Abstract
Prior functional magnetic resonance imaging (fMRI) studies have investigated the neural mechanisms underlying cognitive control in patients with psychosis with findings of both hypo- and hyperfrontality. One factor that may contribute to inconsistent findings is the use of complex and polyfactorial tasks to investigate frontal lobe functioning. In the current study we employed a simple response conflict task during fMRI to examine differences in brain activation between patients experiencing their first-episode of psychosis (n=33) and age- and sex-matched healthy volunteers (n=33). We further investigated whether baseline brain activation among patients predicted changes in symptom severity and treatment response following 12 weeks of controlled antipsychotic treatment. During the task subjects were instructed to press a response button on the same side or opposite side of a circle that appeared on either side of a central fixation point. Imaging data revealed that for the contrast of opposite-side vs. same-side, patients showed significantly greater activation compared with healthy volunteers in the anterior cingulate cortex and intraparietal sulcus. Among patients, greater baseline anterior cingulate cortex, temporal-parietal junction, and superior temporal cortex activation predicted greater symptom reduction and therapeutic response following treatment. All findings remained significant after covarying for task performance. Intact performance on this relatively parsimonious task was associated with frontal hyperactivity suggesting the need for patients to utilize greater neural resources to achieve task performance comparable to healthy individuals. Moreover, frontal hyperactivity observed using a simple fMRI task may provide a biomarker for predicting treatment response in first-episode psychosis.
Keywords: psychosis, schizophrenia, cognitive control, executive function, functional magnetic resonance imaging, treatment response
Introduction
Several prominent theories implicate abnormalities in the lateral prefrontal cortex and anterior cingulate cortex (ACC) as underlying executive dysfunction in psychosis (Jukuri et al. 2015; Minzenberg et al. 2009; Morey et al. 2005; Pedersen et al. 2012; Wilmsmeier et al. 2010). According to one longstanding model of intact executive functioning (Botvinick et al. 2004; Kerns et al. 2004; MacDonald et al. 2000), working memory and top-down regulatory capacities of the dorsolateral prefrontal cortex (DLPFC) modulate the conflict- and performance-monitoring features of ACC that relay back to DLPFC to permit adaptive changes in cognitive control. This reciprocal process allows for flexible thinking and successful decision-making by permitting individuals to attend to pertinent information in their environment and to choose the most fitting behavioral response among competing responses.
Early models of frontal lobe function and cognitive symptoms in schizophrenia suggested that deficits in cognitive control were the result of reduced recruitment of frontal lobe resources (Weinberger and Berman 1996). However, neuroimaging evidence for this model has been inconsistent. Although some functional magnetic resonance imaging (fMRI) studies report hypofrontality in patients during executive functioning tasks, particularly in DLPFC and ACC (Fryer et al. 2018; Laurens et al. 2005; MacDonald and Carter 2003; MacDonald et al. 2005; Rubia et al. 2001; Schneider et al. 2007; Smucny et al. 2018; Voegler et al. 2016), others report hyperfrontality, especially in ACC and ventrolateral prefrontal cortex (Schneider et al. 2007; Tan et al. 2006; Weiss et al. 2003; Wilmsmeier et al. 2010). Meta-analyses of neural dysregulation during cognitive control tasks in schizophrenia and related disorders have revealed both hyper- and hypo-activation in distinct subregions of ACC. Specifically, an area of anterior midcingulate near presupplementary motor cortex showed greater activation and anterior dorsal ACC exhibited decreased activation when compared with healthy volunteers (McTeague et al. 2017). Similarly, a dorsal subregion of ACC adjacent to the supplementary motor area showed greater activation in schizophrenia compared with healthy volunteers, but a more ventral subregion showed decreased activation in schizophrenia compared with healthy volunteers (Minzenberg et al. 2009).
Prior work suggests that inconsistent findings of hyper- and hypo-activation in frontal lobe regions may be due to differences in fMRI task design, including cognitive demands, task complexity, whether the task is event-related or block-design, and whether patient and healthy volunteer groups are matched for performance (Manoach 2003). For example, less recruitment of dorsal ACC in patients compared with healthy volunteers was observed during a Stroop task (Kerns et al. 2005), continuous performance tasks (Smucny et al. 2018; C. S. Carter et al. 2001), and detection of rare target events (J. D. Carter et al. 2010), but increased recruitment of ACC was observed during sustained attention (J. D. Carter et al. 2010) and when successfully shifting cognitive set during a modified Wisconsin Card Sorting Test (WCST) (Wilmsmeier et al. 2010). Moreover, patients’ current symptom state may influence activation; patients experiencing active psychotic symptoms exhibited reduced activation in ACC compared with healthy volunteers during a Stroop task (Weiss et al. 2007), but patients in a remitted state exhibited greater recruitment of ACC compared with healthy volunteers during the same task (Weiss et al. 2003).
It has also been suggested that regions of hypoactivation in ACC are related to reduced gray matter volume in those areas (McTeague et al. 2017), while regions of hyperactivation represent inefficient neural mechanisms or compensatory neural activation to maintain intact performance (Callicott et al. 2003; Manoach 2003; McTeague et al. 2017; Potkin et al. 2009). Alternatively, hyperactivation may reflect the possibility that individuals with psychosis utilize alternate strategies to complete cognitive tasks that engage frontal regions (Gur et al. 2007; M. A. Kim et al. 2010; Minzenberg et al. 2009; Murray et al. 2010; Pedersen et al. 2012). Indeed, behavioral performance during both executive functioning (Polli et al. 2008) and working memory (Callicott et al. 2003; Manoach 2003) tasks has been strongly linked to neural activation in ACC and lateral PFC during these tasks. Moreover, recent observations suggest that as task difficulty is increased during inhibitory tasks, patients with higher error rates and increased symptom presentation do not exhibit typical patterns of increased activation in cognitive control circuitry necessary to meet task demands (Baran et al. 2016). Hence, dysfunction within the brain systems contributing to executive functioning deficits in psychosis and the alternate neural mechanisms leading to successful task performance remain to be fully elucidated.
A critical challenge in the interpretation of tasks assessing executive functioning concerns their polyfactorial nature. Tasks such as the WCST are difficult to interpret, particularly in parsing out the different executive requirements of the task such as working memory, attention, and set-shifting. Considerable ambiguity thus remains in understanding executive functioning deficits in psychosis due to complexities associated with cognitive task design. Therefore, the use of simpler tasks may be better suited for the identification of individual constructs underlying complex cognitive processes and neural mechanisms underlying frontal lobe functioning in psychosis. However, simplified cognitive control tasks that have been utilized in prior neuroimaging studies, such as the Stroop (Kerns et al. 2005), continuous performance task (C. S. Carter et al. 2001; MacDonald et al. 2005), target detection tasks (J. D. Carter et al. 2010), or set-shifting tasks (Dove et al. 2000; Shafritz et al. 2005), are typically event-related designs requiring long scan times that are not ideal for patient populations. Rather, the most suitable task designs for patient groups – and those that may be most easily adaptable to clinical settings for assistance with individual patient care – are likely to be simplified block-design tasks. Such tasks can also result in more power compared to event-related designs depending on the exact nature of the task comparisons used (e.g., how close are the cognitive requirements of the control condition to the experimental condition?).
