Abstract
Urothelial carcinoma, or bladder cancer, is a malignancy, which most commonly affects older patients. The median age at diagnosis is 73 and care of these patients requires consideration not just of the disease-related factors such as stage and histology, but also of patient-related factors. Many of these patients have concurrent medical morbidities and additional changes related to the aging process. Older patients with cancer are a unique population requiring additional considerations and assessment in treatment decision-making. It is important to look beyond chronologic age. The traditional treatment for advanced disease has relied on platinum-based chemotherapy. These multi-agent regimens require consideration of baseline organ function as well as competing conditions that may heighten toxicity. The advent of a new class of cancer therapeutics, the immune checkpoint inhibitors, has changed the care of patients with advanced disease considerably. These immunotherapeutics have been approved for treating patients with disease progression on chemotherapy, or those that are ineligible (or unfit) to receive cisplatin-based therapy. This expansion of the population of patients eligible for treatment has great applicability to the unique considerations in an older patient population. In general, these new immunotherapies are well tolerated and effective in this group of patients.
1. Introduction
Bladder cancer is a disease of the elderly, as 90% of all bladder cancer patients are over 55 years old with a median age at diagnosis of 73 years [1]. This is a common cancer worldwide, and in the United States (US) there were an estimated 79, 000 new cases in 2017 with almost 17, 000 deaths [2]. Nearly three quarters of all cases diagnosed are non-muscle invasive tumors; and despite local treatment, there is a high rate of recurrence and progression to muscle invasive disease [3]. Tobacco use is the greatest risk factor in developing bladder cancer, contributing to 50% of all cases [4]. Bladder cancer is also termed urothelial carcinoma or transitional cell carcinoma; we will use these terms interchangeably. Bladder cancer is generally separated into “invasive” and “non-muscle invasive, or superficial” disease. Patients with invasive disease may have localized and potentially curable disease, or locally advanced and metastatic disease. For the purpose of this review, we will primarily focus on the treatment of advanced disease. For decades, the treatment of advanced bladder cancer has largely relied on platinum-based chemotherapy. Yet in the past year there have been notable changes in the therapies for metastatic disease, as checkpoint inhibitors allowing for immune activation have shown activity in advanced stage disease. The incorporation of these novel therapeutics has had a major impact on the care of patients with bladder cancer, especially those thought to be more vulnerable or frail.
There are minimal data on the current care of older patients with advanced bladder cancer. Of the limited population-based studies available, there are a large proportion of patients that do not receive any systemic therapy [5]. It is unclear if this is due to patient preference to forgo anti-cancer therapy or a lack of physician willingness to prescribe a toxic anti-cancer therapy to a potentially vulnerable patient population. The lack of data on how these decisions are made is a gap in our current knowledge of the treatment of advanced bladder cancer in older patients. There are some data available in the general treatment of advanced disease which we will apply to older patients. Through this review, we will focus on the basic approach to care of older patients with advanced bladder cancer and the available treatment options with a focus on immunotherapy. This is a changing treatment landscape and immunotherapy has significantly altered treatment options for vulnerable patients, such as frail older patients. In this unique and understudied population, we will summarize the current evidence and identify areas of needed additional study.
2. Approach to the care of an older patient with cancer
The bladder cancer population is at increased risk for comorbidities, and these comorbidities can influence the outcomes of surgery and choice of perioperative systemic therapy [6]. In part, this is often due to history of tobacco use, and thus the presence of multiple comorbid conditions can impact the approach and choice of cancer therapy. Patients with advanced bladder cancer are generally offered systemic therapy as palliative treatment. Clinical studies of efficacy and safety of therapeutics in general include patients that are much younger than average cancer patients [7]. In addition, bladder cancer patients may have organ dysfunction, such as kidney disease, based on the site of the primary tumor, which would preclude them from most clinical trial inclusion criteria. As a result, the available drug therapeutic trial data may be difficult to apply to older patients with comorbid conditions and altered organ function. A geriatric assessment can be used to help assess the potential impact of these factors on the older patient.
To determine which elderly patients are “fit” for treatment, the National Comprehensive Cancer Network (NCCN) recommends that all geriatric patients undergo a geriatric assessment (GA) to determine how well/if they can tolerate treatment for their cancer [8]. The main areas of assessment include demographic data, social support services comorbidities, functional status, cognition, depression, nutrition, fatigue, polypharmacy, and various geriatric syndromes [9]. A GA aids in identifying underlying geriatric syndromes or areas that may not be as evident in a standardized oncologic history but are highly influential of a patient’s global health. Older patients are a complicated and variable population and a comprehensive analysis of these listed domains allows for an improved understanding of a patient’s health and healthcare needs in order to better inform a treatment plan. In approaching the care of an older patient with cancer, one must first consider their cognitive status and ability to participate in the care discussion. If patients are able to discuss their goals, and wish to proceed with therapy, the medical team must then assess their fitness for therapy based on multiple indicators. Aligning care with patient overall goals and values, treatment needs, and baseline health is a component of performing a geriatric assessment. This tailored approach to care planning would then allow for standard therapy in fit patients and modified therapies in less fit patients in order to avoid toxicities. Through the geriatric assessment, patients are identified as fit for standard (or unmodified) therapy, vulnerable and in need of modified therapy, or frail and likely to suffer harm with even modified therapy. Approaching the care of older patients through a geriatric assessment has been shown to be feasible and informative [10, 11]. Despite this, there are limited data specific to the population of older patients with bladder cancer.
Multiple resources exist to enable incorporation of GA into practice as described by the International Society of Geriatric Oncology and NCCN older adult oncology guidelines [8, 12]. A geriatric assessment is a multicomponent evaluation of the global health and functioning of an older person. The full geriatric assessment is lengthy and extensive, and not necessary for every patient. To address these issues, several screening tools have been validated to use in the clinical setting. The most well-known and studied are the G8, Triage Risk Screening Tool (TRST), and Vulnerable Elder-13 Survey (VES-13). Any of these can be used to screen for further geriatric assessment; however, the G8 was identified to be “equally or more sensitive” in determining the need for further geriatric assessment when compared to the TRST, VES-13 and other less known screening tools in a recent review of geriatric assessment screening tools [12–15]. If a patient is identified as vulnerable on one of these screens, the patient should undergo a full geriatric assessment to determine eligibility for treatment. This assessment may then identify areas that are in need of additional support or care, which if addressed would allow a patient to initiate therapy with less risk of harm. These screening tools are also more sensitive than the standard assessments (Eastern Cooperative Oncology Group [ECOG] and Karnofsky) in identifying functional deficits [16]. After an initial screen, most patients will have some mild comorbidities or functional deficits which increase their risk of complications from chemotherapy, but not to a degree in which there is a high probability of irreversible harm. Intervening prior to initiating chemotherapy may protect vulnerable patients from treatment toxicity. For example, reviewing medication lists and addressing polypharmacy, or referring patients to physical therapy (“prehab”) to avoid loss of functional independence are treatment modifications which can help mitigate treatment toxicity in vulnerable patients [17, 18]. While there are limited data in bladder cancer specifically, this approach has proven helpful in other populations [16, 19].
