Abstract
As new drugs targeting MYC show clinical activity in AML, understanding MYC expression in AML is of critical importance. We assessed MYC protein expression by immunohistochemistry in bone marrow of patients with untreated AML (n=265). Overall, 90% of patients demonstrated MYC overexpression and MYC-immunopositivity ≤6 % was associated with superior complete remission (CR) duration of 23 months vs. 12 months for MYC-immunopositivity >6 % (p=0.028). Among 241 patients at higher risk for relapse, including those ≥55-years of age and patients with intermediate- and high-risk AML, MYC-immunopositivity ≤6% conferred significantly superior median overall survival (OS) (24 vs.13 months; p=0.042), event-free survival (EFS) (14 vs. 6 months; p=0.048), and relapse-free survival (RFS) (25. vs. 12 months; p=0.024). The prognostic impact of MYC-immunopositivity was retained on multivariate analysis of OS, EFS, and RFS. We conclude that MYC-immunopositivity is an important prognostic factor in patients with untreated AML, particularly those at higher-risk for relapse.
INTRODUCTION
MYC is among the most prevalent oncogenic transcription factors implicated in the genesis of many cancers. MYC deregulation stimulates proliferation and inhibits terminal differentiation [1]. Multiple mechanisms contribute to MYC deregulation including translocations, amplification, and hyperactivated MYC transcription. In hematologic malignancies, MYC overexpression was first recognized in Burkitt lymphoma as a result of translocation with IGH, or less often IGK or IGL [2]. MYC translocations subsequently were recognized in subsets of other lymphoma types. Aberrant MYC expression assessed by immunohistochemistry (IHC) is currently used for lymphoma prognostication [3].
Unlike Burkitt lymphoma, where MYC overexpression is attributed to MYC translocation, the cause of MYC overexpression and deregulation in myeloid neoplasms is ill-defined [1,4]. As a downstream target and regulator of oncogenes [1], MYC maintains tumorigenesis through regulation of miR-17-92, which controls chromatin regulatory and apoptosis genes [5]. MYC-mediated transactivation also involves a variety of epigenetic processes[6] and is dependent upon interactions with histone acetyltransferases[6–8]. MYC regulates histone methylation and controls histone acetylation[7].
The bone marrow (BM) microenvironment is also impacted by MYC-dependent mechanisms. Stroma-mediated protection of both leukemia cell-lines and primary cells can be overcome by using the MYC-inhibitor 10058-F4 [9], providing a rationale for targeting MYC as a treatment for both leukemia and the microenvironment. New agents under development may modify the course of AML by indirectly inhibiting MYC expression (e.g., BET inhibitors) or by blocking MYC transactivation (e.g., small molecules) [1].
Previous preclinical studies demonstrated increased expression of MYC RNA in FLT3-ITD-transduced hematopoietic stem cells [10]. Furthermore, up-regulation of MYC mRNA was observed in CD34+ cells from small numbers of patients with myelodysplastic syndrome (MDS) with trisomy 8 [11]. However, larger clinical studies relating molecular and cytogenetic features to MYC protein expression in AML are lacking.
Although the myeloid leukemogenic activity of MYC was demonstrated in murine models in 1986, the role of MYC in acute myeloid leukemia (AML) has received little attention [12–14]. However, as new drugs targeting MYC show clinical activity in AML, understanding MYC protein expression in AML is of critical importance [15]. Herein, we assessed MYC protein expression by immunohistochemistry (IHC) and explored its prognostic impact in a contemporary cohort of patients with untreated AML.
MATERIALS AND METHODS
We identified 265 untreated patients with AML referred to MD Anderson Cancer Center during 2007–2014. We reviewed BM morphology in all cases and sub-classified using the World Health Organization(WHO)-2016 classification[16–18] for all cases except for the following: 1) those involving CEBPA, for which WHO–2008[19] classification was used (since the biallelic nature of CEBPA mutations could not be determined with certainty) and 2) RUNX1 mutations were not tested for all cases. Multicolor flow cytometry immunophenotypic analysis was performed [20,21]. This study was approved by the Institutional Review Board and conducted according to the Declaration of Helsinki.
MYC-IHC was performed on BM biopsy sections using a pre-diluted rabbit monoclonal antibody specific for MYC (clone Y69, Ventana Medical Systems, Tuscon, AZ, USA) as described previously[22]. Control BM samples obtained from 21 patients without a hematologic neoplasm were also assessed. Two hematopathologists independently assessed MYC expression manually (visually) and using computer-assisted image analysis (Aperio Technologies, Vista, CA, USA) [23]. Inter-observer reliability assessed with a kappa-statistic showed agreement between observations (p<0.001).
MYC-immunopositivity was determined as the mean percentage of blasts showing moderate to strong nuclear MYC expression counted in 5 high-power (400X objective) microscopic fields. Areas were selected based on the presence of sheets of blasts with highest MYC expression. MYC-immunopositivity was considered within normal range if ≤3% BM blasts showed nuclear staining (Figure 1A); and positive if >3% of BM blasts showed moderate to strong nuclear reactivity (Figure 1B) [22]. These ranges were based on assessment of control cases (n=20) demonstrating MYC-immunopositivity in ≤3% blasts.
Figure 1.