An additional challenge, particularly for translational psychiatry, is the identification of patients who may not respond to antipsychotic treatment given that treatment resistance is associated with increased risk for suicide and functional disability. Because patients with psychosis demonstrate deficits in executive functioning, such tasks may serve as promising neuroimaging biomarkers of treatment response. Moreover, an important consideration in the design of translational neuroimaging tasks concerns the use of more parsimonious tasks to enhance replicability of individual differences. Magnetic resonance imaging (MRI) may be particularly suited for integration with clinical trials and the identification of treatment response biomarkers. Although some MRI studies reveal structural or connectivity changes associated with functional outcome and treatment response (Dazzan et al. 2015; Sarpal et al. 2016), few studies have specifically linked task-based functional differences with antipsychotic treatment success or failure. One such study revealed that activation within dorsal frontal-parietal working memory circuitry may predict negative symptom improvement during antipsychotic pharmacotherapy (Nejad et al. 2013). Another showed that DLPFC activation during a practiced and non-practiced version of a working memory task can predict poor treatment response (van Veelen et al. 2011). Most recently, activation during reinforcement learning has been shown to distinguish treatment responders from non-responders (Vanes et al. 2018). Despite these important advances, a simple and brief (i.e., more parsimonious) cognitive control task, is still needed that can assist with predicting subsequent treatment response.
In this study we employed a variant of the Simon task (Hommel 2011; Proctor 2011) to clarify the neurobiological mechanisms underlying a critical cognitive component (i.e., response conflict) believed to play a role in executive functioning deficits among patients with first-episode psychosis. Subjects pressed a response button corresponding to either the same side or opposite side of a white circle appearing to the left or right of fixation. The task was designed to be a simple and straightforward way to tap the executive functioning network of the brain, specifically the dorsal frontal-parietal attentional network and the ACC, via alternating blocks requiring a standard prepotent response (same side) or the inhibition of the prepotent response (opposite side). Importantly, the use of a two-condition block-design task allowed us to directly compare the neural systems implicated in a simple sensorimotor task (same side) with those involved in a task that has a cognitive load related to the presence of a competing prepotent response (opposite side). The combination of a simplified executive function task and the use of a short block-design fMRI paradigm is a unique feature of the current study. Based upon prior findings and theoretical perspectives (Jamadar et al. 2010; Murray et al. 2010; Pedersen et al. 2012; Wilmsmeier et al. 2010), we hypothesized that during the response conflict condition (in comparison to the sensorimotor control condition), patients would demonstrate greater recruitment of ACC compared with healthy volunteers due to a heightened demand on cognitive resources that monitor ongoing performance.
We further investigated BOLD response during the task at the initiation of antipsychotic treatment in relationship to symptom changes and treatment response following 12 weeks of antipsychotic pharmacotherapy, to determine whether BOLD activation could be used to predict response to pharmacotherapy. We hypothesized that greater ACC activation would be associated with reductions in symptom severity and with treatment response following antipsychotic pharmacotherapy, given prior evidence that greater ACC activation is associated with better functional outcome and that antipsychotic medication acts robustly on ACC functions (Ikuta et al. 2012; Murray et al. 2010; Snitz et al. 2005).
Materials/Subjects and Methods
Subjects
An initial sample of forty patients experiencing a first-episode of psychosis were recruited from admissions to the inpatient service at The Zucker Hillside Hospital in Glen Oaks, NY and were enrolled in an NIMH-funded double blind randomized controlled trial comparing aripiprazole versus risperidone (R01MH060004). Seven patients exceeded motion criteria for the neuroimaging scan (see fMRI Task Description and Analysis) and were excluded from analysis. The final sample consisted of thirty-three patients (see Table 1 for sample demographics).
Table 1.
Sample Demographics
| Characteristic | Patient Group Responders (n=22) |
Patient Group Non-Responders (n=11) |
Healthy Volunteers (n=33) |
|---|---|---|---|
| Age (years) | 21.9 (5.5) | 22.3 (3.8) | 22.1 (4.8) |
| Sex (M/F) | 17/5 | 9/2 | 26/7 |
| Education (years) | 12.6 (2.1) | 12.6 (1.7) | 13.0 (2.3) |
| Laterality Quotient1 | .50 (.60) | .90 (.23) | .68 (.49) |
| Baseline BPRS | 41.55 (7.23) | 42.55 (11.00) | N/A |
All individuals were classified as either right or left-handed based on a modified version of the Edinburgh Inventory. The total number of right and left hand items was scored and the laterality quotient was computed: (Total R − Total L)/(Total R + Total L) yielding a range from +1.00 (totally dextral) to −1.00 (totally nondextral).
All patient (lifetime) diagnoses were based on the SCID for Axis I DSM-IV Disorders supplemented by information from clinicians and, when available, family members. Patients with psychosis met DSM-IV criteria for schizophrenia (n=21), schizophreniform disorder (n=9) or psychosis NOS (n=3). Mean age at first psychotic symptoms was 19.9 years (SD=5.7); data were unavailable for 3 patients.
Thirty-three healthy volunteers were recruited from advertisements posted on websites and by word of mouth to match the demographic distribution of the patient group. Inclusion criteria for healthy volunteers included the denial of any lifetime history of a major mood or psychotic disorder as determined by clinical interview using the SCID-NP (First et al. 2002).
Exclusion criteria for all participants included: (a) MRI contraindications; (b) significant medical illness (c) prior psychosurgery; (d) DSM-IV diagnosis of Tourette’s syndrome, developmental disorders, autism and neurological conditions; (e) DSM-IV mental retardation; (f) stroke and (g) pregnancy. The study was approved by the North Shore-LIJ Institutional Review Board. Written informed consent was obtained from all individuals, and from a parent or legal guardian in the case of minors. Written assent was obtained from all minors.
Clinical Assessments
Patients completed the 18-item Brief Psychiatric Rating Scale – Anchored version (BPRS-A) and we derived a total score by summing all items. Mean BPRS score for patients was 42 (SD=8.51) at Week 0 and 26 (SD=7.98) at Week 12, indicating a significant reduction in symptoms, t(18)=6.82, p<.001. BPRS scores were unavailable for 8 patients at the week 12 timepoint due to subject dropout. In addition, all 33 patients were categorized as either treatment responders or nonresponders using survival analysis as described previously in the larger clinical trial (Sarpal et al. 2016; Robinson et al. 2015); treatment response was defined a priori as two consecutive visits with a Clinical Global Impression improvement score of 1 or 2 (much or very much improved) and a rating of 3 (mild) or less on all of the following items of the BPRS-A: conceptual disorganization, grandiosity, hallucinatory behavior, and unusual thought content.
