Abstract
Background
Chronic rhinosinusitis with nasal polyps (CRSwNP) is commonly characterized by type-2 inflammation. It is established that group-2 innate lymphoid cells (ILC2s) are a subset of immune cells important in orchestrating mucosal type-2 response. IL-25 is an epithelial-derived cytokine that is a critical activator of ILC2s. Recent evidence demonstrates that specialized taster epithelial cells, such as solitary chemosensory cells (SCCs), may be producers of IL-25. To elucidate the relationship between SCCs and ILC2s in CRSwNP, we sought to quantify ILC2s and SCCs to determine if these cell types are enriched in nasal polyps compared to healthy sinonasal mucosa.
Methods
We quantified SCCs and ILC2s using multicolor flow cytometry in nasal polyps and non-inflamed turbinate mucosa from seven patients and investigated the role of IL-13 and dexamethasone on SCC frequency using tissue explants of nasal polyps and turbinate mucosa.
Results
SCCs were found to be the primary source of IL-25. Nasal polyps demonstrated higher populations of SCCs (33.0% vs. 5.6%, p <0.001) and ILC2s (2.40% vs. 0.19%, p = 0.008) compared to patient-matched non-polypoid turbinates. In cultured polyp explants, exogenous IL-13 increased the proportion of epithelial SCCs (40.2% IL-13 condition vs. 28.9% untreated, p = 0.012), and this effect was reversed by addition of dexamethasone (40.2% vs. 8.9%, p<0.0005).
Conclusion
These data support SCC and ILC2 expansion as well as increased IL-25 production in nasal polyps and may represent early events in the pathogenesis of CRSwNP. IL-13 stimulates proliferation of SCC in a feed-forward loop, a process that is steroid-sensitive.
Keywords: type-2 inflammation, mucosal immunity, IL-25, IL-13, CRSwNP
Introduction
Chronic rhinosinusitis (CRS) continues to remain a highly prevalent disease, affecting 16% of the American population1, and driving upwards of $12.5 billion in healthcare expenditure annually.1,2 Among these patients, individuals with nasal polyps (CRSwNP) exhibit worse outcomes and higher incidence of comorbid conditions, such as severe asthma and atopy.3,4 Studies have demonstrated that different inflammatory endotypes, or subclassifications, are found within the overall CRSwNP phenotype. In the United States and European countries, type-2 (Th2) inflammation characterized by eosinophilic infiltrate is the underlying pathogenic cause in a vast majority of patients, while a minority exhibit Th1, Th17, or Th22 inflammatory endotypes.5–8 Despite this early work in clustering CRSwNP patients by inflammatory signatures, the key mediators and sequence of events involved in the inflammatory pathways are incompletely understood. Indeed, a more granular understanding of mechanisms behind Th2-mediated nasal polyposis is needed to better refine treatment for the majority of patients who suffer from CRSwNP.
Recent studies in murine models have begun to shed light on the molecular and cellular regulators of type-2 mucosal immunity. In particular, epithelial chemosensory cells in the intestine, called tuft cells, have been shown to release interleukin (IL)-25 in the setting of gut parasitic infection resulting in eosinophilic Th2 inflammation.9–11 Unlike cytokines of hematopoietic origin, IL-25 is derived from epithelium and has been shown in humans to be elevated in type-2 inflammatory conditions, including CRSwNP, asthma, and atopy.12–16 Evidence also demonstrates that IL-25 activates a subset of downstream immune cells, called group-2 innate lymphoid cells (ILC2s),9,17–19 which are analogous to CD4+ Th2 cells.20–22 Unlike Th2 cells however, ILC2s do not express antigen-specific receptors and are engaged as an amplifier of epithelial immunity.23 In this capacity, resident ILC2s serve as a local source of type-2 cytokines—IL-4, IL-5, IL-9, and IL-13 in the mucosal environment24, and have been implicated in sinonasal type-2 inflammation (i.e. CRSwNP).24,25
In the present study, we hypothesized that a parallel process to the Th2 inflammation described in the mouse gut is occurring in the human upper airway. Exhibiting remarkable similarity to intestinal tuft cells, solitary chemosensory cells (SCCs) are a rare and discrete non-ciliated cell population in the respiratory epithelium capable of modulating the immune system.26 Like tuft cells, SCCs express the same taste receptors and downstream taste pathway proteins as gustatory cells in taste bud cells, namely taste-associated G-protein 3 (GNAT3), transient receptor cation channel subfamily M member 5 (TRPM5), and doublecortin like kinase 1 (DCLK1).27–29 It is established that bitter taste receptors (T2Rs) on SCCs are stimulated by microbial byproducts28 resulting in a calcium signal that propagates to the surrounding epithelial cells with subsequent release of antimicrobial peptides.26 We now postulate that SCCs are also capable of secreting IL-25 and, in effect, may stimulate ILC2s to produce pro-type-2 cytokines leading to a CRSwNP phenotype.
