Abstract
Excessive alcohol consumption impairs mucociliary clearance, in part, by compromising ciliary movement. Our previous study found alcohol reduces ciliary beat frequency in Chlamydomonas through a mechanism that involves the β and γ-heavy chains of the outer dynein arm (ODA). Moreover, we identified DC1, a subunit of the ODA-docking complex (ODA-DC), as the first ciliary target for alcohol. DC1 phosphorylation is disrupted in the absence of the central pair and ODA. DC1 phosphorylation is alcohol sensitive and correlates with alcohol-induced ciliary dysfunction (AICD). Furthermore, DC1 phosphorylation is disrupted in the absence of the central pair and ODA. These results implicate a role for DC1 phosphorylation in regulating the ODA activity and mediating AICD. In our current study, we identified 4 alcohol-sensitive phospho-sites in DC1: S33, T73, T351 and S628. Mutations of these sites rescue the assembly of the ODA-DC and ODA resulting in wild-type swimming velocities. When cells were challenged with alcohol, we determined that 3 sites, S33, T351 and S628, are critical for mediating the ciliary slowing effects of alcohol. This result is consistent with our pharmacological studies, which reveal that both PP1 and PKA activities are required for AICD.
Keywords: Cilia, alcohol, dynein, motility
Introduction
Chronic and excessive alcohol consumption impairs lung mucociliary clearance resulting in diseases such as bronchitis, pneumonia, and acute respiratory distress syndrome. Multiple lines of evidence demonstrate that excessive alcohol intake compromises airway ciliary movement to yield a condition termed alcohol-induced ciliary dysfunction (AICD) (Sisson, 2007).
Previous studies in mammalian systems have identified prominent roles for the second messenger nitric oxide (Sisson, 1995) and the kinases PKG and PKA (Wyatt, Spurzem, May, & Sisson, 1998) in mediating alcohol’s effect on airway cilia movement. In addition, protein phosphatase 1 (PP1) inhibition and antioxidant treatment both prevent AICD (M. E. Price et al., 2017; M. E. Price, Pavlik, Sisson, & Wyatt, 2015). These data indicate that alcohol disrupts phospho-regulatory and redox-based pathways for control of ciliary movement.
Cilia (flagella) are highly conserved organelles. Chlamydomonas reinhardtii is a classic model for ciliary studies that has been utilized to identify genes defective in many ciliopathies including Primary Ciliary Dyskinesia, which affects the lung (Pan, 2008). Recently, we demonstrated the use of this protist model organism to study AICD, an “acquired ciliopathy” (M. Price, Yang, Sisson, & Wirschell, 2017). We demonstrated that alcohol reduces ciliary beat frequencies through a mechanism that requires the ODA and its β and γ heavy chains, and more specifically the microtubule binding domain of the β heavy chain (F. Yang et al., 2015). The ODA motor complex is comprised of three heavy chains (α, β and γ), which are the ATPase motor subunits. It primarily contributes to ciliary beat frequency. Mutations or biochemical defects of the ODA result in slower swimming speed of Chlamydomonas, and decreased airway clearance (Pazour, Agrin, Walker, & Witman, 2006). In Chlamydomonas, there is one ODA isoform that assembles throughout the axonemes.
In contrast, human ODAs are comprised of two heavy chain subunits, which are orthologous to β and γ heavy chains in the Chlamydomonas ODA. The β-heavy chain has three human orthologues (DNAH11, DNAH17 and DNAH9), the γ-heavy chain has two orthologues (DNAH5 and DNAH8), while the α-heavy chain is not conserved (Pazour et al., 2006). Mutations in the DNAH5 and DNAH11 genes result in Primary Ciliary Dyskinesia with poor mucociliary clearance, which leads to severe respiratory infections (Hornef et al., 2006; Lai et al., 2016). In human motile airway cilia, there exist two discernable ODA isoforms: proximal ODAs contain DNAH5, but not DNAH9; distally localized ODAs contain both DNAH5 and DNAH9 (Dougherty et al., 2016; Fliegauf et al., 2005).
In Chlamydomonas, the assembly of the ODA on axonemes requires the ODA docking complex (ODA-DC) (Casey et al., 2003; Koutoulis et al., 1997; Takada & Kamiya, 1994; Takada, Wilkerson, Wakabayashi, Kamiya, & Witman, 2002). The ODA-DC contains three subunits (DC1, DC2 and DC3). DC1 and DC2 are coiled-coil proteins that form a heterodimer (Ide, Owa, King, Kamiya, & Wakabayashi, 2013). The entire ODA-DC complex forms an ~24-nm ellipsoidal structure that assembles end to end on microtubules (Owa et al., 2014). This arrangement is thought to contribute to the periodic binding of ODAs along the length of the axonemal microtubules. In addition to the ODA-DC, the ODA motors require the ODA5/8/10 complex and the CCDC103 protein for assembly (Dean & Mitchell, 2013; Desai, Freshour, & Mitchell, 2015; King & Patel-King, 2015; Wirschell et al., 2004). Of these proteins, only DC2 (CCDC114), ODA10 (CCDC151) and CCDC103 (CCDC103) are conserved in humans. Mutations in all three genes also result in Primary Ciliary Dyskinesia (Alsaadi et al., 2014; Hjeij et al., 2014; Knowles et al., 2013; Onoufriadis et al., 2013; Panizzi et al., 2012; Shoemark et al., 2018). In humans, additional genes encode proteins required for ODA assembly including the ARMC4 and TTC25 genes (Hjeij et al., 2013; Wallmeier et al., 2016). TTC25 interacts with CCDC114 and is a newly identified subunit of the human ODA-DC. Defects in the ARMC4 gene result in the absence of ODAs in the distal ciliary axoneme, suggesting distinct docking complexes exist for the two classes of ODAs. c
Pharmacological, biochemical and molecular approaches in Chlamydomonas have revealed pathways controlling ciliary movement that involve the central pair and radial spoke structures, along with conserved kinases and phosphatases anchored to the axoneme (Habermacher & Sale, 1995, 1996; Howard, Habermacher, Glass, Smith, & Sale, 1994; Pazour, Agrin, Leszyk, & Witman, 2005; P. Yang & Sale, 2000). In our previous study, we identified DC1 as the first ciliary target for alcohol. DC1 phosphorylation is reduced by alcohol, and correlates with AICD. Importantly, the central pair structure and the ODA motor are essential for normal DC1 phosphorylation (F. Yang et al., 2015), indicating DC1 is a target of a central pair mechanism for control of ODA activity. These results suggest that DC1 (and the ODA-DC) may regulate the ODA motors through a phospho-regulatory mechanism, which is disrupted by alcohol.