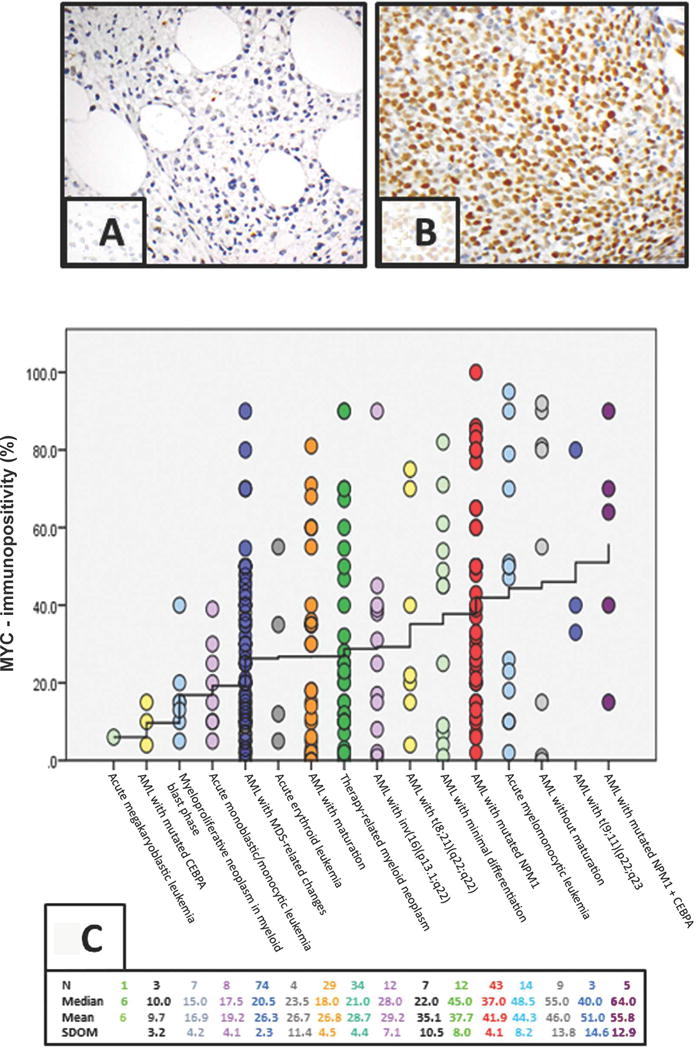
A. Negative MYC-immunopositivity (≤3%) by immunohistochemistry (IHC) in AML patient.
B. Positive MYC-immunopositivity >90% of AML blasts by IHC.
C. MYC-immunopositivity by IHC by WHO categories: MYC values of 265 patients are plotted against WHO classification-categories. Each dot represents one patient. Position of each dot with respect to the X-axis identifies the WHO category to which the patient belongs, and its position with respect to the Y-axis gives the patient’s MYC expression. The WHO categories along the X-axis are arranged in an increasing order of their mean MYC-immunopositivity values. The “stair-step” interpolation line connects the mean MYC-immunopositivity values of WHO categories. The interpolation line is seen to rise from a MYC-immunopositivity value of 6% on the far left to a MYC value of 55.8% on the far right. Additional relevant statics are listed above the plot along four rows. The number of patients (N) in each WHO category is given along the first row. The median MYC-immunopositivity values of each category are listed along the second row. The third row contains the mean MYC-immunopositivity value of each WHO category; and the corresponding standard deviations of the means (SDOM) are listed along the fourth row.
Conventional cytogenetic analysis and fluorescence in situ hybridization (FISH) analysis was performed on BM as previously described [22,24]. Molecular analysis for NPM1, CEBPA and FLT3 mutations was performed by using PCR-based assays. On selected cases, we performed DNA copy number analysis on peripheral blood (PB) or BM, as described elsewhere [25]. Data were analyzed using CytoGenomics 2.9 (Agilent Technologies).
Quantitative reverse-transcriptase PCR was used to assess the levels of MYC mRNA in CD34+ cells sorted by flow cytometry in BM of healthy donors and AML patients as described elsewhere[26].
Statistical Analysis
Summary statistics were used for study population description. Chi-square (or Fisher’s exact test) and t-test (or Wilcoxon’s rank or Kruskal-Wallis) were used to determine differences between groups. Pearson’s correlation test was used to evaluate the correlation between MYC-immunopositivity and markers of proliferation, gene mutations and core-binding factors. Survival was calculated as the months from the start of treatment to event (i.e. death) or last follow-up date; patients who were alive or without event at their last follow-up were then censored. The Kaplan-Meier product limit method [27] was used to estimate median survival. Cox proportional hazards regression models [28] were used to identify any associations with each of the variables and survival outcomes including overall survival (OS), event-free survival (EFS) and relapse-free survival (RFS).
A full multivariate analysis was used to model the association between survival outcomes and MYC, while adjusting for factors with p <0.25 at the univariate analysis and had less than 25 missing values. X-tile software was used to determine a clinically relevant cut-point for MYC-immunopositivity for each survival outcome. This software creates a two-dimensional plot with a log-rank chi-square value at every possible cut-point of the MYC marker. The cut-point that provides the maximum chi-square value is then presented as the “optimal” cut-point [29]. Statistical analysis was performed using Stata/SE version 14.1 statistical software (Stata Corp. LP, College Station, TX).
RESULTS
MYC protein expression varies between WHO categories and impacts remission duration
The study included 142 (54%) men and 123 (46%) women with a median age of 63 years (range, 22-88). Only 17 patients (6%) had trisomy 8, where MYC resides (Table 1A). Overall, 238 of 265 (90%) cases of AML showed MYC overexpression in BM compared with normal BM; the mean number of MYC-immunopositive cells was 32% (range, 0-100). Only 27 patients had normal MYC-immunopositivity of ≤ 3%. Both median and mean MYC expression varied significantly between WHO subtypes and was disease-characterizing (p=0.004) (Figure 1C). Among all 265 patients, MYC-immunopositivity correlated positively with markers of proliferation: serum LDH (p<0.001), WBC count (p=0.001), BM blasts (p<0.001), PB blasts (p<0.001), and FLT3-ITD (p<0.001). MYC-immunopositivity also correlated with the presence of mutated NPM1 (p=0.009) alone or dual NPM1+CEBPA+ mutations (p=0.040). Fifty-two of 253 tested patients harbored FLT3-ITD mutations (median MYC-immunopositivity 46.9%, range, 10-100). Notably, 98% of FLT3-ITD mutated patients (51/52) had MYC-immunopositivity >6%.
Table 1.
The clinical and laboratory features, and results of MYC-immunopositivity by IHC.
A describes the entire 265 patients with AML of all risk types.
B describes the 241 patients with AML who are older (≥55 years of age) and/or intermediate-risk and high-risk.
| Parameters | Table 1A | Table 1B | ||||
|---|---|---|---|---|---|---|
| AML Patients of all Risk Types (n=265) | Mean MYC IHC | p-value | Older and/or Intermediate-risk and High-risk AML (n=241) | Mean MYC IHC | p-value | |
| Age, Median (Range) | 63 (22-88) | 25.0 | 0.165 | 66 (22-88) | 31.0 | 0.315 |
| Sex | 0.105 | 0.095 | ||||
| F | 123 (46) | 34.6 | 109 (45) | 34.0 | ||
| M | 142 (54) | 29.4 | 132 (55) | 28.6 | ||
| Cytogenetic category | 0.614 | 0.686 | ||||
| Core Binding Factor | 19 (7) | 31.4 | 11 (5) | 30.3 | ||
| Diploid | 155 (58) | 32.6 | 141 (59) | 32.1 | ||
| Trisomy 8 | 17 (6) | 38.3 | 16 (7) | 34.4 | ||
| -5/5q- | 10 (4) | 31.0 | 10 (4) | 31.0 | ||
| -5/5q- and -7/7q- | 12 (5) | 22.9 | 12 (5) | 22.9 | ||
| -7/7q- | 12 (5) | 21.5 | 12 (5) | 21.5 | ||
| abnormal 11q | 9 (3) | 45.0 | 9 (4) | 45.0 | ||
| -Y | 1 (0.4) | 25.0 | 1 (0.4) | 25.0 | ||
| 20q- | 1 (0.4) | 2.0 | 1 (0.4) | 2.0 | ||
| Miscellaneous | 24 (9) | 30.8 | 23 (10) | 29.3 | ||
| Insufficient Metaphases | 3 (1) | 26.7 | 3 (1) | 26.7 | ||
| Not Done | 2 (1) | 18.5 | 2 (1) | 18.5 | ||
| Cytogenetic Risk Group | 0.946 | 0.901 | ||||
| Favorable | 19 (7) | 31.4 | 11 (5) | 30.3 | ||
| Adverse (complex, chromosome 5/7) | 45 (17) | 30.9 | 45 (19) | 30.9 | ||
| Diploid | 156 (59) | 32.6 | 142 (59) | 32.1 | ||
| IM*/ND** | 5 (2) | 23.4 | 5 (2) | 23.4 | ||
| Intermediate (including +8, noncomplex, miscellaneous) | 40 (15) | 31.3 | 38 (16) | 28.6 | ||
| De Novo vs. Secondary AML | 0.012 | 0.039 | ||||
| De Novo | 157 (59) | 35.1 | 131 (54) | 34.1 | ||
| Secondary | 108 (41) | 27.1 | 110 (46) | 27.4 | ||
| Prior Chemo/Prior Radiation | 0.114 | 0.913 | ||||
| Yes | 34 (13) | 28.8 | 34 (14) | 28.7 | ||
| No | 231 (87) | 32.3 | 207 (86) | 31.4 | ||
| FLT3-ITD | <0.001 | <0.001 | ||||
| Positive | 52 (21) | 46.8 | 47 (21) | 46.3 | ||
| Negative | 201 (79) | 28.7 | 182 (79) | 27.9 | ||
| FLT3-D835 | 0.420 | 0.226 | ||||
| Positive | 13 (5) | 38.1 | 12 (5) | 40.3 | ||
| Negative | 240 (95) | 32.2 | 217 (95) | 31.2 | ||
| RAS | 0.786 | 0.678 | ||||
| Positive | 34 (14) | 32.9 | 27 (12) | 32.8 | ||
| Negative | 217 (86) | 31.6 | 200 (88) | 30.6 | ||
| NPM1 | 0.001 | 0.003 | ||||
| Positive | 53 (27) | 43.2 | 40 (23) | 43.0 | ||
| Negative | 141 (73) | 29.5 | 133 (77) | 29.2 | ||
| CEPBA | 0.299 | 0.269 | ||||
| Positive | 15 (10) | 38.6 | 12 (10) | 38.0 | ||
| Negative | 130 (90) | 31.5 | 114 (90) | 30.1 | ||
IM= insufficient metaphases;
ND=not done
The clinical and laboratory features, and results of MYC-immunopositivity by IHC for the 265 patients are summarized in Table 1A. Mean MYC-immunopositivity was significantly higher in patients with de novo AML (n=157, 35.1%) compared to those with secondary AML (n=108, 27.1%) (p=0.012). For survival estimation, MYC-immunopositivity >6% was determined as an optimal cut-off point.