Antipsychotic Titration Schedule
Research psychiatrists followed a flexible dosing titration schedule as described in Robinson et al 2015 (Robinson et al. 2015). Briefly, the initial daily dose for patients was 5 mg for aripiprazole and 1 mg for risperidone. The dose was initially increased until the patient improved, developed side effects that precluded a dose increase, or reached a maximum daily dose of 30 mg of aripiprazole or 6 mg of risperidone. Subjects were not allowed to receive antidepressants or mood stabilizers. Patients had a mean of 7.8 days (SD=8.2) of antipsychotic treatment prior to the MR imaging exam. Ten patients were antipsychotic drug-naïve at the time of the scan. Of the remaining 23 patients, 10 were being treated with aripiprazole (mean = 5.5 days; SD = 9.7) and 13 with risperidone (mean days = 6.6; SD = 7.9). In addition, lorazepam was prescribed for anxiety or agitation; four individuals (3 individuals subsequently categorized as nonresponders and 1 individual subsequently categorized as a responder) received lorazepam on the day of the scan.
Magnetic Resonance Imaging Procedures
Magnetic resonance imaging exams were conducted using a GE 3T Signa HDx whole body superconducting imaging system equipped with an 8-channel phased array coil and gradients for echo-planar blood oxygen level-dependent (BOLD) imaging, located at the North Shore University Hospital. T1-weighted anatomical localizer imagers were first collected in the sagittal plane using conventional parameters, followed by a high-resolution (.94mm × 1.00mm × .94mm) 3-D spoiled gradient recalled (SPGR) sequence of 216 images collected in the coronal plane with the following image parameters: TR (repetition time)=7.5 ms, TE (echo time)=3 ms, TI (inversion time)=650 ms, flip angle=8°, matrix=256 × 256, FOV (field of view)=240x192 mm, to use for co-registration of functional images. T2*-weighted BOLD images were then acquired using the following parameters: 26 axial-oblique slices parallel to the anterior-posterior commissural (AC-PC) plane, with a voxel size of 3.75mm × 3.75mm × 5mm (TR=2.0 sec, TE=30 ms, flip angle=77°, matrix=64 × 64, FOV=240 mm). BOLD data were collected in one run lasting 8 minutes and 28 seconds. A radiologist reviewed all scans for gross anatomic pathology that would preclude participation in this study.
fMRI Task Description and Analysis
The fMRI task was presented in a block design using E-Prime software v1.1.3 (www.pstnet.com). Stimuli were reverse-projected onto a screen viewed through a mirror located over the participant’s head. For each trial, a white circle appeared to either the right or left of fixation, with a randomized presentation per trial achieved by using the full randomization option in E-Prime within each block. In the “same-side” condition, participants were instructed to press the response button corresponding to the same side as the white circle. In the “opposite-side” condition, participants were instructed to press the response button corresponding to the opposite side of the white circle. Each block contained an instruction screen (2 seconds) with the words “same side” or “opposite side,” followed by 20 trials of the task: white circle (500 ms) then fixation cross (1500 ms) for a total of 42 seconds. Task blocks were separated by 18 second fixation/rest periods. Therefore, time between onset of each task condition was 60 seconds. Blocks alternated between “same” and “opposite,” with 8 blocks in total and 4 blocks per condition. Figure 1 depicts sample trials for the two task conditions.
Figure 1.
Sample Trials from the Cognitive Task. Illustrated are example trials at the beginning of a “same-side” block (top) and “opposite-side” block (bottom). Participants were required to press a response button corresponding to the same side or opposite side of the white circle presented during each task trial.
BOLD data were analyzed using the SPM8 program (www.fil.ion.ucl.ac.uk/spm). Prior to statistical analysis, images were motion corrected, co-registered to the SPGR image, normalized to the standard Montreal Neurological Institute (MNI) template with 2 mm3 voxel size, and Gaussian-filtered (full-width at half-maximum=8.0 mm). Scans were discarded if movement away from the first collected volume exceeded 3 mm of translational movement in any direction, or 3° of rotation.
Statistical parametric maps of BOLD activation were created by grouping images together corresponding to each task and estimating signal parameters (β-coefficients) for same-side and opposite-side task conditions using the delayed boxcar function convolved with the canonical hemodynamic response function described in SPM8. Single-subject task contrast maps were created comparing activation during same-side with activation during opposite-side trials. Group contrast maps for the comparison of same-side and opposite-side conditions were then created by averaging the signal change for each contrast at each voxel and determining the probability that the percent signal change across subjects was different than zero using a t-test at each voxel (second-level random-effects analysis). A 2×2 (diagnostic group X trial type) ANOVA compared activations between patients and healthy volunteers, and examined a possible interaction between diagnostic group and trial type. Results of this ANOVA were confirmed by independent-samples t-tests.
To determine whether task performance was associated with BOLD activation in task contrasts, reaction time data for correct trials for each participant were included as regressors to produce correlation maps in the second-level random-effects analyses. Similar correlation maps were produced for task accuracy for the opposite-side condition in the patient group only. Due to the restricted range of scores for accuracy data in the opposite-side condition for healthy controls and for the same-side condition in both groups, we could not conduct a similar analyses for those aspects of the task. In cases where performance data correlated with BOLD activation appearing in the primary task contrasts, analyses of covariance were performed to ensure that activations remained after covarying BOLD data with performance data.
To determine whether BOLD activation in the task contrasts predicted symptom severity following antipsychotic treatment, change in BPRS score for each patient from baseline to 12-week follow-up was included as a regressor to produce correlation maps in the second-level random-effects analyses contrasting opposite-side and same-side activation within the patient group. A complementary approach was used to buttress the results of this forward-predictive correlational analysis; to ensure that activation differences could be reliably observed between treatment responders and nonresponders, t-tests directly compared activation between patients classified as responders (n=22) and nonresponders (n=11) using the criteria described above (see Clinical Assessments).
All neuroimaging maps were generated using a voxelwise height threshold of p<.001, corrected for multiple comparisons by calculating the appropriate cluster size for each analysis to attain p<.05 using the spatial autocorrelation function (-acf option) in 3dClustSim of AFNI version 16 (afni.nimh.nih.gov). This procedure correcting for false discovery rates (and the resulting cluster size for an uncorrected voxelwise height threshold of p<.001) has been recently revalidated to correct whole brain alpha to p<.05 (Cox et al. 2017). It is consistent with (or more conservative than) prior simulation techniques (McAvoy et al. 2001) and with many neuroimaging studies with patient groups using direct comparisons of task conditions and two-sample second-level random-effects analyses (Grutzmann et al. 2016; Hummer et al. 2013; Vercammen et al. 2012; Wessa et al. 2007; Wiggins et al. 2016; Wilmsmeier et al. 2010). We considered using family-wise correction, which is possible with event-related designs that compare ongoing background activation to a single event. Such approaches for limiting Type I error are not recommended with the smaller effect sizes resulting from between-groups comparisons of block-design tasks, however, in which active cognitive load conditions are contrasted with tightly controlled sensorimotor comparison conditions (Lieberman and Cunningham 2009).
Results
The groups did not differ significantly in distributions of age, sex, handedness or education (Table 1). Moreover, at the time of baseline scan, there were no significant differences in distributions of age, sex, years of education and total BPRS score between individuals subsequently classified as responders vs. nonresponders. In addition, there was no significant difference in the number of days patients were initially treated with either aripiprazole or risperidone at the time of the scan.
fMRI Task Performance
Accuracy (percent correct) and reaction time (RT) data for opposite side and same side responses are shown in Table 2. RT data include correct trials only. Performance data were not recorded for four individuals in the patient group and for four individuals in the healthy volunteer group due to equipment failure.