To test this idea, we examined the effect of kinase and phosphatase inhibitors and determined that protein phosphatase 1 (PP1) and protein kinase A (PKA) play functional roles in mediating AICD. To further understand the role of DC1 phosphorylation in AICD, we identified 4 alcohol-sensitive phosphosites (S33, T73, T351and S628) and tested their role in AICD. Our results demonstrate that S33, T351and S628 are critical for AICD.
Materials and Methods
Strains and culture conditions
The Chlamydomonas strains used in this study were as follows: Wild-type strains include CC125, a gift from Winfield Sale (Emory University, Atlanta, GA) and g1, a gift from George Witman (University of Massachusetts Medical School, Worcester, MA). The high-efficiency mating type cells CC620 and CC621, as well as CC3935 (oda3-4) were obtained from the Chlamydomonas Resource Center (University of Minnesota, St. Paul, MN). Cells were grown in tris-acetate-phosphate (TAP) media for crude DNA extraction and L or M media for motility analyses. All cultures were grown with aeration plus 1% CO2 on a 14-hr: 10-hr light/dark cycle.
Cilia and axoneme extraction
Cells grown on TAP or L plates were scraped into liquid TAP or L media and cultured for 1–2 hours to allow ciliogenesis to occur. Cilia and axonemes were isolated (Witman, 1986) with slight modifications as described (F. Yang et al., 2015).
Mass spectrometry
Wild-type (CC125) axonemes were isolated from cells grown on L plates. Isolated axonemes were resuspended to 2 μg/μl in motility buffer (30 mM Hepes pH 7.4, 5 mM MgSO4, 1 mM DTT, 1 mM EGTA, 50 mM potassium acetate, 1% PEG [Mr 20,000], and proteinase inhibitors (Roche, Indianapolis, IN)), then diluted with an equal volume of motility buffer, motility buffer plus 2 mM ATP, or motility buffer plus 2 mM ATP and 200 mM alcohol (ethanol), which gave a final alcohol concentration of 1 mM ATP and 100 mM alcohol. The axonemes were incubated at room temperature for 2 min, collected by centrifugation and resuspended to 2 μg/μl in 1× sample buffer. The axoneme samples were separated on 7% polyacrylamide gels, then stained with Coomassie or processed for western blot to detect DC1. Gel slices corresponding to the migration of DC1, as determined by western blot, were excised for analyses of DC1 phosphorylation by tandem mass spectrometry (MS/MS) at the University of Alabama Mass Spectrometry/Proteomics Shared Facility (Birmingham, AL).
Reactivated cell models
Wild-type (CC125) cells were grown in M media to 1×106 ~ 1×107 cells/ml. Reactivated cell models were prepared as described (Gaillard, Fox, Rhea, Craige, & Sale, 2006) with slight modifications. 1 ml of cell culture was collected by centrifugation (1000 rpm, 2 min). Cells were washed with 10 mM Hepes pH 7.4, and resuspended in 100 μl demembranation buffer (motility buffer plus 0.1% NP-40 [Calbiochem], 5 mM creatine phosphate, and 70 U/ml creatine kinase). The cells were extracted in demembranation buffer for ~30 sec. 50 μl demembranated cells were loaded on a microscope to confirm the cessation of motility. The remaining 50 μl was diluted with 150 μl activation buffer (motility buffer with 2 mM EGTA plus 2 mM ATP, 5 mM creatine phosphate, and 70 U/ml creatine kinase). The reactivated motility was immediately observed and recorded. Reactivation was performed in the presence of rabbit, recombinant protein phosphatase inhibitor 2 (I2, Millipore, Burlington, MA), protein kinase A inhibitor fragment 6-22 amide (PKI), or inhibitors plus alcohol. I2 and PKI are inhibitors specific for PP1 and PKA. I2 (0.1 μM, 0.5 μM, 1 μM) or PKI (10 nM, 100 nM, 1 μM, 100 μM) were added to both the demembranation and activation buffers; 133 mM alcohol was added to the activation buffer to give a final concentration of 100 mM.
Measurement of swimming speed
For analysis of intact cells, cultures were grown in L media for 2 days to densities of 1~4×106 cells/ml. Swimming speeds were measured as described (F. Yang et al., 2015). The data are presented as the mean of 30 cells.
AICD analyses were performed as described (F. Yang et al., 2015) with slight modifications. Cells were cultured in M media for 2 days to densities of 1~4×106 cells/ml. 1 ml of cell culture was transferred to a 1.5 ml tube with or without 100 mM alcohol. Swimming speeds were measured immediately. The data are presented as the mean of 3–4 independent trials with 50 cells measured in each trial.
Reactivated cell models were processed for video microscopy immediately after reactivation. Swimming velocities were calculated from 30 cells per condition. The data represent the pooled results from 4–5 independent trials.
For each experiment, error bars represent the standard deviation of each data set. Statistical significance was determined by t-test calculated using Microsoft Excel software.
