Abstract
Aim:
Pulmonary hypertension significantly increases morbidity and mortality in infants with bronchopulmonary dysplasia. The frequency of single nucleotide polymorphisms in arginase-1 (ARG1 rs2781666) and dimethylarginine dimethylaminohydrolase-1 (DDAH1 rs480414) genes have been found to differ in a cohort of bronchopulmonary dysplasia patients with pulmonary hypertension (cases) and without pulmonary hypertension (controls). Therefore, we tested the hypothesis that combining these genotypes with phenotypic data would better predict pulmonary hypertension in bronchopulmonary dysplasia patients.
Methods:
Bronchopulmonary dysplasia patients (n=79) born at <35 weeks gestation were studied. Pulmonary hypertension was diagnosed by echocardiographic criteria (n=20). ROC curves to predict pulmonary hypertension in bronchopulmonary dysplasia were generated from genotype and/or clinical data.
Results:
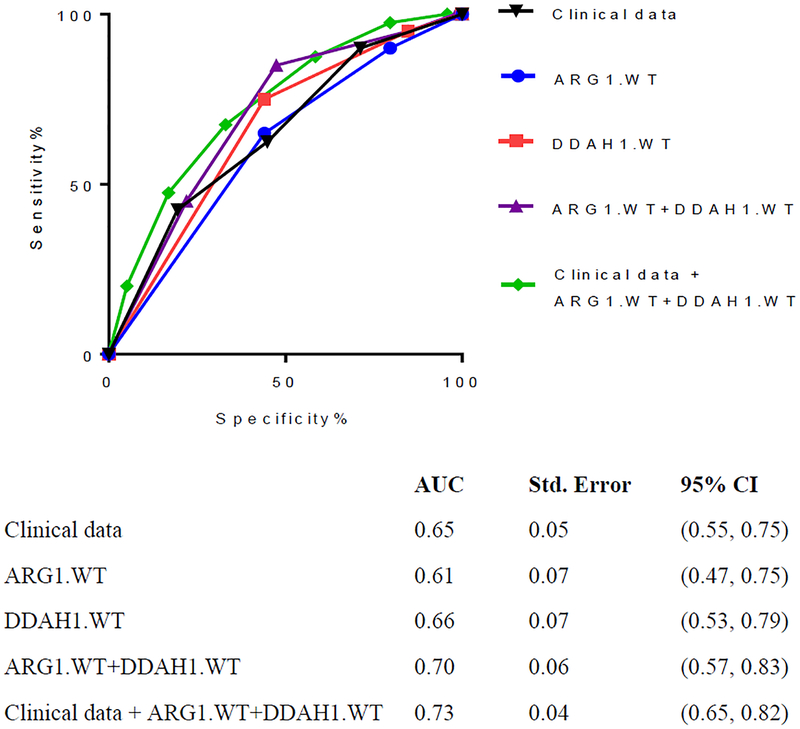
Cases were born at an earlier gestation and weighed less at birth than did controls. ROC curves for rs2781666 had an AUC of 0.61, while rs480414 had an AUC of 0.66. Together, the AUC was 0.70. When clinical data was added to the genetic model, AUC was 0.73.
Conclusion:
These findings demonstrate that ROC predictive modeling of pulmonary hypertension in bronchopulmonary dysplasia improves with inclusion of both genotypic and phenotypic data. Further refinement of these types of models could facilitate the implementation of precision medicine approaches to pulmonary hypertension in bronchopulmonary dysplasia.
Keywords: arginase, dimethylarginine dimethylaminohydrolase, predictive model, prematurity, neonatology
INTRODUCTION
Bronchopulmonary dysplasia (BPD) is the most common morbidity associated with preterm birth and the presence of BPD significantly increases neonatal morbidity and mortality in preterm infants (1). Patients with BPD have impaired gas exchange and pulmonary vasoreactivity, and 20 – 35% of patients with BPD will develop pulmonary hypertension (PH) (2). The presence of PH in BPD patients increases the adjusted odds of mortality by 4.6 times over that of BPD alone patients (3). However, studies of therapies designed to prevent BPD-associated PH have been hampered by the fact that most patients with BPD will never develop PH. Therefore, there is a real need to identify risk strata for developing PH in patients with BPD. In this study, our aim was to develop a model using biomarkers to predict the development of PH in patients with BPD.