Table 2.
Task Performance Data
| Task Condition | Patient Group M (SD) |
Healthy Volunteers M (SD) |
Statistical Test |
|---|---|---|---|
| Accuracy (% Correct) | |||
| Same Side | 94.10 (9.85) | 96.79 (5.14) | n.s. |
| Opposite Side | 86.03 (26.08) | 97.45 (1.88) | p<.05 |
| Reaction Time (ms) | |||
| Same Side | 455.29 (171.80) | 419.59 (109.96) | n.s. |
| Opposite Side | 507.07 (189.92) | 461.83 (123.99) | n.s. |
A 2×2 (task condition × diagnostic group) repeated-measures ANOVA compared the accuracy for same-side and opposite-side conditions for the two participant groups. Results indicated a nonsignificant main effect of task condition, F(1,56)=3.059, p=.086, a significant main effect of diagnostic group, F(1,56)=5.275, p=.025, and a significant task × group interaction, F(1,56)=4.236, p=.044. Post-hoc analyses were computed using independent groups and paired-samples t-tests. Accuracy for opposite side responses was lower in the first-episode group compared with healthy volunteers, t(56)=−2.350, p=.022, but accuracy for same-side responses did not significantly differ between groups, t(56)= −1.304, p=.198. No significant differences were observed when directly comparing same-side with opposite-side accuracy in either group.
A second 2x2 (task condition × diagnostic group) repeated-measures ANOVA compared RT for same-side and opposite-side conditions for the two groups. Results indicated a significant main effect of task condition, F(1,56)=58.141, p<.001, but neither a main effect of diagnostic group, F(1,56)=1.035, p=.313, nor a task × group interaction, F(1,56)=.599, p=.442. Because there were no group differences in RT, data were collapsed across groups, and a paired-samples t-test indicated significantly longer RTs for opposite-side responses compared with same-side responses, t(57)=7.64, p<.001.
Imaging Data
For healthy volunteers, the primary task contrast maps of opposite-side compared with same-side blocks revealed greater activation for opposite-side in superior and middle frontal cortex (Brodmann’s Area 6), intraparietal sulcus (IPS; BA 7,40), posterior superior parietal cortex (BA 7), and temporal-parietal junction (BA 39) (Table 3, Figure 2A). The contrast of same side – opposite side revealed greater activation for same-side in middle frontal cortex (BA 8), posterior cingulate cortex (BA 31), and inferior parietal cortex (BA 19,39) (Table 3, Figure 2B).
Table 3.
Regions of Activation from Task Contrasts, voxelwise threshold p<.001, whole- brain corrected p<.05
| Location of Activation Cluster | Brodmann’s Area | Tmax | Cluster Size (k) |
MNI Coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Opposite-Same Healthy Volunteers | ||||||
| Superior/Middle Frontal, left | 6 | 6.47 | 530 | −20 | 0 | 60 |
| Superior/Middle Frontal, right | 6 | 6.27 | 647 | 24 | −2 | 52 |
| Intraparietal Sulcus, left | 7, 40 | 4.35 | 196 | −30 | −38 | 32 |
| Intraparietal Sulcus, right | 7, 40 | 5.61 | 191 | 32 | −38 | 34 |
| Posterior Superior Parietal, left | 7 | 4.22 | 77 | −16 | −64 | 50 |
| Posterior Superior Parietal, right | 7 | 4.50 | 135 | 22 | −68 | 50 |
| Temporal Parietal Junction, left | 39 | 4.17 | 66 | −28 | −70 | 20 |
| Same-Opposite Healthy Volunteers | ||||||
| Middle Frontal Gyrus, left | 8 | 4.48 | 219 | −22 | 22 | 40 |
| Superior/Middle Frontal, right | 8 | 3.99 | 41 | 26 | 32 | 38 |
| Posterior Cingulate Cortex | 31 | 4.46 | 714 | 8 | −44 | 42 |
| 3.98 | −4 | −40 | 42 | |||
| Inferior Parietal Cortex, left | 19, 39 | 4.36 | 85 | −36 | −74 | 38 |
| Opposite-Same Patient Group | ||||||
| Ventral Prefrontal Cortex/Insula, left | N/A | 4.47 | 67 | −30 | 18 | 16 |
| Ventral Prefrontal Cortex/Insula/ | N/A | 6.17 | 1,811 | 30 | 18 | 14 |
| Striatum/ACC, right | 3.58 | 16 | 8 | 36 | ||
| Intraparietal Sulcus/Precuneus, left | 7, 31, 40 | 5.53 | 795 | −18 | −64 | 34 |
| 4.72 | −30 | −40 | 38 | |||
| Intraparietal Sulcus/Precuneus, right | 7, 31, 40 | 5.30 | 1,977 | 26 | −62 | 28 |
| 5.15 | 40 | −44 | 36 | |||
| Occipital Temporal Junction, left | 19, 37 | 4.05 | 152 | −40 | −48 | −4 |
| Occipital Temporal Junction, right | 19, 37 | 4.10 | 116 | 50 | −46 | −10 |
| Anterior Cingulate Cortex, right | 32 | 3.45 | 45 | 12 | 26 | 34 |
| Same-Opposite Patient Group | ||||||
| Superior Temporal Gyrus, left | 22 | 4.44 | 107 | −56 | −18 | 2 |
| Hippocampus, left | N/A | 3.75 | 25 | −28 | −14 | −20 |
Figure 2.
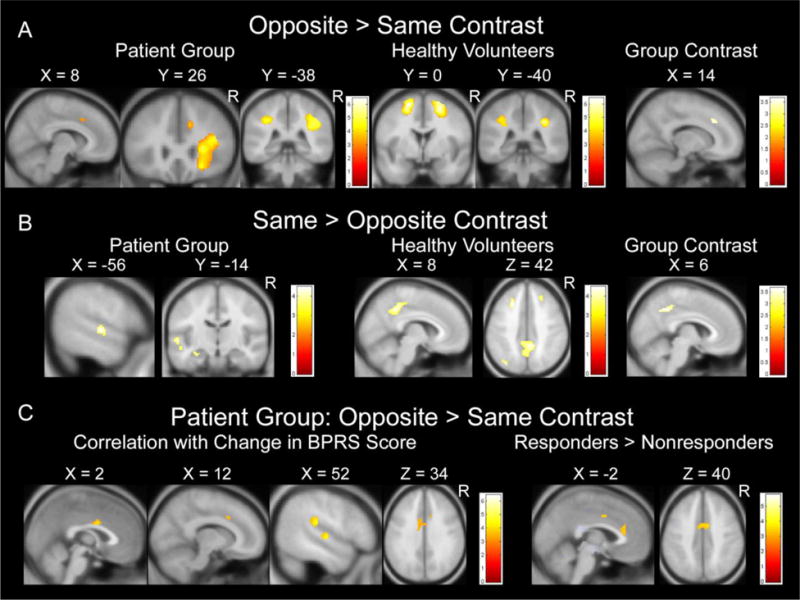
Illustration of Activation Differences between Patients and Healthy Volunteers. Within-group and between-group task contrast maps depicting brain activation for (A) the opposite-side task relative to the same-side task, (B) same-side task relative to the opposite-side task, and (C) opposite-side task relative to same-side task for medication responders compared with nonresponders. Maps show areas of significant signal increase exceeding a voxel height threshold of p=.001, whole-brain corrected to p<.05. Numerical values next to the color bars indicate the t-value for each color. Positions (in mm) in Montreal Neurological Institute (MNI) coordinate space are shown above each slice. The group contrast for opposite-same depicts significant signal increase in the patient group compared with healthy volunteers. The group contrast for same-opposite depicts significant signal increase in the healthy volunteers compared with patients. Correlation maps in (C) show significant negative correlations exceeding a voxel height threshold of p=.001, whole-brain corrected to p<.05.