Western blot analysis
Cilia and axoneme samples were fixed with laemmli sample buffer, separated on standard SDS-PAGE gels and transferred to nitrocellulose (Transblot, Biorad, Hercules, CA). The blots were probed with anti-DC1 1:2,000 (gift from Dr. Ken-ichi Wakabayashi) and anti-IC2 1:10,000 (Sigma, St. Louis, MO).
Glass bead transformation
Autolysin was prepared from CC620 and CC621 strains cultured on TAP or L plates for at least two weeks. For each strain, one plate was resuspended in 150 ml M-N media and cultured in constant light with shaking overnight. Each strain was harvested by centrifugation (4000 rpm, 5 min), and resuspended in 10 ml M-N media. The two strains were mixed to allow large mating clusters to form. After 5 min, cells were separated from the medium by centrifugation. The supernatant containing autolysin was collected and filtered through a 0.2 μM filter. Autolysin was used fresh or stored at −20 °C.
One plate of oda3-4 cells were washed with TAP or L media, then resuspended in 10 ml autolysin. Removal of cell walls was monitored by sensitivity to 0.25% Triton X-100. Autolysin treated oda3-4 cells were transformed using glass beads as described (Kindle, 1990) with slight modifications. Cells were harvested from autolysin by centrifugation, washed with M-N media, and resuspended in 2–3 ml M-N liquid medium. For transformation, 0.3 ml cell culture, 0.3 g glass beads, 3 μg plasmid DNA and 100 μl 20% PEG [Mr 8,000], were added in a 15-ml conical tube, and vortexed for 30 sec. Transformation reactions were diluted with 5 ml TAP or L and cultured in constant light overnight. The next day, cells were collected by centrifugation, resuspended in 250 μl TAP or L and plated on TAP plates containing 10 μg/ml hyromycin B. Plates were incubated at 23°C under a 14-hr light/10-hr dark illumination schedule. Hygromycin resistant transformant colonies were visible after two weeks.
Crude DNA extraction and PCR for the DCC1 gene
Crude DNA was extracted as described (Lin, Miller, Granas, & Dutcher, 2013) with slight modifications. Cell colonies were resuspended in TAP media to a minimum of 106 cells/ml. 10 μl cell culture was mixed with 2 μl Vent buffer (200 mM Tris-HCl, 100 mM (NH4)2SO4, 100 mM KCl, 20 mM MgSO4 and 1% Triton X-100, pH 8.8), 1 μl 20 mg/ml Proteinase K and 7 μl of water. The cells were incubated at 58°C for 30 min to digest proteins and 95°C for 15 min to inactivate the enzyme. Samples were centrifuged (4000 rpm, 5 min) and 1 μl of supernatant was used for PCR with GoTaq polymerase (Promega, Madison, WI) (Sup Fig. 1B and C). The cycling protocol was: 95°C for 2 min, and 34 cycles of 95°C for 30 sec, annealing temperature for 30 sec, and 72°C for 30 sec, followed by a final extension time of 5 min at 72°C.
Cloning of DCC1 mutant constructs
A 1.8-kb EcoRV-PvuII restriction fragment from the pHyg3 plasmid containing the hygromycin-resistance cassette (Berthold, Schmitt, & Mages, 2002) was cloned into the EcoRV site of the DCC1 pCS3.3 plasmid (Brown et al., 2017) to make plasmid pMM4.39. pMM4.39 was digested with SpeI and XbaI and religated to make plasmid pFY-15. This removes an NruI site, making the NruI site in DCC1 unique. 1.5-kb BglII-NruI DCC1 genomic fragments with site-directed mutations (S33A, S33E, T73A or T73E) were synthesized and used to replace the original BglII-NruI DCC1 genomic fragment in pFY-15. The resulting plasmids were pFY-16.1 (S33A), pFY-17.1 (S33E), pFY-18.1 (T73A) and pFY-19.2 (T73E). Similarly, 2.1-kb NruI-AgeI DCC1 genomic fragments with site-directed mutations (T351A, T351E, S628A or S628E) were synthesized and used to replace the original NruI-AgeI DCC1 genomic fragment in pFY-15. The resulting plasmids were pFY-20.1 (T351A), pFY-21.1 (T351E), pFY-22.1 (S628A) and pFY-23.1 (S628E). The synthesized sequences were made by Genscript (Piscataway, NJ). All constructs were sequence verified by the Iowa State DNA Facility (Ames, IA).
Bioinformatics and computer analyses
The COILS program was used to map coiled-coil domains in DC1 (https://embnet.vital-it.ch/software/COILS_form.html) (Sup Fig. 1A). Primer 3 was used for primer design (http://primer3.ut.ee). Snapgene was used for construct design and DNASTAR was used for DNA sequence analyses.
Chemicals and reagents
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless specified otherwise.
Results
PP1 and PKA mediate AICD and regulate motility
In this study, inhibitors specific for PP1 (I2) and PKA (PKI) were tested for their effects on motility and AICD. To determine the effects of these inhibitors, we removed the ciliary membrane with detergent and reactivated motility with ATP. The detergent-treated cells are hereafter referred to as cell models (Kamiya & Witman, 1984). Consistent with our previous findings (F. Yang et al., 2015), swimming velocities of wild-type cell models were reduced in the presence of alcohol (Fig. 1). Pre-treatment of cell models with I2 (0.1 μM) resulted in a failure to reduce swimming speed upon alcohol exposure. At higher concentrations, pre-treatment with I2 was partially protective (0.5 μM, 8% reduction in velocity vs. 17% without I2) or caused a decrease in swimming speed (1 μM) independent of alcohol (Fig. 1A). Notably, alcohol treatment did not induce a strong, additional decrease in forward swimming speed.
Figure 1. PP1 and PKA mediate AICD and regulate motility.
