Abstract
Background
Screening rates for colorectal cancer are below the Healthy People 2020 goal. There are several colorectal cancer screening tests that differ in terms of accuracy, recommended frequency, and administration. In this article, we compare how a set of personal characteristics correlates with preferences for colorectal cancer screening test attributes, past colorectal cancer screening behavior, and future colorectal cancer screening intentions.
Methods
We conducted a discrete-choice experiment survey to assess relative preferences for attributes of colorectal cancer screening tests among adults aged 50–75 years in USA. We used a latent class logit model to identify classes of preferences and calculated willingness to pay for changes in test attributes. A set of personal characteristics were included in the latent class analysis and analyses of self-reported past screening behavior and self-assessed likelihood of future colorectal cancer screening.
Results
Latent class analysis identified three types of respondents. Class 1 valued test accuracy, class 2 valued removing polyps and avoiding discomfort, and class 3 valued cost. Having had a prior colonoscopy and a higher income were predictors of the likelihood of future screening and membership in classes 1 and 2. Health insurance and a self-reported higher risk of developing colorectal cancer were associated with prior screening and higher future screening intentions, but not class membership.
Conclusion
We identified distinct classes of preferences focusing on different test features and personal characteristics associated with reported behavior and intentions. Healthcare providers should engage in a careful assessment of patient preferences when recommending colorectal cancer test options to encourage colorectal cancer screening uptake.
1. Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in USA for men and women combined [1]. Colorectal cancer screening may increase the identification of early-stage disease and the likelihood of successful treatment and survival [2]. The US Preventive Services Task Force recommends colonoscopy, flexible sigmoidoscopy (FlexSig), and a high-sensitivity fecal occult blood test (FOBT) for screening [3]. The US Preventive Services Task Force recommends routine screenings for individuals aged 50–75 years and, in certain circumstances, screenings for individuals aged 75–85 years.
The Healthy People 2020 target for CRC screening is70.5% [4] and the National Colorectal Cancer Roundtable has a goal of 80% screening by 2018 [5], but survey data suggest that only 58.2% of the screening recommended population are up to date with CRC screening [6]. As with other preventive health measures, healthcare and public health professionals are trying to identify barriers to screening and develop measures that will increase the screening rate.
Researchers have published numerous studies on barriers to and perceptions of CRC screening tests. Some of the most common barriers include financial concerns, fear of pain or humiliation, lack of education about the screening, and lack of a screening recommendation from a physician [7–11]. In addition, a number of researchers have used stated-preference surveys, such as conjoint analysis or discrete-choice experiments (DCEs), to study preferences for CRC screening tests [12–14]. Conjoint analysis and DCE surveys can provide a quantitative ranking of the relative importance of selected CRC test attributes. Across multiple countries and for different types of CRC screening tests, the existing studies on CRC screening consistently demonstrate that respondents value better accuracy, lower testing frequency, less discomfort, less burdensome preparation, and lower cost. Previous studies have also found differences in preferences across race [15] and prior testing experience [16, 17]. While some previous studies examined the impact of one or two observable characteristics on preferences, none compared the impact of a large set of personal characteristics on preferences alongside the information on the correlation of these characteristics with past screening behavior and future screening intentions. Learning about how screening preferences and behavior are related to observable personal characteristics could help with the development of more CRC screening targeted interventions and educational materials.
This study aims to provide more detailed information on preferences for the features of CRC screening tests and how those preferences and reported screening behavior correlate with personal characteristics. We used a latent class logit model to estimate the DCE, which allows for the identification of multiple classes of preferences, and included a set of personal characteristics to predict class membership. We also compare the impact of a set of personal characteristics on preferences for CRC screening test attributes, past screening behavior, and future screening intentions. The latent class logit model allows us to test whether responses to the DCE reveal segments of the sample (classes) that have systematically different preferences. Using a set of personal characteristics, we test whether similar characteristics are significant predictors of preferences for test attributes, past screening behavior, and future screening intentions.
2. Methods
2.1. Survey Design and Administration
Discrete-choice experiments provide information about individuals’ willingness to accept tradeoffs among features of hypothetical multi-attribute products, in this case, hypothetical CRC screening tests [18]. Our survey included: (1) questions on CRC risk factors and respondents’ subjective estimates of their own CRC risk; (2) descriptions of and questions about each test attribute used in the DCE; (3) DCE questions; (4) questions about history with CRC screening, self-assessed likelihood of future screening, and perceptions about CRC screening tests; and (5) general questions on health and health behaviors. Questions about CRC screening history came from the 2010 National Health Interview Survey [19]. The survey contained a one- or two-sentence description of each test in case respondents did not know the names of the tests.
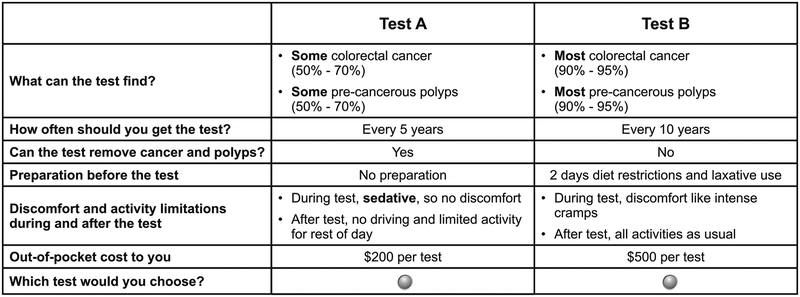
The DCE attributes of CRC screening tests were selected to reflect the characteristics of existing tests [colonoscopy, FlexSig, FOBT, and fecal immunochemical test (FIT)] after a review of the attributes included in other DCE surveys for CRC screening tests [14], and a ranking exercise with Centers for Disease Control and Prevention subject matter experts. Table 1 presents the attributes and levels, along with how the levels match the recommended CRC screening tests. The attribute levels for accuracy were based on Zauber et al. [20] The survey instrument was pretested in nine qualitative interviews. In the survey layout, accuracy and frequency were presented in separate rows for improved respondent clarity. The accuracy and frequency were combined into three levels corresponding to the recommendations for colonoscopy, FlexSig, and FOBT/FIT based on respondent feedback during the pretests. Some respondents did not find it plausible that a less accurate test would be administered less frequently. Figure 1 presents an example of a choice question.
