Fracture healing is a complex process of many coordinated biological pathways. This system can go awry resulting in nonunion, which leads to significant patient morbidity. The Hedgehog (Hh) signaling pathway is upregulated in fracture healing. We hypothesized that the Hh signaling pathway can be pharmacologically modulated to positively affect fracture healing. Diaphyseal femur fractures were created in elderly mice (18 months, C57BL/6 females), which have a blunted and delayed healing response compared to younger mice, and were stabilized with intramedullary pins. To activate the Hh pathway we targeted the receptor Smoothened using an agonist (Hh-Ag1.5 [Hh-Ag]) and compared this to a vehicle control. Expression of Hh target genes were significantly increased in the fracture callus of the agonist group compared to controls, indicating pathway activation. Expression of osteogenic and chondrogenic-related genes was greatly upregulated in fracture callus vs. intact femora, although Hh agonist treatment did not consistently enhance this response. Blindly graded, radiographic callus healing scores were significantly higher in the Hh-Ag groups at post operative day (POD) 14, indicating earlier callus bridging. On microCT, Hh-Ag treatment led to greater callus volume (+40%) and bone volume (+25%) at POD21. By day 14, callus vascularity, as assessed by 3D microCT angiography vessel volume, was 85% greater in the Hh-Ag group. Finally, mechanical strength of the calluses in the Hh-Ag groups was significantly greater than in the control groups at POD21. In conclusion, systemic administration of a Hh agonist appears to improve the osseous and vascular healing responses in a mouse fracture healing-impaired model.
Keywords: mouse femur fracture, hedgehog agonist, aged mice
Introduction
Up to ten percent of the 5.6 million fractures that occur annually in the United States show evidence of impaired healing.1, 2 Fracture nonunion leads to significant patient morbidity, missed work time, prolonged pain, and often multiple surgeries.3 Elucidation of novel strategies to promote reparative osteogenesis is critical and may potentially benefit large numbers of patients. Recombinant bone morphogenetic protein (BMP) 2, BMP 7, and teriparatide have been developed for clinical use as therapeutics that target inherent bone anabolic pathways, but each has disadvantages that have limited their widespread use.4–6
The hedgehog (Hh) signaling pathway is critical to developmental osteogenesis7, 8 and bone homeostasis9. Hh ligands activate signaling through one pathway consisting of two transmembrane receptors: Patched1 (Ptch1) and Smoothened (Smo).10 Hh ligand binding to Ptch1 on the cell surface suppresses the baseline inhibition of Smo by Ptch1, resulting in increased transcription of downstream genes including glioma-associated oncogene (Gli1),11, 12 and Ptch1 itself. Thus, Ptch1 is a negative regulator of Hh signaling through this feedback loop, and Ptch1 expression is a marker of increased Hh pathway activation.13, 14 Bone tissue-specific genetic targets downstream from Gli1 are yet to be determined, however the bone sialoprotein (BSP) gene was found to contain an upstream Gli1 DNA consensus sequence.15 Exogenous modulators of the pathway include the commercially-available Hh inhibitors cyclopamine and GDC-0449 that act directly on Smo.14, 16, 17 Additionally Smoothened agonists, SAG and HhAg-1.5 (Figure 1A), are available and have been used successfully in cell culture and in vivo experiments.18–21
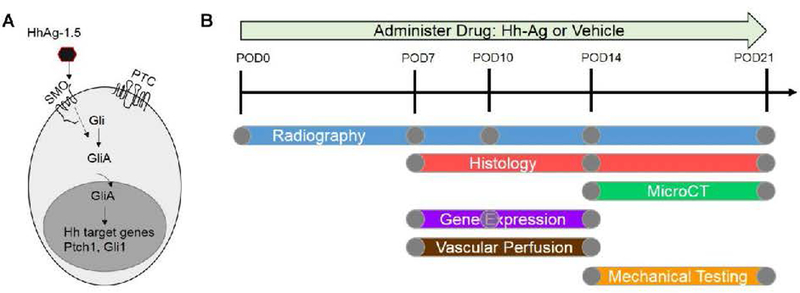
Fig. 1.
A) Hh agonist (Hh-Ag-1.5), working through Smo on the cell surface, leads to expression of Hh target genes Ptch1 and Gli1. B) Overview of the experimental outline. Either Hh-Ag or vehicle drug was administered daily starting at the time of fracture (post-operative day zero, POD0). Mice were sacrificed for analysis outcomes of histology, microCT, gene expression, vascular perfusion or mechanical testing between POD7 and POD21. Radiographs were taken of all mice before sacrifice.
An angiogenic function of the Hh pathway may be partially responsible for its effect on bone formation and development.22–24 These observations suggest a possible role for Hh signaling in osteogenesis, and associated angiogenesis, during fracture healing. In fact, Hh upregulation has been associated with fracture healing,25–28 and a recent study shows that genetic up- and down-regulation of the pathway in osteoblasts enhances and impairs, respectively, fracture healing in mice.29 Moreover, recent data indicate that pharmacological inhibition of Hh signaling impairs intramembranous bone formation and angiogenesis in healing stress fractures in young rats,30 and delays callus mineralization in full fracture in young mice.31 Interestingly, in older rodents there is a blunted upregulation of Indian hedgehog (Ihh)32 and sonic hedgehog33 after fracture in association with delayed healing suggesting that impaired Hh signaling may contribute to delayed fracture healing with aging. Thus, emerging evidence points to a functional role for hedgehog signaling in fracture repair, and raises the question of whether pharmacological upregulation can enhance fracture healing.