The effects of I2 and PKI on reactivated cell models (CC125) in the absence and presence of alcohol are shown. (A) At low concentration (0.1 μM), pre-treatment of cell models with I2 protects cell models against the ciliary slowing effects of alcohol. At higher concentrations, I2 shows less protection (0.5 μM), or results in significant reduction in swimming speed independent of alcohol (1 μM). (B) Similarly, pre-treatment with PKI is protective for AICD (10 nM – 100 nM). At higher doses, PKI treatment results in mild AICD (1 μM) or causes a significant reduction of forward swimming speeds (100 μM) in the absence of alcohol. (*: p<0.01; **: p<0.5)
Similarly, pre-treatment of cell models with PKI (10 nM and 100 nM) rendered cells resistant and swimming speeds did not decline in the presence of alcohol. Like I2, at higher concentrations, PKI was partially protective for the ciliary slowing effects of alcohol (1μM; 5% reduction in velocity vs. 12.5% without PKI) or significantly reduced (100 μM) swimming speed independent of alcohol and alcohol treatment did not further reduce swimming velocity (Fig. 1B). These data suggest that PP1 and PKA regulate ODA function, and demonstrate they play a functional role in generation of AICD.
Identification of alcohol-sensitive phospho-sites in DC1
Previously, we identified DC1 as an alcohol-sensitive phosphoprotein (F. Yang et al., 2015). To further understand the role of DC1 in AICD, we analyzed untreated and alcohol-treated axonemes by mass spectrometry and identified alcohol-sensitive phosphorylation sites in DC1. Four amino acids showed altered phosphorylation in the presence of alcohol. S33, T73 and S628, in the non-coiled-coil domains, showed reduced phosphorylation by alcohol. Within the coiled-coil domain, T351 showed either an increased phosphorylation, decreased phosphorylation, or was undetected in the 3 independent experiments (Sup Fig. 1A). Although there were variable results for T351 phosphorylation, we tested the role of all four phosphosites in regulation of ODA activity and AICD.
Mutations in the alcohol-sensitive phosphosites rescue the assembly and motility defects in a DCC1 mutant
In order to test the role of the alcohol-sensitive phosphosites in regulation of ODA activity and AICD, these four sites were mutated to alanine (A), a non-phosphorylatable residue, or glutamic acid (E) to mimic phosphorylation. To select a DC1 null mutant for transformation, PCR was performed on crude DNA extractions from wild type (g1) and oda3-4 (CC3935), a DCC1 insertional mutant (Koutoulis et al., 1997). With DCC1-1325 and DCC1-1898 primers, oda3-4 yielded a smaller PCR product than wild type, whereas the other two primer pairs did not amplify a product in oda3-4 DNA (Supp. Fig. 1B). These results suggest that in the DCC1 gene of oda3-4, the insertional mutagenesis resulted in a small deletion within 1325bp~1898bp and a disruption within 1898bp~2594bp, which result in a failure to express DC1 protein.
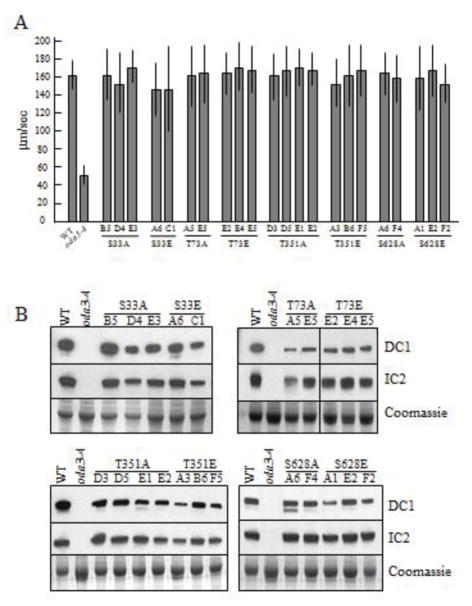
Mutated DCC1 transgenes were transformed into oda3-4. Integration of the DCC1 transgenes was confirmed by PCR with primers DCC1-2134 and DCC1-2594. Representative transformants identified include: S33A-B5, D4 and E3; S33E-A6 and C1; T73A-A5 and E5; T73E-E2, E4 and E5; T351A-D3, D5, E1 and E2; T351E-A3, B6 and F5; S628A-A6 and F4; S628E-A1, E2 and F2 (Sup Fig. 1C). Swimming speed analyses demonstrated that all the mutant DCC1 transgenes rescued the Oda-phenotype and restored wild-type swimming velocities (Fig. 2A). As expected, the wild-type parental cells (g1) swam with an average velocity of 161.7±15.4 μm/sec, whereas oda3-4 cells swam at 53.3±10.2 μm/sec. Consistently, all the mutant DCC1 transgenes restored axonemal assembly of the ODA-DC and ODA (Fig. 2B). Western blots of isolated axonemes showed the presence of DC1 and IC2 in wild-type axonemes and all the transformants, whereas these bands were missing in oda3-4 axonemes. Given that the mutant transgenes rescue the motility and assembly defects, we conclude that the phosphorylation state of these four sites is not critical for assembly and function of the ODA-DC and ODA in the absence of alcohol.
Figure 2. DCC1 mutant transgenes rescue the Oda3 motility and assembly defects.
(A) Swimming speeds of all representative transformants are rescued to wild-type (WT), or near wild-type levels, and are significantly higher than the oda3-4 strain. (B) All representative transformants assemble ciliary DC1 and IC2, indicating successful assembly of the ODA-DC and ODA on the axonemes. Protein stained gels (Coomassie) are used as loading controls.