In conclusion, the approach to the elderly patient with bladder cancer should start with a discussion with the patient about his/her diagnosis. If the patient is cognitively impaired or lacks capacity, this conversation should occur with the patient’s designated decision-maker. This should then be followed by a goals-of-care discussion in order to determine if the patient would like to pursue treatment. If the patient would like to pursue treatment, the use of a geriatric assessment screening tool is the next appropriate step (followed by a full geriatric assessment if the patient fails the screening). Should the patient be determined to be “fit” for therapy, or vulnerable but able to receive therapy with some intervention, the patient should be offered guideline appropriate therapy for their disease stage. In the past, vulnerable geriatric patients with bladder cancer had limited treatment options. Fortunately, developments in immunotherapy may open avenues for treatment in patients who previously would have been determined too vulnerable or frail for standard therapy.
3. Current treatment for Muscle Invasive and Metastatic Disease
Muscle invasive bladder cancer has invaded the muscularis propria resulting in at least T2, or stage II, disease. Stage II and III bladder cancer can still be cured with the use of multimodal approaches to therapy – generally surgery or radiation given in combination with some systemic therapy. These interventions, however, are high risk and require careful selection and consideration of patients. More advanced disease, or disease not amenable to local treatment, is rarely amenable to cure. Patients are then offered systemic therapy which has traditionally been reliant on chemotherapy. In recent years, the development of inhibitors of checkpoints on the immune system (or immunotherapy) has led to the incorporation of another class of active systemic therapy for advanced disease.
3.1. Surgery and radiation
Elderly patients are less likely to be offered definitive therapy with surgery or chemo-radiation than their younger counterparts [20]. Small studies over the past several years, however, have shown that patients should not be discounted from therapy because of their age alone and that definitive treatment is often under-utilized in the older population [21]. The main definitive therapy for muscle invasive disease, radical cystectomy, is a high-risk surgery regardless of age. When considering surgery, with or without neoadjuvant therapy, one must consider the total health of the patient. A retrospective study analyzing “well-selected” (based on physical exam, screening for cardiac and physical performance status, and routine preoperative studies) elderly patients with muscle invasive bladder cancer deemed to be cystectomy candidates concluded that there were similar rates of operative mortality and early/late complications when comparing the elderly patients with younger patients [22]. A review of complications following radical cystectomy in the elderly summarized that the elderly have increased perioperative morbidity and mortality following cystectomy [23]. Additionally, a retrospective analysis also came to the conclusion that 90-day mortality and morbidity remains relatively high at 9% in elderly bladder cancer patients undergoing cystectomy, but that Charlson comorbidity index and body mass index (BMI) were also predictive of complication development, implying that appropriate geriatric assessment could potentially decrease these risks [24]. For example, if a patient is able to adjust their nutrition in order to improve BMI, then they may have improved outcomes – a “prehab” approach. Alternatively, geriatric assessment that considers comorbidities and age could identify patients less likely to derive a survival benefit from a high-risk surgery. Patients in this subset may choose to focus on symptom management rather than curative resection. Thus, despite well founded concerns for complications and mortality associated with bladder cancer treatment, data suggests that appropriately selected, “fit” elderly patients have similar mortality and complication rates to younger patients, and should be offered definitive treatment. A geriatric assessment will aid in defining the fitness of a patient as well as identifying areas of vulnerability in need of additional support or attention.
Radiation is an option for patients not eligible for cystectomy, or who opt for organ preservation. Trimodal therapy of radiation given with radiosensitizing chemotherapy after an adequate resection of bladder tumor is an effective curative option for the treatment of muscle invasive disease. However, many studies still require that salvage cystectomy is a feasible option for patients treated with chemoradiation. Thus, this is still a treatment for healthier patients. Definitive therapy, whether surgery or radiation therapy, is underutilized in older patients as only half of patients over the age of 70 undergo curative intent therapy [1, 25]. Yet data suggest an appropriately selected population derives similar benefit to younger patients undergoing chemotherapy and radiation [26–30]. Combined chemoradiation can be altered to allow for either a high chance of cure or long-term disease control. In a population with limited life expectancy due to comorbid conditions or chronologic age, a therapy which may palliate any symptoms of local disease and offer long term control could prove useful [31]. As such, many elderly patients may benefit from chemo-radiation without the additional risks of radical cystectomy. Clinicians mainly prescribe cisplatin as a chemosensitizer, but the trial by James et al utilized 5FU and mitomycin C with a 5 year overall survival of 48% with combined modality therapy versus 35% with radiation alone [32]. A single institution evaluation of older patients treated with combined modality therapy disease-specific survival was 60% at 5 years and 56% at 10 years [33]. This is a favorable comparison to radical cystectomy.
For non-metastatic muscle-invasive cancers, therapy consists of either surgical resection through radical cystectomy or definitive radiation therapy. Neoadjuvant cisplatin-based chemotherapy has improved the survival rate and decreased the rate of relapse [34]. Combined modality therapy has also improved disease control rates in comparison to radiation alone. However, the ability to safely administer chemotherapy to many patients - due to tobacco-related comorbidities, and impairment of renal function - has long been a challenge in this complex patient population [35]. Cisplatin-based chemotherapy remains a cornerstone of these treatments, which can also result in considerable morbidity. Immunotherapy is under exploration in this disease stage as well, to be used in combination with definitive therapies in order to potentially decrease toxicities and improve outcomes.
3.2. Chemotherapy
The medical treatment of metastatic bladder cancer has changed little in recent decades. This is reflected by 5 year survival rates reported in the Surveillance, Epidemiology and End Results (SEER) database that have remained largely stable since the 1980’s [36]. This is a chemotherapy-sensitive tumor that often responds initially to cytotoxic therapy and then recurs or progresses. Recently, an improved understanding of tumor biology and immunology has led to the development of a novel class of immunotherapies that have shown promise in advanced bladder cancer, expanding treatment options beyond cisplatin-combination therapy.
Currently, cytotoxic platinum-based chemotherapy is the foundation of metastatic bladder cancer therapy. These multi-drug combinations have changed little over decades. There are two primary regimens: methotrexate, vinblastine, Adriamycin, and cisplatin (MVAC) and gemcitabine, cisplatin (GC) that have been used both in the neoadjuvant and metastatic settings. In the neoadjuvant setting, MVAC was shown to improve median overall survival (mOS) from 44 to 77 months and the rate of complete pathologic response to therapy at the time of cystectomy (pT0) also improved in the arm receiving neoadjuvant MVAC from 15% to 38% (p<0.001) [34]. The MVAC regimen has also been shown to result in improved response rates and survival in comparison to single-agent cisplatin in the treatment of metastatic disease [37]. The regimen is notable for significant hematologic, gastrointestinal and infectious toxicities but a dose-dense regimen has improved many of these toxicities and enhanced tolerability [38–40]. The dose-dense MVAC (ddMVAC) regimen shortened the treatment cycle from 4 weeks to 2 weeks with mandated hematologic growth factor support and resulted in an improved overall response rate (62% v 50%) and complete response rate (31% v 9%) in comparison to traditional 4-week MVAC dosing [41]. As such, the dose-dense approach allows for increased dose density, lower toxicity when given in combination with growth factor support, and improved responses and subsequent survival when compared to the standard dosing and schedule. Another cisplatin-based regimen is the combination of cisplatin and gemcitabine (GC), which in the stage IV setting has been shown to have similar response rate to traditional MVAC (49% v 45%) with a similar impact on survival (15.2 mos MVAC v 14.0 mos with GC p=0.66) and has less high-grade hematologic toxicity [42]. There has not been a large-scale trial of GC in the neoadjuvant setting, but a single institution series compared GC to MVAC historical controls and found a pT0 rate of 26% with GC compared to the historical control rate of 28% with MVAC [43]. The authors of this institutional study applied an intention to treat analysis to the cooperative group study of neoadjuvant chemotherapy and included all patients that received MVAC (regardless of surgical status) and thus reported a revised pT0 rate of 28% in comparison to the reported 38% [34]. The GC regimen is in widespread use for advanced disease as well, and may be the most commonly used regimen in the US [44].