The goal of this study was to use a systemically-administered Hh pathway agonist, Hh-Ag1.5, in a fracture healing-challenged mouse model (aged mice) to determine if the fracture healing process could be altered. The model we chose was aged (18mo) mice, which have diminished healing. Our central hypothesis was that Hh signaling pathway can be pharmacologically modulated to positively affect fracture healing.
Methods
Study Overview
This study used 235 female, C57BL/6 wild-type mice from the National Institute of Aging (NIA, Bethesda, MD). The mice were acquired at 17 months old and housed in a standard environment (12hr light/12hr dark cycles). Mice were used at 18-months age, which is considered the onset of old age34 and when the long bones exhibit age-related changes similar to human, including expanded medullary cavity and decreased cortical thickness compared to young-adult (4–6 mo) old mice.35–37 Importantly, old mice are reported to have delayed fracture healing compared to young mice.38, 39 The mice were randomly divided into two treatment groups for various experimental outcomes (Figure 1B). The treatment group received a hedgehog agonist (Hh-Ag1.5, XcessBio, 20 mg/kg, 20 mg/ml dissolved in DMSO then diluted 1:6 with PBS), hereafter referred to as Hh-Ag, once every weekday by oral gavage until time of sacrifice. The control group received an equivalent volume of DMSO vehicle (1mL/kg, diluted 1:6 with PBS), hereafter referred to as vehicle. Additional drug and animal details are given in the supplemental methods. All protocols were approved by IACUC.
Femur Fracture Surgery
The left femur (LF) was uninjured and used as a control while the right femur (RF) was fractured. Additional surgical details are given in the supplemental methods. Day of surgery was defined as post-operative day 0 (POD0). The first dosing of Hh-Ag or vehicle was given on POD0.
Radiography
After the fracture surgery, the lower extremities were radiographed at 3x (Faxitron UltraFocus 100; Tuscon, Az). Radiographs were also taken at time of sacrifice (POD7, 10, 14, or 21) to ensure pin placement across the fracture site and to assess healing. These time points correspond to the span of unhealed to near complete healing.40 Radiographs were independently graded in a blind manner using a subset of the Goldberg Scale based on stage of mineralization (0=nonunion; 1 = possible union; 2 = radiographic union)41. Two investigators blindly graded each image, with discrepancies resolved by the senior investigator (MJG).
MicroCT
On POD14 or 21, mice were euthanized and both femurs processed for microCT. Additional details are given in the supplemental methods.
Gene Expression
On POD7, 10 and 14 mice were euthanized and both femurs were dissected with the surrounding tissue removed. Femurs were prepared for qPCR as previously described (Table S1).31 Additional details are given in the supplemental methods.
Histology
On POD7, 14, and 21 femurs were dissected for histological analysis. Tissues were stained for hematoxylin/eosin, picrosirius red/Alcian blue or tartrate-resistant acid phosphatase (TRAP). Additional details are given in the supplemental methods.
Vascular Perfusion
At POD7 and 14 mice were anesthetized and perfused with a radiopaque silicon solution in order to evaluate the vascular network around the callus. The vasculature was imaged using high resolution microCT. Additional details are given in the supplemental methods.
Mechanical Testing
Both femurs were dissected from mice at POD14 and 21. Femurs were potted and tested in torsion at 1 deg/sec until failure. Additional details are given in the supplemental methods.
Sample Size
Sample sizes (n) for each experimental group were chosen based on a priori sample size calculation (alpha [significance] = 0.05, and beta [type II error] = 0.2). Previous data were used to estimate variance, and effect sizes were based on evaluation of biological importance. Target sample sizes were as follows: qPCR, n = 8; histology, n=4; microCT, n = 10; torsion, n = 10; and vascular perfusion, n = 6. Actual group sizes are stated in the Results.
Statistical Analysis
A Chi-Square test was used to compare radiographic union assessment based on the Goldberg scale at each time point. A paired t-test was used to compare left and right femur relative qPCR gene expression (2−ΔCT) for each time point and treatment separately. A two-factor ANOVA with Bonferroni multiple comparisons test (with time and treatment as independent factors) was used to analyze fold change gene expression (2−ΔΔCT), histological analysis of cartilage and callus, TRAP analysis of osteoclast activity, vascular perfusion analysis, and torsion analysis. An unpaired t-test between treatment groups was used to analyze tibia cortical and cancellous bone. Outcomes that did not meet the assumption for parametric statistical comparison (normality [D’Agonistino & Pearson or Shapiro-Wilk test] or variance [F test]) were further analyzed using a non-parametric t-test (Mann Whitney test, <20%). For all tests, p<0.05 was considered significant. All analysis was done using GraphPad software (Prism 7, CA).
Results
Expression of Hh target genes in intact and fractured femurs is increased with Hh agonist
Hh pathway activity was assessed by expression of Gli1 and Ptch1 in the left (intact control) and right (fractured) femurs using qPCR at POD7, 10 and 14. First, we observed increased expression of Gli1 and Ptch1 in fractured vs. intact femurs for both treatment groups, confirming induction of Hh pathway during fracture repair (Table S2). Second, at POD7 and POD10 we observed significantly greater expression of Gli1 and Ptch1 in the Hh-Ag groups for both the intact and fractured femurs, indicating that the Hh pathway was effectively upregulated following systemic administration of Hh-Ag1.5. Importantly, in the fractured femur Ptch1 and Gli1 were increased by over 100% at POD7 and by 60% on POD10 (Fig. 2). By POD14 there was no longer a significant increase in Gli1 or Ptch1 expression with Hh-Ag treatment.