S33, T351 and S628 are important for AICD
To test the role of the alcohol-sensitive phosphosites in AICD, we quantitated swimming velocities in wild-type, oda3-4 and DCC1 mutant strains in the absence and presence of 100 mM alcohol. As expected, wild-type cells (g1) showed a significant reduction (19%) in swimming velocity after alcohol treatment, whereas oda3-4 cells were resistant to alcohol (Fig. 3). S33A-B5 showed a mild reduction (9%) in swimming speed in response to alcohol, while the other S33 transformants (S33A-D4, S33A-E3, S33E-A6 and S33E-C1) were resistant to the ciliary slowing effects of alcohol (Fig. 3A). Like S33, mutations in T351 and S628 resulted in alcohol resistance (Fig. 3B–C). These results demonstrate that the S33, T351 and S628 are alcohol-sensitive phosphosites important for mediating AICD. All the T73 transformant strains showed reduced swimming velocities in response to 100 mM alcohol (T73A-A5, 12%; T73A-E5, 17%; T73E-E2, 9%; T73E-E4, 7%; T73E-E5, 15%) (Fig. 3D). Lower doses of alcohol showed no difference in sensitivity to alcohol between wild-type and the T73A- or T73E-expressing strains (data not shown). Thus, the T73 alcohol-sensitive phosphosite is not required for mediating the ciliary slowing effects of alcohol.
Figure 3. S33, T351 and S628 in DC1 are important for AICD.
(A) Wild-type cells (WT) show a significant motility reduction (19%) after alcohol treatment; whereas oda3-4 cells have greatly reduced velocities and no AICD. In the presence of 100mM alcohol, S33A-B5 shows a mild reduction (9%) of motility, while none of other S33 transformants (S33A-D4 and E3; S33E-A6 and C1) display AICD. (B) The swimming speeds of the T73 transformants are reduced by alcohol to various degrees (T73A-A5, 12%; T73A-E5, 17%; T73E-E2, 9%; T73E-E4, 7%; T73E-E5, 15%). (C) and (D) T351 or S628 mutants do not show AICD upon alcohol treatment. In summary, these results demonstrate S33, T351and S628 are important sites for mediating AICD, whereas the phosphorylation state of T73 is dispensible for AICD. (*: p-Value<0.01)
Discussion
We previously demonstrated that alcohol exposure reduces swimming velocities by reducing ciliary beat frequencies resulting in AICD (F. Yang et al., 2015). We also identified DC1, of the ODA-DC, as the first ciliary target of alcohol; DC1 phosphorylation is reduced by alcohol treatment. Moreover, we showed that DC1 phosphorylation was reduced or absent in mutant strains lacking the central pair or ODA motor respectively. Previous genetic screens isolated suppressor mutations in the β and γ motor subunits of the ODA (Huang, Ramanis, & Luck, 1982). These extragenic mutations restored motility in central pair and radial spoke mutants and established the role of these structures in regulation of ODA activity. These data suggest that DC1 is a component of the central pair-ODA regulatory pathway, that this pathway involves phosphorylation of DC1 and this pathway is a target for alcohol.
In this study, we determined PP1 and PKA activities and specific alcohol-sensitive phosphosites in DC1 are required for AICD. Our pharmacological studies, using PP1 and PKA inhibitors, revealed a role for PP1 and PKA in ODA function and the AICD mechanism. Inhibition at low doses resulted in cell models that are largely resistant to the ciliary slowing effects of alcohol. It is of interest that high doses of the inhibitors resulted in a decrease in forward swimming speed in the absence of alcohol. In these conditions, alcohol treatment only mildly reduced (I2) or did not further reduce (PKI) swimming velocities of cell models. Since alcohol primarily targets the ODA (F. Yang et al., 2015), it is likely that the ODA motors are compromised by the high doses of inhibitors and are not further susceptible to the effects of alcohol.
Importantly, these effects are observed in cell models, where the ciliary membrane has been removed. Thus, the AICD mechanism is integrated into the ciliary axoneme structure along with the dynein motors that drive motility. This is consistent with the axonemal localization of kinases and phosphatases that have been shown to regulate motility (Gaillard, Diener, Rosenbaum, & Sale, 2001; Habermacher & Sale, 1995, 1996; Howard et al., 1994; P. Yang, Fox, Colbran, & Sale, 2000).
In mammalian cell or tissue models, AICD is PP1-dependent and alcohol treatment inactivates axonemal kinases. Prolonged, chronic alcohol exposure activates axonemal PP1 resulting in inactivation of axonemal PKA. This is consistent with our findings that low doses of I2 renders Chlamydomonas cell models resistant to alcohol. Interestingly, inhibition of PKA also blocks alcohol’s ciliary slowing effects. It is possible that active PKA is required to phosphorylate other sites in DC1 or other ciliary targets to mediate AICD in Chlamydomonas. Future studies will be required to identify additional ciliary targets of alcohol.
Using mass spectrometry, we identified four sites in DC1 with alcohol-sensitive alterations in phosphorylation. S33, T73 and S628 all show a reproducible decrease in phosphorylation in the presence of alcohol. The T351 results vary in the 3 independent experiments (Sup Fig. 1A). It is possible that alcohol activates a phosphatase (like PP1) and/or inactivates a kinase (like PKA) to result in dephosphorylation of these sites. These sites were mutated to alanine, a non-phosphorylatable residue or glutamic acid to mimic phosphorylation. All mutant DCC1 transgenes restore motility to wild-type levels and rescue assembly of the ODA-DC and ODA in the axoneme (Fig. 2). Thus, the phosphorylation status of the four sites is not critical for assembly or function of the ODA in the absence of alcohol. Future studies are required to determine if mutation of these sites in combination have a more significant impact on ODA assembly or function.
We directly tested the role of these four sites in the AICD mechanism. All T73A and T73E transformants were sensitive to alcohol (Fig. 3D). Thus, while T73 was identified as an alcohol-sensitive phosphosite, this site is not important for AICD. In contrast, S33, T351 and S628 are important for AICD (Fig. 3A–C). Notably, multiple independent transformants for each specific mutation were analyzed, demonstrating that the results obtained are not caused by an insertion of the transgene into a specific locus. The results indicate that S33, T351 and S628 need to be competent for both phosphorylation and dephosphorylation activities in order for AICD to occur. These findings are consistent with the requirement of both kinase (PKA) and phosphatase (PP1) activities for effective AICD (Fig. 1). It is possible that PKA and PP1 are directly involved in regulation of DC1 at these sites to mediate AICD. Mass spectrometry of axonemes pre-treated with PKI or I2, will be required to determine if PKA and PP1 inhibition changes the phosphorylation state of these sites upon alcohol exposure.
