Abstract
Objectives
To evaluate large-vessel (LV) abnormalities on serial imaging in patients with giant cell arteritis (GCA) and discern predictors of new lesions.
Methods
Clinical and imaging data from patients with GCA (including subjects diagnosed by LV imaging) enrolled in a prospective, multicenter, longitudinal study and/or a randomized clinical trial were included. New arterial lesions were defined as a lesion in a previously unaffected artery.
Results
The study included 187 patients with GCA, 146 (78%) female, mean (±SD) age at diagnosis 68.5 ± 8.5 years; 39% diagnosed by LV imaging. At least one arterial lesion was present in 123 (66%) on the first study. The most frequently affected arteries were subclavian (42%), axillary (32%), and thoracic aorta (20%). In 106 patients (57%) with serial imaging, new arterial lesions were noted in 41 patients (39%), all of whom had a baseline abnormality, over a mean (±SD) follow-up of 4.39 (2.22) years. New abnormalities were observed in 33% patients by year 2; clinical features of active disease were present at only 50% of these cases. There were no differences in age, sex, temporal artery biopsy positivity, or disease activity in patients with or without new lesions.
Conclusions
In this cohort of patients with GCA, LV abnormalities on first imaging were common. Development of new arterial lesions occurred in patients with arterial abnormalities at first imaging, often in the absence of symptoms of active disease. Arterial imaging should be considered in all patients with GCA at diagnosis and serial imaging at least in patients with baseline abnormalities.
Keywords: Giant cell arteritis, Computed tomography angiography, Magnetic resonance angiography, Large-artery stenosis, Aortic aneurysm, Disease activity
Giant cell arteritis (GCA) is one of the most common forms of vasculitis with an estimated incidence of 15–30 cases per 100,000 people ≥50 years [1]. Involvement of the temporal and craniofacial arteries in GCA lead to the characteristic symptoms of headache, scalp tenderness, tongue and jaw claudication, and visual changes. It is now well recognized that vascular inflammation in GCA often extends beyond cranial arteries. Large-vessel (LV) manifestations in GCA include aortic involvement with aortitis, aneurysms, aortic dissections, and large-artery stenoses [1–10]. Furthermore, prospective imaging studies in patients with newly diagnosed GCA have found evidence of possible subclinical inflammation of the aorta and its branches in a significant number of patients depending on the imaging modality used [10–18]. Large-artery stenosis can lead to significant morbidity while in one study mortality was increased in patients with aortic manifestations [6].
Studies evaluating longitudinal LV imaging findings in patients with GCA are few [19–23]. The aim of this study is to evaluate presence of large-artery lesions based on imaging studies in a longitudinal cohort of patients with GCA, and to evaluate if any clinical variables were associated with the development of new arterial lesions.
Patients and methods
Patients with GCA enrolled in a prospective, multicenter, longitudinal study (Vasculitis Clinical Research Consortium (VCRC) Longitudinal Study of GCA) and/or a randomized clinical trial of abatacept for treatment of GCA (the VCRC AGATA trial) were included. The study was approved by Institutional Review Boards at each participating site. All participants provided informed consent.
All patients in this cohort met the 1990 ACR classification criteria for GCA [24], modified to include patients with giant cell arteritis diagnosed by large-vessel angiography or biopsy. Inclusion criteria were age above 50 years with presence of ≥2 of the following features: (1) new localized headache, (2) temporal artery abnormality on examination, (3) ESR > 40 mm/h by Westergren method, (4) abnormal temporal artery biopsy, and (5) LV vasculitis by imaging [catheter based angiography, computed tomography angiography (CTA), magnetic resonance angiography (MRA)] or biopsy. All subjects were followed prospectively with standardized clinical assessments, including symptoms attributed to vasculitis (since last visit, in the prior 28 days, and on the day of evaluation), physical examination, and laboratory tests. Disease activity was defined as any symptom attributable to vasculitis since the last visit.
Subjects with at least one imaging study were included. Imaging reports were completed by the investigator using standardized forms, based on the clinical reports received from radiologists at centers with expertise in vasculitis. Abstracted data included details on type of study (catheter based angiography, CTA, MRA), date of study, arterial beds imaged, type of lesion (stenosis, aneurysm, occlusion, stent, angioplasty, or graft), whether the lesion was new, worse, unchanged, or improved.
For the longitudinal study, the decision regarding timing and type of imaging study was left to the discretion of the treating physician. For the clinical trial, all patients without contraindications underwent magnetic resonance angiography of the aorta and branches at study entry. In patients found to have involvement of the large vessels, this imaging was repeated at 6-month intervals and at the time of early termination/common close. In patients with GCA enrolled in the clinical trial without LV abnormalities on baseline imaging, subsequent imaging was only performed if they developed symptoms or signs suggestive of LV disease.
In subjects with more than one study, all available reports were reviewed. A new vascular lesion was defined as any area of stenosis, occlusion, or aneurysm in a previously unaffected artery. Worsening stenosis or intervention to a previously affected artery was not considered a new lesion.