Figure 2.
Gene expression of Hh signaling target genes showing the activation of the Hh pathway with Hh-Ag treatment. At POD7 and 10, the expression of Gli1 and Ptch1 were significantly upregulated with Hh-Ag treatment in the fractured femur (p<0.05, two-way ANOVA; n=7–8/group).
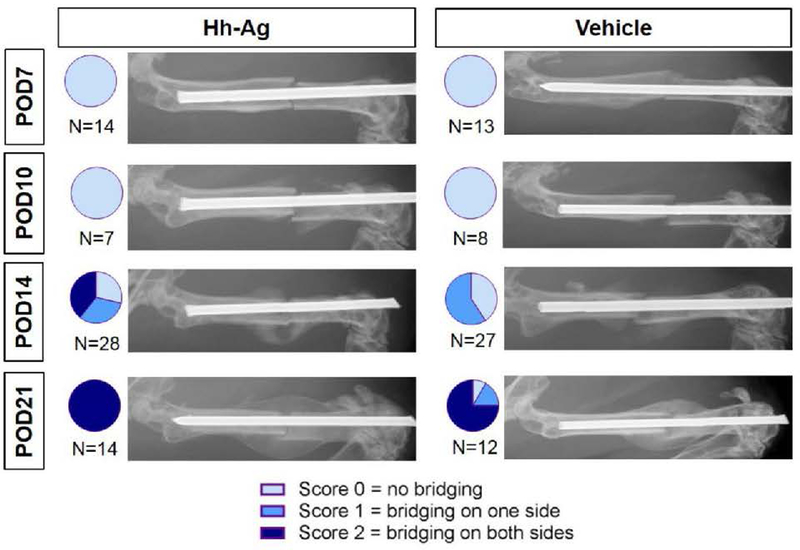
Hh treatment leads to earlier radiographic fracture healing
Radiographs were taken at four time points from POD7 to POD21, and the Goldberg score was determined to assess healing progression. At POD14, 39% (11 of 28 specimens) in the Hh-Ag group had a score of 2 (full bridging), compared to 0% (0 of 27) in the vehicle group. Additionally, the average Goldberg score was significantly greater in the Hh-Ag group (p = 0.0012). By POD21, 100% (14 of 14) of the Hh-Ag treated were fully healed, compared to 75% (9 of 12) in the vehicle group (Figure 3).
Figure 3.
Representative radiographic images showing the time course of healing. In the first 10 days post-fracture, no bridging is seen in either treatment group. By POD14, almost 40% of Hh-Ag treated mice are fully bridged (Score 2) while no vehicle treated mice are fully bridged. By POD21, almost all mice were fully bridged in both treatment groups. Radiographs were scored based on a modified Goldberg scale to assess the degree of callus bridging, shown in the pie graph inserts. Chi-squared analysis showed significant differences between treatments at POD14 (p<0.05).
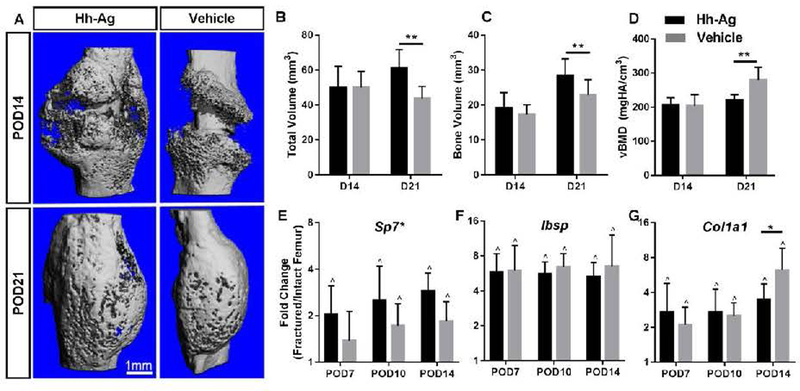
Hh agonist treatment led to greater total callus volume and bone volume on microCT, but few changes in osteogenic gene expression
Ex vivo microCT was performed on POD14 and 21 to quantify callus formation in the fracture region (Fig. 4, Table S3). On POD14, there were no differences in total volume, bone volume or vBMD between the agonist and vehicle groups. On POD21, total volume and bone volume were 40 and 25% greater, respectively, in the Hh-Ag group compared to vehicle (p < 0.01,Fig 4B, 4C). BV/TV and vBMD were lower in the Hh-Ag group. Thus, Hh-Ag treatment resulted in increase in callus size, both non-mineralized and mineralized volumes, at POD21.
Fig. 4.
A) Representative microCT reconstructions based on average TV at POD14 (n = 10–11) and POD21 (n = 10–11) for Hh-Ag and vehicle treated fractures. B-C) At POD14, no significant differences in total volume or bone volume are seen between treatment groups. At POD21, both the total and bone volumes were significantly increased with Hh-Ag treatment. D) The density increased for both groups over time, and was significantly lower in the treatment group at POD21. E) Osteogenic gene Osterix (Sp7) was significantly increased with Hh-Ag treatment. F) Bone siaoloprotein (Ibsp) expression was increased with fracture, but not affected by treatment G) Expression of Co1a1 was significantly reduced with Hh-Ag treatment at POD14. * treatment significant p<0.05, ** treatment significant p<0.01, two-way ANOVA; ^ LF vs RF significant p<0.05, paired t-test
Osteogenic genes were upregulated approximately 2–6 fold in fractured vs. intact control limbs, consistent with robust bone formation after fracture. Of the osteogenic genes analyzed, Sp7 was significantly upregulated with treatment (overall +47%, 2-way ANOVA), whereas Col1a1 expression was reduced at POD14 with Hh-Ag treatment (Fig. 4 D–F, Table S2). To assess the effects of Hh-Ag on osteoclasts TRAP staining was performed histologically. Osteoclast number (Oc.N/BS) and surface (Oc.S/BS) were not different with time or treatment (Table S4).