In an effort to avoid toxicity and preserve activity, many studies have evaluated the substitution of carboplatin for cisplatin [45]. In a single institution, retrospective study, older patients receive carboplatin or cisplatin in similar rates, suggesting that many providers substitute carboplatin in practice [46]. In a cohort of cisplatin-ineligible patients, gemcitabine and carboplatin were compared to methotrexate, carboplatin and vinblastine and resulted in similar overall survival and less toxicity [47]. Additionally, providers unable to prescribe cisplatin to patients may also consider single agent chemotherapy to palliate symptoms. Despite many attempts, there are no second-line chemotherapy agents approved by the Food and Drug Administration (FDA). Providers have offered taxanes or pemetrexed as agents that have exhibited activity and are tolerable [48, 49]. These single agents are likely more tolerable than combination therapy but also have not demonstrated survival advantage in advanced disease. Newer immunotherapy agents have filled this gap as the first FDA approved therapy for second-line treatment of bladder cancer. This class is tolerable and effective, showing a survival advantage in many of the reported second line studies.
Chemotherapy use in older bladder cancer patients is limited by multiple factors. Cisplatin is known to cause nephrotoxicity, neurotoxicity, and ototoxicity. It is also a highly emetogenic therapy. Cisplatin is oftentimes the limiting factor in the ability to safely prescribe chemotherapy to patients with bladder cancer, as physicians must consider patient’s age-related comorbidities (hearing loss, renal dysfunction). In addition, as a tobacco related disease, patients may also have neuropathy or nephropathy due to vascular changes. Yet limited data suggest that older patients with bladder cancer derive similar benefit from chemotherapy as their younger counterparts and may tolerate the regimen well if they meet initial inclusion criteria [28, 29]. For patients with adequate organ function and physical function to tolerate chemotherapy, this remains a viable option in an older population. Yet, one must certainly monitor the impact of development of neuropathy on an older person’s mobility and balance as this could lead to falls that result in added morbidity [50]. In addition, minimizing the impact of a highly emetogenic regimen relies on adequate hydration, a knowledge of how to take “as needed” medications appropriately, and additional resources to get to frequent clinical checkups. However, many patients will not be able to tolerate cisplatin, or multi-agent chemotherapy. While there are altered chemotherapy regimens, many may forego therapy. Checkpoint immune therapy has expanded options for patients that may not be cisplatin-eligible but otherwise interested in pursuing anticancer treatment.
3.3. Immunotherapy
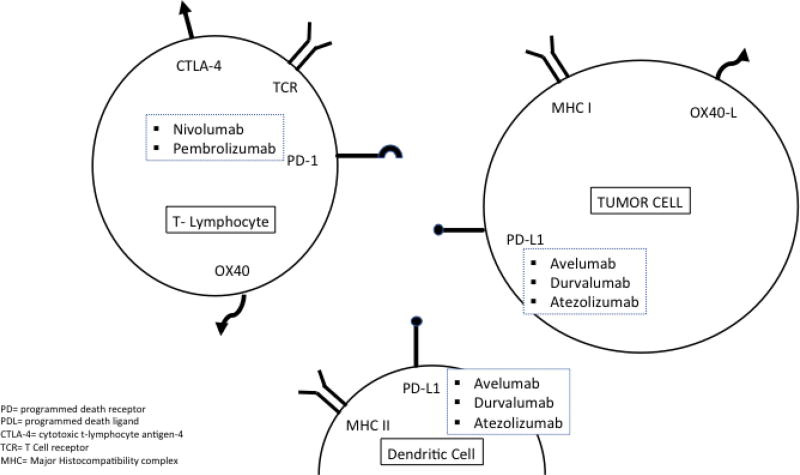
In a very short time, five immunotherapies have been approved by the FDA for use in urothelial carcinoma: atezolizumab (May 18, 2016), nivolumab (February 2, 2017), durvalumab (May 1, 2017), avelumab (May 9, 2017), and pembrolizumab (May 18, 2017) (Table 1). These medications affect the programmed cell death-1 (PD-1) pathway. When the PD-1 pathway is activated by the binding of programmed cell death ligand-1 (PD-L1), T-cell activity is inhibited, terminating the inflammatory response. PD-L1 expression also causes peripheral tolerance of tissue to the immune system [51]. When PD-L1 is expressed on tumor cells, it allows the tumor to go undetected by the immune system and escape destruction [52]. The immunotherapies approved thus far by the FDA for advanced urothelial carcinoma are monoclonal antibodies that block the interaction between PD-1 and PD-L1, allowing the immune system to detect tumor cells and stimulate an immune response against the tumor cells (Figure 1). Atezolizumab, avelumab, and durvalumab bind to PD-L1, blocking the interaction with PD-1 and B7.1; whereas nivolumab and pembrolizumab bind to the PD-1 receptor blocking PD-L1 and PD-L2 from interacting with PD-1. Agents in this class are generally referred to as “checkpoint inhibitors” and are part of a new generation of therapeutics which harness the action of the immune system to impede tumor growth.
Table I.
Trial data of currently approved PD and PD-L1 inhibitors
| Trial | Phase | Objective Response Rate |
12 Month Overall Survival |
Median Overall Survival, Months |
|---|---|---|---|---|
| Atezolizumab Following Cisplatin [53] | Phase 2 N=315 | Total: 15 ≥5% PD1+: 26% 5%>PD1+≥1%: 10% PD1+<1%: 8% | Total: 36% ≥5% PD1+: 48% 5%>PD1+≥1%: 30% PD1+<1%: 29% | Total: 7.9 ≥5% PD1+: 11.4 5%>PD1+≥1%: 6.7 PD1+<1%: 6.5 |
| Atezolizumab in cisplatinum-ineligible (IMvigor 210) [54] | Phase 2 N=119 | Total: 24% ≥5% PD1+: 28% 5%>PD1+≥1%: 21% PD1+<1%: 21% | ≥5% PD1+: 57% 5%>PD1+≥1%: 52% PD1+<1%: 59% | ≥5% PD1+: 15.9 5%>PD1+≥1%: 12.3 PD1+<1%: 19.1 |
| Atezolizumab compared to chemotherapy, 2nd line (IMvigor211) [55] | Phase 3 N=931 | 23% in IC 2/3 selected | 39.2% | IC2/3: 11.1 mos |
| Nivolumab Following cisplatin (Checkmate 275) [56] | Phase 2 N=265 | Total: 19.6% ≥5% PD-L1+: 28.4% PD-L1+≥1%: 23.8% PD-L1+<1%: 16.1% | N/A | Total: 8.74 PD-L1<1%: 5.95 PD-L1≥1%: 11.30 |
| Durvalumab Following cisplatin [57] | Phase 1/2 N=191 | Total: 17.8% PD-L1≥25%: 27.6% PD-L1<25%: 5.1% | Total: 55% PD-L1≥25%: 63% PD-L1<25%: 41% | Total: 18.2 PD-L1≥25%: 20.0 PD-L1<25%: 8.1 |
| Pembrolizumab Following cisplatin (Keynote 045) [58] | Phase 3 N=542 | Total: 21.1 | Total: 43.9% | Total: 10.3 |
| Pembrolizumab In cisplatinum-ineligible (Keynote 052) [59] | Phase 2 N=100 | Total: 24% PDL1 selected: 28% | NR | NR |
| Avelumab Following cisplatin JAVELIN [62] | Phase 1 N=226 | 13.3% | NR | NR |
Figure 1.