Our group has previously shown that a single nucleotide polymorphism (SNP) in the arginase-1 gene (ARG1 rs2781666) occurs less frequently in BPD patients with PH (BPDPH) than in BPD patients with no PH (BPD-alone) (4). Similarly, we have found that a SNP in the dimethylarginine dimethylaminohydrolase-1 (DDAH1 rs480414) gene is also less common in BPD-PH than BPD-alone patients (5). Therefore, these SNPs in ARG1 and DDAH1 appear to be independently protective against the development of PH in BPD patients, while the wildtype ARG1 rs2781666 and DDAH1 rs480414 appear to be independent risk factors for the development of PH in BPD patients. ARG1 and DDAH1 genes were initially studied as potential BPD-PH genetic markers because of their role in regulating the production of nitric oxide (NO), a key endogenous vasodilator, and often used in the treatment of PH as inhaled NO (6–9). Arginase catalyzes the hydrolysis of L-arginine to L-ornithine and urea and competes with NO synthase (NOS) for its common substrate, L-arginine. Nitric oxide produced by NOS in endothelial cells that line the pulmonary arteries diffuses to pulmonary smooth muscle cells causing relaxation (10). DDAH is an enzyme that degrades asymmetric dimethylarginine (ADMA), an endogenous NOS inhibitor, and ADMA is increasingly recognized as a key regulator of cardiopulmonary endothelial NO production (9, 11).
In the present study, we tested the hypothesis that by combining genotypes from both ARG1 rs2781666 (G>T) and DDAH1 rs480414 (G>A) loci, as well as patient clinical data, we could develop a model that better predicts the development of PH in BPD patients than any of those factors alone.
METHODS
The Institutional Review Board at Nationwide Children’s Hospital (NCH) approved this study. All patients admitted to the Nationwide Children’s Hospital NICU after September 1, 2009 with the diagnosis of bronchopulmonary dysplasia (BPD) were eligible for this study and parental consent was obtained. BPD was defined according to the NICHD consensus statement as a supplemental oxygen requirement at 28 days of life (12). Enrollment, clinical data abstraction, and specimen collection were completed through the Ohio Perinatal Research Network (OPRN) and Perinatal Research Repository (PRR) at The Research Institute at Nationwide Children’s Hospital.
Patient Cohort:
Patients with BPD who were born at <35 weeks gestation and enrolled in the OPRN/PRR were eligible for study. Patients were included in the study cohort if they had genotype data for both ARG1 rs2781666 (13) and DDAH1 rs480414 (5). Therefore, all of the patients in the present study have been enrolled in previous research studies (4, 5, 11, 13). We studied a total of 79 BPD patients, 20 (25%) of whom developed PH. BPD patients with congenital heart disease were excluded from the study. However, BPD patients with isolated atrial septal defect, ventricular septal defect, or patent ductus arteriosus were included in this study. Patients with anatomical causes of PH, including congenital diaphragmatic hernia and lung hypoplasia, were excluded from this study.
Pulmonary Hypertension Definition:
PH in our BPD cohort was defined by the presence of abnormally elevated pulmonary arterial pressure on echocardiography in a structurally normal heart after 28 days of age (cases). BPD patients who did not have PH were considered controls. Elevated pulmonary arterial pressure on echocardiography was defined as commonly done by the presence of any of the following four criteria: 1) right ventricular hypertrophy; 2) flattening of the intraventricular septum; and/or 3) tricuspid regurgitant jet velocity greater than 3 m/s and/or; 4) pulmonary regurgitation (2, 3, 5, 14).
Clinical Characteristics:
Clinical characteristics were compared between BPD-PH (cases) and BPD-alone (controls) groups. Clinical data analyzed included birthweight, gestational age, admission weight, discharge weight, 1 minute Apgar, 5 minute Apgar, admission age, discharge age, presence of a patient ductus arteriosus, presence of a central line, oxygen at discharge, and death. Clinical data was selected for the final ROC model if it was statistically different between cases and controls on t-test and occurred prior to onset of PH.
Single Nucleotide Polymorphisms (SNP) Assay:
Patient blood samples were collected and analyzed by Agena MassArray (Agena, San Diego, CA) as previously described (5, 13). SNP verses wildtype genotype was known for both ARG1 rs2781666 (G>T) (13) and DDAH1 rs480414 (G>A) (5) in each of the 79 BPD patients studied. Since our previous studies had determined that ARG1 SNP rs2781666 and DDAH1 SNP rs480414 were protective against PH in BPD (5, 13), ARG1 wildtype rs2781666 (homozygote or heterozygote) and/or DDAH1 wildtype rs480414 (homozygote or heterozygote) were considered a positive genetic test for echocardiographic PH.