Descriptive statistics, including means and medians were used. Subset analyses were also performed evaluating patients who were diagnosed with GCA by LV imaging to the remainder of the cohort. Clinical variables were compared using Fisher’s exact test for categorical variables and two independent samples t-test for continuous variables. Kaplan-Meier analysis was used to evaluate presence of new lesions on follow-up imaging. In order to account for the variability in the number of serial imaging studies, patients were censored at time of last available imaging study. Log-rank test was used to compare clinical variables of age, sex, temporal artery biopsy disease duration, cumulative months of glucocorticoid use, use of adjunctive immunosuppression at entry into the cohort between patients with and without new lesions, again censoring for last available imaging study.
Results
The cohort
The study included 187 subjects (146, 78% female) with GCA and at least 1 imaging study; 50 patients (27%) were enrolled in the AGATA clinical trial. Mean (±SD) age at diagnosis was 68.5 (±8.5) years. Temporal artery biopsy was positive in 97 of 130 patients (75%) in whom it was performed. GCA was diagnosed by LV imaging in 73 patients (39%); 29 of whom also had a temporal artery biopsy which was positive in 14 patients. The median (25th, 75th percentile) time from diagnosis of GCA to first imaging was 6.5 (0.5, 24.5) months. Clinical symptoms at diagnosis of GCA was different in the subset of patients diagnosed with LV imaging (73 patients) compared to the remainder of the cohort (114 patients) (Table 1).
Table 1.
Symptoms at diagnosis and arterial involvement in the subset of patient diagnosed with giant cell arteritis by large-vessel (LV) imaging compared to the remainder of the cohort
| Variable | LV imaging (N = 73) | Remainder cohort (N = 114) | p Value |
|---|---|---|---|
| Positive temporal artery biopsy, N (%) | 14/29 (48%) | 83/101 (82%) | <0.001 |
| Cranial symptoms, N (%) | 38 (52%) | 100 (88%) | <0.001 |
| Ocular manifestations, N (%) | 9 (12%) | 46 (40%) | <0.001 |
| Polymyalgia rheumatica, N (%) | 31 (52%) | 48 (68%) | 1.00 |
| Upper extremity claudication, N (%) | 33 (45%) | 21 (18%) | <0.001 |
| Lower extremity claudication, N (%) | 13 (18%) | 7 (6%) | 0.015 |
| Stroke, transient ischemic attack, N (%) | 10 (14%) | 11 (10%) | 0.478 |
| Baseline study with any abnormality, N(%) | 66 (90%) | 57 (50%) | <0.001 |
| Serial imaging | 56 (77%) | 50 (44%) | <0.001 |
N = number.
Imaging findings at baseline and follow-up
Data available on 187 patients included 737 imaging studies; 465 (63%) of the aorta and branches, 38 (5%) abdominal arteries, 44 (6%) cerebral arteries, 44 (6%) coronary arteries; 11 (1.5%) right upper extremity, 14 (2%) left upper extremity, and 51 (7%) right lower extremity and 52 (7%) left lower extremity. Baseline imaging modality was MRA in 63.2%, CTA in 36.2% and conventional angiography in 0.6%. The overall distribution of type of imaging study was MRA (72%), CTA (27%) and conventional angiography (1%). At least one arterial lesion was noted in 123 patients (66%) on the first available imaging. When restricted to patients enrolled in AGATA who were systematically evaluated with imaging, 28 patients (56%) had at least 1 abnormality on first imaging.