To assess systemic effects of Hh-Ag at non-fracture sites, microCT analysis was performed on intact tibiae after 21 days. There was a significant increase in cancellous TV (+10%), with Hh-Ag treatment, but an increase in trabecular separation (+46%) and a decrease cortical thickness (−7%, p<0.05, Table S6). Other bone parameters were unchanged due to Hh-Ag treatment.
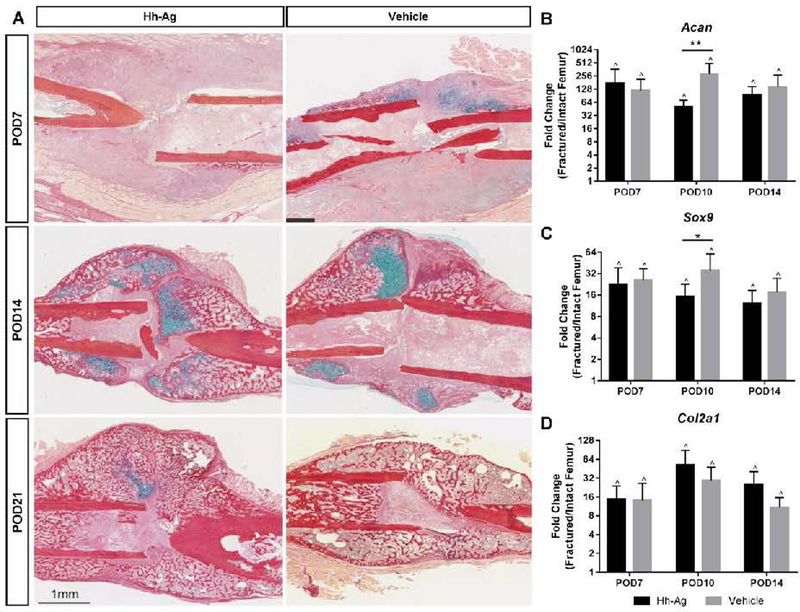
Chondrogenesis is unaffected by Hh-Ag treatment
A review of callus sections from POD7, 10 and 14 (n=3–6/treatment/time point) demonstrated the expected temporal stages of callus healing (Fig. 5A). Quantification of cartilage area revealed no differences between treatments (Table S5). Gene expression for chondrogenesis-related genes (Acan, Sox9, and Col2a1) revealed markedly higher expression in fracture vs. intact control limbs at each time point (Fig. 5B, Table S2). Among the chondrogenic genes, both Acan and Sox 9 were less up-regulated in the Hh-Ag group compared to the vehicle group at POD10.
Fig. 5.
A) Representative Picrosirius Red/Alcian Blue histology images showing the healing time course (POD7–21). Collagen and bone stain red while glucosaminoglycans and cartilage stain blue. At POD7, the callus shows small pockets of cartilage and no woven bone formation. Cartilage area peaked at POD14, and woven bone can be seen at the distal and proximal edges of the callus. By POD21, all calluses show predominantly woven bone with very little evidence of cartilage. B-D) Gene expression of chondrogenic markers was robustly upregulated in the fractured femur (RF) at all time points. At POD10, both Acan and Sox9 expression in Hh-Ag treated mice were lower compared to vehicle. *p<0.05 **p<0.01, two-way ANOVA; ^p<0.05 LF vs RF, paired t-test
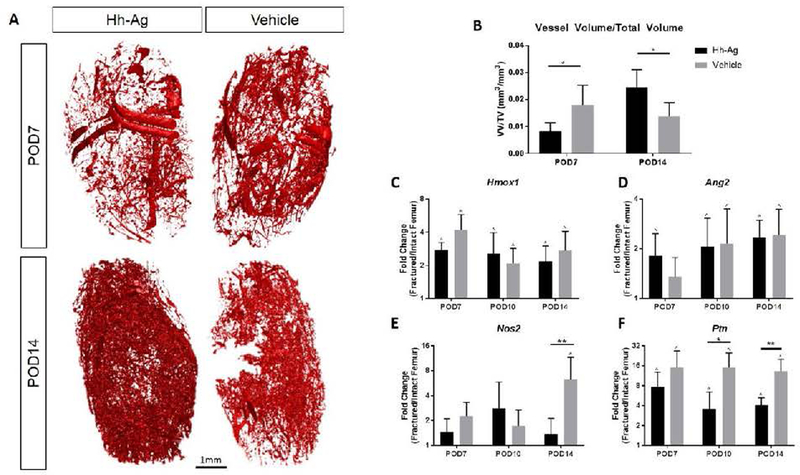
Hh agonist led to increased neovascularization by POD14
A cardiac perfusion technique was used to infiltrate the callus microvascularity, followed by high-resolution microCT to visualize and quantify the extent of vascularization. At POD7, there was a less dense vessel plexus in the calluses of Hh-Ag specimens. However, by POD14, this had reversed and the Hh-Ag group had nearly two-fold greater vessel volume/total volume ratio (Fig. 6). To further analyze the vascular effects of Hh agonist treatment, multiple angiogenic genes were assessed on qPCR (Fig. 6 C-F, Table S2). Most of these genes were upregulated in fracture callus (right femur) compared to intact bone (left femur). This upregulation was blunted in several of these genes in the Hh-Ag groups at POD7 (Hif1a), POD10 (Ptn) and POD14 (Ang, Nos2, Hif1a, Fgf2, Ptn). Thus, the increased vascularity seen on POD 14 in the Hh-Ag treated group occurred despite the decreased expression of several angiogenesis-related genes.