Table 1.
Attributes and levels for the discrete-choice experiment
| Attributes | Levels | Levels mapped to existing CRC screening testsa |
|---|---|---|
| What can the test find and how often do you take the test? | 1. Most colorectal cancer (90–95%)/most pre-cancerous polyps (90–95%)/once every 10 y 2. Some colorectal cancer (50–70%)/some pre-cancerous polyps (50%−70%)/once every 5 y 3. Some colorectal cancer (50–70%)/very few pre-cancerous polyps (10–25%)/once every year |
Colonoscopy: 1 FlexSig: 2 FOBT: 3 FIT: 3 |
| Can the test remove cancer and polyps? | 1. Yes 2. No |
Colonoscopy: 1 FlexSig: 1 FOBT: 2 FIT: 2 |
| Preparation before the test | 1. No preparation 2. 2 days of diet restrictions 3. 2 days of diet restrictions and laxative use |
Colonoscopy: 3 FlexSig: 3 FOBT: 2 FIT: 1 |
| Discomfort and activity limitations during and after the test | 1. No pain or discomfort during the test/all activities as usual after the test 2. Discomfort such as intense cramps during the test/all activities as usual after the test 3. During test, sedative so no discomfort/after test, no driving, and limited activity rest of day |
Colonoscopy: 3 FlexSig: 2 FOBT: 1 FIT: 1 |
| Out-of-pocket cost to you per test | 1. US$0 (no cost to you) 2. US$10 3. US$50 4. US$200 5. US$500 |
CRC colorectal cancer, FIT fecal immunochemical test, FlexSig flexible sigmoidoscopy, FOBT fecal occult blood test
Number for level in column 2 that corresponds to test
Fig. 1.
Example of a choice question from the survey. Choice questions display hypothetical tests created by the experimental design
Given the attributes and levels included in this study (Table 1), we created an efficient experimental design of 16 sets of five DCE questions using experimental design software (NGene, 2012; ChoiceMetrics, Sydney, NSW, Australia). The design was optimized for a multinomial logit model in which the underlying utility was assumed to be additively separable in attribute levels with no attribute interaction effects (i.e., a main-effects model). The final design had the best relative D-efficiency (inverse of the estimated variance-covariance matrix) for a logit model [21]. Each respondent was randomly assigned to one of the 16 sets of DCE questions.
As a secondary objective, we tested the impact on preferences and reported past behavior of two CRC screening information sheets developed by the Centers for Disease Control and Prevention as part of the Screen for Life campaign materials (available at http://www.cdc.gov/cancer/colorectal/sfl/print_materials.htm). Before the DCE questions, respondents were randomly assigned an information sheet about colonoscopies, an information sheet about CRC screening and screening tests, or no additional information.
The target sample size was 2000 respondents based on the needs of the DCE model. Optimal sample sizes for DCE surveys are challenging to calculate. Most published choice experiments have a sample size from 100 to 300 respondents [22]. The survey was administered online to a sample of US adults aged 50–75 years drawn from the GfK KnowledgePanel®. The survey and data collection were approved by the RTI International Institutional Review Board and by the Office of Management and Budget (OMB control number 0920-1023).
2.2. Data Analysis
We analyzed the DCE data using a latent class logit model [23]. The latent class logit model is an extension of the conditional logit model that identifies classes of respondents based on unobserved or “latent” heterogeneity in preferences [24]. Rather than estimating a single set of average coefficients for the entire sample, the latent class model uses the data to identify patterns in the responses (classes of respondents), and personal characteristics can be included in the model to predict class membership. The model allows one to assess the impact of a large set of characteristics on preferences, rather than specifying an interaction term for a single specific subgroup. Latent class models have been used to analyze DCE data in a variety of applications [25, 26].
After specification tests using Bayesian information criteria [27] and the Akaike information criteria [28] for models with up to five classes, the final model specified three latent classes. The variables for the DCE attribute levels were effects coded except for the cost variable, which was modeled as a linear continuous variable, and interacted with the natural log of a respondent’s reported household income. For the omitted level of each attribute, the preference weight was calculated as the negative sum of the estimated preference weights on the non-omitted categories. For each preference weight, we calculated a 95% confidence interval. If the confidence intervals of preference weights for two levels of the same attribute did not overlap, then the preference weights were statistically different at the 5% level of confidence. If the confidence intervals of the preference weights of two levels of an attribute overlapped, a Wald χ2 test was used to determine the statistical significance of differences between adjacent attribute levels.
Using the parameters for the latent class logit model, we estimated willingness to pay (WTP) for changes in attribute levels for each of the three classes. Willingness to pay was calculated as the difference between the preference weights for two levels of an attribute divided by the preference weight for cost. In the model, cost was interacted with the natural log of a respondent’s reported household income. The WTP values were calculated for the average income for the sample. Confidence intervals around WTP estimates were calculated using the delta method [29]. The parameters were also used to predict the probability that the average respondent in each class who would prefer a test with characteristics similar to a colonoscopy or a FIT at different costs (see Table 1 for characteristics defining each test).