For the entire cohort of 265 patients, complete remission (CR) duration for patients with MYC-immunopositivity ≤6% (n=27) was significantly longer than for those with MYC-immunopositivity >6% (n=154), (23 vs. 12 months; p=0.028). Relapse-free survival was marginally superior for patients with MYC-immunopositivity ≤6% compared to those with MYC-immunopositivity >6% (25 vs. 13 months; p=0.078). However, we found no statistically significant difference in OS (16 vs. 24, p=0.156) or EFS (14 vs. 8 months, p=0.173).
Given the increasing complexity of mutation-based prognostication models for patients with AML who are older or who have intermediate-/high-risk disease, we sought to assess whether MYC-immunopositivity by IHC could be a rapid, cost-effective prognostication tool. Consequently, we focused on older patients (≥55-years) of all risk groups and on patients of all ages with intermediate- or high-risk AML (n=241). These patients are known to be at higher risk for relapse [30–34]. Patients with AML who are ≥55-years old have generally been considered “older” in the literature [33,35–39] and because ≥55-years is an established age group associated with more adverse cytogenetics and higher risk for relapse, that was the cutoff selected to denote “older” for this analysis [30–34]. Furthermore, many molecular biomarkers either lose their prognostic value or have not been assessed in this age group because patients ≥55-years were historically excluded from clinical trials [40–43]; thus, the impact of MYC-immunopositivity warrants further exploration in these patients. To analyze the impact of MYC-immunopositivity in patients at higher risk for relapse, we excluded patients <55-years with favorable-risk AML (n=24) from the original 265 patients.
MYC protein expression is prognostic in patients ≥55-years of age and in all patients with intermediate-risk or high-risk disease
Among patients ≥55-years-old of all risk types and patients of all ages with intermediate- and high-risk AML (n=241), those with MYC-immunopositivity ≤6% (n=40) demonstrated statistically significantly longer median OS (p=0.042), EFS (p=0.048), RFS (p=0.024) (Figures 2A-2C) and CR duration [23 (n=25) vs. 10 months (n=121); p=0.007]. Cytogenetic, molecular, and clinical data of these patients are shown in Table 1B. In addition to MYC-immunopositivity ≤6%, factors on univariate analysis associated with longer OS included high-dose cytarabine (HiDAC)-based therapy compared to hypomethylating agents (HMA) and other regimens (p<0.001), Auer rods (p=0.029), and AML with inv(16) (p=0.039). No statistically significant advantage was observed in patients with AML with mutated NPM1. Patients with complex karyotypes (17 vs. 6 months (p<0.001)), prior chemotherapy (15 vs. 7 months (p=0.014)) and history of radiation therapy (15 vs. 7 months (p=0.026) showed inferior survival. On multivariate analysis, when considering age, WBC count, PB blasts, BM monocytes, treatment types (HiDAC, HMA, and other regimens), cytogenetic risk groups, Auer rods, PS>2, FLT3-ITD status, prior chemotherapy, and prior radiation therapy, MYC-immunopositivity >6% retained prognostic significance for median OS (p=0.047) (Table 2A), EFS (p=0.010) and RFS, (p=0.012) (Table 2A). Among patients with available European Leukemia Network (ELN) data (n=194) (Table 2B), the prognostic impact of MYC-immunopositivity >6% was retained for OS (p= 0.012), EFS (p=0.003), and RFS (p=0.003).
Figure 2.
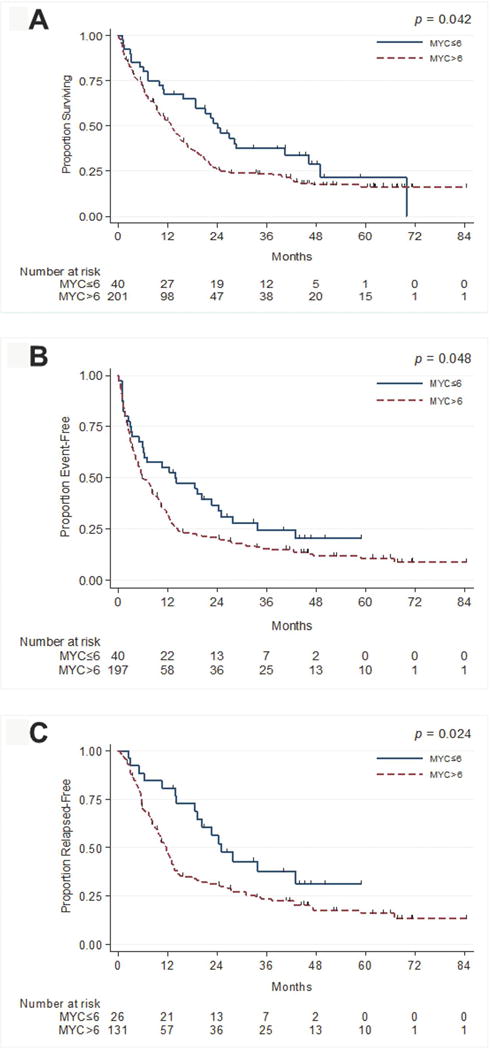
A. Overall survival as a function of MYC-immunopositivity for older and/or intermediate- and high-risk AML patients.
B. Event free survival as a function of MYC-immunopositivity for older and/or intermediate- and high-risk AML patients.
C. Relapse free survival as a function of MYC-immunopositivity for older and/or intermediate- and high-risk AML patients.
Table 2.