For the patient group, primary task contrast maps of opposite-side compared with same-side blocks revealed greater activation for opposite-side in ventral prefrontal/insular cortex, IPS (BA 7,40), anterior cingulate cortex (ACC; BA 32), precuneus and cuneus (BA 31), and occipital-temporal junction (BA 19,37) (Table 3, Figure 2A). The contrast of same side – opposite side revealed greater activation for same side in left superior temporal gyrus (BA 22) and left hippocampus (Table 3, Figure 2B).
Directly comparing activations between the two groups revealed significantly greater activation for the patient group compared with healthy volunteers in ACC and IPS for the contrast of opposite-same side. Significantly greater activation for healthy volunteers compared with the first-episode group was observed in posterior cingulate cortex for the contrast of same-opposite (Table 4, Figure 2).
Table 4.
Regions of Activation Difference Between Patients and Healthy Volunteers, and Between Treatment Responders and Nonresponders, voxelwise threshold p<.001, whole-brain corrected p<.05
| Location of Activation Cluster | Brodmann’s Area | Tmax | Cluster Size |
MNI Coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Opposite-Same (Patients>Healthy Volunteers) | ||||||
| Anterior Cingulate Cortex, right | 32 | 3.55 | 37 | 14 | 28 | 34 |
| Intraparietal Sulcus, right | 7, 40 | 3.46 | 174 | 26 | −50 | 34 |
| Same-Opposite (Healthy Volunteers>Patients) | ||||||
| Posterior Cingulate Cortex | 31 | 3.60 | 192 | 6 | −46 | 42 |
| Opposite-Same (Responders>Nonresponders) | ||||||
| Anterior Cingulate Cortex, left | 24, 32 | 3.64 | 66 | −2 | 26 | 28 |
| Anterior Cingulate Cortex | 24, 32 | 3.60 | 85 | 8 | 0 | 40 |
| 3.60 | −6 | −2 | 40 | |||
Correlations between imaging data and task performance
For healthy volunteers, positive correlations between RT and BOLD activation during both opposite and same conditions were observed in dorsal ACC (BA 24, coordinates of local maximum [8, 4, 40] for opposite, r(27)=.65, p<.001, and [6, 4, 42] for same, r(27)=.58, p<.001) and in left IPS ([−24, −42, 56] for opposite, r(27)=.81, p<.001, and [−18, −40, 56] for same, r(27)=.84, p<.001). No positive correlations were observed for the patient group, and no negative correlations were observed in either group. For patients, a negative correlation between accuracy and BOLD activation for the opposite-same contrast was observed in left lateral PFC (BA 10/46, [−32, 42, 10], r(27)= −.66, p<.001). Analyses of covariance revealed that all primary task activations remained after covarying for task performance.
Correlations between imaging data and clinical measures
In the patient group, negative correlations between change in BPRS scores and BOLD activation for the contrast of opposite-same were observed in two regions of ACC (BA 24/32, [12, 16, 36], r(23)= −.60, p<.001, and BA 24, [2, 2, 30], r(23)= −.63, p<.001, k=55) (Figure 2C), plus temporal-parietal junction (BA 22/40, [58, −28, 22], r(23)= −.80, p<.001, k=319) and superior temporal gyrus/superior temporal sulcus (BA 22, [50, −16, 2], r(23)= −.69, p<.001, k=79). Moreover, significantly greater activation among responders compared to nonresponders was observed in ACC for the contrast of opposite-same (Table 4, Figure 2C). Importantly, baseline BPRS scores did not differ between treatment responders (M=41.55, SD=7.23) and nonresponders (M=42.55, SD=11.00), t(31)=.314, p=.756, ruling out the possibility that response to medication and neural activation differences occurred simply because responders had a less severe form of illness. Additionally, baseline BPRS scores were not significantly correlated with opposite-side task accuracy, r(27)= −.237, p=.216, indicating that task performance did not simply reflect severity of illness at time of scan. Change in BPRS scores was not significantly correlated with opposite-side task accuracy, r(19)= −.112, p= −.630, indicating that task performance alone cannot reliably predict symptom changes.
Discussion
This study examined the neural systems underlying response conflict, a critical component of executive functioning, in patients with first-episode psychosis and its relationship to antipsychotic treatment response. Patients demonstrated significantly greater activation compared with healthy volunteers in ACC and IPS during response conflict (i.e., opposite side – same side). Moreover, baseline BOLD activation within the ACC significantly predicted changes in symptom severity and treatment response among patients following antipsychotic pharmacotherapy. Specifically, those patients demonstrating greater ACC activation at baseline had the greatest reduction in symptom severity following 12-weeks of antipsychotic treatment. Strengths of the current study compared to prior work include the large and well-matched samples and use of patients studied with either minimal or no antipsychotic treatment and then again following controlled treatment. Additionally, the use of a short and relatively simple block-design task assessing behavioral response inhibition and set-shifting may make it more likely to be implemented in a clinical setting.
The finding of greater activation in dorsal ACC among patients compared with healthy volunteers continues to challenge assumptions regarding consistent hypofrontality in schizophrenia. Although early studies suggested that schizophrenia is associated with lower ACC activity (C. S. Carter et al. 2001; Kerns et al. 2005), the current results are consistent with a recent fMRI study demonstrating higher ACC activity in patients compared with healthy volunteers following correct shifts of cognitive sets (Wilmsmeier et al. 2010). A subsequent study revealed a linear trend between ACC activation in patients and learning potential on the WCST, such that individuals who readily learned the task demonstrated the greatest ACC activation. In contrast, individuals who did not learn the task demonstrated minimal or no ACC activation (Pedersen et al. 2012). Other studies reveal similar compensatory activation in patients with intact cognitive performance (Ettinger et al. 2011; Gur et al. 2007; Jamadar et al. 2010; Potkin et al. 2009). Of particular relevance, dorsal ACC exhibits “extra” or compensatory activation in psychosis that specifically reflects enhanced task performance in patients, but not healthy volunteers (Murray et al. 2010). In the current study, despite a small reduction in task accuracy among patients compared with healthy volunteers, patient performance was still high. Our findings are thus consistent with the hypothesis that greater brain activity in patients may reflect the requirement for patients to use greater (or compensatory) neural resources and/or alternate strategies to accomplish cognitive control comparable to healthy volunteers (Callicott et al. 2003; Gur et al. 2007; Minzenberg et al. 2009; Murray et al. 2010; Pedersen et al. 2012).