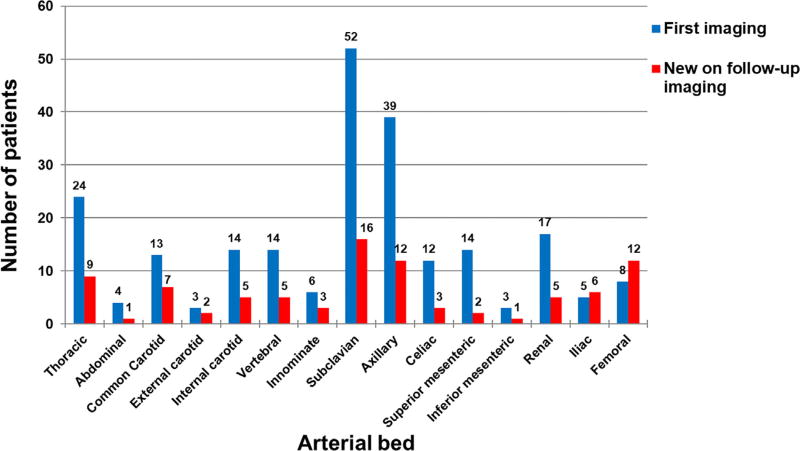
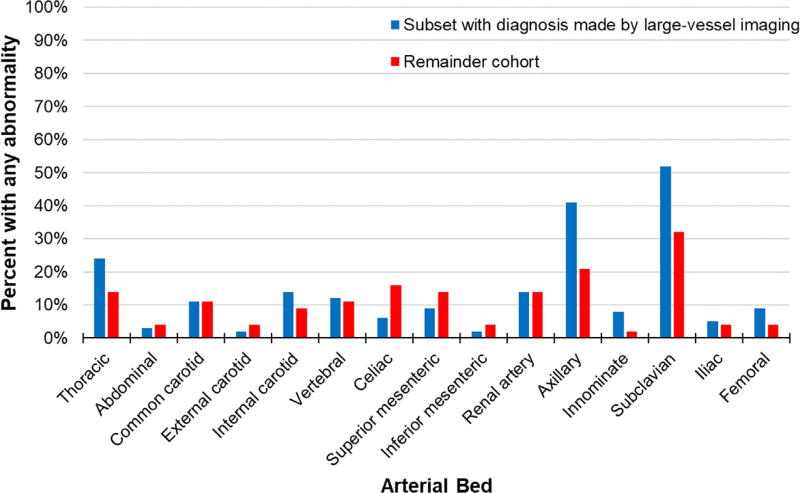
The most frequently observed lesions were subclavian (52 patients, 42%), axillary (39 patients, 32%), and thoracic aortic (24 patients, 20%). The frequency and distribution of arterial lesions at baseline is shown in Figure 1. The frequency and distribution of arterial lesions on first imaging in the subset of patients with GCA diagnosed by LV imaging versus the remainder of the cohort is shown in Figure 2. There were no differences in mean age, sex, frequency of positive temporal artery biopsy, disease duration between the patients with or without baseline lesions (p > 0.05, data not shown).
Fig. 1.
Frequency of arterial involvement (Y-axis) by anatomic location (X-axis) in patients with giant cell arteritis at baseline (N = 123 with any involvement) and at follow-up (N = 41 with new lesion).
Fig. 2.
Comparison of frequency of arterial involvement (Y-axis) by anatomic location (X-axis) in the subset of patients diagnosed with giant cell arteritis by large-vessel imaging (N = 73) compared to the remainder of the cohort (N = 114). Involvement of the axillary and subclavian arteries was significantly higher in the subset diagnosed by large-vessel imaging compared to the remainder of the cohort (p = 0.02 and p = 0.03, respectively).
Serial imaging was available in 106 patients (57% of all patients); 29 patients (59%) enrolled in AGATA and 77 patients (56%) in the longitudinal cohort. This included imaging in 88 of the 123 subjects (72%) with at least one arterial lesion on first imaging and 18 of 64 patients (28%) without any abnormality on first study. The median (25th, 75th percentile) number of follow-up studies was 4 (3, 6). At least 1 new lesion in a previously unaffected area was noted in 41 patients (39%) on a subsequent study. The majority of new lesions were in the subclavian (16 patients, 39%), axillary (12 patients, 29%), and femoral (12 patients, 29%) arteries, and new thoracic aortic involvement was observed in 9 patients (22%) (Fig. 1). All 41 patients with new lesions during serial imaging had arterial involvement at first imaging. All 18 patients without any abnormality on first imaging had no abnormality on a subsequent study with a median (25th, 75th percentile) number of studies of 3 (2, 3). 18 patients (43%) had multiple serial imaging studies with a new lesion; all of whom belonged to the subset of patients diagnosed with GCA by LV imaging.
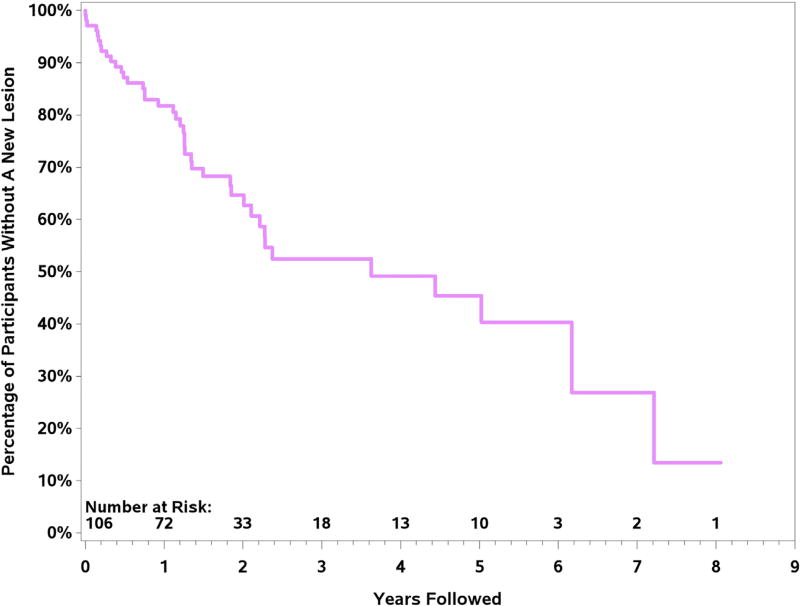
Kaplan-Meier curve evaluating the number of patients without any new lesion over time is shown in Figure 3. By 2 years, 33% of patients had developed a new arterial lesion. Using the log-rank test and censoring for last available study, there were no differences in age, sex, disease duration, or cumulative months of use of glucocorticoids between patients with new lesions or those without new lesions (p > 0.05). However, use of immunosuppressive therapy at entry into the cohort was associated with lower risk of new arterial lesions (p = 0.038).