General interaction between the immune system and tumor cells.
3.3.1. Atezolizumab
Atezolizumab was the first immunotherapy approved by the FDA for use in metastatic urothelial carcinoma. It was initially approved as second-line therapy following platinum-based chemotherapy based on a trial done by Rosenberg et al. This trial included patients aged 32–91 with a median of 66 years who had an ECOG performance status of 0 or 1. In this trial, all patients, regardless of their PD-L1 status, had an objective response rate (ORR) of 15% in an unselected population. While there was no subgroup analysis done during the trial, patients with >5% of PD-L1 positive immune cells had an ORR of 27% [53]. The median age of patients in this study reflects the older age of bladder cancer patients, and enrollment of patients into their 90’s allows for some evaluation of the activity of this agent in older patients.
The FDA also approved atezolizumab for first line use in platinum-ineligible patients. The definition of “platinum ineligible” refers to patients with impaired renal function, ECOG 2, hearing loss of >25 decibels, and/or grade 2 or greater peripheral neuropathy. In this trial, patients aged 51–92 with a median age of 73 years and ECOG 0–2 received atezolizumab as first line therapy, with an ORR of 23%. This is more reflective of the average patient with bladder cancer in that the age and performance status are higher, and most of these patients also had organ dysfunction precluding the use of cisplatin. They additionally reported the ORR in patients older than 80 years of age (25/119 patients) as 28% versus 23% in the entire study population [54]. The most common adverse events in the above trials were fatigue, nausea, decreased appetite, diarrhea, and pruritus. In both trials, grade 3/4 adverse events occurred in 16% of the patients, with the most common grade 3/4 adverse events being fatigue, transaminitis, and anemia. Immune-mediated adverse events (including colitis, pneumonitis, and transaminitis) occurred in 7% of patients who received atezolizumab following cisplatin therapy and 12% of patients who received atezolizumab as first line therapy. This rate of significant adverse events is lower than rates generally seen in chemotherapeutic combinations and expands the treatments which may be offered to older, or more vulnerable, patients.
A later study, IMvigor 211, of atezolizumab compared to physician’s choice of chemotherapy in patients that had progressed after platinum therapy, enrolled 931 patients [55]. This study failed to meet its primary endpoint of longer overall survival in comparison to chemotherapy in patients overexpressing PD-L1 with an immunohistochemical stain of 2/3. However, exploratory analysis of the intention to treat population did reveal an OS at 12 months of 39.2% (95% CI 34.8–43.7) with atezolizumab and 32.4% (28.0–36.8) with chemotherapy. The adverse event profile was similar to previous atezolizumab studies. The authors were unable to fully explain the lack of statistically significant benefit, but there is a general sense that the selection of a group of patients with overexpression of PD-L1 likely complicated the results. PD-L1 analysis is affected by variation from primary tumor and metastatic site analysis, requires a highly accurate PD-L1 assay, and entails a clear understanding of the predictive nature of the assay. The current understanding of proper assay and cutoff levels has not yet been determined for the field, and the results of this study illustrate the need for improved understanding of PD-L1 as a predictive and prognostic biomarker. Thus, while this study confirmed atezolizumab is a well-tolerated agent, interpretation of its use in comparison to chemotherapy remains complicated.
3.3.2. Nivolumab
Nivolumab was approved for use in urothelial carcinoma following platinum therapy based on the results of the Checkmate 275 trial. In this trial, 265 patients aged 38–90 with a median age of 66 years were evaluated for response to nivolumab. Notably, 110 patients were ages 65–75, 35 patients were ages 75–85, and 3 patients were older than 85, indicating that over half the patients were older than 65 years in the study. In this fit population (ECOG 0–1), the overall response rate based on radiographic analysis was 19.6%. An analysis of the correlation of response to PD-L1 status, demonstrated that patients with >5% PD-L1 expression had an ORR of 28.4%, >1% PD-L1 expression had an ORR of 23.8%, and <1% PD-L1 expression had an ORR of 16.1%. This study defined “PD-L1 positivity” differently from the IMvigor studies. Each trial discussed here uses slightly different definitions which complicates the ability to compare response rates based on PD-L1 expression. Thus, at this time, there are no approvals based on PD-L1 selected patients and no guidelines suggesting that PD-L1 status should guide the use or avoidance of this class of therapy. In the Checkmate 275 study, there was no subgroup analysis based on age, but it did enroll patients reflective of the bladder cancer population. The most common adverse events were fatigue, pruritus, diarrhea, decreased appetite, and hypothyroidism. The most common grade 3 adverse events were fatigue or diarrhea, reported at 16%. There were no grade 4 adverse events, however, 3 patients died from treatment-related pneumonitis, acute renal failure, and cardiovascular failure respectively [56]. As the use of these agents increases, we now understand that these autoimmune toxicities can be managed in many cases, but patients must be aware of warning symptoms and seek prompt care. Lack of familiarity on the part of the provider or patient can result in organ failure with potential life-threatening consequences. While understanding an older patient’s overall health and support system through a geriatric assessment will aid providers in care planning, life-threatening autoimmune complications may still arise. Prompt management may prevent escalation of toxicity.
3.3.3. Durvalumab
Durvalumab was approved as second-line therapy following platinum chemotherapy based on data from 191 patients aged 34–88 years (median 67) with ECOG scores 0–1. Patients were enrolled into the UC cohort of a phase I/II study of the activity of durvalumab in advanced solid tumors [57]. The study required submission of archival or fresh tumor tissue for PD-L1 testing and defined high as ≥25% of tumor cells/tumor infiltrating immune cells staining for PD-1, and low as <25% staining for PD-1. PD-L1 expression was high in 98 of 191 (51.3%), low or negative in 79 of 191 (41.4%), and unknown in 14 of 191 (7.3%). In these patients, total ORR was 17.7%, ORR for patients with high PD-L1 tumors was 27.6%, and ORR for PD-L1 low/negative was 5.1%. There were 7 complete responses and a median overall survival of 18.2 months after a median follow up of 5.78 months (0.4–25.9). Again, this trial enrolled patients with an average age reflective of the population, although the number of patients in the older age range was not described. The most common adverse reactions of all grades, occurring in >5% of patients, were fatigue, decreased appetite, diarrhea, rash, nausea, arthralgia, fever, and pruritus. Grade 3 or 4 adverse reactions occurred in 6.8% of patients. Two treatment-related deaths occurred in urothelial carcinoma patients receiving durvalumab, due to autoimmune hepatitis and pneumonitis respectively [57]. Thus, while less toxic than chemotherapy, the risk of treatment-related adverse events is still present and largely due to the autoimmune toxicities associated with these checkpoint inhibitor therapies. Durvalumab is currently approved post-platinum therapy in the United States but not yet approved by the European Medicines Agency.