Model Selection and Development:
ARG1 and DDAH1 loci were analyzed separately and then combined. Since wildtype (G) allele was considered a positive PH test for both loci, separate analysis of ARG1 rs2781666 (G>T) included ARG1.GT (wildtype heterozygote) and ARG1.GG (wildtype homozygote). Separate analysis of DDAH1 rs480414 (G>A) included DDAH1.GA (wildtype heterozygote) and DDAH1.GG (wildtype homozygote). Combined analysis of ARG1 rs2781666 (G>T) and DDAH1 rs480414 (G>A) included four possible genetic wildtype (G-allele) combinations, labeled Groups 1–4 (table 3). Of note, Group 3 contains Groups 1&2 and Group 4, contains Groups 1, 2, &3. Both the separate and combined analysis did not include the SNP homozygote, since previous studies had determined that these SNPs were protective and the aim of the study was to predict PH in BPD.
TABLE 3: ARG1 and DDAH1 loci analyzed by genotype combinations.
Combinations of ARG1 WT rs2781666 and DDAH1 WT rs480414 were considered a positive genetic test for echocardiographic PH in BPD as in Groups 1–4 above. Sensitivity, specificity, positive likelihood ratio (+LR), negative likelihood ratio (-LR), positive predictive value (PPV), and negative predictive value (NPV) were calculated for ARG1 SNP rs2781666 and DDAH1 SNP rs480414, for each combined group (Groups 1–4). ARG1 and DDAH1 loci were calculated as binary variables for each genotype status (SNP or wildtype allele). Wildtype allele (G) was considered a positive genetic PH test for echocardiographic BPD-PH.
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| ARG1.GT+DDAH1.GG | X | X | X | |
| ARG1.GG+DDAH1.GA | X | X | X | |
| ARG1.GG+DDAH1.GG | X | X | X | |
| ARG1.GT+DDAH1.GA | X | |||
| Sensitivity | 45% | 40% | 85% | 85% |
| Specificity | 78% | 75% | 53% | 34% |
| +LR | 2.04 | 1.57 | 1.79 | 1.29 |
| −LR | 0.71 | 0.80 | 0.28 | 0.44 |
| PPV | 41% | 35% | 38% | 30% |
| NPV | 81% | 79% | 91% | 87% |
Statistical Analysis:
This is a case-control study, with 20 cases (BPD-PH patients) and 59 controls (BPD-alone patients). Genotype status for each patient at two loci: ARG1 rs2781666 (G>T) and DDAH1 rs480414 (G>A) was determined. Frequency distribution for wildtype genotype status of cases and controls was analyzed by chi-square analysis. Cases and controls were evaluated by genotype, both independent and combined, for sensitivity, specificity, positive likelihood ratio (+LR), negative likelihood ratio (-LR), positive predictive value (PPV), and negative predictive value (NPV) for the ability of the positive genetic test to predict PH diagnosed by echocardiography. ROC curves were generated independently for: 1) clinical data; 2) ARG1.WT (homozygote+heterozygote); 3) DDAH1.WT (homozygote+heterozygote); 4) ARG1.WT+DDAH1.WT (homozygote+heterozygote for each locus); and 5) clinical data (gestational age and birthweight) + ARG1.WT+DDAH1.WT (homozygote+heterozygote for each locus). Independent ROC curve calculations included AUC, standard error, and 95% confidence intervals. Statistical analyses for these studies were completed by GraphPad Prism 7. A test for equality of ROC areas was performed with Stata IC/13.
RESULTS
Clinical characteristics of the study cohort are presented in Table 1. We found no differences in chorioamnionitis or maternal hypertension between BPD-PH patients and BPD patients. BPD-PH patients had a younger gestational age, lower birthweight, older age at discharge, and greater discharge weight than in BPD patients (p<0.05). BPD-PH patients were more often on CMV at 36 weeks CGA than BPD patients (p<0.05) and had greater percent ROP than BPD patients (p<0.05). We found no difference in Apgar scores, admission age or weight, presence of patent ductus arteriosus, NEC or perforation, IVH, use of a central line, oxygen at discharge, or death between BPD-PH and BPD-alone patients.