Using past studies, we identified a set of personal characteristics that we hypothesized would influence preferences for screening test attributes, and predict past screening behavior and future screening intentions. The characteristics included age; sex; race (black, white, and other); income; health insurance status; college education; married; live in a metropolitan statistical area; past screening experience (a colonoscopy, stool test, or Flex-Sig); subjective CRC risk perceptions; and whether the respondent received one of the two CRC screening information sheets. All the variables were dummy coded, except age and income, which were continuous.
The latent class model included the set of respondent characteristics as predictors of the likelihood that respondents were in one of the three classes. To estimate the impact of respondent characteristics on past screening behavior, two logit models were estimated for respondents who reported (1) having had a colonoscopy and (2) having had any CRC screening test (colonoscopy, FlexSig, FOBT, FIT, CT colonography or virtual colonoscopy, or stool DNA test). To estimate the impact of respondent characteristics on future screening intentions, we used responses to the question about the likelihood that a respondent would complete a CRC screening test in the future measured through a four-point Likert scale (see Table 2 for question wording and response categories). The responses were estimated using an ordered logit model.
Table 2.
Sample characteristics and experience with cancer screening tests, unweighted (n = 2073)
| Characteristic | Value |
|---|---|
| Mean age (SD), years | 61.2 (6.9) |
| Education (highest degree received) | |
| Less than high school | 129 (6.2%) |
| High school graduate—high school diploma or equivalent | 626 (30.2%) |
| Some college, no degree | 606 (29.2%) |
| Bachelors’ degree or higher | 712 (34.3%) |
| Race/ethnicity | |
| White, non-Hispanic | 1659 (80.0%) |
| Black, non-Hispanic | 171 (8.2%) |
| Other, non-Hispanic | 60 (2.9%) |
| Hispanic | 124 (6.0%) |
| 2 + races, non-Hispanic | 59 (2.8%) |
| Sex | |
| Male | 996 (48.0%) |
| Female | 1077 (52.0%) |
| Household income, US$ | |
| Under 25,000 | 309 (14.9%) |
| 25,000–49,999 | 440 (21.2%) |
| 50,000–74,999 | 425 (20.5%) |
| 75,000 and above | 899 (43.4%) |
| Marital status | |
| Married | 1383 (66.7%) |
| Widowed | 112 (5.4%) |
| Divorced | 274 (13.2%) |
| Separated | 34 (1.6%) |
| Never married | 191 (9.2%) |
| Living with partner | 79 (3.8%) |
| MSA status | 1383 (66.7%) |
| Current employment status | |
| Working | 1009 (48.7%) |
| Not working, retired | 706 (34.1%) |
| Not working, disabled | 193 (9.3%) |
| Not working, other | 165 (8.0%) |
| What type of health insurance do you have? Check all that apply (n = 2,069)a | |
| Private health insurance | 1325 (63.9%) |
| Medicaid, medical assistance, or any government low-income/disability assistance plan | 193 (9.3%) |
| Medicare | 690 (33.3%) |
| Tricare, VA, or other military healthcare | 121 (5.8%) |
| Indian Health Services | 5 (0.2%) |
| Other | 83 (4.0%) |
| I do not have health insurance | 75 (3.6%) |
| Not sure/don’t know | 23 (1.1%) |
| Experience with cancer screening tests | |
| Have you ever been told by a doctor or another healthcare professional that you are at increased risk of developing colorectal cancer in the future? (n = 2072)a | |
| Yes | 198 (9.7%) |
| No | 1849 (90.3%) |
| Compared to the average man or woman of your age, would you say that you are more likely to develop colorectal cancer, less likely, or about as likely? If you are a colorectal cancer survivor, we are asking about developing colorectal cancer again in the future (n = 2070)a | |
| More likely | 110 (5.3%) |
| About as likely | 767 (37.1%) |
| Less likely | 672 (32.5%) |
| I don’t know | 521 (25.2%) |
| Below is a list of screening tests for other types of cancer (not colorectal cancer). Please check off all the tests you have ever had. | |
| Mammogram for breast cancer (women) | 1961 (94.6%) |
| Pap test for cervical cancer (women) | 1942 (93.7%) |
| PSA test for prostate cancer (men) | 1349 (65.1%) |
| Skin cancer screening by a doctor | 938 (45.2%) |
| X-ray or CT scan for lung cancer | 370 (17.8%) |
| Other cancer screening | 264 (12.7%) |
| No cancer screening | 267 (12.9%) |
| Has a doctor or other health professional ever recommended that you have a test for colorectal cancer? (n = 2072)a | |
| Yes | 1450 (70.0%) |
| No | 567 (27.4%) |
| I don’t know | 55 (2.7%) |
| Have you ever had a colonoscopy? | |
| Yes | 1446 (69.8%) |
| No | 605 (29.2%) |
| I don’t know | 22 (1.1%) |
| There are other tests besides colonoscopies that look for colorectal cancer. Please check off all the other tests that you have had for colorectal cancerb (n = 2072)a | |
| Stool blood or fecal occult blood test | 725 (35.0%) |
| Fecal immunochemical test | 128 (6.2%) |
| Flexible sigmoidoscopy | 248 (12.0%) |
| CT colonography or virtual colonoscopy | 94 (4.5%) |
| Stool DNA test | 52 (2.5%) |
| Other | 26 (1.3%) |
| I have never had any of these tests for colorectal cancer | 963 (46.5%) |
| I don’t know | 181 (8.7%) |
| In the future, how likely are you to have a colonoscopy if you have never had one or to have another colonoscopy if you have had one? (n = 2072)a | |
| Very likely | 1072 (51.7%) |
| Somewhat likely | 595 (28.7%) |
| Somewhat unlikely | 165 (8.0%) |
| Very unlikely | 93 (4.5%) |
| I’m not sure | 147 (7.1%) |
CT computerized tomography, MSA metropolitan statistical area, PSA prostate-specific antigen, SD standard deviation, VA Department of Veterans Affairs
n is the number who answered the question if different from 2073
Survey instrument contained a short description of each test
We used NLOGIT 5 (Econometric Software, Inc., Plainview, NY, USA) [30] for the latent class model and Stata Version 14 (StataCorp LP, College Station, TX, USA) for the remaining analyses [31]. Respondents with missing values were dropped.