Multivariate Analysis (MVA) for overall survival (OS), event-free survival (EFS), and relapse-free survival (RFS) for patients with AML who are older and/or intermediate-risk and high-risk
| OSa | 2A. OS for Entire Cohort (n=241); MYC ≤6 (n=40); MYC >6% (n=201) | 2B. OS accounting for ELN (n=194); MYC ≤6% (n=26); MYC >6% (n=168) | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| MYC>6 vs. MYC ≤6 | 1.67(1.01-2.77) | 0.047 | 2.25 (1.19-4.23) | 0.012 |
| EFSb | EFS for Entire Cohort (n=205); MYC≤6% (n=34); MYC >6% (n=171) | EFS accounting for ELN (n=167); MYC ≤6% (n=22); MYC >6% (n=145) | ||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| MYC>6 vs. MYC ≤6 | 1.81 (1.15-2.83) | 0.010 | 2.39 (1.35-4.21) | 0.003 |
| RFSc | RFS for Entire Cohort (n=139); MYC ≤6% (n=22); MYC >6% (n=117) | RFS accounting for ELN (n=120); MYC ≤6% (n=17); MYC >6% (n=103) | ||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| MYC>6 vs. MYC ≤6 | 2.23 (1.19-4.17) | 0.012 | 2.93 (1.45-5.90) | 0.003 |
MVA shown for OS, accounting for age, WBC, peripheral blood blasts, bone marrow monocytes, treatment type (HMA vs. HiDAC and other vs. HiDAC), cytogenetic risk groups (diploid/intermediate vs. adverse; favorable vs. adverse), Auer rods, PS, FLT3-ITD, prior malignancy, prior XRT and prior chemotherapy. Table 2B also accounts for ELN groups.
MVA shown for EFS, accounting for age, WBC, bone marrow monocytes, treatment type (HMA vs. HiDAC and other vs. HiDAC), cytogenetic risk groups (diploid/intermediate vs. adverse; favorable vs. adverse), Auer rods, FLT3-ITD, prior XRT, and prior chemotherapy. Table 2B also accounts for ELN groups.
MVA shown for RFS, when accounting for age, WBC, treatment type (HMA vs. HiDAC and other vs. HiDAC), cytogenetic risk groups (diploid/intermediate vs. adverse; favorable vs. adverse), Auer rods, FLT3-ITD, prior XRT, and prior chemotherapy. Table 2B also accounts for ELN groups.
HMA=Hypomethylating Agent-based Regimens; HiDAC=High-dose Cytarabine-based Regimens
MYC ≤6 % identifies a diverse subgroup of AML patients with superior outcomes
Given the more favorable outcomes of older, intermediate- and high-risk patients with ≤6% MYC-immunopositivity (n=40), we sought to identify clinical and molecular features that distinguished these patients from those with MYC-immunopositivity >6% (Table 3). AML with MYC-immunopositivity >6% had significantly higher rates of proliferative features: increased BM blasts (p=0.003), increased PB blasts (p<0.001), increased PB monocytes (p<0.005), elevated LDH (p<0.001), and a higher frequency of FLT3-ITD mutations (p=0.003). No statistically significant differences were noted in NPM1 mutation, CEBPA mutation or karyotype between MYC-immunopositivity ≤6% or >6%.
Table 3.
Patient Characteristics by MYC-IHC Above and Below 6%
| MYC ≤6% (n=40) | MYC>6 (n=201) | ||||
|---|---|---|---|---|---|
| Patient Characteristics | Total number of Patients | Median (IQR) | Total number of Patients | Median (IQR) | p-value |
| White Blood Cell Count | 40 | 2.0 (1.4-7.2) | 200 | 6.3 (2.3-25.5) | 0.001 |
| Peripheral Blood Blasts | 40 | 1.0 (0.0-15.0) | 197 | 17.0 (3.0-49.0) | <0.001 |
| LDH | 40 | 503.5 (456.0-776.5) | 194 | 879.0 (549.0-1527.0) | <0.001 |
| Bone Marrow Blasts | 40 | 35.0 (22.0-51.0) | 200 | 44.0 (30.0-69.5) | 0.003 |
| Patient Characteristics | Total number of Patients | % | Total number of Patients | % | p-value |
| Cytogenetic Risk | |||||
| Adverse | 5 | 12.8 | 40 | 20.3 | 0.621 |
| Diploid/Intermediate | 32 | 82.1 | 148 | 75.1 | |
| Favorable | 2 | 5.1 | 9 | 4.6 | |
| Treatment | |||||
| HiDAC* | 14 | 35.9 | 88 | 45.1 | 0.233 |
| HMA** | 4 | 10.3 | 31 | 15.9 | |
| Other | 21 | 53.9 | 76 | 39.0 | |
| Complex | |||||
| No | 34 | 87.2 | 161 | 81.7 | 0.411 |
| Yes | 5 | 12.8 | 36 | 18.3 | |
| FLT3-ITD | |||||
| Negative | 35 | 97.2 | 147 | 76.2 | 0.003 |
| Positive | 1 | 2.8 | 46 | 23.8 | |
| NPM1 | |||||
| Negative | 23 | 92.0 | 110 | 74.3 | 0.071 |
| Positive | 2 | 8.0 | 38 | 25.7 | |
| CEPBA | |||||
| Negative | 16 | 94.1 | 98 | 89.8 | 0.999 |
| Positive | 1 | 5.9 | 11 | 10.1 | |
| t (8;21) | |||||
| No | 39 | 97.5 | 198 | 98.5 | 0.519 |
| Yes | 1 | 2.5 | 3 | 1.5 | |
| inv(16) | |||||
| No | 39 | 97.5 | 195 | 97.0 | 0.999 |
| Yes | 1 | 2.5 | 6 | 3.0 | |
HiDAC=High-dose Cytarabine-based Regimens
HMA=Hypomethylating Agent-based Regimens
Even in patients with adverse pathologic, molecular or cytogenetic features, MYC-immunopositivity ≤6% conferred better outcomes, suggesting that MYC protein expression influenced disease biology in ways beyond the molecular/cytogenetic features. Among the 40 patients with MYC-immunopositivity ≤6%, 19/40 (48%) had adverse-risk features whereas 5/40 (13%) had favorable-risk features. The remaining 16/40 (40%) were intermediate-risk.
Among the 201 patients with MYC-immunopositivity >6%, 41 (20%) had favorable-risk features, whereas 101 (50%) had adverse-risk features and 59 (29%) had intermediate-risk AML.
MYC-immunopositivity >6% portends a poor outcome in older patients
Patients with AML who are ≥55-years-old have generally been considered “older”[33,35–39] and have historically been excluded from many clinical trials [34,35]. Thus, we focused on AML patients ≥55 years of age, as they have higher rates of relapse[30–34], early death [32–34], unfavorable cytogenetics [36,44,45], and given that the clinical significance of commonly assessed molecular biomarkers are already well-established in younger AML patients [40–43], particularly <55-years-old [46]. Our patient cohort contained 194 patients ≥55 years of age (median age 68.5 years; range, 55–88).
These older patients with MYC-immunopositivity ≤6% (n=33) had a superior median OS (p=0.035) (Figure 3A) and RFS (p=0.018) compared to those with MYC-immunopositivity >6% (n=161). This survival advantage was retained on multivariate analysis (Supplemental Table 1A), even when accounting for ELN groups (Supplemental Table 1B). In evaluable patients, the duration of CR for patients with MYC-immunopositivity ≤6% (n=19) was superior to that of the 100 patients with MYC-immunopositivity >6%, 25 months vs. 9 months (p=0.002). The EFS for patients with MYC-immunopositivity ≤6% (n=33) was marginally superior to those with MYC-immunopositivity >6% (n=157) (p=0.076).