Baseline ACC activation was significantly associated with symptom reductions and changes in symptom severity following antipsychotic pharmacotherapy. Importantly, baseline symptom severity scores were not correlated with task performance, and treatment response could not be predicted from task performance alone. Therefore, this activation may represent a novel biomarker to gauge treatment response in psychosis. In prior work, aberrant ACC activation predicted functional disability in patients with schizophrenia (Ikuta et al. 2012), suggesting that relatively simple constructs (e.g., resolving response conflict) may have implications for real world functioning and could potentially serve as a target for cognitive remediation. Our data also converge with the hypothesis that learning potential on executive functioning tasks is positively associated with ACC activation in patients with psychosis (Murray et al. 2010; Pedersen et al. 2012) and prior work indicating that antipsychotic medication can act robustly on ACC functions (Snitz et al. 2005). The finding that greater ACC brain activation during response conflict predicts treatment response in patients following antipsychotic pharmacotherapy is also consistent with the hypothesis that some functional preservation of the ACC (albeit still abnormal) portends a better outcome compared to less activation in the face of task demands. The relative preservation of frontal functioning observed in the current study has been similarly observed in schizotypal personality disorder, which may serve to prevent the onset of frank psychosis (Hazlett et al. 2012). It is also similar to how cortical “reserve” is associated with enhanced response to cognitive therapy (Keshavan et al. 2011).
In the contrast of same-side vs. opposite-side, we observed increased posterior cingulate cortex activation among healthy volunteers compared to patients. In other words, the posterior cingulate cortex exhibited task-induced “deactivation” (McKiernan et al. 2003; Shulman et al. 1997) for the opposite-side task relative to the same-side task among healthy volunteers, but not among patients. This region has been demonstrated to show activation in block-designs when a simple baseline sensorimotor task is compared with a task requiring cognitive effort (McKiernan et al. 2003) and when a well-rehearsed or highly practiced task is compared with a more novel task (Mason et al. 2007). The posterior cingulate is also an integral part of the default mode network (DMN) (Raichle et al. 2001). Interestingly, although the DMN shows greater activation when a resting condition is compared with a cognitively-demanding task, it does not show increased activation when rest is compared with an easier (passive) sensory task (Greicius and Menon 2004). Thus, the lack of activation in the same-side vs. opposite-side contrast for the patient group, coupled with the increased activation in healthy volunteers, strongly implicates DMN dysregulation in psychosis and converges with prior work indicating a failure of DMN to deactivate during cognitive tasks in patients (Hasenkamp et al. 2011; Jeong and Kubicki 2010; D. I. Kim et al. 2009; Metzak et al. 2012; Salgado-Pineda et al. 2011).
Because activation in posterior cingulate has been associated with mind-wandering and self-referential thought during well-rehearsed (i.e., easier) tasks (Mason et al. 2007), it is conceivable that the healthy volunteers engaged in appropriate stimulus-independent thought during the same-side task, but the patients did not. Thus, one interpretation of these findings is that healthy volunteers found the same-side task to be “mindless” (Greicius and Menon 2004), whereas the patients did not. Alternatively, patients may have been unable to appropriately suppress mind-wandering or stimulus-independent thought during the opposite-side task. Both these possibilities are equally plausible, however, and we did not directly measure mind wandering in the current study. Therefore, interpretation of a mental state associated with the lack of activation in posterior cingulate should be made cautiously pending further investigation.
Several limitations should be acknowledged in this study. Reaction time was positively correlated with dorsal ACC and IPS activation in the healthy volunteers; however, reaction time did not differ significantly between groups and all primary task activations remained significant after covarying BOLD signal associated with task performance. Therefore, the between-group activation differences cannot be accounted for simply by performance differences. Rather, the activation differences likely represent underlying neural mechanisms related to psychosis separate from cognitive performance. We further acknowledge that it is not possible to fully isolate a single neurocognitive component in a neuroimaging study, whether block-design or event-related. Our two task conditions had the same sensory, mnemonic, and attentional demands, however, and were distinguished by the requirement for an ipsilateral or contralateral response. Therefore, the contrast of “opposite” with “same” most likely reflects the enhanced requirement to monitor performance during response conflict (Hommel 2011; Kerns et al. 2004), combined with transient cognitive set-shifting when remapping stimulus-response rules (Shafritz et al. 2005).
We also acknowledge the small sample sizes when patients were categorized as responders vs. nonresponders. It is important to note, however, that these groups did not differ at the time of the baseline scan in distributions of age, sex, education and BPRS total score, ruling out the possibility that nonresponders simply had a more severe illness at the onset of treatment that could predispose them to a more severe ACC activation profile. Because the current findings were collapsed across two antipsychotic medications, they do not directly provide information regarding which antipsychotic treatment regimen could potentially be most useful for the prediction of response in the context of the current fMRI paradigm. However, neither positive symptom response rate nor time to response differed between patients treated with aripiprazole or risperidone from the larger clinical trial in which they participated (Robinson et al. 2015). In this regard, the current results would be bolstered by future studies that prospectively predict apriori treatment response from baseline BOLD data. Nevertheless, the use of parsimonious tasks with straightforward single-subject data analysis procedures should help facilitate this goal.
An additional study limitation is that not all scans were conducted immediately prior to the initiation of antipsychotic medication in patients and that several individuals received lorazepam at the time of the scan. It is conceivable that these psychotropic medications affected the brain activation patterns observed herein. Furthermore, it should be acknowledged that resting-state fMRI has been demonstrated to predict antipsychotic treatment response (Kraguljac et al. 2016; Sarpal et al. 2016), and this might circumvent issues associated with task performance and reaction time, but may be less reliable for activating specific networks (Kristo et al. 2014). The comparison and complementary use of these approaches for investigating treatment response will be an important future research goal. We further acknowledge that the net result of the large voxel size in combination with the smoothing kernel may not provide optimal spatial resolution. Also, for block designs, the -acf cluster threshold algorithm in 3DClustSim may not always fully reduce whole brain alpha to .05 (Cox et al. 2017). Thus, the potential exists for a slightly inflated risk of Type I error, particularly for the activation in hippocampus, which rests just above the suggested threshold of k>24 voxels. Finally, although a later version of SPM (SPM12) was released at the time of this study, we had already begun data analysis using SPM8, and continued to use that version for all analyses as recommended by the software developers.
In sum, our data provide evidence for ACC hyperactivity in patients with first-episode psychosis compared to healthy volunteers during the performance of a simple response conflict test and that patients with the greatest activation at treatment baseline may benefit the most from antipsychotic medication. These findings also suggest that patients may need to utilize greater neural resources to achieve executive functioning comparable to healthy volunteers and that such abnormalities may serve as a biomarker for subsequent antipsychotic treatment response.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health to Dr. Szeszko (R01 MH076995), the NSLIJ Research Institute General Clinical Research Center (M01 RR018535), an Advanced Center for Intervention and Services Research (P30 MH090590) and a Center for Intervention Development and Applied Research (P50 MH080173), and by grants to Dr. Shafritz from Hofstra University. We thank Daniel Bentley for assistance with data analysis.