3.3.4. Pembrolizumab
Pembrolizumab was approved for second-line therapy in advanced urothelial carcinoma based on the results of the Keynote-045 trial. Patients with progressive or recurrent disease despite platinum therapy were randomized to either receive pembrolizumab or the investigator’s choice of chemotherapy (docetaxel, paclitaxel, or vinflunine). This is the only approved agent which has shown a statistically significant survival benefit when directly compared to chemotherapy. There were 542 patients enrolled, aged 26–88 with a median age of 67 and 65 in the pembrolizumab and chemotherapy arms respectively. ECOG scores were predominantly 0–1, though 6 patients in the study had an ECOG of 2. In the pembrolizumab arm, the ORR was 21.1% versus 11.4% in the chemotherapy arm. The responses were generally long lasting; an estimated 68% of responders to pembrolizumab were still responding after 12 months. Median overall survival in the total pembrolizumab population was 10.3 months compared to 7.4 months in the chemotherapy arm with a hazard ratio (HR) of 0.73 (p=0.002). In a subgroup analysis based on age, the overall survival was not statistically significant, with an HR in patients < 65 years of 0.75 (CI 0.53–1.05) and 0.76 (CI 0.56–1.02) in patients > 65 years. Therefore, this therapy seems to have similar effect regardless of age. In looking at tolerability, there were fewer treatment-related adverse effects in the pembrolizumab group compared to chemotherapy as well as fewer grade 3–5 events. The most common adverse events in the pembrolizumab group were pruritus, fatigue, nausea, diarrhea, and decreased appetite. The only grade 3–4 adverse events that occurred more than twice were colitis and pneumonitis, and there was only one grade 5 adverse event (pneumonitis) [58].
Another trial, Keynote-052, led the FDA to approve pembrolizumab as first-line therapy in patients that were ineligible for cisplatin chemotherapy [59]. Cisplatin ineligibility was defined according to standard criteria [60]. In this single arm, phase II trial, 370 patients with a median age of 74 were treated with pembrolizumab. Notably, 42% of the patients had an ECOG of 2. The ORR was 29% at median follow up of 7.8 months [61]. The subgroup of patients over 65 years had a response rate of 35% (25–46) in comparison to a response rate of 50% (30–70) in the younger patients. Nearly one quarter (24%) of patients had a response to therapy and 23% had stable disease. The study was published with a relatively short follow up interval, with 37% of patients still on therapy and 83% of responses ongoing. The most common grade 3 or 4 treatment-related adverse events were fatigue, elevated alkaline phosphatase, colitis and muscle weakness, occurring at a frequency of 2% or less. Ten percent of all patients enrolled experienced a serious treatment-related adverse event. One treatment-related death occurred as well, with notable immune complications. This study included a number of elderly patients with only 18% of the patients enrolled less than 65 years old and 48% over 75 years. Despite enrolling a more vulnerable population, rates of adverse events were not increased, and this study did not uncover any new toxicity in comparison to other trials of pembrolizumab.
3.3.5. Avelumab
Avelumab is the most recent US approved immunotherapy for advanced UC as a treatment option after platinum chemotherapy based on the UC cohorts in the JAVELIN Solid Tumor Trial. There were 226 patients in the cohort with a median age of 68 and an ECOG of 0–1. Of these patients, the radiographic ORR at 13 weeks was 13.3% (30 patients). Of the 30 patients that had a response, 26 of those patients continued to have a response at 6 months. In patients 65 years or older, 14% (22/153) had a response at 13 weeks. The most common adverse events (occurring in >15% of patients) were fatigue, nausea, musculoskeletal pain, urinary tract infection, and decreased appetite. The most common grade 3–4 adverse reactions were fatigue, hypertension, urinary tract infection, anemia, hyponatremia, and musculoskeletal pain, with 59% of patients experiencing a grade 3 or 4 adverse event. Of note, 14 patients receiving avelumab died from pneumonitis, sepsis, stroke, gastrointestinal events, or respiratory failure. Many of these deaths were likely attributable to disease progression rather than treatment toxicity. Additionally, 58% of patients 65 years of age or older experienced grade 3–4 adverse events, which was comparable to younger patients [62]. This is another agent with a prolonged duration of effect for those patients that initially respond. In a disease state where the mOS is 8 months when initiating second line therapy, a continued response at 6 months is noteworthy.
3.4 Immunosenescence
One of the concerns with the approval of PD-L1 inhibitors is their efficacy and safety in the elderly population. There are concerns that these medications will not be as efficacious in this population due to the phenomena of immunosenescence, or the functional decline of the immune system as people age. Studies have shown that multiple aspects of the immune system change with age. These changes include and are not limited to a decrease in the number of dendritic cells, a decrease in dendritic cell phagocytic and migratory function, impaired naïve CD4+ T cell function, a decrease in CD8+ naïve T cells, increased PD-1 expression, and several other changes [63]. Given this known decline in the immune system, there is concern that PD-L1 inhibitors, which utilize the patient’s own immune system to kill cancer cells, will not be as effective in the elderly population. There are no specific data in these clinical populations, and thus far response rates seem similar. This will be an area of interest moving forward.