Here, we report that human SCCs and ILC2s are enriched in nasal polyps compared to healthy, non-polypoid sinonasal mucosa. We also demonstrate that the SCC population frequency increases in the presence of type-2 cytokine, IL-13. Together, our results support that IL-25 is produced by SCCs in the epithelium and increases in response to IL-13, presumed to be produced by resident ILC2s24, thus further amplifying the type-2 inflammatory cascade driving CRSwNP.
Materials and Methods
Patient population & Tissue Acquisition
Tissue was obtained from patients recruited from the Department of Otorhinolaryngology – Head and Neck Surgery, Division of Rhinology, University of Pennsylvania and the Philadelphia Veterans Administration Medical Center, with informed consent and full approval of both Institutional Review Boards. The study patient population met the diagnosis of CRSwNP in accordance to clinical diagnostic standards.30,31 Selected patients were refractory to medical management for CRSwNP and were therefore undergoing functional endoscopic sinus surgery and polypectomy from the ethmoid sinuses. To obtain healthy control mucosa from the same patient, an 8 mm by 4 mm mucosal strip was taken from the medial aspect of the middle turbinate to induce scarring to the septum (i.e., bolgerization) in attempts to prevent lateralization of the middle turbinate after surgery.32,33 Middle turbinates were inspected for any polypoid degeneration, and patients demonstrating polypoid degeneration of their middle turbinate were excluded from this study. Patients were excluded if they carried an existing diagnosis of systemic disease that involve nasal polyposis as a clinical manifestation, such as granulomatosis with polyangiitis, sarcoidosis, cystic fibrosis, and disorders of ciliary motility (e.g., primary ciliary dyskinesia).
Single-cell tissue preparation
Nasal polyps (n=7) and control mucosa from middle turbinates (n=7) were each taken from the same patient. Tissue was cut into 5 mm pieces and incubated for 5 hours at 37 °C in Dulbecco's Modified-Eagle Medium (Lonza) with 5μg/mL Brefeldin A (Sigma-Aldrich). Tissue was transferred and minced into 1-2 mm pieces in solution containing 0.26 Wurtsch units/mL Liberase TM (Sigma-Aldrich) with 100 μg/mL DNAse I (Sigma-Aldrich), 10 mM HEPES buffer (ThermoFisher), 5 mM KCl (Sigma-Aldrich), 1 mM MgCl2, (ThermoFisher), 1.8 mM CaCl2 (ThermoFisher). Tissue was incubated at 37°C with intermittent vortexing for 45 minutes and passed through a 5 mL syringe to release cells into solution. EDTA (Sigma-Aldrich) at final concentration 2 mM was added to stop the digestion. Debris was removed using a 40 μm cell strainer and red blood cells were removed using an RBC lysis buffer (Miltenyi Biotech). Cells were serially washed in 2% bovine serum albumin in PBS (Sigma-Aldrich).
Cell Staining & Flow Cytometry
Single-cell suspensions were each split to stain for markers of SCCs and ILCs. Single cell suspensions (each >1.0 million cells) were stained with viability dye Aqua at 1:1000 dilution (Thermofisher) for dead cell exclusion. Fc receptors were blocked with TruStain FcX TM (Biolegend). For each marker, fluorescence minus one (FMO) controls were also prepared. Isotype control antibodies were used in FMOs for GNAT3 and IL-25.
For SCCs, cell surface markers were stained using anti-EpCAM-PECy7 (eBiosciences) to mark epithelial cells and anti-CD45-eFlour450 (eBiosciences) to mark lymphocytes. After fixation and permeabilization, intracellular markers were stained overnight at 4°C using anti-DCLK1-AF647 (AbCAM), anti-GNAT3-PE (LS Biosciences), anti-IL25-FITC (Invitrogen). For ILC2s, cell surface markers were stained using anti-CD45-PECy7 (eBiosciences), anti-human cocktail lineage 1-FITC (contains anti-CD3, CD14, CD16, CD19, CD20, CD56) (BD Biosciences), and anti-KLRG-1-PE. Intracellular marker anti-GATA-3-PerCPeFlour710 was used as described above.
Antibody-labelled cell suspensions were analyzed on an LSR Fortessa TM using FACSDiva Software 8 (BD Biosciences). At least 250,000 live events were acquired per sample. Data were analyzed using FlowJo 10 (Treestar). Samples were FSC and SSC gating was used to identify singlets and exclude debris. Positive gates were determined using <1% of events on FMO samples. SCCs were identified as Singlets, Aqua Live, EpCAM+CD45-IL25+GNAT3+DCKL1+ events and represented as a frequency of Aqua Live, EpCAM+ events. ILC2s were identified as Singlets, Aqua Live, CD45+Lin1-KLRG1+GATA3+ events and represented as a frequency of Aqua Live, CD45+ events.