TABLE 1: Clinical characteristics of BPD-PH patients verses BPD patients.
Continuous variables were calculated using a t-test and are presented mean ± standard deviation. Categorical variables were evaluated with chi-square test and are presented number (percentage). #APGAR at 5 minutes was calculated with 58 BPD alone patients. *significant values at p<0.05.
| Clinical characteristics | BPD (n=59) | BPD-PH (n=20) | p-value |
|---|---|---|---|
| Chorioamnionitis, n (%) | 4 (7) | 3 (15) | 0.37 |
| Maternal hypertension, n (%) | 13 (22) | 5 (25) | 0.82 |
| Gestational age, weeks | 28 ± 4 | 26 ± 3 | 0.03* |
| Birthweight, grams | 1088 ± 554 | 819 ± 335 | 0.01* |
| Small for gestational age, n (%) | 11(19) | 4 (20) | 0.92 |
| APGAR at 1 minute# | 5 ± 3 | 4 ± 3 | 0.21 |
| APGAR at 5 minute | 7 ± 3 | 5 ± 3 | 0.13 |
| Age at admission, days | 22 ± 29 | 21 ± 45 | 0.93 |
| Admission weight, grams | 1530 ± 818 | 1281 ± 1060 | 0.34 |
| CPAP at 36 weeks CGA, n (%) | 9 (15) | 6 (30) | 0.22 |
| CMV at 36 weeks CGA, n (%) | 5 (8) | 8 (40) | 0.01* |
| Patent ductus arteriosus, n (%) | 33 (56) | 14 (70) | 0.40 |
| ROP, n (%) | 34 (58) | 18 (90) | <0.01* |
| NEC or perforation, n (%) | 11(19) | 4 (20) | 0.92 |
| IVH, n (%) | 20 (34) | 12 (60) | 0.06 |
| Central line, n (%) | 45 (76) | 17 (85) | 0.61 |
| Oxygen at discharge, n (%) | 25 (42) | 14 (70) | 0.06 |
| Age at discharge, days | 105 ± 54 | 152 ± 80 | 0.02* |
| Discharge weight, grams | 3325 ± 1176 | 4215 ± 1583 | 0.03* |
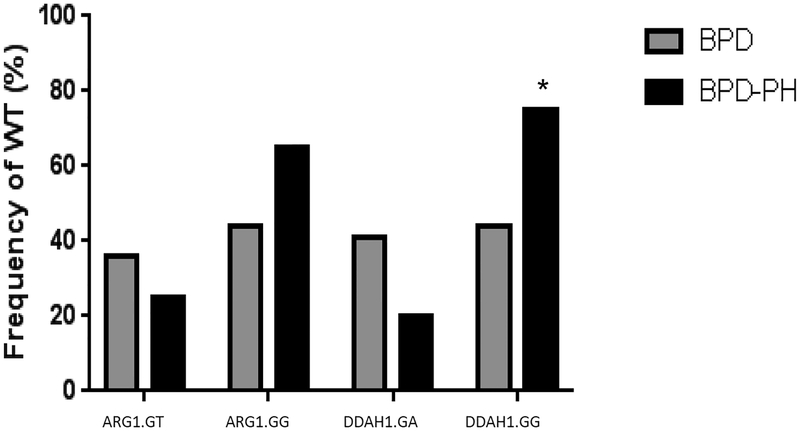
Figure 1 is a frequency histogram of independent genetic tests (ARG1 or DDAH1 wildtype G-allele heterozygote or homozygote) for PH in BPD analyzed independently. The frequency of the homozygous wildtype DDAH1.GG in the entire cohort was 52% (41/79). Of the independent positive genetic tests studied, only the DDAH1.GG test was different between BPD-PH (15/20 (75%)) and BPD-alone patients (26/59 (44%), p=0.02). The frequency of the homozygous wildtype ARG1.GG in the entire cohort was 49% (39/79). ARG.GG test was not different between BPD-PH (13/20 (65%)) and BPD-alone patients (26/59 (44%) (p=0.11). As shown in Table 2, we calculated sensitivity, specificity, positive likelihood ratio (+LR), negative likelihood ratio (-LR), positive predictive value (PPV), and negative predictive value (NPV) for each individual genotype. We found that all of the single genotype tests had low sensitivity and specificity.
FIGURE 1: Frequency distribution for wildtype (G-allele) genotype status for ARG1 or DDAH1 independently.