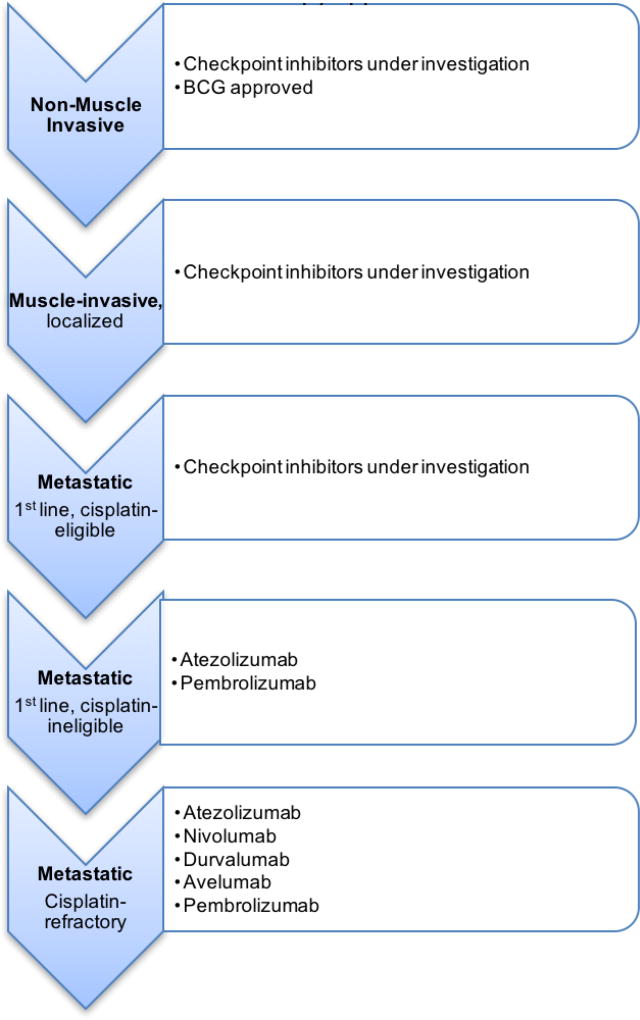
In terms of efficacy of checkpoint inhibitors in older urothelial carcinoma patients, only the studies of atezolizumab, pembrolizumab, and avelumab included a subgroup analysis in patients > 65 years. In each of these studies, it was determined that PD-L1 efficacy population rates were comparable in younger and older patients [54, 58]. Other studies of immune checkpoint point inhibitors in the elderly population, though in different cancers, have similar conclusions. In a meta-analysis of the use of PD-L1 inhibitors in older patients with melanoma, non-small cell lung cancer, or renal cell carcinoma, it was determined that there was a statistically significant survival benefit in patients aged 65–75 compared to patients < 65 years, but a non-statistically significant benefit in patients older than 75 years [64]. Additionally, a review of PD-L1 inhibitors in a variety of different malignancies concluded that efficacy of these medications in the elderly populations is likely comparable to the total population [65]. Small sample sizes currently limit data analysis and the ability to draw more broad conclusions. There are ongoing studies of immune checkpoint inhibitors in nearly every stage of bladder cancer therapy (non-invasive, muscle invasive perioperative, and advanced disease) (Figure 2). These should provide additional data on the older patients enrolled and their subsequent clinical course. Ultimately, more data are needed to better determine if there is a difference in the efficacy of these medications based on age, but at this time, it appears similar.
Figure 2.
Urothelial carcinoma disease states and checkpoint immunotherapy approvals
3.5 Cisplatin-ineligible patients
As noted previously, cisplatin is a highly toxic agent which is particularly difficult to administer to older patients. Studies of modified chemotherapy have produced results clearly inferior to cisplatin. Of note, the immune checkpoint inhibitor class of therapy has allowed for enrollment of patients not eligible for cisplatin therapy, with excellent results. The Keynote-052 and IMvigor 210 study specifically limited enrollment to patients ineligible for cisplatin. The natural process of aging is associated with reduced organ function. As such, renal dysfunction is a natural part of the aging process, the result of a reduction in creatinine clearance associated with age. Similarly, loss of hearing is also a known condition of aging, and may preclude the safe administration of cisplatin. Given these considerations, patients who are otherwise functional and in reasonable health, may not be eligible to receive highly effective cisplatin-based therapy. At the same time, patients with other areas of frailty or normal organ function but reduced functional status, may also not tolerate cytotoxic chemotherapy. The vulnerable population of patients enrolled in these cisplatin-ineligible studies is reflective of the population of older patients with urothelial carcinoma. The immune checkpoint inhibitor class appears to be an effective treatment option for a patient population with previously limited options.
3.6 Toxicity in an older population
There are fewer data on the adverse events of PD-L1 inhibitors specific to the elderly population in urothelial carcinoma patients. The JAVELIN trial was the only study that broke down the AE rate based on age, and reported that the frequency of grade 3–4 adverse reactions (58%) for older patients taking avelumab were similar to that of the total population [66]. Based on Keynote-045, immune checkpoint inhibitors appear less toxic than typical chemotherapy, which is encouraging [58]. Consequently, more patients may consider treatment as the potential risks and benefits become acceptable with immunotherapy. Some special considerations to the adverse event profile, however, should be noted in the elderly population. One of the most common side effects of all the PD-L1 inhibitors was diarrhea. The elderly are at significantly higher risk of experiencing symptoms of dehydration from diarrhea, leading to higher risk of falls and syncope than the general population. Also, the risk for infection was not insignificant, especially in atezolizumab and avelumab. Cases of urosepsis in the elderly population could be significantly more devastating in this population compared to younger patients. Additionally, a case of immune-mediated pneumonitis/colitis has the potential to be more debilitating for the elderly in comparison to younger patients, as elderly patients tend to decompensate rapidly while in the hospital and require more time for healing. We know that even “non-serious” grade 2 adverse events may be intolerable to an older population at higher risk of disequilibrium [67]. Lastly, the large majority of patients across all of these studies had ECOG score of 0–1. The elderly population is more likely to be less functional than the general population. More data on the efficacy and safety of immunotherapy in patients with an ECOG of 2 would help determine if immunotherapy is a valid option for patients who have worse functional status at baseline.
4 Conclusion
The care of patients with bladder cancer is rapidly changing as the field incorporates immune system checkpoint inhibitors and looks at additional genomic targets for therapy. The use of platinum based chemotherapy still holds a place. This is an effective treatment modality that can offer fairly quick symptom control in responsive patients. However, the short and long-term toxicity are oftentimes a barrier to safe administration. This is of special consideration in older patients that may have compromised organ function, physical function, or a lower personal threshold of acceptable treatment-related toxicity. Thus, the emerging immune therapy agents are likely to expand the population of patients receiving treatment for this common cancer. In summary, the FDA registration trials for many of these agents enrolled a generally older population, enrolled patients that would have previously been excluded based on cisplatin-ineligibility, and subgroup analysis of older patients revealed similar toxicity and efficacy profiles. While the care of older patients with cancer still requires adequate assessment and intervention of areas of vulnerability, immunotherapy should be considered as an active treatment for these patients.
Key points.
Bladder cancer is a condition of older persons and thus requires a geriatric-oncology specific approach to treatment planning
Treatment of advanced disease has generally relied on chemotherapy, but the approval of multiple new immunotherapies has greatly altered our treatment options and toxicities to consider.
Immune checkpoint inhibitors have demonstrated considerable activity and good tolerability in older patients.
Acknowledgments
Funding
No external funding was used in the preparation of this manuscript.
Footnotes
Compliance with Ethical Standards
Conflict of interest
Gray Jodon, Stacy Fischer, and Elizabeth Kessler declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
References
- 1.Gray PJ, Fedewa SA, Shipley WU, Efstathiou JA, Lin CC, Zietman AL, et al. Use of Potentially Curative Therapies for Muscle-invasive Bladder Cancer in the United States: Results from the National Cancer Data Base. Eur Urol. 2013 May;63(5):823–9. doi: 10.1016/j.eururo.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 2. [cited 2017]; https://seer.cancer.gov/statfacts/html/urinb.html. Available from.