In Chlamydomonas, multiple mechanisms exist for docking the ODA to the ciliary microtubules. Two complexes, the heterotrimeric ODA-DC and the ODA5/8/10 complex, and CCDC103 are required for ODA docking in the axoneme. The CCDC103 protein forms oligomers that are important for ODA docking (King & Patel-King, 2015; Shoemark et al., 2018). Of the 7 proteins named here, only DC2, ODA10 and CCDC103 are conserved. ODA docking in humans requires two additional proteins, ARMC4 and TTC25. TTC25 is a component of the human ODA-DC and interacts with the DC2 orthologue, CCDC114. ARMC4 is required for docking of the distally-localized ODA isoforms. It is possible, in humans, that these proteins serve functional roles equivalent to DC1 in Chlamydomonas. Aberrant phosphorylation of DC1 may alter the structure of the ODA-DC, leading to compromised ODA function through a pathway that ultimately alters the microtubule-binding domains of the ODA heavy chains. AICD in humans may similarly alter ODA heavy chain function via post-translational modifications that alter docking complex conformation. High-resolution structural analyses of alcohol-treated cilia compared to untreated cilia will be required to test these ideas. Regardless of the lack of a human DC1 orthologue, our studies shed light into the molecular underpinnings of AICD.
In summary, our studies define mechanisms for AICD and reveal a novel regulatory pathway for control of the ODA motors that involves the PP1, PKA, and DC1. While the AICD mechanism involves DC1, it is likely the mechanism also involves alteration in phosphorylation of multiple axonemal proteins that require the activities of both kinases and phosphatases like PP1 and PKA, in Chlamydomonas.
Supplementary Material
(A) The locations of alcohol-sensitive phospho-sites in DC1 are shown along with the coiled-coil domain. (B) The locations of primers used for PCR are shown relative to the DCC1 gene structure. Primer pair 5 yields a smaller PCR product in oda3-4, whereas primer pairs 6 and 7 fail to amplify a product in oda3-4 DNA. (C) Primer pair 7 was used to screen transformant strains. Amplification of a band at the wild-type size demonstrates the successful integration of the mutant DCC1 transgenes. Wild type (WT) and oda3-4 (CC3935) are shown as positive and negative controls respectively.
Protein phosphatase 1 and protein kinase A mediate alcohol-induced ciliary dysfunction and regulate cell motility.
4 residues (S33, T73, T351 and S628) in DC1 showed alcohol-sensitive phosphorylation.
Mutations of the alcohol-sensitive residues in DC1 rescue the assembly and motility defects in a DC1-null mutant.
Three alcohol-sensitive residues (S33, T351 and S628) in DC1 are important for alcohol-induced ciliary dysfunction.
Acknowledgments
FY performed experimental protocols and data collection. CS assisted with motility analyses, culture of cells and experimental advice. JHS helped in the development of the hypotheses for this project and was involved in the translation of the Chlamydomonas AICD findings to mammalian models for AICD. JHS, FY and MW wrote the manuscript. We acknowledge Dr. George Witman (University of Massachusetts) and Winfield S. Sale (Emory University) for critical reading of the manuscript. This work was supported by the National Institutes of Health (NIAAA 5R01AA008769-22 to JHS; sub-contract 34-5237-2020-016 to MW) and the University of Mississippi Medical Center Department of Cell and Molecular Biology.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Alsaadi MM, Erzurumluoglu AM, Rodriguez S, Guthrie PA, Gaunt TR, Omar HZ, … Day IN. Nonsense mutation in coiled-coil domain containing 151 gene (CCDC151) causes primary ciliary dyskinesia. Hum Mutat. 2014;35(12):1446–1448. doi: 10.1002/humu.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold P, Schmitt R, Mages W. An engineered Streptomyces hygroscopicus aph 7′ gene mediates dominant resistance against hygromycin B in Chlamydomonas reinhardtii. Protist. 2002;153(4):401–412. doi: 10.1078/14344610260450136. [DOI] [PubMed] [Google Scholar]
- Brown JM, Mosley M, Montes-Berrueta D, Hou Y, Yang F, Scarbrough C, … Wirschell M. Characterization of a new oda3 allele, oda3-6, defective in assembly of the outer dynein arm-docking complex in Chlamydomonas reinhardtii. PLoS One. 2017;12(3):e0173842. doi: 10.1371/journal.pone.0173842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DM, Inaba K, Pazour GJ, Takada S, Wakabayashi K, Wilkerson CG, … Witman GB. DC3, the 21-kDa subunit of the outer dynein arm-docking complex (ODA-DC), is a novel EF-hand protein important for assembly of both the outer arm and the ODA-DC. Mol Biol Cell. 2003;14(9):3650–3663. doi: 10.1091/mbc.E03-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AB, Mitchell DR. Chlamydomonas ODA10 is a conserved axonemal protein that plays a unique role in outer dynein arm assembly. Mol Biol Cell. 2013;24(23):3689–3696. doi: 10.1091/mbc.E13-06-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai PB, Freshour JR, Mitchell DR. Chlamydomonas axonemal dynein assembly locus ODA8 encodes a conserved flagellar protein needed for cytoplasmic maturation of outer dynein arm complexes. Cytoskeleton (Hoboken) 2015;72(1):16–28. doi: 10.1002/cm.