Funding: This work was funded by grants from the National Institute of Mental Health to Dr. Szeszko (R01 MH076995), the NSLIJ Research Institute General Clinical Research Center (M01 RR018535), an Advanced Center for Intervention and Services Research (P30 MH090590) and a Center for Intervention Development and Applied Research (P50 MH080173), and by grants to Dr. Shafritz from Hofstra University.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: All authors declare that they have no conflicts of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- Baran B, Karahanoglu FI, Agam Y, Mantonakis L, Manoach DS. Failure to mobilize cognitive control for challenging tasks correlates with symptom severity in schizophrenia. Neuroimage Clin. 2016;12:887–893. doi: 10.1016/j.nicl.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160(12):2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, 3rd, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158(9):1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Carter JD, Bizzell J, Kim C, Bellion C, Carpenter KL, Dichter G, et al. Attention deficits in schizophrenia–preliminary evidence of dissociable transient and sustained deficits. Schizophr Res. 2010;122(1–3):104–112. doi: 10.1016/j.schres.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect. 2017;7(3):152–171. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzan P, Arango C, Fleischacker W, Galderisi S, Glenthoj B, Leucht S, et al. Magnetic resonance imaging and the prediction of outcome in first-episode schizophrenia: a review of current evidence and directions for future research. Schizophr Bull. 2015;41(3):574–583. doi: 10.1093/schbul/sbv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res Cogn Brain Res. 2000;9(1):103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Williams SC, Fannon D, Premkumar P, Kuipers E, Moller HJ, et al. Functional magnetic resonance imaging of a parametric working memory task in schizophrenia: relationship with performance and effects of antipsychotic treatment. Psychopharmacology (Berl) 2011;216(1):17–27. doi: 10.1007/s00213-011-2214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, Research Version, Non-patient edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fryer SL, Roach BJ, Ford JM, Donaldson KR, Calhoun VD, Pearlson GD, et al. Should I Stay or Should I Go? FMRI Study of Response Inhibition in Early Illness Schizophrenia and Risk for Psychosis. Schizophr Bull. 2018 doi: 10.1093/schbul/sbx198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16(9):1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Grutzmann R, Endrass T, Kaufmann C, Allen E, Eichele T, Kathmann N. Presupplementary Motor Area Contributes to Altered Error Monitoring in Obsessive-Compulsive Disorder. Biol Psychiatry. 2016;80(7):562–571. doi: 10.1016/j.biopsych.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Loughead J, Snyder W, Kohler C, Elliott M, et al. Visual attention circuitry in schizophrenia investigated with oddball event-related functional magnetic resonance imaging. Am J Psychiatry. 2007;164(3):442–449. doi: 10.1176/ajp.2007.164.3.442. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W, James GA, Boshoven W, Duncan E. Altered engagement of attention and default networks during target detection in schizophrenia. Schizophr Res. 2011;125(2–3):169–173. doi: 10.1016/j.schres.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, Goldstein KE, Kolaitis JC. A review of structural MRI and diffusion tensor imaging in schizotypal personality disorder. Curr Psychiatry Rep. 2012;14(1):70–78. doi: 10.1007/s11920-011-0241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B. The Simon effect as tool and heuristic. Acta Psychol (Amst) 2011;136(2):189–202. doi: 10.1016/j.actpsy.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Hummer TA, Hulvershorn LA, Karne HS, Gunn AD, Wang Y, Anand A. Emotional response inhibition in bipolar disorder: a functional magnetic resonance imaging study of trait- and state-related abnormalities. Biol Psychiatry. 2013;73(2):136–143. doi: 10.1016/j.biopsych.2012.06.036. doi:S0006-3223(12)00598-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta T, Szeszko PR, Gruner P, DeRosse P, Gallego J, Malhotra AK. Abnormal anterior cingulate cortex activity predicts functional disability in schizophrenia. Schizophr Res. 2012;137(1–3):267–268. doi: 10.1016/j.schres.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamadar S, Michie P, Karayanidis F. Compensatory mechanisms underlie intact task-switching performance in schizophrenia. Neuropsychologia. 2010;48(5):1305–1323. doi: 10.1016/j.neuropsychologia.2009.12.034. [DOI] [PubMed] [Google Scholar]
- Jeong B, Kubicki M. Reduced task-related suppression during semantic repetition priming in schizophrenia. Psychiatry Res. 2010;181(2):114–120. doi: 10.1016/j.pscychresns.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukuri T, Kiviniemi V, Nikkinen J, Miettunen J, Maki P, Mukkala S, et al. Central executive network in young people with familial risk for psychosis–the Oulu Brain and Mind Study. Schizophr Res. 2015;161(2–3):177–183. doi: 10.1016/j.schres.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Johnson MK, Stenger VA, Aizenstein H, et al. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162(10):1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Eack SM, Wojtalik JA, Prasad KM, Francis AN, Bhojraj TS, et al. A broad cortical reserve accelerates response to cognitive enhancement therapy in early course schizophrenia. Schizophr Res. 2011;130(1–3):123–129. doi: 10.1016/j.schres.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DI, Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG, et al. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum Brain Mapp. 2009;30(11):3795–3811. doi: 10.1002/hbm.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MA, Tura E, Potkin SG, Fallon JH, Manoach DS, Calhoun VD, et al. Working memory circuitry in schizophrenia shows widespread cortical inefficiency and compensation. Schizophr Res. 2010;117(1):42–51. doi: 10.1016/j.schres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, White DM, Hadley N, Hadley JA, Ver Hoef L, Davis E, et al. Aberrant Hippocampal Connectivity in Unmedicated Patients With Schizophrenia and Effects of Antipsychotic Medication: A Longitudinal Resting State Functional MRI Study. Schizophr Bull. 2016;42(4):1046–1055. doi: 10.1093/schbul/sbv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristo G, Rutten GJ, Raemaekers M, de Gelder B, Rombouts SA, Ramsey NF. Task and task-free FMRI reproducibility comparison for motor network identification. Hum Brain Mapp. 2014;35(1):340–352. doi: 10.1002/hbm.22180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens KR, Kiehl KA, Ngan ET, Liddle PF. Attention orienting dysfunction during salient novel stimulus processing in schizophrenia. Schizophr Res. 2005;75(2–3):159–171. doi: 10.1016/j.schres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4(4):423–428. doi: 10.1093/scan/nsp052. doi:nsp052 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Carter CS. Event-related FMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. J Abnorm Psychol. 2003;112(4):689–697. doi: 10.1037/0021-843X.112.4.689. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, et al. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005;162(3):475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60(2–3):285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy MP, Ollinger JM, Buckner RL. Cluster size thresholds for assessment of significant activation in fMRI. Neuroimage. 