Fig. 3.
Kaplan-Meier curve evaluating absence of a new lesion over time in subset of patients with giant cell arteritis with serial imaging. Patients were censored at the time of last available imaging study.
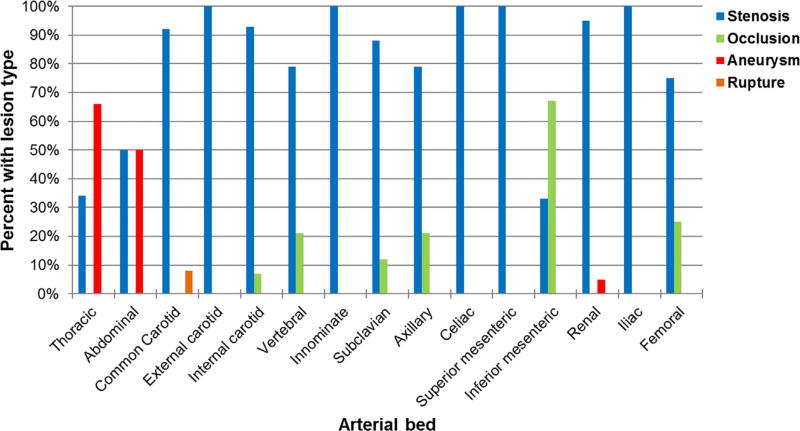
Stenoses were the most frequently observed lesions at the arterial beds both at baseline and during follow-up. Occlusive lesions were most frequently observed at the inferior mesenteric artery while aneurysms were only observed at the aorta or renal arteries. The type of arterial lesion by vascular bed is shown in Figure 4.
Fig. 4.
Type of lesion noted by arterial beds in patients with giant cell arteritis. All available studies with any abnormality in the above vascular beds were used.
Disease activity at time of new imaging findings
Disease activity and symptoms were evaluated in the 41 patients (73 encounters) with new arterial lesions on serial imaging. In almost all patients, the imaging study preceded the clinical evaluation by a median (25th, 75th) of 59 (36, 147) days. The evaluating physician systematically documented any GCA related disease activity since last visit, in the past 28 days, and on the day of evaluation. Any disease activity since last visit was present in 20 (27%) encounters where a new arterial lesion was noted with 13 encounters of active disease the day of evaluation. Only 9 of 20 patients (45%) with new upper extremity arterial lesions were symptomatic on the day of assessment.
When disease activity assessment was restricted to 15 encounters (21%) with clinical evaluation within 30 days of imaging, 6 encounters (40%) were marked as having any active disease since prior visit. The physician-rated disease activity for the prior 28 days for patients with new lesions was remission in 10 encounters (67%), and active in in 5 cases (33%) with new lesions: low disease activity in 1 encounter, moderate disease activity in 3 encounters, and high disease activity in 1 encounter. Medication use at the time of new lesion included prednisone in 13 encounters, azathioprine in 1 encounter, methotrexate in 3 encounters, abatacept in 1 encounter. Only 2 of the 6 encounters (33%) with new upper extremity arterial lesions were symptomatic while none of the 6 patients with new lower extremity arterial lesions were symptomatic.
Predictors of new arterial lesions during follow-up
The clinical characteristics of patients with and without new arterial lesions were compared (Table 2). There were no differences in sex, proportion of patients with positive temporal artery biopsy or disease duration between the two groups. All of the new lesions in this study occurred among patients who had abnormalities on first imaging. A greater proportion of patients with new lesions on serial imaging belonged to the subset with diagnosis of GCA based on LV imaging (Table 2).
Table 2.