Figure 6.
(A) Representative reconstructions of vascular networks in and around the fracture callus. (B) At POD7 there was significantly less vasculature in Hh-Ag treated mice, but by POD14 there was significantly more vasculature (p<0.05). (C-F) Gene expression of angiogenic markers show no increase from the Hh-Ag treatment. (E) At POD14 Nos2 expression is higher for the vehicle treated specimen. (F) At POD10 Ptn expression is higher for the vehicle treated specimen. * treatment significant p<0.05, ** treatment significant p<0.01 (two-way ANOVA), ^ LF vs RF significant p<0.05 (t-test).
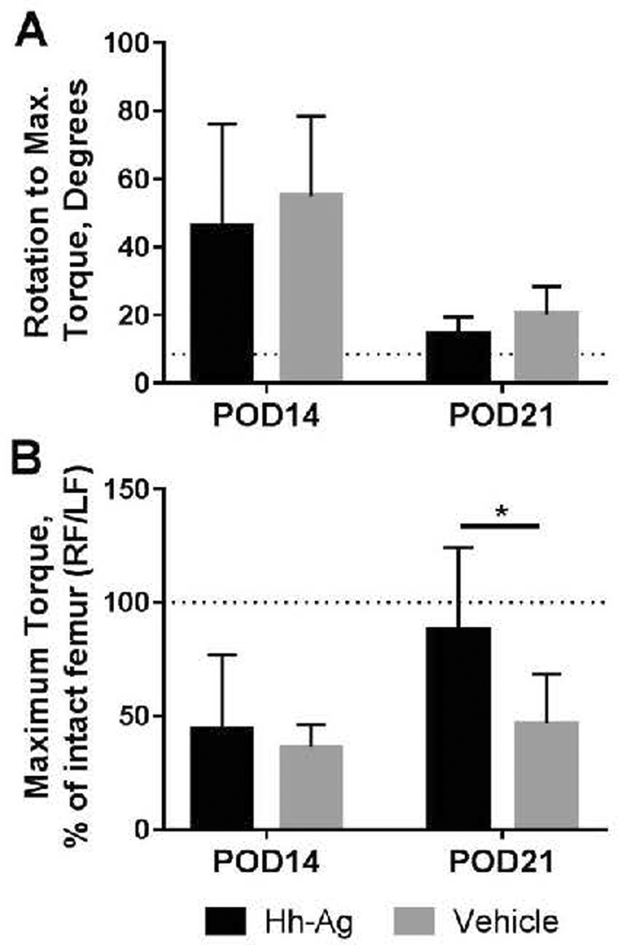
Treatment with Hh agonist led to a mechanically stronger repair at POD 21
Biomechanical testing (torsion) was performed on fractured specimens and intact femurs at two time points, POD14 (n=8–9) and POD21 (n=9–10), and revealed a positive effect of Hh treatment on strength recovery. Rotation at maximum torque was determined as a functional measure of callus maturation, with greater rotation indicative of a less mineralized, more compliant callus. From POD14 to 21, the rotation at maximum torque decreased in both treatment groups, consistent with increasing callus mineralization independent of Hh-Ag treatment (Fig. 7A). On the other hand, maximum torque (a measure of strength) did not change with time in the vehicle group, and the average value remained at 50% of intact level at POD21 (Fig. 7B). By contrast, maximum torque increased with time in the Hh-Ag group; the average value was 90% of intact level, and was significantly greater than the vehicle group at POD21. Additionally, 40% (4 of 10) of the Hh-Ag specimens were stronger than their intact contralateral control, while this occurred in only 11% (1 of 9) of WT.
Fig. 7.
A) The rotation to reach max torque decreased with time for both treatment groups. The dotted line represents the pooled average rotation of all intact femurs. B) At POD21, maximum torque of the fractured femurs in the Hh-Ag group averaged about 90% of their contralateral (intact) control value, whereas the vehicle group averaged about 50% of intact (p<0.05, 2-way ANOVA). The dotted line represents a fractured limb with the same maximum torque as its contralateral intact limb.
Discussion
Uneventful fracture healing requires a precise and complex orchestration of biological events. This can get disrupted by a variety of local and systemic dysregulations. These disruptions manifest clinically with fractures that do not proceed to timely union. The cumulative effect of fracture nonunion is significant societal burden and patient morbidity. The magnitude of this problem is evidenced by the multiple investigations into therapeutics to accelerate, augment, or rescue the fracture healing process. In this study, we used a known healing-challenged mouse model to determine if systemic upregulation of the Hh pathway could improve the healing response. We found that, in general, this intervention was modestly successful. Oral administration of a Hh pathway agonist led to upregulation of Hh activity at the fracture site. This led to larger calluses and increased bone volume on microCT, earlier radiographic fracture bridging, and mechanically stronger calluses.