Figure 3.
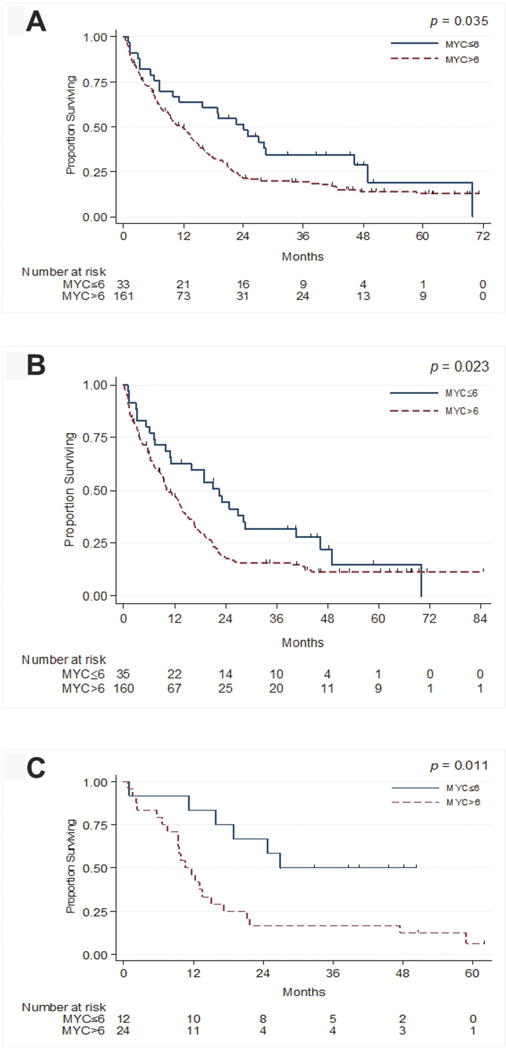
A. Overall survival as a function of MYC-immunopositivity for older AML patients.
B. Overall survival as a function of MYC-immunopositivity for patients of all ages with intermediate- and high-risk AML.
C. Overall survival for older and/or intermediate- and high-risk AML patients treated on a protocol of clofarabine low dose cytarabine alternating with decitabine.
MYC-immunopositivity >6% portends a worse outcome in patients with intermediate-risk and high-risk disease
For patients of all ages (median, 66 years; range, 22-86 years) with intermediate- and high-risk AML (n=195), those with MYC-immunopositivity ≤6% (n=35) had a superior median OS (23 vs. 10 months; p=0.023) (Figure 3B), EFS [(11 vs. 5 months; p=0.033), RFS (24 vs. 10 months; p=0.018) (Supplemental Table 2), and CR duration (21 vs. 9 months (n=96); p=0.003)] compared to patients with MYC-immunopositivity >6%. On multivariate analysis, MYC-immunopositivity >6% retained prognostic significance for median OS (p=0.012) and RFS (p=0.030); the difference for EFS approached significance (p=0.066) (Supplemental Table 2A). When accounting for ELN data (available for 153/195 patients), MYC-immunopositivity ≤6% retained significance for median OS (p=0.015), EFS (p=0.049), and RFS (p=0.031) (Supplemental Table 2B).
MYC-immuno positivity >6% portends poor outcomes in subgroups of patients undergoing less intense therapies, but is not predictive for patients receiving intensive HiDAC-based regimens
As treatment has substantial bearing on AML patient outcomes, we examined the prognostic impact of MYC-immunopositivity >6% in subgroups of patients ≥ 55 years of age (of all risk types) and patients of all ages with intermediate- and high-risk AML who were treated similarly: intensive HiDAC-based regimens (n=102), hypomethylator-based regimens (n=35), and other therapies (n=97). Patients who were considered too elderly or frail for HiDAC therapy were offered hypomethylator-based regimens or other therapies. Other therapies consisted of a diverse range of generally less intensive regimens without HiDAC, including mono-therapies, various combinations of investigational agents combined with low-intensity chemotherapy, low-intensity combination regimens (e.g., the combination of clofarabine, low-dose cytarabine (LDAC), and decitabine[47]), as well as “7+3” combined with investigational agents. Among patients on HiDAC-based regimens, there were no statistically significant differences in median OS (24 vs. 21 months; p=0.679), median EFS (20 vs. 12 months; p=0.345), or median RFS (23 vs. 14 months; p=0.603) between patients with MYC-immunopositivity ≤6% versus >6%. Among the patients on hypomethylator-based regimens, those with MYC-immunopositivity ≤6% had a longer median OS (7 vs. 6 months; p=0.032); however, patient numbers were small. There were no significant differences in median EFS (3 vs. 2.83 months; p=0.078) or RFS (not reached vs. 5 months; p=0.122). Among the 97 patients on other regimens without HiDAC, those with MYC-immunopositivity ≤6% had marginally superior median OS (23 vs.11 months; p=0.050) and significantly superior EFS (14 vs. 6 months; p=0.043), and RFS (34 vs. 10 months; p=0.005).
The largest proportion of favorable-risk WHO groups (23/102, 23%) was in the HiDAC group. Fewer patients (16/97, 16%) on “other” regimens without HiDAC had favorable-risk AML. Among the 35 patients on hypomethylator-based regimens, 20% had favorable features. These findings demonstrate the potential prognostic impact of MYC-immunopositivity in patients who are not candidates for standard HiDAC-based intensive chemotherapy. Thus, the impact of MYC-immunopositivity is context-dependent.
In patients with secondary AML, including therapy-related myeloid neoplasm (t-MN) cases and AML with myelodysplasia-related changes (AML-MRC) (n=108), patients with MYC-immunopositivity ≤6% had longer median OS compared to those with MYC-immunopositivity >6% (11 vs. 8 months, p=0.031). Furthermore, although the patient numbers are small, in t-MN patients, those with MYC-immunopositivity ≤6% (n=5) had a longer median OS compared to those with MYC-immunopositivity >6% (n=29) (46 vs. 5 months, p=0.024).
To validate our findings in patients receiving the same therapy, we analyzed a subgroup of patients (n=36) uniformly treated on the prospective clinical trial of clofarabine, low-dose cytarabine (LDAC) and decitabine [47]. In this homogeneously treated group of older, intermediate- and high-risk AML patients, MYC-immunopositivity ≤6% (n=12) conferred longer OS (27 vs. 11 months; p=0.011) (Figure 3C), EFS (24 vs. 8 months; p=0.035), and RFS (43 vs. 10 months; p=0.010), suggesting that on this therapy MYC-immunopositivity is a clinically significant prognostic factor.
MYC DNA Copy Number and mRNA Analysis
In this study, data on MYC DNA copy number, estimated by whole exome sequencing, was available for 54 patients. Only two patients had segmental mean copy number values high enough to suggest copy number gain or amplification (cut-off set at >0.5 based on previous studies [48,49]). No association was noted between MYC DNA copy number changes and MYC-immunopositivity. This observation prompted us to prospectively explore MYC amplification by array comparative genomic hybridization (array CGH) in 10 additional patients. Only 3 of these patients showed MYC amplification: two with DNA copy number gain manifesting as trisomy 8 and one with isochromosome 8q associated with gain of 1 extra copy of MYC, confirmed by FISH (Supplementary Figures 1A and 1B). Median MYC-immunopositivity in these 3 AML patients with MYC amplification was 40% versus 35% 7 in AML patients without gene amplification (p=0.569), although the numbers are small. Further, in the Cancer Genome Atlas [50], MYC DNA copy number gain data showed a small subset of AML patients (17/154) demonstrating MYC amplification with a segmental mean value >0.5, further supporting our observation that MYC amplification is rare in AML.