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty GW, Loges NT, Klinkenbusch JA, Olbrich H, Pennekamp P, Menchen T, … Omran H. DNAH11 Localization in the Proximal Region of Respiratory Cilia Defines Distinct Outer Dynein Arm Complexes. Am J Respir Cell Mol Biol. 2016;55(2):213–224. doi: 10.1165/rcmb.2015-0353OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf M, Olbrich H, Horvath J, Wildhaber JH, Zariwala MA, Kennedy M, … Omran H. Mislocalization of DNAH5 and DNAH9 in respiratory cells from patients with primary ciliary dyskinesia. Am J Respir Crit Care Med. 2005;171(12):1343–1349. doi: 10.1164/rccm.200411-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard AR, Diener DR, Rosenbaum JL, Sale WS. Flagellar radial spoke protein 3 is an A-kinase anchoring protein (AKAP) J Cell Biol. 2001;153(2):443–448. doi: 10.1083/jcb.153.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard AR, Fox LA, Rhea JM, Craige B, Sale WS. Disruption of the A-kinase anchoring domain in flagellar radial spoke protein 3 results in unregulated axonemal cAMP-dependent protein kinase activity and abnormal flagellar motility. Mol Biol Cell. 2006;17(6):2626–2635. doi: 10.1091/mbc.E06-02-0095. E06-02-0095 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermacher G, Sale WS. Regulation of dynein-driven microtubule sliding by an axonemal kinase and phosphatase in Chlamydomonas flagella. Cell Motil Cytoskeleton. 1995;32(2):106–109. doi: 10.1002/cm.970320207. [DOI] [PubMed] [Google Scholar]
- Habermacher G, Sale WS. Regulation of flagellar dynein by an axonemal type-1 phosphatase in Chlamydomonas. J Cell Sci. 1996;109(Pt 7):1899–1907. doi: 10.1242/jcs.109.7.1899. [DOI] [PubMed] [Google Scholar]
- Hjeij R, Lindstrand A, Francis R, Zariwala MA, Liu X, Li Y, … Omran H. ARMC4 mutations cause primary ciliary dyskinesia with randomization of left/right body asymmetry. Am J Hum Genet. 2013;93(2):357–367. doi: 10.1016/j.ajhg.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjeij R, Onoufriadis A, Watson CM, Slagle CE, Klena NT, Dougherty GW, … Mitchison HM. CCDC151 mutations cause primary ciliary dyskinesia by disruption of the outer dynein arm docking complex formation. Am J Hum Genet. 2014;95(3):257–274. doi: 10.1016/j.ajhg.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornef N, Olbrich H, Horvath J, Zariwala MA, Fliegauf M, Loges NT, … Omran H. DNAH5 mutations are a common cause of primary ciliary dyskinesia with outer dynein arm defects. Am J Respir Crit Care Med. 2006;174(2):120–126. doi: 10.1164/rccm.200601-084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DR, Habermacher G, Glass DB, Smith EF, Sale WS. Regulation of Chlamydomonas flagellar dynein by an axonemal protein kinase. J Cell Biol. 1994;127(6 Pt 1):1683–1692. doi: 10.1083/jcb.127.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Ramanis Z, Luck DJ. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for Flagellar function. Cell. 1982;28(1):115–124. doi: 10.1016/0092-8674(82)90381-6. 0092-8674(82)90381-6 [pii] [DOI] [PubMed] [Google Scholar]
- Ide T, Owa M, King SM, Kamiya R, Wakabayashi K. Protein-protein interactions between intermediate chains and the docking complex of Chlamydomonas flagellar outer arm dynein. FEBS Lett. 2013;587(14):2143–2149. doi: 10.1016/j.febslet.2013.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya R, Witman GB. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J Cell Biol. 1984;98(1):97–107. doi: 10.1083/jcb.98.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle KL. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1990;87(3):1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Patel-King RS. The oligomeric outer dynein arm assembly factor CCDC103 is tightly integrated within the ciliary axoneme and exhibits periodic binding to microtubules. J Biol Chem. 2015;290(12):7388–7401. doi: 10.1074/jbc.M114.616425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles MR, Leigh MW, Ostrowski LE, Huang L, Carson JL, Hazucha MJ … Genetic Disorders of Mucociliary Clearance, C. Exome sequencing identifies mutations in CCDC114 as a cause of primary ciliary dyskinesia. Am J Hum Genet. 2013;92(1):99–106. doi: 10.1016/j.ajhg.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutoulis A, Pazour GJ, Wilkerson CG, Inaba K, Sheng H, Takada S, Witman GB. The Chlamydomonas reinhardtii ODA3 gene encodes a protein of the outer dynein arm docking complex. J Cell Biol. 1997;137(5):1069–1080. doi: 10.1083/jcb.137.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M, Pifferi M, Bush A, Piras M, Michelucci A, Di Cicco M, … Pistello M. Gene editing of DNAH11 restores normal cilia motility in primary ciliary dyskinesia. J Med Genet. 2016;53(4):242–249. doi: 10.1136/jmedgenet-2015-103539. [DOI] [PubMed] [Google Scholar]
- Lin H, Miller ML, Granas DM, Dutcher SK. Whole genome sequencing identifies a deletion in protein phosphatase 2A that affects its stability and localization in Chlamydomonas reinhardtii. PLoS Genet. 2013;9(9):e1003841. doi: 10.1371/journal.pgen.1003841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoufriadis A, Paff T, Antony D, Shoemark A, Micha D, Kuyt B, … Mitchison HM. Splice-site mutations in the axonemal outer dynein arm docking complex gene CCDC114 cause primary ciliary dyskinesia. Am J Hum Genet. 2013;92(1):88–98. doi: 10.1016/j.ajhg.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owa M, Furuta A, Usukura J, Arisaka F, King SM, Witman GB, … Wakabayashi K. Cooperative binding of the outer arm-docking complex underlies the regular arrangement of outer arm dynein in the axoneme. Proc Natl Acad Sci U S A. 2014;111(26):9461–9466. doi: 10.1073/pnas.1403101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J. Cilia and ciliopathies: from Chlamydomonas and beyond. Sci China C Life Sci. 2008;51(6):479–486. doi: 10.1007/s11427-008-0071-3. [DOI] [PubMed] [Google Scholar]