3. Results
The survey was administered in USA from September 2014 to February 2015. GfK sent out 3263 invitations resulting in 2073 completed surveys (64% completion rate). Six respondents did not answer any DCE questions, providing a sample size of 2067 for the DCE analysis. Table 2 presents the unweighted characteristics of the sample. More than 90% of the women in the sample reported having a mammogram and a Pap test. Among male respondents, 65% reported having a prostate-specific antigen test for prostate cancer. Only 13% reported never having been screened for any type of cancer. Seventy percent reported having had a colonoscopy and 81% reported having had at least one of the CRC tests (a colonoscopy or another test) [data not shown]. Data for USA collected in 2013 indicated that screening rates for CRC, breast, and cervical cancers were 58.2, 72.6, and 80.7%, respectively [6]. Compared to data from the Current Population Survey [32] (March 2014 Supplement) for the age group sampled, the sample has more white individuals (70% compared with 80% in the sample), is more educated (10% have no high school degree and 31% have a college degree compared with 6 and 34%, respectively, in the sample) and has a higher income (18% have income under US$25,000 and 40% have income over US$75,000 compared with 17 and 46%, respectively, in the sample).
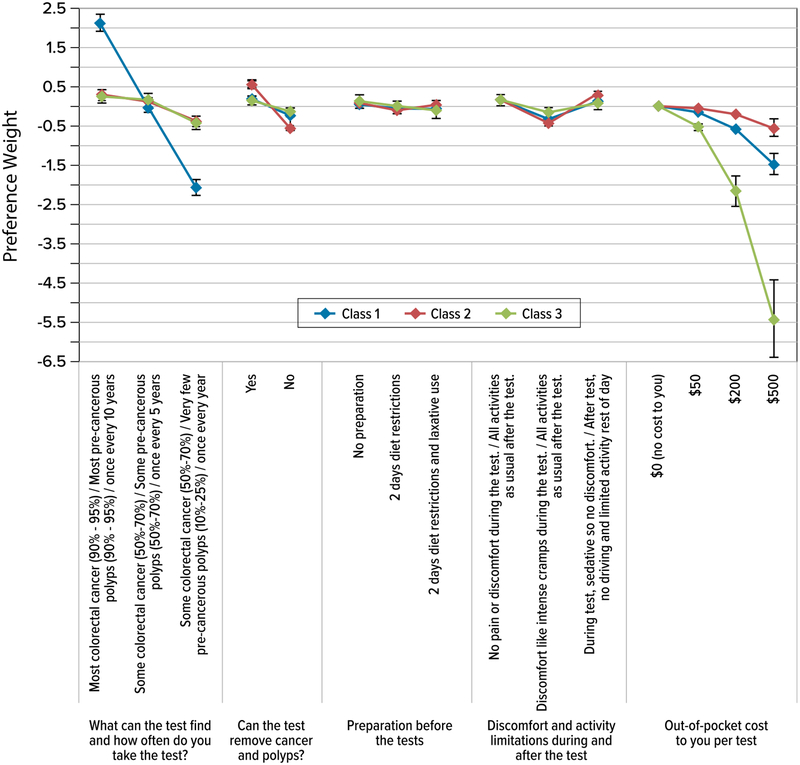
Figure 2 presents the normalized coefficients or preference weights from the latent class model for the three classes (results from the latent class analysis are contained in the Electronic Supplementary Material). The preference weights indicate the relative strength of preference for each attribute level, where larger positive numbers indicate greater preference and smaller numbers indicate less preference. The vertical distance between any two levels of an attribute indicates the relative importance of a change from one level to another. The average probability that respondents would be in each class was 49% for class 1, 28% for class 2, and 23% for class 3.
Fig. 2.
Discrete-choice experiment (DCE) preference weights from a latent class logit model, normalized. Classes 1–3 are the classes from the latent class analysis. The cost levels were estimated as a linear continuous variable with an interaction between the cost attribute and the natural log of the respondent’s income. The values were calculated using the natural log of the average income for the sample (US$75,639.53). The axis for the cost attribute is not to scale. The vertical bars surrounding each mean preference weight denote the 95% confidence interval about the point estimate. The levels of the non-cost attributes were effects coded so the preference weights are estimated relative to the mean effect of each attribute, which is normalized at zero. CRC colorectal cancer
The three classes displayed some important differences. Class 1 placed the most importance on higher accuracy and longer intervals between screening tests relative to other test features. A change in accuracy from the highest level (similar to a colonoscopy) to the middle level (similar to a FlexSig) was relatively more important than a change in any other attribute from the best to the worst level. The second class placed the most weight on tests that could remove polyps. The next most important change was avoiding discomfort such as intense cramps. Respondents in this class were less sensitive to cost than were respondents in the other two classes. Class 3 placed the most importance on changes in cost relative to other attributes. A change from a test with no cost to a test with the highest cost (US$500) was a more important predictor of choice than changes from the best to worst levels of any other attributes.