Similar to earlier reports of up-regulation of MYC mRNA in CD34+ cells from patients with MDS with trisomy 8 [11], our findings suggest that MYC amplification is not the only driver of MYC expression in AML. Hence, we analyzed MYC mRNA expression in 9 AML patients (selected based on a broad range of MYC-immunopositivity, 0-100%) and an additional 4 controls. There was significant over-expression of MYC mRNA in AML patients compared to controls (p=0.015) (Figure 2B). In 7 of 9 cases, elevated MYC-immunopositivity corresponded with elevated MYC mRNA (Figure 2C).
DISCUSSION
Despite new molecular diagnostic technologies, prognostication for intermediate-risk, high-risk, and older AML patients remains challenging and complex. These challenges are due to multiple biologic and technical factors including co-occurring molecular/cytogenetic abnormalities and varying allelic burdens. Thus, MYC-IHC represents a rapid and inexpensive tool that could provide clinically valuable information. Besides the association with FLT3-ITD, mutated NPM1, or dual NPM1+CEBPA mutations, there were no other significant associations between MYC-immunopositivity and other molecular abnormalities. However, MYC was an independent prognostic factor even when accounting for multiple factors including FLT3-ITD status, cytogenetic risk groups, and ELN groups (which incorporate cytogenetic and molecular data).
Previously, Mughal et al. used a different method (tissue microarray) to assess the impact of MYC protein expression in AML, but their analysis lacked molecular data and they did not show a significant survival impact on multivariate analysis [51]. In contrast, our dataset was larger, contained ample cytogenetic and molecular data, and we showed that MYC-immunopositivity ≤6% is associated with superior remission duration when assessing the entire heterogeneous group of 265 untreated AML patients, as well as superior survival in subgroups of older, intermediate-, and high-risk patients. MYC-immunopositivity >6% is an important prognostic factor for OS, EFS, RFS, and CR duration when considering older, intermediate-, and high-risk patients.
The finding in this study of relatively higher MYC-immunopositivity in the core-binding factor leukemias (Figure 1C) (although not statistically significant) are consistent with previous data showing elevated MYC mRNA in core binding factor AML [52]. The association observed in our study between higher MYC-immunopositivity and mutated NPM1 may be attributable to the preclinical observations; others have shown that NPM1 dislocation into the cytoplasm allows nuclear transcription sites previously occupied by wild-type NPM1 to become re-occupied by BRD4, which then upregulates MYC expression [53].
The literature on MYC protein expression in AML is limited. This is the largest study of MYC expression in AML to date. Our comprehensive assessment of MYC-immunopositivity according to WHO groups shows that MYC-immunopositivity is distinct and disease characterizing for each WHO category, suggesting its potential role for sub-classification of AML. While MYC-immunopositivity was significantly prognostic for duration of CR when combining all categories of untreated AML patients, including all ages and risk-groups, higher MYC levels in certain favorable-risk AML categories (e.g. core-binding factor and AML with NPM1) likely contribute to the context-dependent prognostic effect of MYC-immunopositivity on survival, potentially limiting its survival impact in the context of the HiDAC-based treatment group, which had higher proportions of favorable-risk patients. Nevertheless, higher MYC was consistently associated with poorer survival in older, intermediate-, and high-risk AML patients. Larger studies exploring uniformly treated patient populations with similar risk-status are needed to determine whether the prognostic impact of MYC overexpression might be treatment-specific. A limitation of this study is that post-treatment MYC-IHC values were not available for analysis; however, we are now investigating post-therapy samples as well as the potential impact of MYC-immunopositivity on outcome after hematopoietic stem cell transplantation.
Patients with AML who are ≥55-years-old have historically been considered “older”[33–39] in the AML literature. For this reason, we chose 55 years as the cutoff to denote older patients. However, given that 60 years and older is also commonly considered as “older” in AML research[54], we also examined this cutoff (n=165) and observed that MYC-immunopositivity ≤ 6 %(n=30) was marginally prognostic for superior OS (p=0.05) and EFS (p=0.069), while significantly prognostic for superior CR duration (p=0.001).
It is notable that our cut-off for MYC-immunopositivity of ≤6%, just marginally above the normal range of MYC-immunopositivity (≤3%), was prognostic; this observation is consistent with pre-clinical observations that slight changes in MYC mRNA and protein expression substantially impact prognosis in transgenic mouse lines, where low MYC protein expression in hematopoietic precursors leads to myeloid leukemias and high MYC protein expression results in rapid onset T-cell lymphomas. OS of the mice was inversely related to MYC protein expression [55].
MYC overexpression leads to deregulation of a variety of cellular functions, including induction of genomic instability, cell immortalization, inhibition of differentiation [1,12], and maintenance of pluripotency[1]–all of this can contribute to adverse outcomes in patients with AML. MYC also maintains leukemic proliferation through multiple mechanisms including regulation of target genes, favoring cell cycle progression by repressing cyclin dependent kinase (CDK) inhibitors and activating CDKs[1].
Furthermore, MYC is well established as a regulator of microRNAs that are critical to metabolism, signal transduction, and anti-apoptotic pathways[56]. Many mechanisms may contribute to MYC overexpression in the absence of translocations, such as DNA amplification, protein stabilization[1], or cytogenetic abnormalities in AML. Multiple leukemogenic transcription factors are generated by recurrent translocations in AML including RUNX-RUNX1T1 [17,57,58] and they induce MYC expression [17,57,58]. MYC amplification in AML has manifested as double minute chromosomes (dmins) and homogeneous staining regions [1]. In our previous study of 22 patients with myeloid neoplasms with MYC amplification manifesting as dmins, MYC-immunopositivity was overexpressed, but varied between 15–90%, suggesting mechanisms beyond amplification contribute to MYC-immunopositivity [22]. Only one previously reported patient[24] in this analysis had t(8;14)(q24;q32); thus the prognostic impact of this abnormality in AML could not be assessed. AML with t(8;14) (q24;q32) is extremely rare. In the few reports available in the literature, AML with t(8;14)(q24;q32) is associated with poor outcomes[24,59]. In two previously reported cases of AML with MYC rearrangement from our group, MYC-immunopositivity was only marginally increased[24] and in the present study, MYC rearrangement was not present by FISH in 8 tested cases of normal karyotype AML with variable MYC-immunopositivity (0-70%), suggesting that MYC rearrangement is not a driver of MYC-immunopositivity in AML. This observation highlights the fact the regulators of MYC protein expression are quite complex and future studies are needed to understand these processes, particularly since targeting MYC therapeutically in AML is becoming increasingly possible [15].
In conclusion, MYC overexpression by IHC is a strong prognostic factor for remission duration in AML patients of all ages and risk types. MYC overexpression is also an important biomarker for survival in untreated patients ≥55 years of age (all risk groups) and intermediate- and high-risk AML patients of all ages, with higher MYC-immunopositivity conferring an inferior prognosis. MYC IHC is rapid, inexpensive, and could be easily adopted for standard evaluation of AML patients. Further studies are warranted to validate these findings and understand the drivers of MYC protein expression.