- 3.Burger M, Grossman HB, Droller M, Schmidbauer J, Hermann G, Dragoescu O, et al. Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: a meta-analysis of detection and recurrence based on raw data. Eur Urol. 2013 Nov;64(5):846–54. doi: 10.1016/j.eururo.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 4.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. Jama. 2011 Aug 17;306(7):737–45. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lara P, Brunson A, White Rd, Dall’Era MA, Evans CP, Yap SA, et al. Survival of patients (pts) with metastatic bladder cancer (MBC) in the past quarter century: Sobering results from the California Cancer Registry (CCR) Journal of Clinical Oncology. 2016;34(15_suppl) e16033-e. [Google Scholar]
- 6.Goossens-Laan CA, Leliveld AM, Verhoeven RH, Kil PJ, de Bock GH, Hulshof MC, et al. Effects of age and comorbidity on treatment and survival of patients with muscle-invasive bladder cancer. Int J Cancer. 2014 Aug 15;135(4):905–12. doi: 10.1002/ijc.28716. [DOI] [PubMed] [Google Scholar]
- 7.Singh H, Beaver JA, Kim G, Pazdur R. Enrollment of older adults on oncology trials: An FDA perspective. J Geriatr Oncol. 2017 May;8(3):149–50. doi: 10.1016/j.jgo.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 8.VanderWalde N, Jagsi R, Dotan E, Baumgartner J, Browner IS, Burhenn P, et al. NCCN Guidelines Insights: Older Adult Oncology, Version 2.2016. J Natl Compr Canc Netw. 2016 Nov;14(11):1357–70. doi: 10.6004/jnccn.2016.0146. [DOI] [PubMed] [Google Scholar]
- 9.Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Aug 20;32(24):2595–603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ommundsen N, Wyller TB, Nesbakken A, Jordhoy MS, Bakka A, Skovlund E, et al. Frailty is an independent predictor of survival in older patients with colorectal cancer. Oncologist. 2014 Dec;19(12):1268–75. doi: 10.1634/theoncologist.2014-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soubeyran P, Fonck M, Blanc-Bisson C, Blanc JF, Ceccaldi J, Mertens C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 May 20;30(15):1829–34. doi: 10.1200/JCO.2011.35.7442. [DOI] [PubMed] [Google Scholar]
- 12.Decoster L, Van Puyvelde K, Mohile S, Wedding U, Basso U, Colloca G, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendationsdagger. Ann Oncol. 2015 Feb;26(2):288–300. doi: 10.1093/annonc/mdu210. [DOI] [PubMed] [Google Scholar]
- 13.Kenis C, Decoster L, Van Puyvelde K, De Greve J, Conings G, Milisen K, et al. Performance of two geriatric screening tools in older patients with cancer. J Clin Oncol. 2014 Jan 01;32(1):19–26. doi: 10.1200/JCO.2013.51.1345. [DOI] [PubMed] [Google Scholar]
- 14.Soubeyran P, Bellera C, Goyard J, Heitz D, Cure H, Rousselot H, et al. Screening for vulnerability in older cancer patients: the ONCODAGE Prospective Multicenter Cohort Study. PLoS One. 2014;9(12):e115060. doi: 10.1371/journal.pone.0115060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Tapia C, Canoui-Poitrine F, Bastuji-Garin S, Soubeyran P, Mathoulin-Pelissier S, Tournigand C, et al. Optimizing the G8 Screening Tool for Older Patients With Cancer: Diagnostic Performance and Validation of a Six-Item Version. Oncologist. 2016 Feb;21(2):188–95. doi: 10.1634/theoncologist.2015-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Repetto L, Fratino L, Audisio RA, Venturino A, Gianni W, Vercelli M, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002 Jan 15;20(2):494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 17.Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014 Nov;121(5):937–47. doi: 10.1097/ALN.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. 2013 Apr;27(4):1072–82. doi: 10.1007/s00464-012-2560-5. [DOI] [PubMed] [Google Scholar]
- 19.Kalsi T, Babic-Illman G, Ross PJ, Maisey NR, Hughes S, Fields P, et al. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. Br J Cancer. 2015 Apr 28;112(9):1435–44. doi: 10.1038/bjc.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prout GR, Jr, Wesley MN, Yancik R, Ries LA, Havlik RJ, Edwards BK. Age and comorbidity impact surgical therapy in older bladder carcinoma patients: a population-based study. Cancer. 2005 Oct 15;104(8):1638–47. doi: 10.1002/cncr.21354. [DOI] [PubMed] [Google Scholar]
- 21.Noon AP, Albertsen PC, Thomas F, Rosario DJ, Catto JW. Competing mortality in patients diagnosed with bladder cancer: evidence of undertreatment in the elderly and female patients. Br J Cancer. 2013 Apr 16;108(7):1534–40. doi: 10.1038/bjc.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark PE, Stein JP, Groshen SG, Cai J, Miranda G, Lieskovsky G, et al. Radical cystectomy in the elderly: comparison of clincal outcomes between younger and older patients. Cancer. 2005 Jul 01;104(1):36–43. doi: 10.1002/cncr.21126. [DOI] [PubMed] [Google Scholar]
- 23.Froehner M, Brausi MA, Herr HW, Muto G, Studer UE. Complications following radical cystectomy for bladder cancer in the elderly. Eur Urol. 2009 Sep;56(3):443–54. doi: 10.1016/j.eururo.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Berger I, Martini T, Wehrberger C, Comploj E, Ponholzer A, Wolfgang M, et al. Perioperative complications and 90-day mortality of radical cystectomy in the elderly (75+): a retrospective, multicentre study. Urologia internationalis. 2014;93(3):296–302. doi: 10.1159/000357127. [DOI] [PubMed] [Google Scholar]
- 25.Clayman RH, Shipley WU, Galland-Girodet S, Niemierko A, Gray PJ, Paly J, et al. Outcomes of Selective Bladder Preservation in the Elderly Treated With Conservative Surgery and Chemoradiation. Int J Radiat Oncol. 2013 Oct 1;87(2):S83-S. [Google Scholar]
- 26.Mak RH, Hunt D, Shipley WU, Efstathiou JA, Tester WJ, Hagan MP, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Dec 01;32(34):3801–9. doi: 10.1200/JCO.2014.57.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McPherson VA, Rodrigues G, Bauman G, Winquist E, Chin J, Izawa J, et al. Chemoradiotherapy in octogenarians as primary treatment for muscle-invasive bladder cancer. Canadian Urological Association journal = Journal de l’Association des urologues du Canada. 2017 Jan-Feb;11(1–2):24–30. doi: 10.5489/cuaj.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chau C, Wheater M, Geldart T, Crabb SJ. Clinical outcomes following neoadjuvant cisplatin-based chemotherapy for bladder cancer in elderly compared with younger patients. Eur J Cancer Care (Engl) 2015 Mar;24(2):155–62. doi: 10.1111/ecc.12282. [DOI] [PubMed] [Google Scholar]
- 29.Galsky MD, Moshier E, Krege S, Lin CC, Hahn N, Ecke T, et al. Posttreatment prognostic nomogram for patients with metastatic urothelial cancer completing first-line cisplatin-based chemotherapy. Urol Oncol. 2014 Jan;32(1):48 e1–8. doi: 10.1016/j.urolonc.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Guancial EA, Roussel B, Bergsma DP, Bylund KC, Sahasrabudhe D, Messing E, et al. Bladder cancer in the elderly patient: challenges and solutions. Clinical interventions in aging. 2015;10:939–49. doi: 10.2147/CIA.S74322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Efstathiou JA, Spiegel DY, Shipley WU, Heney NM, Kaufman DS, Niemierko A, et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience. Eur Urol. 2012 Apr;61(4):705–11. doi: 10.1016/j.eururo.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 32.James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, et al. Radiotherapy with or without Chemotherapy in Muscle-Invasive Bladder Cancer. New Engl J Med. 2012 Apr 19;366(16):1477–88. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 33.Efstathiou JA, Spiegel DY, Shipley WU, Heney NM, Kaufman DS, Niemierko A, et al. Long-Term Outcomes of Selective Bladder Preservation by Combined-Modality Therapy for Invasive Bladder Cancer: The MGH Experience. Eur Urol. 2012 Apr;61(4):705–11. doi: 10.1016/j.eururo.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003 Aug 28;349(9):859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 35.Cowan NG, Chen Y, Downs TM, Bochner BH, Apolo AB, Porter MP, et al. Neoadjuvant chemotherapy use in bladder cancer: a survey of current practice and opinions. Adv Urol. 2014;2014:746298. doi: 10.1155/2014/746298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SEER Stat Fact Sheets: Bladder Cancer. seercancergov/statfacts/html/urinbhtml 2016 [cited; Available from.