Most of the preference weights were significantly different from the other levels within attributes (p < 0.05 for a test of differences between attribute levels). All three classes valued a test that could also remove polyps over a test that could not and wanted to avoid discomfort such as intense cramps. Preference weights for classes 1 and 2 for no discomfort and sedation with activity limitations were not significantly different from each other, while class 3 only had a significant preference for no discomfort over intense cramps. For preparation, class 1 preferred no preparation to the other two levels, but showed no significant difference between the other two levels, while classes 2 and 3 had no difference in preference weights across the preparation attribute. Preference weights for the two higher levels of accuracy and reduced frequency were not significantly different for classes 2 and 3.
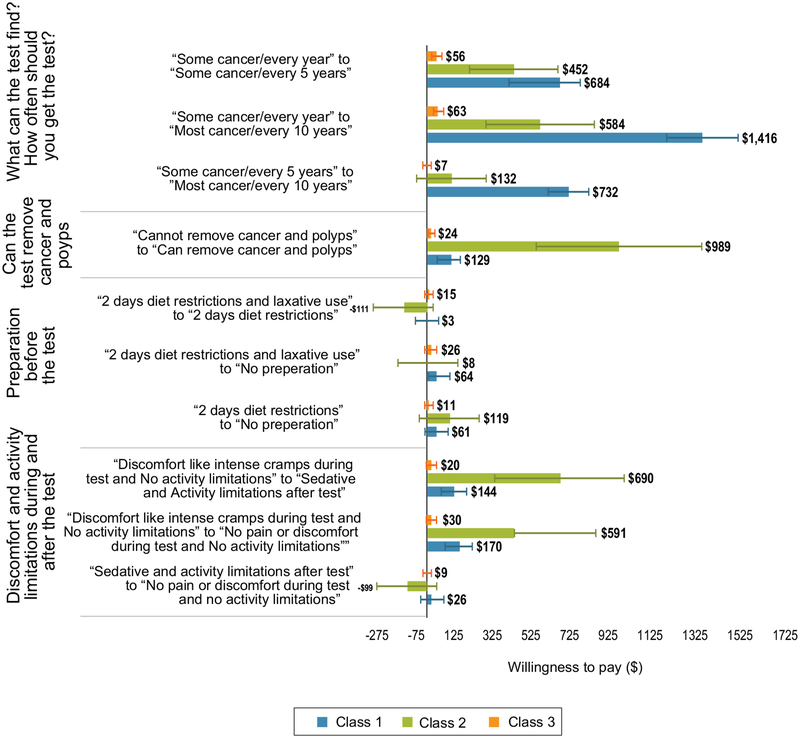
Class 1 had a higher WTP for more accurate and lower frequency tests than the other two classes (Fig. 3). Class 1 had a WTP of US$1416 to go from a test that found “some cancer” to a test that found “most cancer” compared with US$584 and US$63 for classes 2 and 3, respectively. Class 2 had a significantly higher WTP for a test that removed polyps (US$989 compared with US$129 for class 1 and US$24 for class 3) and for avoiding discomfort. Class 2 had a WTP of US$690 to go from a test with discomfort such as intense cramps to a test that used a sedative compared with US$144 for class 1 and US$20 for class 3.
Fig. 3.
Willingness to pay (WTP) for changes in attribute levels comparing accuracy class and cost class (with 95% confidence intervals). Classes 1–3 are the predicted classes analysis. The horizontal bars surrounding each WTP value denote the 95% confidence interval about the point estimate. CRC colorectal from the latent class cancer
Comparing predicted preference shares for a choice between tests with features such as colonoscopy and FIT, class 3 displayed the largest difference in preference shares when the cost of the colonoscopy increased. For class 3, the probability of selecting a test with attribute levels like a colonoscopy over a FIT was 64% when the colonoscopy was free and 17% when the colonoscopy cost US$200. The colonoscopy was preferred by 99% and 98% of class 1 for the no cost colonoscopy and the US$200 colonoscopy, respectively. For class 2, the numbers selecting colonoscopy were 87% and 84% for the two colonoscopy costs.
Table 3 lists the results for the models examining the impact of respondent characteristics on class membership for classes 1 and 2 relative to class 3. Being white, higher income, and having had a colonoscopy were associated with an increased likelihood of class 1 membership, while being black was associated with a decreased likelihood (column 2). Older age, higher income, and having had a colonoscopy in the past were associated with an increased likelihood of membership in class 2.
Table 3.
Personal characteristics and membership in latent classes relative to cost class (coefficients [standard errors])
| Characteristica | Membership in class 1 in DCE (latent class logit model) | Membership in class 2 in DCE (latent class logit model) |
|---|---|---|
| Age, years | 0.017 [0.013] | 0.040*** [0.014] |
| Male | −0.019 [0.165] | −0.360* [0.187] |
| White | 0.548** [0.251] | −0.283 [0.262] |
| Black | −0.962** [0.384] | −0.389 [0.348] |
| Income (thousands US$) | 0.019*** [0.003] | 0.014*** [0.003] |
| Health insurance | 0.789* [0.409] | 0.456 [0.437] |
| College educated | 0.282 [0.176] | −0.214 [0.194] |
| Married | −0.043 [0.187] | 0.110 [0.212] |
| Live in an MSA | −0.097 [0.204] | −0.123 [0.231] |
| Information sheet about colonoscopies | 0.018 [0.206] | −0.278 [0.230] |
| CRC screening information sheet | 0.718D−4 [0.202] | −0.212 [0.221] |
| More likely than average person my age to develop CRC | −0.232 [0.401] | 0.136 [0.433] |
| Had colonoscopy | 0.907*** [0.180] | 0.843*** [0.205] |
| Had CRC screening stool test | 0.185 [0.181] | 0.237 [0.200] |
| Had FlexSig | 0.470 [0.323] | 0.507 [0.337] |
| Constant | −3.360*** [0.902] | −3.406*** [0.981] |
| Observations | 20,512 observations; 2067 respondents | 20,512 observations; 2067 respondents |
CRC colorectal cancer, DCE discrete-choice experiment, FlexSig flexible sigmoidoscopy, MSA metropolitan statistical area
p<0.01;
p<0.05;
p < 0.10 measuring difference from 0 for coefficients in the latent class model
Age and income coded as continuous variables, all other variables were dummy coded. Excluded categories are: female, other race, no health insurance or did not know, no college, not married, do not live in an MSA, was not shown an information sheet on colonoscopies, was not shown a CRC screening information sheet, same risk or less risk of developing CRC than someone my age, has not had a colonoscopy, has not had a CRC screening stool test, has not had a FlexSig
Table 4 presents the results for the logit and ordered logits models of past screening behavior and future screening intentions. Past screening experience, older age, higher income, having health insurance, and believing one was at higher-than-average risk of developing CRC were positively associated with the probability that the respondent had any test in the past and had a colonoscopy in the past (columns 3 and 4). In addition, respondents with a college education and respondents who received the CRC screening test information sheet to read before the DCE were more likely to report having had any screening test in the past. Respondents who were black and respondents who were married were more likely to report having had a colonoscopy in the past.