ARG1 SNP rs2781666 and DDAH1 SNP rs48041 were evaluated by chi-square analysis. DDAH1.GG at locus rs480414 has a significantly greater frequency in BPD-PH patients (75%) than did BPD alone patients (44%, *p<0.05). BPD, bronchopulmonary dysplasia; BPD-PH, bronchopulmonary dysplasia-associated pulmonary hypertension; ARG1, arginase-1; DDAH1, dimethylarginine dimethylaminohydrolase-1. ARG1.GT, wildtype heterozygote; ARG1.GG, wildtype homozygote; DDAH1.GA, wildtype heterozygote; DDAH1.GG, wildtype homozygote.
TABLE 2: ARG1 and DDAH1 loci analyzed by independent genotype.
Sensitivity, specificity, positive likelihood ratio (+LR), negative likelihood ratio (-LR), positive predictive value (PPV), and negative predictive value (NPV) were calculated for ARG1 SNP rs2781666 and DDAH1 SNP rs480414, separately. ARG1 and DDAH1 loci were calculated as binary variables for each genotype status (SNP or wildtype allele). Wildtype allele (G) was considered a positive genetic PH test for echocardiographic BPD-PH.
| ARG1.GT | ARG1.GG | DDAH1.GA | DDAH1.GG | |
|---|---|---|---|---|
| Sensitivity | 25% | 65% | 20% | 75% |
| Specificity | 64% | 56% | 59% | 56% |
| +LR | 0.70 | 1.47 | 0.49 | 1.70 |
| −LR | 1.17 | 0.63 | 1.36 | 0.45 |
| PPV | 19% | 33% | 14% | 37% |
| NPV | 72% | 83% | 69% | 87% |
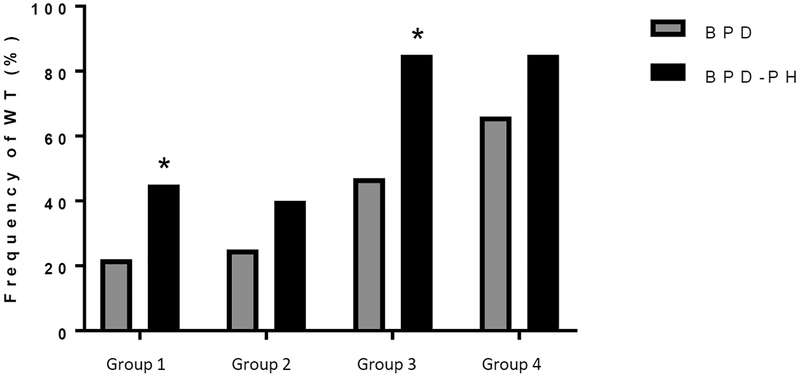
Table 3 shows the model analysis combining the ARG1 and DDAH1 SNP genotypes, represented by Groups 1–4. When evaluating combined genotypes, we found that Groups 3 and 4 performed the best in terms of sensitivity, while Group 1 performed the best in terms of specificity. When examining the likelihood ratios, the +LR were relatively low, while the −LR was 0.28 for Group 3. Similarly, when considering the PPV, all of the groups had relatively low PPV, suggesting that there were many false positives. However, the NPV were higher, with a NPV of 91% in Group 3, suggesting that when negative, the test is more likely to be a true negative. The frequency of the wild-type allele in the combined genotype groups is shown in Figure 2. For the entire cohort there were 28% (22/79) of patients in Group 1.
FIGURE 2: Frequency distribution for wildtype (G-allele) genotype status for ARG1 and DDAH1 combined.
ARG1 SNP rs2781666 and DDAH1 SNP rs48041 were evaluated by chi-square analysis for differences in frequency of wildtype (WT) allele. Groups 1–4 as defined in table 3. ARG1+DDAH1 group 1 has a significantly greater frequency in BPD-PH patients (45%) than did BPD alone patients (22%, *p<0.05). ARG1+DDAH1 group 3 has a significantly greater frequency in BPD-PH patients (85%) than did BPD alone patients (47%, *p<0.05).
Figure 3 compares the AUC’s of the ROC curve for the SNPs independently, the SNPs combined, and the combined SNPs with phenotypic (clinical) data. DDAH1.WT alone had an AUC of 0.66, however when ARG1.WT+DDAH1.WT were combined, the AUC was 0.70. We also evaluated how many patients were correctly identified into case and control groups by our combined model and found that the overall combined clinical and genetic model correctly identified 100% of controls (59/59) and 70% of cases (14/20).