2001;13(6):S198. Supplement. [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of Common Neural Circuit Disruptions in Cognitive Control Across Psychiatric Disorders. Am J Psychiatry. 2017;174(7):676–685. doi: 10.1176/appi.ajp.2017.16040400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzak PD, Riley JD, Wang L, Whitman JC, Ngan ET, Woodward TS. Decreased efficiency of task-positive and task-negative networks during working memory in schizophrenia. Schizophr Bull. 2012;38(4):803–813. doi: 10.1093/schbul/sbq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. doi:66/8/811 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Arch Gen Psychiatry. 2005;62(3):254–262. doi: 10.1001/archpsyc.62.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GK, Corlett PR, Fletcher PC. The neural underpinnings of associative learning in health and psychosis: how can performance be preserved when brain responses are abnormal? Schizophr Bull. 2010;36(3):465–471. doi: 10.1093/schbul/sbq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejad AB, Madsen KH, Ebdrup BH, Siebner HR, Rasmussen H, Aggernaes B, et al. Neural markers of negative symptom outcomes in distributed working memory brain activity of antipsychotic-naive schizophrenia patients. Int J Neuropsychopharmacol. 2013;16(6):1195–1204. doi: 10.1017/S1461145712001253. [DOI] [PubMed] [Google Scholar]
- Pedersen A, Wilmsmeier A, Wiedl KH, Bauer J, Kueppers K, Koelkebeck K, et al. Anterior cingulate cortex activation is related to learning potential on the WCST in schizophrenia patients. Brain Cogn. 2012;79(3):245–251. doi: 10.1016/j.bandc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Thakkar KN, Greve DN, Goff DC, Rauch SL, et al. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131(Pt 4):971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, Glover GH, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009;35(1):19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor RW. Playing the Simon game: use of the Simon task for investigating human information processing. Acta Psychol (Amst) 2011;136(2):182–188. doi: 10.1016/j.actpsy.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Gallego JA, John M, Petrides G, Hassoun Y, Zhang JP, et al. A Randomized Comparison of Aripiprazole and Risperidone for the Acute Treatment of First-Episode Schizophrenia and Related Disorders: 3-Month Outcomes. Schizophr Bull. 2015;41(6):1227–1236. doi: 10.1093/schbul/sbv125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Russell T, Bullmore ET, Soni W, Brammer MJ, Simmons A, et al. An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophr Res. 2001;52(1–2):47–55. doi: 10.1016/s0920-9964(00)00173-0. [DOI] [PubMed] [Google Scholar]
- Salgado-Pineda P, Fakra E, Delaveau P, McKenna PJ, Pomarol-Clotet E, Blin O. Correlated structural and functional brain abnormalities in the default mode network in schizophrenia patients. Schizophr Res. 2011;125(2–3):101–109. doi: 10.1016/j.schres.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Sarpal DK, Argyelan M, Robinson DG, Szeszko PR, Karlsgodt KH, John M, et al. Baseline Striatal Functional Connectivity as a Predictor of Response to Antipsychotic Drug Treatment. Am J Psychiatry. 2016;173(1):69–77. doi: 10.1176/appi.ajp.2015.14121571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Habel U, Reske M, Kellermann T, Stocker T, Shah NJ, et al. Neural correlates of working memory dysfunction in first-episode schizophrenia patients: an fMRI multi-center study. Schizophr Res. 2007;89(1–3):198–210. doi: 10.1016/j.schres.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Kartheiser P, Belger A. Dissociation of neural systems mediating shifts in behavioral response and cognitive set. Neuroimage. 2005;25(2):600–606. doi: 10.1016/j.neuroimage.2004.12.054. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. Common Blood Flow Changes across Visual Tasks: II. Decreases in Cerebral Cortex. J Cogn Neurosci. 1997;9(5):648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Smucny J, Lesh TA, Newton K, Niendam TA, Ragland JD, Carter CS. Levels of Cognitive Control: A Functional Magnetic Resonance Imaging-Based Test of an RDoC Domain Across Bipolar Disorder and Schizophrenia. Neuropsychopharmacology. 2018;43(3):598–606. doi: 10.1038/npp.2017.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, MacDonald A, 3rd, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. Am J Psychiatry. 2005;162(12):2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- Tan HY, Sust S, Buckholtz JW, Mattay VS, Meyer-Lindenberg A, Egan MF, et al. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry. 2006;163(11):1969–1977. doi: 10.1176/ajp.2006.163.11.1969. [DOI] [PubMed] [Google Scholar]
- van Veelen NM, Vink M, Ramsey NF, van Buuren M, Hoogendam JM, Kahn RS. Prefrontal lobe dysfunction predicts treatment response in medication-naive first-episode schizophrenia. Schizophr Res. 2011;129(2–3):156–162. doi: 10.1016/j.schres.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Vanes LD, Mouchlianitis E, Collier T, Averbeck BB, Shergill SS. Differential neural reward mechanisms in treatment-responsive and treatment-resistant schizophrenia. Psychol Med. 2018:1–10. doi: 10.1017/S0033291718000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen A, Morris R, Green MJ, Lenroot R, Kulkarni J, Carr VJ, et al. Reduced neural activity of the prefrontal cognitive control circuitry during response inhibition to negative words in people with schizophrenia. J Psychiatry Neurosci. 2012;37(6):379–388. doi: 10.1503/jpn.110088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voegler R, Becker MP, Nitsch A, Miltner WH, Straube T. Aberrant network connectivity during error processing in patients with schizophrenia. J Psychiatry Neurosci. 2016;41(2):E3–12. doi: 10.1503/jpn.150092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF. Prefrontal function in schizophrenia: confounds and controversies. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1495–1503. doi: 10.1098/rstb.1996.0135. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Golaszewski S, Mottaghy FM, Hofer A, Hausmann A, Kemmler G, et al. Brain activation patterns during a selective attention test-a functional MRI study in healthy volunteers and patients with schizophrenia. Psychiatry Res. 2003;123(1):1–15. doi: 10.1016/s0925-4927(03)00019-2. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Siedentopf C, Golaszewski S, Mottaghy FM, Hofer A, Kremser C, et al. Brain activation patterns during a selective attention test–a functional MRI study in healthy volunteers and unmedicated patients during an acute episode of schizophrenia. Psychiatry Res. 2007;154(1):31–40. doi: 10.1016/j.pscychresns.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Wessa M, Houenou J, Paillere-Martinot ML, Berthoz S, Artiges E, Leboyer M, et al. Fronto-striatal overactivation in euthymic bipolar patients during an emotional go/nogo task. Am J Psychiatry. 2007;164(4):638–646. doi: 10.1176/appi.ajp.164.4.638. doi:164/4/638 [pii] [DOI] [PubMed] [Google Scholar]
- Wiggins JL, Brotman MA, Adleman NE, Kim P, Oakes AH, Reynolds RC, et al. Neural Correlates of Irritability in Disruptive Mood Dysregulation and Bipolar Disorders. Am J Psychiatry. 2016;173(7):722–730. doi: 10.1176/appi.ajp.2015.15060833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmsmeier A, Ohrmann P, Suslow T, Siegmund A, Koelkebeck K, Rothermundt M, et al. Neural correlates of set-shifting: decomposing executive functions in schizophrenia. J Psychiatry Neurosci. 2010;35(5):321–329. doi: 10.1503/jpn.090181. doi:jpn.090181 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]