We chose a healing-challenged mouse model for this study. Because of the robust and resilient fracture healing capacity of mice, it is difficult to demonstrate healing acceleration from interventions in young, normal mice. Several healing-challenged models have been described in the literature, which have mostly consisted of nonunion models based on periosteal ablation.42, 43 The downside of these models is they are relatively artificial and divergent from clinical situations, where patients have systemic pathology that impairs fracture healing. Several studies have described the notably poor healing capacity of elderly mice. Lopas et al.38 created tibial fractures in 5 mo and 25 mo old mice, and found that the elderly mice produced a significantly less robust healing response based on callus volume and density, although the proportion of cartilage to callus size was the same. Yukata et al.39 used a similar elderly mouse tibial fracture model, and attributed the blunted healing response to decreased proliferation and differentiation of periosteal stem cells. Similarly, compared to our recent data in 10-week old vehicle-treated mice31, we found 18 mo mice had delayed radiographic bridging (80% fully bridged in 10-wk mice at POD14, versus 0% fully bridged in 18-mo mice), and reduced callus density (169 mgHA/cm3 at 10-wk versus 100 mgHA/cm3 at 18-mo) at POD14 compared to 10 wk mice. The healing deficiencies and clinical applicability of older rodent studies led us to use 18 wk mice for our healing-challenged fracture study.
The skeletal cell type which is most responsive to post-natal changes in the Hh pathway has not been fully elucidated. Chondrogenesis is dependent upon Hh during embryogenesis and development,44–47 however data regarding this association during fracture healing is less conclusive. Baht et al. 29 found no effect on fracture healing when Hh signaling was genetically inactivated in chondrocytes, and concluded that the Hh pathway in chondrocytes was dispensable for fracture healing. Consistent with this, a previous study using Gli1 reporter mice found Hh activity in cartilagenous callus regions but more in the osseous callus regions.31 Moreover, treatment with a systemic Hh antagonist (GDC-0449) had no effect on expression of chondrogenic genes during healing.31 The current study similarly found no substantial differences in callus cartilage regions histologically and no increases in chondrogenic gene expression with Hh agonist treatment. Conversely, Kashiwagi et al.28 found increased SOX9-positive cells and larger cartilage regions in calluses treated with direct injection of an Hh agonist into murine fractures. It is possible that these authors achieved a greater local concentration of the agonist with injection than we achieved via oral dosing. Nonetheless, we did observe changes in osseous outcomes with systemic Hh agonist treatment, and likewise Baht et al.29 reported enhanced bone matrix formation when they genetically activated Hh signaling in osteoblasts. Thus, while we do not exclude a role for agonist treatment in the chondrogenic phase of healing, current and previous findings29, 31 support the view that osteogenic cell lines are most responsive to Hh modulation in a fracture healing situation.
Radiographic results indicate accelerated healing due to Hh-Ag treatment. Supporting that, microCT showed increased total and bone volume at POD21. vBMD was reduced at POD21, reflecting the greater proportional increase in total volume than bone volume. Interestingly, vascularity was also impacted in a time-dependent manner, with fewer vessels on POD7 but an increased number by POD14. The mechanism underlying this difference is unclear, but perhaps the initial blunted response of the angiogenic genes led to a compensatory rebound effect that led to a burst of neovascularization as fracture healing progressed. The significance of these angiogenic gene expression findings are consistent with the CT angiography results, which demonstrated less developed callus microvascular networks at POD7. Taken together, the results of our comprehensive evaluation of Hh upregulation demonstrated the largest effects on osteogenesis and angiogenesis at later timepoints, with few changes in chondrogenesis during healing.
Previous research has focused on direct application of pharmacologic interventions at the fracture site to increase healing.28, 48 A recent study demonstrated that direct application of SAG, a small molecule Hh pathway agonist, was shown to enhance callus formation in a mouse tibial osteotomy model.28 However, there are significant clinical drawbacks to therapeutics that require direct administration. Invasive treatments typically require an additional surgical procedure, the drawbacks of which include, cost, time, and patient morbidity related to anesthesia and infection. BMP-2 and BMP-7 demonstrated evidence of fracture healing augmentation, but both require direct surgical implantation, and their use is typically reserved for an adjunct during recalcitrant nonunion surgery.49 To explore an alternative approach, the current study utilized a systemic administration approach for upregulating the Hh pathway.
There were some limitations to our study. Although this was not a rigorous safety study, up to four weeks of treatment did not show any detrimental side effects. Animals from both treatment groups lost an average of <8% body weight over the course of the study and were active in their cages (Supplemental Figure 1). There were indications in the non-fractured (left femur) microCT that Hh-Ag treatment was detrimental to bone in older rodents. Additional studies would need to be performed to determine if this was recoverable after drug is withdrawn. Moreover, we did not attempt to identify an optimal dosing regimen, but focused on establishing proof-of-concept that a systemic administration of a bone anabolic agent could alter fracture healing. Nevertheless, in various pilot experiments we did validate that Gli1 and Ptch1 could be upregulated similarly in both young and old mice, with two separate Hh agonist drugs (Hh-Ag1.5 and commonly used smoothened agonist [SAG]). Finally, a human study looking at the presence of sonic hedgehog (SHH) post fracture found no role for SHH post fracture.50 While this does not indicate that there is no role for the Hh pathway in human fractures more work needs to be done in human studies prior to using a Hh agonist to promote healing.