Table 4.
Personal characteristics and influence on the likelihood of completing tests in the future and having been screened in the past (odds ratios [standard errors])
| Characteristica | More likely to have a test in the future (ordered logit, odds ratios) | Had any CRC screening test in the past (logit model, odds ratios) | Had colonoscopy in the past (logit model, odds ratios) |
|---|---|---|---|
| Age, y | 0.955*** [0.007] | 1.100*** [0.011] | 1.081*** [0.009] |
| Male | 1.184* [0.114] | 0.975 [0.117] | 1.021 [0.106] |
| White | 1.008 [0.153] | 0.807 [0.152] | 1.274 [0.197] |
| Black | 1.550* [0.354] | 1.507 [0.435] | 1.915*** [0.451] |
| Income (thousands US$) | 1.004*** [0.001] | 1.005*** [0.002] | 1.005*** [0.001] |
| Health insurance | 2.987*** [0.673] | 2.726*** [0.621] | 4.327*** [1.063] |
| College educated | 1.085 [0.114] | 1.356** [0.174] | 1.164 [0.130] |
| Married | 0.928 [0.104] | 1.307* [0.179] | 1.331** [0.158] |
| Live in an MSA | 0.980 [0.120] | 1.200 [0.175] | 1.149 [0.148] |
| Information sheet about colonoscopies | 1.043 [0.122] | 1.216 [0.174] | 1.097 [0.138] |
| CRC screening information sheet | 1.116 [0.130] | 1.506*** [0.221] | 1.103 [0.138] |
| More likely than average person my age to develop CRC | 1.824** [0.440] | 3.074*** [1.170] | 3.877*** [1.239] |
| Had colonoscopy | 10.100*** [1.199] | n/a | n/a |
| Had CRC screening stool test | 1.222* [0.126] | n/a | n/a |
| Had FlexSig | 1.828*** [0.306] | n/a | n/a |
| Constant | n/a | 0.002*** [0.001] | 0.002*** [0.001] |
| Cut-off point 1 | −3.191 [0.521] | n/a | n/a |
| Cut-off point 2 | −1.845 [0.512] | n/a | n/a |
| Cut-off point 3 | 0.368 [0.511] | n/a | n/a |
| Observations | 1921 observations; 1921 respondents | 2067 observations; 2067 respondents | 2067 observations; 2067 respondents |
CRC colorectal cancer, FlexSig flexible sigmoidoscopy, MSA metropolitan statistical area, n/a not applicable
p < 0.01;
p < 0.05;
p < 0.10 measuring difference from 1 for odds ratios
Age and income coded as continuous variables, all other variables were dummy coded. Excluded categories are: female, other race, no health insurance or did not know, no college, not married, do not live in an MSA, was not shown an information sheet on colonoscopies, was not shown a CRC screening information sheet, same risk or less risk of developing CRC than someone my age, has not had a colonoscopy, has not had a CRC screening stool test, has not had a FlexSig
The self-assessed likelihood of undergoing testing in the future (column 2) increased with income, having health insurance, a past colonoscopy or FlexSig, and believing one was at higher risk of CRC. The largest positive impact came from a previous test, followed by having health insurance. Age had a slightly negative effect on the reported likelihood of completing a test in the future.
4. Discussion
This study identified patterns in choices for CRC screening tests using a latent class model; linked those patterns to personal characteristics; and qualitatively compared the association between personal characteristics and preferences, past testing behavior, and future testing intentions. The latent class model indicated that respondents could be separated into three classes, each with systematically different preferences—a class that placed the greatest relative importance on test accuracy, a class that placed greater relative importance on tests that can remove polyps and on avoiding discomfort, and a class that placed the greatest relative importance on costs. Our results corroborate the results from other DCE studies on CRC screening tests [12–14]. However, using the latent class model, we were able to identify distinct classes of preferences within the sample that may be masked by models that estimate average preferences across the sample. Similar to our study, Hawley et al. [15] found that white respondents placed greater weight on accuracy. Hol et al. [16] found no differences in preferences between men and women, as did our latent class results. Other studies have also found that people with previous screening experience had a more positive view of screening [16, 17].