Comparison of clinical variables between patients with giant cell arteritis and new arterial lesions on follow-up imaging and those without any new lesions
| Variable | New lesions (n = 41) | No new lesions (n = 65) | p Value |
|---|---|---|---|
| Mean (±SD) age, years | 68 (7.9) | 68 (6.8) | 1.00 |
| Mean (±SD) disease duration, weeks | 72 (107) | 71 (95) | 0.960 |
| Median (25th, 75th) disease duration, weeks | 31 (4.2, 113.9) | 26 (6, 100) | 0.72 |
| Disease duration ≤1 year, number | 27 (65%) | 41 (63%) | 0.837 |
| Mean (±SD) duration of follow-up, years | 4.0 (2) | 4.6 (2.3) | 0.172 |
| Female sex, number | 34 (82%) | 56 (86%) | 0.782 |
| Positive temporal artery biopsy, number | 15/19 (78%) | 27/40 (68%) | 0.540 |
| Median (25th, 75th) number studies | 6 (4, 10) | 3 (2, 5) | <0.010 |
| Any lesion at first imaging, number | 41 (100%) | 47 (72%) | <0.001 |
| Diagnosis of giant cell arteritis by large-vessel imaging, number | 30 (73%) | 26 (40%) | 0.001 |
| Type of study for aorta and branches | |||
| CT angiography | 36 (22%) | 27 (19%) | |
| MR angiography | 124 (76%) | 112 (81%) | |
| Any disease activity | 14 (34%) | 29 (45%) | 0.316 |
| Aspirin use at last follow-up | 25 (61%) | 31 (48%) | 0.232 |
| Prednisone use at last follow-up | 33 (80%) | 34 (52%) | 0.004 |
| Azathioprine use at last follow-up | 2 (5%) | 2 (3%) | 0.640 |
| Methotrexate use at last follow-up | 10 (24%) | 12 (18%) | 0.472 |
SD: standard deviation; CT: computerized tomography; MR: magnetic resonance.
p Value: p-value of difference between “New lesions” and “No new lesions”.
Discussion
In this large, prospective, longitudinal, multi-center study of patients with cranial or LV manifestations from GCA, 66% of patients with GCA had at least one arterial lesion on first imaging study. However, even when restricting to the data from the clinical trial, where all patients underwent systematic imaging of the entire aorta, 56% had a baseline abnormality. The subclavian arteries (42%), and axillary arteries (32%) were the most frequently affected sites at baseline and follow-up. A novel finding in this study is the observation of new arterial lesions on serial imaging in 39% of patients. Furthermore, nearly half of the visits associated with imaging demonstrating new arterial lesions did not have any clinical findings of active disease in the preceding months.
This cohort also allowed inclusion of patients with predominantly large-artery manifestations from GCA and 39% subjects had LV vasculitis as confirmed on imaging. This subset differed with respect to symptoms at presentation of GCA which is consistent with previous reports [5,22,23,25]. When evaluating patients who underwent systematic imaging of the entire aorta as part of the protocol for a clinical trial, 56% of patients had at least one abnormality at first imaging. In prospective studies large-artery involvement was seen in 29–83% of patients with a new diagnosis of GCA [10,11,13–16]. The distribution of arterial lesions and the predominance of stenotic lesions as seen in the current study is consistent with prior reports [2,4,5,10,13–18,25–29]. The reported prevalence of aortic involvement in studies with a systematic protocol for imaging in patients with GCA is between 45% and 65% [11,12,16]. The lower percentage of aortic involvement in the current study may be related to differences in the imaging modalities and definitions used. The present study did not evaluate aortic wall thickening. Unlike previous reports, stenotic lesions were observed at the aorta (especially abdominal aorta) in addition to aneurysms [8,12,20].
In this study with serial imaging in patients with GCA, 57% patients had at least 1 follow-up study. This study defined a new arterial lesion as one appearing in a previously unaffected artery and not merely worsening stenosis. New arterial lesions were present in 39%, a much higher proportion of patients than reported in the previous studies [19,22]. A recent large, multicenter study of 549 patients with GCA and LV imaging only evaluated aortic abnormalities and majority of the LV imaging studies were PET [23]. New aortic dilation was significantly higher in patients with LV abnormalities on first imaging (21%) compared to those without (7%) and (94%) of the new aortic dilations occurring in a previously inflamed segment [23]. In our study, all of the new lesions were in patients who had a baseline abnormality, particularly patients with GCA who were diagnosed by LV imaging. Furthermore, all 18 patients with multiple imaging studies where new lesions were found belonged to this subset. While this may reflect the clinical practice of following these patients with serial imaging, it raises important questions about imaging as part of disease activity assessment.
Importantly, in the current study only 40–50% of visits with a new lesion had any symptoms of active disease in the preceding months even when restricted to encounters where the clinical evaluation and imaging were performed within 1–2 months. This points to the current limitations in assessment of disease activity in patients with GCA [30,31]. In a recent study using positron emission tomography (PET) in patients with LV vasculitis, fluorodeoxyglucose uptake suggesting active disease was noted in 58% of patients whose disease was clinically assessed as remission [32]. Furthermore, disease activity by PET during clinical remission was a predictor of relapse in the future [32]. It is unclear if treatment strategies need to be modified in patients with clinically asymptomatic new lesions incidentally noted on serial imaging. The recent European League Against Rheumatism recommendations for imaging in LVV in clinical practice suggest regular screening with imaging for LV abnormalities in patients with GCA with signs or symptoms of stenosis, occlusion, aneurysms, and, those with recurrent or persistent inflammation of the large arteries and the aorta [33]. Given the paucity of data, frequency of imaging assessments could not be determined and it was recommended it be individualized [33]. They did not recommend routine use of LV imaging in patients who are in clinical remission [33]. However, the frequency of clinically undetected lesions in our study and the findings of the recent PET study by Grayson and colleagues raise questions about whether LV imaging should be included in the disease assessment of GCA [32]. We recommend serial imaging should be strongly considered in patients with GCA, especially those with LV abnormalities.