FIGURE 3. ROC curves for clinical and SNP data:
Clinical characteristics included birthweight and gestational age. SNP data included ARG1.WT, DDAH1.WT, and ARG1.WT+DDAH1.WT. ROC curves were generated and AUC was calculated for clinical and SNP data, separately and combined. AUC was greatest (0.74) for clinical data + ARG1.WT+DDAH1.WT group. Clinical data was recorded as a 0–3 variable (quartiles) and genotype data was recorded as a 0–4 variable dependent on number of WT alleles. Wildtype allele (G) was considered a positive genetic PH test for echocardiographic BPD-PH.
DISCUSSION
The objective of this study was to develop a model including both genetic and phenotypic data to predict which patients with BPD develop PH. The major findings of this study were that 1) birthweight and gestational age were significant predictors of PH in BPD, 2) of the studied genotypes DDAH1.GG had a relatively high −LR and NPV, 3) the combined Group 3, had a higher −LR, NPV and sensitivity then any single genotype, and 4) combining clinical and genetic data resulted in an AUC of 0.73. These findings demonstrate that there are clinical and genetic data that can be used in predictive models for the development of PH in BPD patients. Although using the clinical and genetic data available to us resulted in a best AUC of 0.73, we speculate that continued refinement of this type of model by adding additional data will result in an improved performance of the model. Furthermore, these results are consistent with the notion that the development of PH in BPD is a complex process resulting from an array of physiological and genetic disturbances to normal lung vascular development, suggesting that predictive models will have to include a variety of data to accurately predict the development of PH in BPD patients.
These findings suggest that adding rs2781666 and rs480414 data to clinical data elements results in a better prediction of risk for PH in BPD. This finding is consistent with our hypothesis, and represents a first step in creating a predictive test for risk of PH in patients with BPD. Developing biomarkers to stratify risk of PH in BPD has been identified as an important unmet challenge in the field (14, 15) with great potential to alleviate morbidity and maximize patient outcomes. A biomarker panel might include clinical factors, such as gestational age and birthweight, as well as cord blood genetic biomarkers that could include multiple genetic loci. This type of biomarker panel for PH could be used to stratify risk for the development of BPD-PH. For example, if a clinician found a positive Group 3 PH biomarker panel from neonatal cord blood, especially in the clinical context of larger birthweight and gestational age, this would indicate a relatively low risk of developing BPD-PH. By identifying low risk patients one could exclude these from future trials of preventative therapies for PH in BPD, as well as allowing for precision medicine approaches to BPD-associated PH. For example, low risk patients might get one echocardiogram at 36 weeks and if negative may not need routine echocardiographic screening for PH as has been suggested (16–18).
There are a multitude of candidate biomarkers that have been identified through pre-clinical and clinical studies as directly involved in the pathophysiology of PH by disrupting pulmonary endothelium, including nitric oxide, prostaglandins, and endothelins, while some biomarker candidates target inflammation and/or cardiac function (19). Nitric oxide (NO) metabolites in urine at day of life 3 and decreased citrulline at day of life 7, both have been reported as significant predictors of PH severity at 36 weeks corrected gestational age (20). Also, in a small cohort of extremely low birth weight infants with BPD-PH, B-type natriuretic peptide (BNP) levels were lower in infants that survived, demonstrating potential as a biomarker for mortality in this patient population (14, 21, 22). Similarly, endostatin (ES): angiopoietin-1 (Ang-1) ratio on day of life 7 may also be useful as a biomarker for early PH-risk prediction in patients with severe BPD (14, 23). Lastly, placental growth factor (PIGF) (24), and NT-proBNP have also shown potential as biomarkers for PH in BPD (25). Furthermore, there are likely biomarkers that have yet been identified, so there is likely a place for unbiased evaluation of genome wide associations in the development of PH in BPD patients, although given the large number of subjects needed for this kind of study, this approach can only be successful in a multi-institutional setting given the relatively small numbers of patients in any given center.
There are several limitations to the present study that need to be acknowledged including the small cohort size. Our study was also limited by its narrow inclusion of clinical and genetic biomarkers which was due to the available data at hand. Therefore, validation of the use of this model or an expanded model (i.e. with additional data points) for PH in BPD in a larger independent prospective BPD cohort is needed. Furthermore, in addition to SNPs and clinical data, miRNAs (26), metabolomics, and/or endothelial progenitor cells may also be useful contributors to a predictive model for PH in BPD patients (27).