- Panizzi JR, Becker-Heck A, Castleman VH, Al-Mutairi DA, Liu Y, Loges NT, … Drummond IA. CCDC103 mutations cause primary ciliary dyskinesia by disrupting assembly of ciliary dynein arms. Nat Genet. 2012;44(6):714–719. doi: 10.1038/ng.2277. doi: http://www.nature.com/ng/journal/v44/n6/abs/ng.2277.html - supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170(1):103–113. doi: 10.1083/jcb.200504008. jcb.200504008 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Agrin N, Walker BL, Witman GB. Identification of predicted human outer dynein arm genes: candidates for primary ciliary dyskinesia genes. J Med Genet. 2006;43(1):62–73. doi: 10.1136/jmg.2005.033001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M, Yang F, Sisson JH, Wirschell M. Ciliary dynein dysfunction caused by chronic alcohol exposure. In: King SM, editor. Dyneins: Structrure, Biology and Disease, 2nd edition. Academic Press; 2017. [Google Scholar]
- Price ME, Pavlik JA, Liu M, Ding SJ, Wyatt TA, Sisson JH. Alcohol drives S-nitrosylation and redox activation of protein phosphatase 1, causing bovine airway cilia dysfunction. Am J Physiol Lung Cell Mol Physiol. 2017;312(3):L432–L439. doi: 10.1152/ajplung.00513.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ME, Pavlik JA, Sisson JH, Wyatt TA. Inhibition of protein phosphatase 1 reverses alcohol-induced ciliary dysfunction. Am J Physiol Lung Cell Mol Physiol. 2015;308(6):L577–585. doi: 10.1152/ajplung.00336.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemark A, Moya E, Hirst RA, Patel MP, Robson EA, Hayward J, … Mitchison HM. High prevalence of CCDC103 p.His154Pro mutation causing primary ciliary dyskinesia disrupts protein oligomerisation and is associated with normal diagnostic investigations. Thorax. 2018;73(2):157–166. doi: 10.1136/thoraxjnl-2017-209999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JH. Ethanol stimulates apparent nitric oxide-dependent ciliary beat frequency in bovine airway epithelial cells. Am J Physiol. 1995;268(4 Pt 1):L596–600. doi: 10.1152/ajplung.1995.268.4.L596. [DOI] [PubMed] [Google Scholar]
- Sisson JH. Alcohol and airways function in health and disease. Alcohol (Fayetteville, NY) 2007;41(5):293–307. doi: 10.1016/j.alcohol.2007.06.003. S0741-8329(07)00128-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S, Kamiya R. Functional reconstitution of Chlamydomonas outer dynein arms from alpha-beta and gamma subunits: requirement of a third factor. J Cell Biol. 1994;126(3):737–745. doi: 10.1083/jcb.126.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S, Wilkerson CG, Wakabayashi K, Kamiya R, Witman GB. The outer dynein arm-docking complex: composition and characterization of a subunit (oda1) necessary for outer arm assembly. Mol Biol Cell. 2002;13(3):1015–1029. doi: 10.1091/mbc.01-04-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallmeier J, Shiratori H, Dougherty GW, Edelbusch C, Hjeij R, Loges NT, … Omran H. TTC25 Deficiency Results in Defects of the Outer Dynein Arm Docking Machinery and Primary Ciliary Dyskinesia with Left-Right Body Asymmetry Randomization. Am J Hum Genet. 2016;99(2):460–469. doi: 10.1016/j.ajhg.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirschell M, Pazour G, Yoda A, Hirono M, Kamiya R, Witman GB. Oda5p, a novel axonemal protein required for assembly of the outer dynein arm and an associated adenylate kinase. Mol Biol Cell. 2004;15(6):2729–2741. doi: 10.1091/mbc.E03-11-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman GB. Isolation of Chlamydomonas flagella and flagellar axonemes. Methods Enzymol. 1986;134:280–290. doi: 10.1016/0076-6879(86)34096-5. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Spurzem JR, May K, Sisson JH. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am J Physiol. 1998;275(4 Pt 1):L827–835. doi: 10.1152/ajplung.1998.275.4.L827. [DOI] [PubMed] [Google Scholar]
- Yang F, Pavlik J, Fox L, Scarbrough C, Sale WS, Sisson JH, Wirschell M. Alcohol-induced ciliary dysfunction targets the outer dynein arm. Am J Physiol Lung Cell Mol Physiol. 2015;308(6):L569–576. doi: 10.1152/ajplung.00257.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Fox L, Colbran RJ, Sale WS. Protein phosphatases PP1 and PP2A are located in distinct positions in the Chlamydomonas flagellar axoneme. J Cell Sci. 2000;113(Pt 1):91–102. doi: 10.1242/jcs.113.1.91. [DOI] [PubMed] [Google Scholar]
- Yang P, Sale WS. Casein kinase I is anchored on axonemal doublet microtubules and regulates flagellar dynein phosphorylation and activity. J Biol Chem. 2000;275(25):18905–18912. doi: 10.1074/jbc.M002134200. M002134200 [pii] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The locations of alcohol-sensitive phospho-sites in DC1 are shown along with the coiled-coil domain. (B) The locations of primers used for PCR are shown relative to the DCC1 gene structure. Primer pair 5 yields a smaller PCR product in oda3-4, whereas primer pairs 6 and 7 fail to amplify a product in oda3-4 DNA. (C) Primer pair 7 was used to screen transformant strains. Amplification of a band at the wild-type size demonstrates the successful integration of the mutant DCC1 transgenes. Wild type (WT) and oda3-4 (CC3935) are shown as positive and negative controls respectively.