Factors such as disease duration, sex, a positive temporal artery biopsy, or use of adjunctive immunosuppressive therapy did not differ between patients with or without new arterial lesions. A greater proportion of patients with GCA diagnosed by LV imaging developed new lesions which again may reflect the clinical practice of serial imaging to assess disease activity in these patients.
Eighty percent of the patients with new lesions, versus only 52% of patients with no new lesions, were on prednisone at their last follow-up. However, this finding may be confounded by the possibility that physicians treated new lesions as active disease in our patients and physicians were more reluctant to discontinue prednisone therapy when a new arterial lesion were found. A recent study did find that patients with LV abnormalities on imaging were less likely to discontinue glucocorticoids and more likely to be put on steroid-sparing therapies even though similar frequency of relapses were observed [23]. Twenty-four percent of patients were on methotrexate and 5% on azathioprine at the time of a new lesion, perhaps reflecting the poor performance of currently available so-called “steroid-sparing” medications. Tocilizumab has been shown to be efficacious in patients with GCA, and is now FDA approved for this diagnosis, but long-term studies are needed to assess its efficacy on outcomes such as vessel damage and large-artery complications [34].
Strengths of this study include standardized serial clinical evaluations by specialists expert in the care of patients with vasculitis. Longitudinal data on multiple aspects of interest was available for patients in this cohort allowing a comprehensive study of the research question. Importantly, this longitudinal cohort also includes patients with GCA diagnosed by LV imaging, an approach that has been used in recent clinical trials as well [34,35]. The imaging modalities used to detect LVV provided assessment of the full range of aortic and first-order branch arterial lesions.
There are several limitations to the study. Imaging was not standardized in the longitudinal cohort and the timing, type of study and interval was left to the discretion of the treating physician. This may bias our findings about which patients develop new lesions during follow-up since not all patients with GCA were followed with serial imaging. However, this also reflects clinical practice since LV imaging is not routinely performed in all patients with GCA. Kaplan-Meier analysis was used to evaluate new lesions over time censoring for last available imaging study to partly mitigate the variability in number of imaging studies with similar findings. However, as observed in our cohort, many of the LV lesions were asymptomatic and therefore, the lack of protocolized imaging may underestimate the extent of LV abnormalities in GCA. Majority of the studies were MRA which can overestimate stenosis. Information on imaging abnormalities were abstracted from radiology reports but the studies were all done and evaluated by radiologists at centers with expertise in vascular imaging and vasculitis. Data on wall thickening, ectasia, vessel wall edema were not abstracted but these are often subjective and not well defined. Given the age of the patients, it is possible that some of the imaging findings were due to atherosclerosis. However, the distribution of lesions at baseline and follow-up is consistent with what has been reported in patients with GCA. The majority of the imaging studies were of the thoracic aorta and branches which may have biased results toward the predominance of upper extremity arterial lesions.
In summary, LV involvement in GCA is an increasingly recognized clinical entity. The current study furthers our current knowledge regarding LV involvement in GCA as documented by imaging by providing long term longitudinal data. Baseline imaging, especially of the thoracic aorta and its branches, should be considered for all newly diagnosed patients with GCA. New lesions may appear even in subjects without evidence of active disease as measured by current clinical variables, highlighting limitations in disease assessment but also in the ability of current treatments to induce true remission and prevent further vascular damage. These results highlight the importance of conducting serial imaging, at least in patients with any abnormality on baseline imaging even in the absence of typical signs and symptoms of active disease, and, particularly in the subset of patients with GCA who present with large-artery manifestations. This study cannot comment on the utility of surveillance imaging in patients with GCA without any baseline LV abnormalities. Given the lack of standardized recommendations, practice patterns regarding surveillance, the optimal frequency, and optimal imaging modality needs further investigation. Imaging of the aorta and its branches should be considered in future prospective clinical trials of GCA as a part of the clinical assessment.
Acknowledgments
Funding: This work was supported by the Vasculitis Clinical Research Consortium (VCRC) (U54 AR057319) which is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Science (NCATS). The VCRC is funded through collaboration between NCATS, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and has received funding from the National Center for Research Resources (U54 RR019497).
Abbreviations
- ACR
American College of Rheumatology
- CTA
computed tomography angiography
- ESR
erythrocyte sedimentation rate
- GCA
giant cell arteritis
- LV
large-vessel
- MRA
magnetic resonance angiography
Footnotes
Conflict of interest: The authors have no conflict of interest to report relevant to this manuscript.