Conclusion:
In conclusion, development of a specific and sensitive early biomarker panel for PH in BPD is needed to advance the field. This is the first study to utilize a model combining both clinical and SNP data to create a predictive model of PH in patients with BPD. We found that the model presented herein is relatively robust at predicting patients with BPD at low risk for developing PH. We speculate that further refinement of this type of predictive model with the addition of more data could facilitate the implementation of precision medicine approaches to PH in BPD patients and thereby improve outcomes. Furthermore, being able to accurately stratify risk for PH in BPD patients would significantly improve our ability to develop and implement therapies to prevent PH in BPD patients.
Key Notes:
Bronchopulmonary dysplasia-associated pulmonary hypertension (BPD-PH) is a potentially devastating disease, and there are currently no preventative therapies.
We developed a predictive model for BPD-PH based on clinical and genetic data available early in the disease course.
We found that a combination of genetic and clinical data better predicted BPD-PH than any of those factors alone, demonstrating the feasibility of developing predictive models for PH risk to facilitate precision medicine approaches.
Acknowledgements
The authors would like to thank the patients and parents who participated in this study, the research staff of the Ohio Perinatal Research Network (OPRN) for clinical data collection, the Perinatal Research Repository for biospecimen collection, and Mark Klebanoff MD, MPH, and Julie Gastier-Foster PhD at the Research Institute at Nationwide Children’s Hospital for initial discussions of the project.
Funding
This research was funded by the National Heart, Lung, and Blood Institute (Grant K08HL129080), and by an intra-mural grant from the Center for Clinical and Translational Research at The Research Institute, Nationwide Children’s Hospital (CTSA grant UL1TR001070).
Abbreviations:
- ADMA
asymmetric dimethylarginine
- BPD
bronchopulmonary dysplasia
- DDAH
dimethylarginine dimethylaminohydrolase
- NO
nitric oxide
- PH
pulmonary hypertension
- rs
NCBI dbSNP number
- SNP
single nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Baker CD, Abman SH, Mourani PM. Pulmonary Hypertension in Preterm Infants with Bronchopulmonary Dysplasia. Pediatr Allergy Immunol Pulmonol 2014; 27 1:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkelhamer SK, Mestan KK, Steinhorn RH. Pulmonary hypertension in bronchopulmonary dysplasia. Semin Perinatol 2013; 37 2:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slaughter JL, Pakrashi T, Jones DE, South AP, Shah TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol 2011; 31 10:635–40. [DOI] [PubMed] [Google Scholar]
- 4.Trittmann JK, Jin Y, Chicoine LG, Liu Y, Chen B, Nelin LD. An arginase-1 SNP that protects against the development of pulmonary hypertension in bronchopulmonary dysplasia enhances NO-mediated apoptosis in lymphocytes. Physiol Rep 2016; 4 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trittmann JK, Gastier-Foster JM, Zmuda EJ, Frick J, Rogers LK, Vieland VJ, et al. A single nucleotide polymorphism in the dimethylarginine dimethylaminohydrolase gene is associated with lower risk of pulmonary hypertension in bronchopulmonary dysplasia. Acta Paediatr 2016; 105 4:e170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chicoine LG, Paffett ML, Young TL, Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 2004; 287 1:L60–8. [DOI] [PubMed] [Google Scholar]
- 7.Stanley KP, Chicoine LG, Young TL, Reber KM, Lyons CR, Liu Y, et al. Gene transfer with inducible nitric oxide synthase decreases production of urea by arginase in pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 2006; 290 2:L298–306. [DOI] [PubMed] [Google Scholar]