Supplementary Material
Supplementary Figure 1A. MYC Amplification shown by array CGH with DNA copy number gain manifesting as trisomy 8 and one with isochromosome 8q.
Supplementary Figure 1B. Amplification is shown by FISH. One extra copy of the MYC gene is confirmed by FISH.
Supplementary Figure 2A. MYC mRNA expression in normal control CD34+ cells and AML patient cells.
Supplementary Figure 2B. MYC IHC and MYC mRNA in AML patients.
Acknowledgments
This work was supported in part by Leukemia SPORE Grant P50CA100632 from the National Institutes of Health.
We acknowledge Lynne Abruzzo for her important input on cytogenetic data analysis and MYC expression analysis.
SMP was supported by a National Cancer Institute/National Institutes of Health Award (R01CA207204).
Footnotes
Authorship Contributions:
MO collected and assembled the data, analyzed and interpreted the data, and wrote the manuscript.
XH, GNG, and UR performed the statistical analysis.
SMP collected and assembled the data and analyzed MYC mRNA by qRT-PCR which was supported by a National Cancer Institute/National Institutes of Health Award (R01CA207204).
UR, RK-S, SL, CBR, LJM, JC, MA, GNG, PR, HK, AF, ZZ, PH, SMP, LA, YH, JZ, KT, and MJH analyzed data and edited the manuscript.
ZE, HMK, RK-S, SV, XH, GGM, LJM, FR, SK, GB, KB, CBR, KB and JEC reviewed and approved the manuscript
Conflict of Interest Disclosures:
None of the authors have any relevant conflicts of interest to disclose.
References
- 1.Delgado MD, Albajar M, Gomez-Casares MT, Batlle A, Leon J. MYC oncogene in myeloid neoplasias. Clin Transl Oncol. 2013;15:87–94. doi: 10.1007/s12094-012-0926-8. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Beato M, Sanchez-Aguilera A, Piris MA. Cell cycle deregulation in B-cell lymphomas. Blood. 2003;101:1220–1235. doi: 10.1182/blood-2002-07-2009. [DOI] [PubMed] [Google Scholar]
- 3.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schick M, Habringer S, Nilsson JA, Keller U. Pathogenesis and therapeutic targeting of aberrant MYC expression in haematological cancers. Br J Haematol. 2017;179:724–738. doi: 10.1111/bjh.14917. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Choi PS, Casey SC, Dill DL, Felsher DW. MYC through miR-17-92 suppresses specific target genes to maintain survival, autonomous proliferation, and a neoplastic state. Cancer cell. 2014;26:262–272. doi: 10.1016/j.ccr.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortiguera M, Batlle-López A, Albajar M. MYC as therapeutic target in leukemia and lymphoma. Blood and Lymphatic Cancer: Targets and Therapy Dovepress. 2015;2015:75–91. [Google Scholar]
- 7.Luscher B, Vervoorts J. Regulation of gene transcription by the oncoprotein MYC. Gene. 2012;494:145–160. doi: 10.1016/j.gene.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Eilers M, Eisenman RN. Myc’s broad reach. Genes Dev. 2008;22:2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia B, Tian C, Guo S, et al. c-Myc plays part in drug resistance mediated by bone marrow stromal cells in acute myeloid leukemia. Leukemia research. 2015;39:92–99. doi: 10.1016/j.leukres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Piloto O, Kim KT, et al. FLT3/ITD expression increases expansion, survival and entry into cell cycle of human haematopoietic stem/progenitor cells. Br J Haematol. 2007;137:64–75. doi: 10.1111/j.1365-2141.2007.06525.x. [DOI] [PubMed] [Google Scholar]
- 11.Sloand EM, Pfannes L, Chen G, et al. CD34 cells from patients with trisomy 8 myelodysplastic syndrome (MDS) express early apoptotic markers but avoid programmed cell death by up-regulation of antiapoptotic proteins. Blood. 2007;109:2399–2405. doi: 10.1182/blood-2006-01-030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coppola JA, Cole MD. Constitutive c-myc oncogene expression blocks mouse erythroleukaemia cell differentiation but not commitment. Nature. 1986;320:760–763. doi: 10.1038/320760a0. [DOI] [PubMed] [Google Scholar]
- 13.Prochownik EV, Kukowska J. Deregulated expression of c-myc by murine erythroleukaemia cells prevents differentiation. Nature. 1986;322:848–850. doi: 10.1038/322848a0. [DOI] [PubMed] [Google Scholar]
- 14.Dmitrovsky E, Kuehl WM, Hollis GF, Kirsch IR, Bender TP, Segal S. Expression of a transfected human c-myc oncogene inhibits differentiation of a mouse erythroleukaemia cell line. Nature. 1986;322:748–750. doi: 10.1038/322748a0. [DOI] [PubMed] [Google Scholar]
- 15.Dawson M, Stein E, Huntly B, Karadimitris A. In: A Phase I Study of GSK525762, a Selective Bromodomain (BRD) and Extra Terminal Protein (BET) Inhibitor: Results from Part 1 of Phase I/II Open Label Single Agent Study in Patients with Acute Myeloid Leukemia (AML) Acute Myeloid Leukemia: Novel Therapy eTPI, editor. American Society of Hematology (ASH); 2017. Abstract 2017. [Google Scholar]
- 16.Hong M, He G. 2016 Revision to the WHO Classification of Acute Myeloid Leukemia. J Transl Int Med. 2017;5:69–71. doi: 10.1515/jtim-2016-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arber D, Orazi A, Hasserjian RP. Introduction and overview of the classification of the myeloid neoplasms. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th. IARC: Lyon; 2017. pp. 16–27. 2017. [Google Scholar]
- 18.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th. IARC: Lyon; 2017. 2017. [Google Scholar]
- 19.Swerdlow S, Camp E, Harris NL. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. 2008. [Google Scholar]
- 20.Huh YO, Keating MJ, Saffer HL, Jilani I, Lerner S, Albitar M. Higher levels of surface CD20 expression on circulating lymphocytes compared with bone marrow and lymph nodes in B-cell chronic lymphocytic leukemia. Am J Clin Pathol. 2001;116:437–443. doi: 10.1309/438N-E0FH-A5PR-XCAC. [DOI] [PubMed] [Google Scholar]
- 21.Ravandi F, Jorgensen J, Borthakur G, et al. Persistence of minimal residual disease assessed by multiparameter flow cytometry is highly prognostic in younger patients with acute myeloid leukemia. Cancer. 2017;123:426–435. doi: 10.1002/cncr.30361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huh YO, Tang G, Talwalkar SS, et al. Double minute chromosomes in acute myeloid leukemia, myelodysplastic syndromes, and chronic myelomonocytic leukemia are associated with micronuclei, MYC or MLL amplification, and complex karyotype. Cancer Genet. 2016;209:313–320. doi: 10.1016/j.cancergen.2016.05.072. [DOI] [PubMed] [Google Scholar]
- 23.Loghavi S, Al-Ibraheemi A, Zuo Z, et al. TP53 overexpression is an independent adverse prognostic factor in de novo myelodysplastic syndromes with fibrosis. Br J Haematol. 2015;171:91–99. doi: 10.1111/bjh.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohanian M, Bueso-Ramos C, Ok CY, et al. Acute myeloid leukemia with MYC rearrangement and JAK2 V617F mutation. Cancer Genet. 2015;208:571–574. doi: 10.1016/j.cancergen.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehrotra M, Luthra R, Ravandi F, et al. Identification of clinically important chromosomal aberrations in acute myeloid leukemia by array-based comparative genomic hybridization. Leuk Lymphoma. 2014;55:2538–2548. doi: 10.3109/10428194.2014.883073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallardo M, Lee HJ, Zhang X, et al. hnRNP K Is a Haploinsufficient Tumor Suppressor that Regulates Proliferation and Differentiation Programs in Hematologic Malignancies. Cancer cell. 2015;28:486–499. doi: 10.1016/j.ccell.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 28.Cox D. Regression models and life tables (with discussion) J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 29.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 30.Hiddemann W, Kern W, Schoch C, et al. Management of acute myeloid leukemia in elderly patients. J Clin Oncol. 1999;17:3569–3576. doi: 10.1200/JCO.1999.17.11.3569. [DOI] [PubMed] [Google Scholar]