References
- 1.Gonzalez-Gay MA, Vazquez-Rodriguez TR, Lopez-Diaz MJ, Miranda-Filloy JA, Gonzalez-Juanatey C, Martin J, et al. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum. 2009;61:1454–61. doi: 10.1002/art.24459. [DOI] [PubMed] [Google Scholar]
- 2.Klein RG, Hunder GG, Stanson AW, Sheps SG. Large artery involvement in giant cell (temporal) arteritis. Ann Intern Med. 1975;83:806–12. doi: 10.7326/0003-4819-83-6-806. [DOI] [PubMed] [Google Scholar]
- 3.Lie JT. Aortic and extracranial large vessel giant cell arteritis: a review of 72 cases with histopathologic documentation. Semin Arthritis Rheum. 1995;24:422–31. doi: 10.1016/s0049-0172(95)80010-7. [DOI] [PubMed] [Google Scholar]
- 4.Nuenninghoff DM, Hunder GG, Christianson TJH, McClelland RL, Matteson EL. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum. 2003;48:3522–31. doi: 10.1002/art.11353. [DOI] [PubMed] [Google Scholar]
- 5.Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum. 1999;42:311–7. doi: 10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Kermani TA, Warrington KJ, Crowson CS, Ytterberg SR, Hunder GG, Gabriel SE, et al. Large-vessel involvement in giant cell arteritis: a population-based cohort study of the incidence-trends and prognosis. Ann Rheum Dis. 2013;72:1989–94. doi: 10.1136/annrheumdis-2012-202408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans JM, O'Fallon W, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis: a population-based study. Ann Intern Med. 1995;122:502–7. doi: 10.7326/0003-4819-122-7-199504010-00004. [DOI] [PubMed] [Google Scholar]
- 8.García-Martínez A, Hernández-Rodríguez J, Arguis P, Paredes P, Segarra M, Lozano E, et al. Development of aortic aneurysm/dilatation during the followup of patients with giant cell arteritis: a cross-sectional screening of fifty-four prospectively followed patients. Arthritis Care Res (Hoboken) 2008;59:422–30. doi: 10.1002/art.23315. [DOI] [PubMed] [Google Scholar]
- 9.Robson JC, Kiran A, Maskell J, Hutchings A, Arden N, Dasgupta B, et al. The relative risk of aortic aneurysm in patients with giant cell arteritis compared with the general population of the UK. Ann Rheum Dis. 2015;74:129–35. doi: 10.1136/annrheumdis-2013-204113. [DOI] [PubMed] [Google Scholar]
- 10.Ghinoi A, Pipitone N, Nicolini A, Boiardi L, Silingardi M, Germanò G, et al. Large-vessel involvement in recent-onset giant cell arteritis: a case–control colour-Doppler sonography study. Rheumatology (Oxford) 2012;51:730–4. doi: 10.1093/rheumatology/ker329. [DOI] [PubMed] [Google Scholar]
- 11.Blockmans D, de Ceuninck L, Vanderschueren S, Knockaert D, Mortelmans L, Bobbaers H. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Care Res (Hoboken) 2006;55:131–7. doi: 10.1002/art.21699. [DOI] [PubMed] [Google Scholar]
- 12.Agard C, Barrier J-H, Dupas B, Ponge T, Mahr A, Fradet G, et al. Aortic involvement in recent-onset giant cell (temporal) arteritis: a case–control prospective study using helical aortic computed tomodensitometric scan. Arthritis Care Res (Hoboken) 2008;59:670–6. doi: 10.1002/art.23577. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt WA, Seifert A, Gromnica-Ihle E, Krause A, Natusch A. Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large-vessel giant cell arteritis. Rheumatology (Oxford) 2008;47:96–101. doi: 10.1093/rheumatology/kem322. [DOI] [PubMed] [Google Scholar]
- 14.Aschwanden M, Kesten F, Stern M, Thalhammer C, Walker UA, Tyndall A, et al. Vascular involvement in patients with giant cell arteritis determined by duplex sonography of 2 × 11 arterial regions. Ann Rheum Dis. 2010;69:1356–9. doi: 10.1136/ard.2009.122135. [DOI] [PubMed] [Google Scholar]
- 15.Czihal M, Zanker S, Rademacher A, Tatò F, Kuhlencordt PJ, Schulze-Koops H, et al. Sonographic and clinical pattern of extracranial and cranial giant cell arteritis. Scand J Rheumatol. 2012;41:231–6. doi: 10.3109/03009742.2011.641581. [DOI] [PubMed] [Google Scholar]
- 16.Prieto-González S, Arguis P, García-Martínez A, Espígol-Frigolé G, Tavera-Bahillo I, Butjosa M, et al. Large vessel involvement in biopsy-proven giant cell arteritis: prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis. 2012;71:1170–6. doi: 10.1136/annrheumdis-2011-200865. [DOI] [PubMed] [Google Scholar]
- 17.Diamantopoulos AP, Haugeberg G, Hetland H, Soldal DM, Bie R, Myklebust G. Diagnostic value of color Doppler ultrasonography of temporal arteries and large vessels in giant cell arteritis: a consecutive case series. Arthritis Care Res (Hoboken) 2014;66:113–9. doi: 10.1002/acr.22178. [DOI] [PubMed] [Google Scholar]