The results suggest that some individuals may prefer tests that are less expensive, even though they are less accurate. Financial support or education about existing subsidies for CRC screening might increase uptake of more accurate tests such as colonoscopy among this group. Medicare covers the cost of CRC screening tests for individuals aged 65? years; however, Medicare beneficiaries may incur costs for diagnostic procedures such as the removal of polyps during a screening colonoscopy and other costs associated with the tests, including having time off of work, hiring caregivers for children or dependent adults, and paying for transportation to and from the testing facility. Taking the full out-of-pocket cost and time required for the tests into account, colonoscopies may be too expensive for respondents with preferences similar to those exhibited in class 3.
We found that a college education was associated with a higher likelihood of having had a past test. Efforts to reach individuals with lower levels of education or designing materials targeted for these individuals might increase uptake of all tests. Having had a colonoscopy was associated with an increased likelihood of membership in classes 1 and 2, both which had less cost-sensitive preferences, and having had any test in the past had a strong positive association with future testing behavior. Encouraging individuals to complete their first test could be an important threshold. Health insurance was also correlated with having had a test in the past and intentions to have a test in the future, which has been found in other studies [33].
The latent class results have implications for testing recommendations. While physicians may be more likely to recommend a colonoscopy, some patients may prefer a stool test [34, 35]. Studies show that people who are able to pick the test they prefer are more likely to complete the test, and the Centers for Disease Control and Prevention suggests that to increase receipt of CRC screening, health systems can offer all recommended test options with advice about each, and match patients with the test they prefer and are most likely to complete [36].
The results presented need to be interpreted in light of several limitations. Although the online panel from which the sample was drawn is designed to be representative of the US population, the survey respondents were better educated and wealthier, and the sample contained a higher percentage of white respondents. The reported cancer screening rates in this survey were higher than in other surveys. In addition, black respondents were more likely to report having had a colonoscopy, while some studies have found no difference in colonoscopy uptake by race [33]. Even though the survey contained a short description of each test, responses on prior testing behavior were not verified by medical records. The screening information sheet contained a longer description of CRC tests and respondents who saw this information sheet were more likely to report having had a CRC test in the past. Respondents who received the information sheet may have been reminded of tests they had in the past. Because a large percentage of the sample had already had a colonoscopy and one of the informational sheets presented information only on colonoscopies, it is possible that respondents may have been primed to favor attribute levels associated with colonoscopies, although a subset of the sample was clearly price sensitive. Finally, because the accuracy and frequency of the test were a composite attribute, we do not have independent estimates of the impact of these two items.
5. Conclusion
To the extent that we can identify subgroups of the population with distinct preferences for different testing attributes, education and awareness campaigns about CRC screening test options could include messages that reflect the range of preferences for screening test attributes observed in the population. Results suggesting distinct classes of preferences focusing on different test features reinforce guidance that healthcare providers engage in a careful assessment of patient preferences when recommending CRC test options for better CRC screening uptake.
Data Availability Statement
The dataset generated during the current study is available from the corresponding author on reasonable request.
Supplementary Material
Key Points for Decision Makers.
This study used latent class analysis to identify three distinct classes of preferences for colorectal cancer (CRC) screening tests. One class was most concerned about efficacy, one on removing polyps and avoiding discomfort, and the other class was most concerned about cost.
Factors such as income and previous CRC testing experience impacted preferences for test features and the reported likelihood of completing a future test.
Research on the impact of patient preferences, characteristics, and past behavior on the uptake of CRC screening may help the public health community improve CRC education and awareness and help healthcare providers guide patients to an acceptable CRC test option.
Acknowledgments
Funding Work on this project by RTI International was supported by the Centers for Disease Control and Prevention Contract Number 200-2008-27958, Task Order 25.
Compliance with Ethical Standards
Conflict of interest Carol Mansfield, Donatus U. Ekwueme, Florence K. L. Tangka, Derek S. Brown, Judith Lee Smith, Gery P. Guy, Jr., Chunyu Li, and Brett Hauber have no conflicts of interest directly relevant to the content of this article.
Ethics approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the RTI International Institutional Review Board and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s40271-018-0308-6) contains supplementary material, which is available to authorized users.
References
- 1.US Cancer Statistics Working Group. United States cancer statistics: 1999–2012 Incidence and mortality web-based report. Atlanta (GA): Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2015. [Google Scholar]
- 2.National Institutes of Health. Cancer screening overview (PDQ®). 2013. Bethesda (MD): National Cancer Institute; Available from: http://www.cancer.gov/cancertopics/pdq/screening/overview/HealthProfessional#Section_25. Accessed 14 Apr 2015. [Google Scholar]
- 3.Agency for Healthcare Research and Quality. The guide to clinical preventive services. US Preventive Services Task Force. 2014. Available from: http://www.uspreventiveservicestaskforce.org/Page/Name/tools-and-resources-for-better-preventive-care. Accessed 14 Apr 2015. [PubMed]
- 4.US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy people 2020: disparities in clinical preventive services. 2013. Available from: http://www.healthypeople.gov/2020/LHI/clinicalPreventive.aspx?tab=data. Accessed 3 June 2014.
- 5.National Colorectal Cancer Roundtable. 2015. Available from: http://nccrt.org/. Accessed 4 Nov 2015.