- 31.Goldstone AH, Burnett AK, Wheatley K, et al. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1302–1311. doi: 10.1182/blood.v98.5.1302. [DOI] [PubMed] [Google Scholar]
- 32.Erba HP. Has there been progress in the treatment of older patients with acute myeloid leukemia? Best Pract Res Clin Haematol. 2010;23:495–501. doi: 10.1016/j.beha.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Estey EH. General approach to, and perspectives on clinical research in, older patients with newly diagnosed acute myeloid leukemia. Semin Hematol. 2006;43:89–95. doi: 10.1053/j.seminhematol.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97:1916–1924. doi: 10.3324/haematol.2012.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peffault de Latour R, Labopin M, Cornelissen J, et al. In patients older than 55 years with AML in first CR, should we search for a matched unrelated donor when an old sibling donor is available? Bone Marrow Transplant. 2015;50:1411–1415. doi: 10.1038/bmt.2015.180. [DOI] [PubMed] [Google Scholar]
- 36.Stone RM. Should older adults with AML receive post-remission therapy? Best Pract Res Clin Haematol. 2015;28:106–111. doi: 10.1016/j.beha.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Yanada M, Ohtake S, Miyawaki S, et al. The demarcation between younger and older acute myeloid leukemia patients: a pooled analysis of 3 prospective studies. Cancer. 2013;119:3326–3333. doi: 10.1002/cncr.28212. [DOI] [PubMed] [Google Scholar]
- 38.Damiani D, Tiribelli M, Franzoni A, et al. BAALC overexpression retains its negative prognostic role across all cytogenetic risk groups in acute myeloid leukemia patients. Am J Hematol. 2013;88:848–852. doi: 10.1002/ajh.23516. [DOI] [PubMed] [Google Scholar]
- 39.Barba P, Martino R, Zhou Q, et al. CD34+ Selection Vs. Reduced-Intensity Conditioning and Unmodified Graft for Allogeneic Hematopoietic Cell Transplantation in Patients with AML and MDS > 50 Years. Biol Blood Marrow Transplant. 2018 doi: 10.1016/j.bbmt.2017.12.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falini B, Mecucci C, Tiacci E, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 41.Dohner K, Schlenk RF, Habdank M, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005;106:3740–3746. doi: 10.1182/blood-2005-05-2164. [DOI] [PubMed] [Google Scholar]
- 42.Schnittger S, Schoch C, Kern W, et al. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood. 2005;106:3733–3739. doi: 10.1182/blood-2005-06-2248. [DOI] [PubMed] [Google Scholar]
- 43.Thiede C, Koch S, Creutzig E, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML) Blood. 2006;107:4011–4020. doi: 10.1182/blood-2005-08-3167. [DOI] [PubMed] [Google Scholar]
- 44.Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–1163. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- 45.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ostronoff F, Othus M, Lazenby M, et al. Prognostic significance of NPM1 mutations in the absence of FLT3-internal tandem duplication in older patients with acute myeloid leukemia: a SWOG and UK National Cancer Research Institute/Medical Research Council report. J Clin Oncol. 2015;33:1157–1164. doi: 10.1200/JCO.2014.58.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faderl S, Ravandi F, Huang X, et al. Clofarabine plus low-dose cytarabine followed by clofarabine plus low-dose cytarabine alternating with decitabine in acute myeloid leukemia frontline therapy for older patients. Cancer. 2012;118:4471–4477. doi: 10.1002/cncr.27429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Fujimoto J, Zhang J, et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science. 2014;346:256–259. doi: 10.1126/science.1256930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olshen AB, Venkatraman ES, Lucito R, Wigler M. Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics. 2004;5:557–572. doi: 10.1093/biostatistics/kxh008. [DOI] [PubMed] [Google Scholar]
- 50.National Cancer Institute. The Cancer Genoma Atlas Genomic Data Commons Data Portal. 2017 Aug 2; < https://portal.gdc.cancer.gov/>. August 2, 2017.
- 51.Mughal MK, Akhter A, Street L, Pournazari P, Shabani-Rad MT, Mansoor A. Acute myeloid leukaemia: expression of MYC protein and its association with cytogenetic risk profile and overall survival. Hematol Oncol. 2016 doi: 10.1002/hon.2279. [DOI] [PubMed] [Google Scholar]
- 52.Bagger F, Rapin N, Theilgaard-Monch K, et al. HemaExplorer: MYC Messenger RNA Data. 2017 Jul 25; < http://servers.binf.ku.dk/hemaexplorer/>. July 25, 2017.
- 53.Dawson MA, Gudgin EJ, Horton SJ, et al. Recurrent mutations, including NPM1c, activate a BRD4-dependent core transcriptional program in acute myeloid leukemia. Leukemia. 2014;28:311–320. doi: 10.1038/leu.2013.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Finn L, Dalovisio A, Foran J. Older Patients With Acute Myeloid Leukemia: Treatment Challenges and Future Directions. Ochsner J. 2017;17:398–404. [PMC free article] [PubMed] [Google Scholar]
- 55.Smith DP, Bath ML, Metcalf D, Harris AW, Cory S. MYC levels govern hematopoietic tumor type and latency in transgenic mice. Blood. 2006;108:653–661. doi: 10.1182/blood-2006-01-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muller-Tidow C, Steffen B, Cauvet T, et al. Translocation products in acute myeloid leukemia activate the Wnt signaling pathway in hematopoietic cells. Molecular and cellular biology. 2004;24:2890–2904. doi: 10.1128/MCB.24.7.2890-2904.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rice KL, Hormaeche I, Doulatov S, et al. Comprehensive genomic screens identify a role for PLZF-RARalpha as a positive regulator of cell proliferation via direct regulation of c-MYC. Blood. 2009;114:5499–5511. doi: 10.1182/blood-2009-03-206524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker A, Mrozek K, Kohlschmidt J, et al. New recurrent balanced translocations in acute myeloid leukemia and myelodysplastic syndromes: cancer and leukemia group B 8461. Genes Chromosomes Cancer. 2013;52:385–401. doi: 10.1002/gcc.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1A. MYC Amplification shown by array CGH with DNA copy number gain manifesting as trisomy 8 and one with isochromosome 8q.
Supplementary Figure 1B. Amplification is shown by FISH. One extra copy of the MYC gene is confirmed by FISH.
Supplementary Figure 2A. MYC mRNA expression in normal control CD34+ cells and AML patient cells.
Supplementary Figure 2B. MYC IHC and MYC mRNA in AML patients.