In summary, we used an aged, healing-challenged mouse model to examine the effects of systemic Hh pathway upregulation on fracture healing. We analyzed multiple outcomes, and found a relatively substantial effect. Hh agonist treatment led to larger calluses with earlier bridging, increased neovascularization on 3D microCT angiography, and mechanically stronger bones during the late healing stages. This intervention holds promise to biologically alter fracture healing, particularly in healing-impaired situations, such as osteoporosis.
Supplementary Material
Acknowledgments
Grant Support: Washington University Musculoskeletal Research Center (NIH/NIAMS P30 AR057235 P&F to MJG), NIH/NIAMS (R21 AR066798 to MJG and MJS), and the Orthopaedic Research and Education Foundation (CDA to MJG)
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/jor.23913]
Additional Supporting Information may be found in the online version of this article.
References
- 1.Einhorn TA. Enhancement of fracture-healing. J Bone Joint Surg Am. June1995;77(6):940–956. [DOI] [PubMed] [Google Scholar]
- 2.Tzioupis C, Giannoudis PV. Prevalence of long-bone non-unions. Injury. May 2007;38Suppl 2:S3–9. [DOI] [PubMed] [Google Scholar]
- 3.Brinker MR, Hanus BD, Sen M, O’Connor DP. The devastating effects of tibial nonunion on health-related quality of life. J Bone Joint Surg Am. December 18 2013;95(24):2170–2176. [DOI] [PubMed] [Google Scholar]
- 4.Boraiah S, Paul O, Hawkes D, Wickham M, Lorich DG. Complications of recombinant human BMP-2 for treating complex tibial plateau fractures: a preliminary report. Clin Orthop Relat Res. December 2009;467(12):3257–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brannan PS, Gaston RG, Loeffler BJ, Lewis DR. Complications With the Use of BMP-2 in Scaphoid Nonunion Surgery. J Hand Surg Am. May 2016;41(5):602–608. [DOI] [PubMed] [Google Scholar]
- 6.Epstein NE. Complications due to the use of BMP/INFUSE in spine surgery: The evidence continues to mount. Surg Neurol Int. 2013;4(Suppl 5):S343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. December 1 2001;15(23):3059–3087. [DOI] [PubMed] [Google Scholar]
- 8.McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. [DOI] [PubMed] [Google Scholar]
- 9.Mak KK, Bi Y, Wan C, et al. Hedgehog signaling in mature osteoblasts regulates bone formation and resorption by controlling PTHrP and RANKL expression. Dev Cell May 2008;14(5):674–688. [DOI] [PubMed] [Google Scholar]
- 10.Day TF, Yang Y. Wnt and hedgehog signaling pathways in bone development. J Bone Joint Surg Am. February 2008;90Suppl 1:19–24. [DOI] [PubMed] [Google Scholar]
- 11.Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem. March 19 1999;274(12):8143–8152. [DOI] [PubMed] [Google Scholar]
- 12.Vokes SA, Ji H, McCuine S, et al. Genomic characterization of Gli-activator targets in sonic hedgehog-mediated neural patterning. Development. May 2007;134(10):1977–1989. [DOI] [PubMed] [Google Scholar]
- 13.Agren M, Kogerman P, Kleman MI, Wessling M, Toftgard R. Expression of the PTCH1 tumor suppressor gene is regulated by alternative promoters and a single functional Gli-binding site. Gene. April 14 2004;330:101–114. [DOI] [PubMed] [Google Scholar]
- 14.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. November 1 2002;16(21):2743–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon JW, Kita Y, Frank DJ, et al. Gene expression profiling leads to identification of GLI1-binding elements in target genes and a role for multiple downstream pathways in GLI1-induced cell transformation. J Biol Chem. February 15 2002;277(7):5548–5555. [DOI] [PubMed] [Google Scholar]
- 16.Robarge KD, Brunton SA, Castanedo GM, et al. GDC-0449-a potent inhibitor of the hedgehog pathway. Bioorg Med Chem Lett. October 1 2009;19(19):5576–5581. [DOI] [PubMed] [Google Scholar]
- 17.Rudin CM, Hann CL, Laterra J, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. September 17 2009;361(12):1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A. October 29 2002;99(22):14071–14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Shen J, Pan HC, et al. Calvarial Defect Healing Induced by Small Molecule Smoothened Agonist. Tissue Eng Part A. 2016;22(23-24)(December):1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank-Kamenetsky M, Zhang XM, Bottega S, et al. Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists. J Biol. November 6 2002;1(2):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hojo H, Ohba S, Yano F, et al. Gli1 protein participates in Hedgehog-mediated specification of osteoblast lineage during endochondral ossification. J Biol Chem. May 18 2012;287(21):17860–17869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pola R, Ling LE, Silver M, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. June 2001;7(6):706–711. [DOI] [PubMed] [Google Scholar]
- 23.Rivron NC, Raiss CC, Liu J, et al. Sonic Hedgehog-activated engineered blood vessels enhance bone tissue formation. Proc Natl Acad Sci U S A. March 20 2012;109(12):4413–4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song K, Rao NJ, Chen ML, Huang ZJ, Cao YG. Enhanced bone regeneration with sequential delivery of basic fibroblast growth factor and sonic hedgehog. Injury. August 2011;42(8):796–802. [DOI] [PubMed] [Google Scholar]