- 6.Sabatino SA, White MC, Thompson TD, Klabunde CN. Cancer screening test use: United States, 2013. MMWR Morbid Mortal Weekly Rep. 2013;64(17):464–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Knight JR, Kanotra S, Siameh S, Jones J, Thompson B, Thomas-Cox S. Understanding barriers to colorectal cancer screening in Kentucky. Prev Chronic Dis. 2015;12:E95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman RM, Rhyne RL, Helitzer DL, Stone SN, Sussman AL, Bruggeman EE, Viera R, Warner TD. Peer reviewed: barriers to colorectal cancer screening: physician and general population perspectives, New Mexico, 2006. Prev Chronic Dis. 2011;8:(2)A35. [PMC free article] [PubMed] [Google Scholar]
- 9.Jilcott Pitts SB, Lea CS, May CL, et al. Fault-line of an earthquake: a qualitative examination of barriers and facilitators to colorectal cancer screening in rural, Eastern North Carolina. J Rural Health. 2013;29(1):78–87. [DOI] [PubMed] [Google Scholar]
- 10.Medina GG, McQueen A, Greisinger AJ, Bartholomew LK, Vernon SW. What would make getting colorectal cancer screening easier? Perspectives from screeners and nonscreeners. Gastroenterol Res Pract. 2012;2012:895807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman Wallace DA, Baltrus PT, Wallace TC, Blumenthal DS, Rust GS. Black and white disparities in receiving a physician recommendation for colorectal cancer screening and reasons for not undergoing screening. J Health Care Poor Underserv. 2013;24(3):1115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall D, McGregor SE, Currie G. Measuring preferences for colorectal cancer screening: what are the implications for moving forward? Patient. 2010;3(2):79–89. [DOI] [PubMed] [Google Scholar]
- 13.Ghanouni A, Smith SG, Halligan S, Plumb A, Boone D, Yao GL, et al. Public preferences for colorectal cancer screening tests: a review of conjoint analysis studies. Expert Rev Med Devices. 2013;10(4):489–99. [DOI] [PubMed] [Google Scholar]
- 14.Mansfield C, Tangka F, Ekwueme D, Smith J, Guy G, Li C, Hauber AB. Stated preference for cancer screening: a systematic review of the literature, 1990–2013. Prev Chronic Dis. 2016;13:E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, Kneuper S. Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients. Med Care. 2008;46(9 Suppl. 1):S10–6. [DOI] [PubMed] [Google Scholar]
- 16.Hol L, de Bekker-Grob EW, van Dam L, Donkers B, Kuipers EJ, Habbema JD, et al. Preferences for colorectal cancer screening strategies: a discrete choice experiment. Br J Cancer. 2010;102(6):972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dam L, Hol L, de Bekker-Grob EW, Steyerberg EW, Kuipers EJ, Habbema JD, et al. What determines individuals’ preferences for colorectal cancer screening programmes? A discrete choice experiment. Eur J Cancer. 2010;46(1):150–9. [DOI] [PubMed] [Google Scholar]
- 18.Hensher DA, Rose JM, Greene WH. Applied choice analysis. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). 2010 NHIS questionnaire: sample adult diet and nutrition. 2011. Available from: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Survey_Questionnaires/NHIS/2010/English/qcancer.pdf. Accessed 14 Aug 2015.
- 20.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson FR, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13. [DOI] [PubMed] [Google Scholar]
- 22.Marshall D, Bridges JFP, Hauber AB, Cameron R, Donnalley L, Fyie K, et al. Discrete-choice experiment applications in health: how are studies being designed and reported? An update on current practice in the published literature between 2005 and 2008. Patient. 2010;3(4):249–56. [DOI] [PubMed] [Google Scholar]
- 23.Hauber AB, González JM, Groothuis-Oudshoorn CG, Prior T, Marshall DA, Cunningham C, Ijzerman MJ, Bridges JF. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19(4):300–15 [DOI] [PubMed] [Google Scholar]
- 24.Greene WH, Hensher DA. A latent class model for discrete choice analysis: contrasts with mixed logit. Transp Res Part B Methodological. 2003;37(8):681–98. [Google Scholar]
- 25.Brown DS, Poulos C, Johnson FR, Chamiec-Case L, Messonnier ML. Adolescent girls’ preferences for HPV vaccines: a discrete choice experiment. Adv Health Econ Health Serv Res. 2014;24:93–121. [PubMed] [Google Scholar]
- 26.Deal K Segmenting patients and physicians using preferences from discrete choice experiments. Patient. 2014;7:5–21. [DOI] [PubMed] [Google Scholar]
- 27.Hurvich M, Tsai C. Regression and time series model selection in small samples. Biometrika. 1989;76(2):297–307. [Google Scholar]
- 28.McLachlan G, Peel D. Finite mixture models. New York (NY): Wiley; 2000. [Google Scholar]
- 29.Greene WH. Econometric analysis. 5th ed Englewood Cliffs: Prentice Hall; 2003. [Google Scholar]
- 30.Econometric Software, Inc. 2012. NLOGIT 5 Plainview; Econometric Software, Inc. [Google Scholar]
- 31.StataCorp LP. 2015. Stata statistical software: release 14. College Station: StataCorp LP [Google Scholar]
- 32.Center for Economic and Policy Research. 2016. March CPS Uniform Extracts, Version 1.0. Washington, DC. [Google Scholar]
- 33.Swan JL, Breen N, Graubard BI, McNeel TS, Blackman D, Tangka FK, Ballard-Barbash R. Data and trends in cancer screening in the United States: results from the 2005 National Health Interview Survey. Cancer. 2010;116(20):4872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zapka J, Klabunde CN, Taplin S, Yuan G, Ransohoff D, Kobrin S. Screening colonoscopy in the US: attitudes and practices of primary care physicians. J Gen Intern Med. 2012;27:1150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lafata JE, Cooper GS, Divine G, et al. Patient-physician colorectal cancer screening discussions: delivery of the 5A’s in practice. Am J Prev Med. 2011;41:480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klabunde CN, Djenaba AJ, King JB, White A, Plescia M. Vital signs: colorectal cancer screening test use: United States, 2012. MMWR Morbid Mortal Weekly Rep. 2013;62(44)881–8. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset generated during the current study is available from the corresponding author on reasonable request.