- 18.Prieto-González S, Depetris M, García-Martínez A, Espígol-Frigolé G, Tavera-Bahillo I, Corbera-Bellata M, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case–control study. Ann Rheum Dis. 2014;73:1388–92. doi: 10.1136/annrheumdis-2013-204572. [DOI] [PubMed] [Google Scholar]
- 19.Assie C, Janvresse A, Plissonnier D, Levesque H, Marie I. Long-term follow-up of upper and lower extremity vasculitis related to giant cell arteritis: a series of 36 patients. Medicine (Baltimore) 2011;90:40–51. doi: 10.1097/MD.0b013e318206af16. [DOI] [PubMed] [Google Scholar]
- 20.García-Martínez A, Arguis P, Prieto-González S, Espígol-Frigolé G, Alba MA, Butjosa M, et al. Prospective long term follow-up of a cohort of patients with giant cell arteritis screened for aortic structural damage (aneurysm or dilatation) Ann Rheum Dis. 2014;73:1826–32. doi: 10.1136/annrheumdis-2013-203322. [DOI] [PubMed] [Google Scholar]
- 21.Marie I, Proux A, Duhaut P, Primard E, Lahaxe L, Girszyn N, et al. Long-term follow-up of aortic involvement in giant cell arteritis: a series of 48 patients. Medicine (Baltimore) 2009;88:182–92. doi: 10.1097/MD.0b013e3181a68ae2. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt WA, Moll A, Seifert A, Schicke B, Gromnica-Ihle E, Krause A. Prognosis of large-vessel giant cell arteritis (Oxford) Rheumatology. 2008;47:1406–8. doi: 10.1093/rheumatology/ken258. [DOI] [PubMed] [Google Scholar]
- 23.de Boysson H, Daumas A, Vautier M, Parienti JJ, Liozon E, Lambert M, et al. Large-vessel involvement and aortic dilation in giant-cell arteritis. A multi-center study of 549 patients. Autoimmun Rev. 2018;17:391–8. doi: 10.1016/j.autrev.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 24.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–8. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 25.Muratore F, Kermani TA, Crowson CS, Green AB, Salvarani C, Matteson EL, et al. Large-vessel giant cell arteritis: a cohort study. Rheumatology (Oxford) 2015;54:463–70. doi: 10.1093/rheumatology/keu329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Hello C, Lévesque H, Jeanton M, Cailleux N, Galateau F, Peillon C, et al. Lower limb giant cell arteritis and temporal arteritis: followup of 8 cases. J Rheumatol. 2001;28:1407–12. [PubMed] [Google Scholar]
- 27.Stanson AW, Klein RG, Hunder GG. Extracranial angiographic findings in giant cell (temporal) arteritis. Am J Roentgenol. 1976;127:957–63. doi: 10.2214/ajr.127.6.957. [DOI] [PubMed] [Google Scholar]
- 28.Grayson PC, Maksimowicz-McKinnon K, Clark TM, Tomasson G, Cuthbertson D, Carette S, et al. Distribution of arterial lesions in Takayasu’s arteritis and giant cell arteritis. Ann Rheum Dis. 2012;71:1329–34. doi: 10.1136/annrheumdis-2011-200795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grayson PC, Tomasson G, Cuthbertson D, Carette S, Hoffman GS, Khalidi NA, et al. Association of vascular physical examination findings and arteriographic lesions in large vessel vasculitis. J Rheumatol. 2012;39:303–9. doi: 10.3899/jrheum.110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aydin SZ, Direskeneli H, Sreih A, Alibaz-Oner F, Gul A, Kamali S, et al. Update on outcome measure development for large vessel vasculitis: report from OMERACT 12. J Rheumatol. 2015;42:2465–9. doi: 10.3899/jrheum.141144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Direskeneli H, Aydin SZ, Kermani TA, Matteson EL, Boers M, Herlyn K, et al. Development of outcome measures for large-vessel vasculitis for use in clinical trials: opportunities, challenges, and research agenda. J Rheumatol. 2011;38:1471–9. doi: 10.3899/jrheum.110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grayson PC, Alehashemi S, Bagheri AA, Civelek AC, Cupps TR, Kaplan MJ, et al. Positron emission tomography as an imaging biomarker in a prospective, longitudinal cohort of patients with large vessel vasculitis. Arthritis Rheumatol. 2018;70:439–49. doi: 10.1002/art.40379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77:636–43. doi: 10.1136/annrheumdis-2017-212649. [DOI] [PubMed] [Google Scholar]
- 34.Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med. 2017;377:317–28. doi: 10.1056/NEJMoa1613849. [DOI] [PubMed] [Google Scholar]
- 35.Langford CA, Cuthbertson D, Ytterberg SR, Khalidi N, Monach PA, Carette S, et al. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheum. 2017;69:837–45. doi: 10.1002/art.40044. [DOI] [PMC free article] [PubMed] [Google Scholar]