- 25.Ahmad CS, Cohen ZA, Levine WN, Ateshian GA, Mow VC. Biomechanical and topographic considerations for autologous osteochondral grafting in the knee. American Journal of Sports Medicine. 2001;29(2):201–206. [DOI] [PubMed] [Google Scholar]
- 26.Murakami S, Noda M. Expression of Indian hedgehog during fracture healing in adult rat femora. Calcif Tissue Int. April 2000;66(4):272–276. [DOI] [PubMed] [Google Scholar]
- 27.Miyaji T, Nakase T, Iwasaki M, et al. Expression and distribution of transcripts for sonic hedgehog in the early phase of fracture repair. Histochem Cell Biol. March 2003;119(3):233–237. [DOI] [PubMed] [Google Scholar]
- 28.Kashiwagi M, Hojo H, Kitaura Y, et al. Local administration of a hedgehog agonist accelerates fracture healing in a mouse model. Biochem Biophys Res Commun. October 28 2016;479(4):772–778. [DOI] [PubMed] [Google Scholar]
- 29.Baht GS, Silkstone D, Nadesan P, Whetstone H, Alman BA. Activation of hedgehog signaling during fracture repair enhances osteoblastic-dependent matrix formation. J Orthop Res. April 2014;32(4):581–586. [DOI] [PubMed] [Google Scholar]
- 30.Kazmers NH, McKenzie JA, Shen TS, Long F, Silva MJ. Hedgehog signaling mediates woven bone formation and vascularization during stress fracture healing. Bone. December 2015;81:524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, McKenzie JA, Maschhoff CW, Gardner MJ, Silva MJ. Exogenous hedgehog antagonist delays but does not prevent fracture healing in young mice. Bone. 2017;October(103):241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer RA, Jr., Meyer MH, Tenholder M, Wondracek S, Wasserman R, Garges P. Gene expression in older rats with delayed union of femoral fractures. J Bone Joint Surg Am. July 2003;85-a(7):1243–1254. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto K, Shimo T, Kurio N, et al. Expression and Role of Sonic Hedgehog in the Process of Fracture Healing with Aging. In Vivo. 2016;30(2)(March-April):99–105. [PubMed] [Google Scholar]
- 34.Flurkey KCJ, Harrison DE. Mouse models in aging research. Vol 2nd ed. San Diego: Academic Press; 2007. [Google Scholar]
- 35.Halloran BP, Ferguson Vl Fau - Simske SJ, Simske Sj Fau - Burghardt A, Burghardt A Fau-Venton LL, Venton Ll Fau - Majumdar S, Majumdar S. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J Bone Miner Res. 2002;17(6):1044–1050. [DOI] [PubMed] [Google Scholar]
- 36.Piemontese M, Almeida M, Robling AG, et al. Old age causes de novo intracortical bone remodeling and porosity in mice. JCI Insight. 2017;7(2(17)):pii:93771 doi:93710.91172/jci.insight.93771 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willinghamm MD, Brodt MD, Lee KL, Stephens AL, Ye J, Silva MJ. Age-related changes in bone structure and strength in female and male BALB/c mice. Calcif Tissue Int. June 2010;86(6):470–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopas LA, Belkin NS, Mutyaba PL, Gray CF, Hankenson KD, Ahn J. Fractures in geriatric mice show decreased callus expansion and bone volume. Clin Orthop Relat Res. November 2014;472(11):3523–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yukata K, Xie C, Li TF, et al. Aging periosteal progenitor cells have reduced regenerative responsiveness to bone injury and to the anabolic actions of PTH 1–34 treatment. Bone. May 2014;62:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manigrasso MB, O’Connor JP. Characterization of a closed femur fracture model in mice. J Orthop Trauma. Nov-Dec 2004;18(10):687–695. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg Vm Fau - Powell A, Powell A Fau - Shaffer JW, Shaffer Jw Fau - Zika J, Zika J Fau - Bos GD, Bos Gd Fau - Heiple KG, Heiple KG. Bone grafting: role of histocompatibility in transplantation. J Orthop Res. 1985;3(4):389–404. [DOI] [PubMed] [Google Scholar]
- 42.Garcia P, Holstein JH, Maier S, et al. Development of a reliable non-union model in mice. J Surg Res. June 01 2008;147(1):84–91. [DOI] [PubMed] [Google Scholar]
- 43.Garcia P, Histing T, Holstein JH, Pohlemann T, Menger MD. Femoral non-union models in the mouse. Injury. October 2010;41(10):1093–1094. [DOI] [PubMed] [Google Scholar]
- 44.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. August 15 1999;13(16):2072–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. December 2001;128(24):5099–5108. [DOI] [PubMed] [Google Scholar]
- 46.Iwasaki M, Jikko A Fau - Le AX, Le AX. Age-dependent effects of hedgehog protein on chondrocytes. J Bone Joint Surg Br. 1999;81(6)(November):1076–1082. [DOI] [PubMed] [Google Scholar]
- 47.Long F, Chung UI, Ohba S, McMahon J, Kronenberg HM, McMahon AP. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development. March 2004;131(6):1309–1318. [DOI] [PubMed] [Google Scholar]
- 48.Tevlin R, Seo EY, Marecic O, et al. Pharmacological rescue of diabetic skeletal stem cell niches. Sci Transl Med. January 11 2017;9(372). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedlaender GE, Perry CR, Cole JD, et al. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 2):S151–158. [PMC free article] [PubMed] [Google Scholar]
- 50.Eipeldauer S, Thomas A, Hoechtl-Lee L, et al. Is sonic Hedgehog involved in human fracture healing? --a prospective study on local and systemic concentrations of SHH. 20141216 DCOM- 20151001 (1932–6203 (Electronic)). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.