- 8.Talavera MM, Nuthakki S, Cui H, Jin Y, Liu Y, Nelin LD. Immunostimulated Arginase II Expression in Intestinal Epithelial Cells Reduces Nitric Oxide Production and Apoptosis. Front Cell Dev Biol 2017; 5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luneburg N, Harbaum L, Hennigs JK. The endothelial ADMA/NO pathway in hypoxia-related chronic respiratory diseases. Biomed Res Int 2014; 2014:501612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris SM, Jr. Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol 2009; 157 6:922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trittmann JK, Peterson E, Rogers LK, Chen B, Backes CH, Klebanoff MA, et al. Plasma asymmetric dimethylarginine levels are increased in neonates with bronchopulmonary dysplasia-associated pulmonary hypertension. J Pediatr 2015; 166 2:230–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001; 163 7:1723–9. [DOI] [PubMed] [Google Scholar]
- 13.Trittmann JK, Nelin LD, Zmuda EJ, Gastier-Foster JM, Chen B, Backes CH, et al. Arginase I gene single-nucleotide polymorphism is associated with decreased risk of pulmonary hypertension in bronchopulmonary dysplasia. Acta Paediatr 2014; 103 10:e439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bui CB, Pang MA, Sehgal A, Theda C, Lao JC, Berger PJ, et al. Pulmonary hypertension associated with bronchopulmonary dysplasia in preterm infants. J Reprod Immunol 2017; 124:21–9. [DOI] [PubMed] [Google Scholar]
- 15.Vijlbrief DC, Benders MJ, Kemperman H, van Bel F, de Vries WB. Use of cardiac biomarkers in neonatology. Pediatr Res 2012; 72 4:337–43. [DOI] [PubMed] [Google Scholar]
- 16.Nelin LD, Abman SH, Panitch HB. A physiology-based approach to the respiratory care of children with severe bronchopulmonary dysplasia The Newborn Lung: Neonatology Questions and Controversies. 3rd ed. editor: Bancalari Eduardo, Elsevier Saunders, Philadelphia, PA: in press. [Google Scholar]
- 17.Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis”. Am J Respir Crit Care Med 2001; 164 10 Pt 1:1755–6. [DOI] [PubMed] [Google Scholar]
- 18.Thebaud B, Lacaze-Masmonteil T. If your placenta doesn’t have it, chances are your lungs don’t have it either: the “vascular hypothesis” of bronchopulmonary dysplasia starts in utero. J Pediatr 2010; 156 4:521–3. [DOI] [PubMed] [Google Scholar]
- 19.Colvin KL, Dufva MJ, Delaney RP, Ivy DD, Stenmark KR, Yeager ME. Biomarkers for pediatric pulmonary arterial hypertension - a call to collaborate. Front Pediatr 2014; 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connor MG, Suthar D, Vera K, Slaughter JC, Maitre NL, Steele S, et al. Pulmonary hypertension in the premature infant population: Analysis of echocardiographic findings and biomarkers. Pediatr Pulmonol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuna A, Kandasamy J, Sims B. B-type natriuretic peptide and mortality in extremely low birth weight infants with pulmonary hypertension: a retrospective cohort analysis. BMC Pediatr 2014; 14:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konig K, Guy KJ, Nold-Petry CA, Barfield CP, Walsh G, Drew SM, et al. BNP, troponin I, and YKL-40 as screening markers in extremely preterm infants at risk for pulmonary hypertension associated with bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2016; 311 6:L1076–L81. [DOI] [PubMed] [Google Scholar]
- 23.Kim DH, Kim HS. Serial changes of serum endostatin and angiopoietin-1 levels in preterm infants with severe bronchopulmonary dysplasia and subsequent pulmonary artery hypertension. Neonatology 2014; 106 1:55–61. [DOI] [PubMed] [Google Scholar]
- 24.Mestan KK, Gotteiner N, Porta N, Grobman W, Su EJ, Ernst LM. Cord Blood Biomarkers of Placental Maternal Vascular Underperfusion Predict Bronchopulmonary Dysplasia-Associated Pulmonary Hypertension. J Pediatr 2017; 185:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montgomery AM, Bazzy-Asaad A, Asnes JD, Bizzarro MJ, Ehrenkranz RA, Weismann CG. Biochemical Screening for Pulmonary Hypertension in Preterm Infants with Bronchopulmonary Dysplasia. Neonatology 2016; 109 3:190–4. [DOI] [PubMed] [Google Scholar]
- 26.Kheyfets VO, Sucharov CC, Truong U, Dunning J, Hunter K, Ivy D, et al. Circulating miRNAs in Pediatric Pulmonary Hypertension Show Promise as Biomarkers of Vascular Function. Oxid Med Cell Longev 2017; 2017:4957147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borghesi A, Garofoli F, Cabano R, Tzialla C, Bollani L, Stronati M. Circulating endothelial progenitor cells and diseases of the preterm infant. Minerva Pediatr 2010; 62 3 Suppl 1:21–3. [PubMed] [Google Scholar]