- 37.Saxman SB, Propert KJ, Einhorn LH, Crawford ED, Tannock I, Raghavan D, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997 Jul;15(7):2564–9. doi: 10.1200/JCO.1997.15.7.2564. [DOI] [PubMed] [Google Scholar]
- 38.Logothetis CJ, Dexeus FH, Sella A, Amato RJ, Kilbourn RG, Finn L, et al. Escalated therapy for refractory urothelial tumors: methotrexate-vinblastine-doxorubicin-cisplatin plus unglycosylated recombinant human granulocyte-macrophage colony-stimulating factor. J Natl Cancer Inst. 1990 Apr 18;82(8):667–72. doi: 10.1093/jnci/82.8.667. [DOI] [PubMed] [Google Scholar]
- 39.Loehrer PJ, Sr, Einhorn LH, Elson PJ, Crawford ED, Kuebler P, Tannock I, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1992 Jul;10(7):1066–73. doi: 10.1200/JCO.1992.10.7.1066. [DOI] [PubMed] [Google Scholar]
- 40.Sternberg CN, de Mulder PH, Schornagel JH, Theodore C, Fossa SD, van Oosterom AT, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001 May 15;19(10):2638–46. doi: 10.1200/JCO.2001.19.10.2638. [DOI] [PubMed] [Google Scholar]
- 41.Sternberg CN, de Mulder P, Schornagel JH, Theodore C, Fossa SD, van Oosterom AT, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006 Jan;42(1):50–4. doi: 10.1016/j.ejca.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 42.von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000 Sep;18(17):3068–77. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 43.Dash A, Pettus JAt, Herr HW, Bochner BH, Dalbagni G, Donat SM, et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: a retrospective experience. Cancer. 2008 Nov 1;113(9):2471–7. doi: 10.1002/cncr.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zargar-Shoshtari K, Zargar H, Lotan Y, Shah JB, van Rhijn BW, Daneshmand S, et al. A Multi-Institutional Analysis of Outcomes of Patients with Clinically Node Positive Urothelial Bladder Cancer Treated with Induction Chemotherapy and Radical Cystectomy. J Urol. 2016 Jan;195(1):53–9. doi: 10.1016/j.juro.2015.07.085. [DOI] [PubMed] [Google Scholar]
- 45.De Santis M, Bellmunt J, Mead G, Kerst JM, Leahy M, Maroto P, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012 Jan 10;30(2):191–9. doi: 10.1200/JCO.2011.37.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonpavde G, Watson D, Tourtellott M, Cowey CL, Hellerstedt B, Hutson TE, et al. Administration of cisplatin-based chemotherapy for advanced urothelial carcinoma in the community. Clin Genitourin Cancer. 2012 Mar;10(1):1–5. doi: 10.1016/j.clgc.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Bellmunt J, von der Maase H, Mead GM, Skoneczna I, De Santis M, Daugaard G, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol. 2012 Apr 1;30(10):1107–13. doi: 10.1200/JCO.2011.38.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choueiri TK, Ross RW, Jacobus S, Vaishampayan U, Yu EY, Quinn DI, et al. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol. 2012 Feb 10;30(5):507–12. doi: 10.1200/JCO.2011.37.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sweeney CJ, Roth BJ, Kabbinavar FF, Vaughn DJ, Arning M, Curiel RE, et al. Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J Clin Oncol. 2006 Jul 20;24(21):3451–7. doi: 10.1200/JCO.2005.03.6699. [DOI] [PubMed] [Google Scholar]
- 50.Ward PR, Wong MD, Moore R, Naeim A. Fall-related injuries in elderly cancer patients treated with neurotoxic chemotherapy: a retrospective cohort study. J Geriatr Oncol. 2014;5(1):57–64. doi: 10.1016/j.jgo.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med. 2016 Nov 3;375(18):1767–78. doi: 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proceedings of the National Academy of Sciences of the United States of America. 2002 Sep 17;99(19):12293–7. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (London, England) 2016 May 07;387(10031):1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet (London, England) 2017 Jan 07;389(10064):67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Powles T, Duran I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet (London, England) 2018 Feb 24;391(10122):748–57. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 56.Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. The Lancet Oncology. 2017 Mar;18(3):312–22. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 57.Powles T, O’Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA oncology. 2017 Sep 14;3(9):e172411. doi: 10.1001/jamaoncol.2017.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017 Mar 16;376(11):1015–26. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. The Lancet Oncology. 2017 Nov;18(11):1483–92. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 60.Galsky MD, Hahn NM, Rosenberg J, Sonpavde G, Hutson T, Oh WK, et al. A consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. The Lancet Oncology. 2011 Mar;12(3):211–4. doi: 10.1016/S1470-2045(10)70275-8. [DOI] [PubMed] [Google Scholar]
- 61.Keytruda [package insert] Merck & Co. I, Whitehouse Station; NJ: 2017. [cited 11/1/17]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125514s017s018lbl.pdf. [Google Scholar]
- 62.Palou J, Wood D, Bochner BH, van der Poel H, Al-Ahmadie HA, Yossepowitch O, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Urothelial carcinoma of the prostate. Eur Urol. 2013 Jan;63(1):81–7. doi: 10.1016/j.eururo.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 63.Elias R, Karantanos T, Sira E, Hartshorn KL. Immunotherapy comes of age: Immune aging & checkpoint inhibitors. J Geriatr Oncol. 2017 May;8(3):229–35. doi: 10.1016/j.jgo.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 64.Nishijima TF, Muss HB, Shachar SS, Moschos SJ. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: A systematic review and meta-analysis. Cancer treatment reviews. 2016 Apr;45:30–7. doi: 10.1016/j.ctrv.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 65.Marrone KA, Forde PM. Cancer Immunotherapy in Older Patients. Cancer journal (Sudbury, Mass) 2017 Jul-Aug;23(4):219–22. doi: 10.1097/PPO.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 66.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002 Oct;57(10):B359–65. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- 67.Kalsi T, Babic-Illman G, Fields P, Hughes S, Maisey N, Ross P, et al. The impact of low-grade toxicity in older people with cancer undergoing chemotherapy. Br J Cancer. 2014 Dec 9;111(12):2224–8. doi: 10.1038/bjc.2014.496. [DOI] [PMC free article] [PubMed] [Google Scholar]