Explant Cultures
Nasal polyp and turbinate tissue were obtained as described above and immediately cut into 5 mm-10 mm tissue explants. Care was taken to preserve the epithelium overlying the stroma for each tissue chunk. Explants were submerged in 500 μL of bronchial epithelial cell growth media containing penicillin, streptomycin, and amphotericin B (Clonetics). Each explant was treated with either final concentration 500 ng/mL recombinant human IL-13 (Cell Signaling), 0.02 nM dexamethasone, or no treatment. Explant cultures were incubated at 37°C with 5% CO2 and fed with treated media every 48 hours for a total of 7 days. On day 8, explants were prepared for flow cytometry as described above.
Statistical Methods
Stata version 13 (StataCorp, College Station, TX) software was used for statistical analysis, with p < 0.05 considered statistically significant. Pairwise cell frequencies of SCCs and ILC2s, represented as medians, were compared with Kruskal-Wallis test to account for non-parametric data, as normality assumptions do not apply to flow cytometry event populations. Likewise, non-parametric Mann-Whitney test was applied to SCC populations calculated in the explant experiments.
Results
Enrichment of SCCs and ILC2s in CRSwNP
Representative gating to identify SCCs is demonstrated in nasal polyps versus non-inflamed turbinate mucosa (Figure 1A). The SCC cell population is represented as a percentage of total respiratory epithelial cells, which are identified by selecting for epithelial cell adhesion molecule (EpCAM+) events and excluding CD45+ events, which represent lymphoid cells. Among the EpCAM+CD45- cells, we gated for positive IL-25 expression and found a subpopulation of IL-25 producing cells which was not appreciably present in turbinate mucosa (Figure 1A). Moreover, among the EpCAM+CD45-IL25+ events, an overwhelming majority demonstrated the taster cell phenotype, consistent with SCCs. Namely, EpCAM+CD45-IL25+ events were nearly all (82.9% and 95.6% in representative gating strategies Figure 1A) positive for both SCC markers, DCLK1 and GNAT3. Epithelial cells in nasal polyps demonstrate a higher percentage of SCCs compared to paired turbinate tissue taken from the same patient (median 33.0%, range 17.0-41.0% vs. 5.6%, 1.00-10.2%, p <0.001) (Figure 1C).
Figure 1.
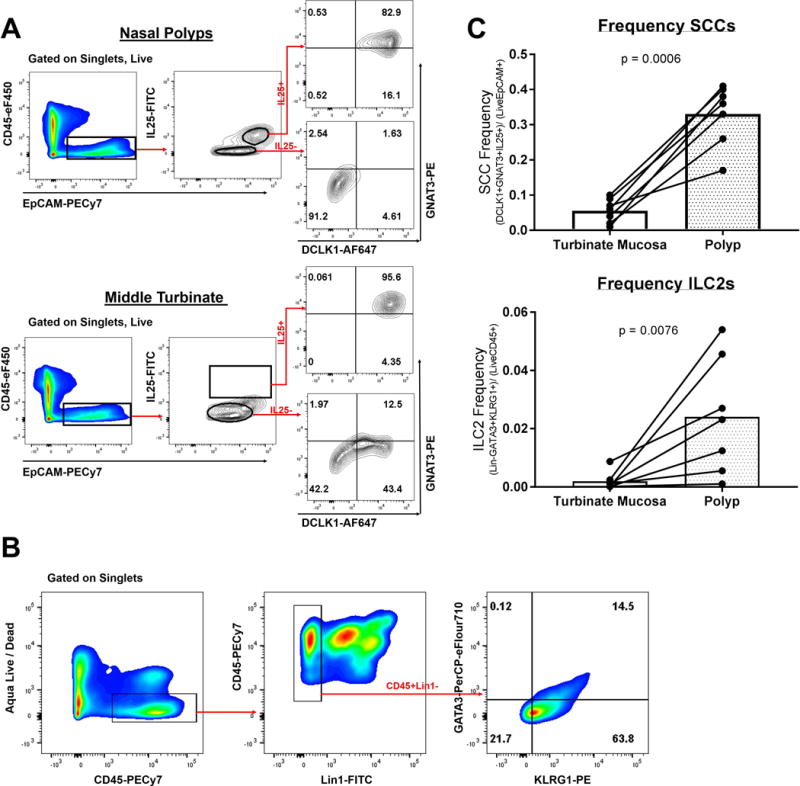
(A) Representative gating strategy of nasal polyp and control middle turbinate tissue samples to identify solitary chemosensory cells, which are defined as Live, EpCAM+, CD45-, IL-25+, DCLK1+, GNAT3+ events. Cells were first gated for singlets and viability (data not shown). Gating was set by FMO negative controls. (B) Representative gating of a nasal polyp sample to identify group-2 innate lymphoid cells, which are defined as Live, CD45+, Lin1-, KLRG1+, GATA3+ events. (C) Comparison of SCC and ILC2 cell frequencies in nasal polyps (n=7) versus turbinate mucosa (n=7) with each line representing a single patient.
Like all lymphoid derived cells, ILC2s express CD45; however, they are negative for mature lymphoid markers— CD3, CD14, CD16, CD19, CD20, CD56—collectively labelled in human cocktail lineage 1 (Lin1).25 After identification of the CD45+Lin1- subset, we identified dual positive events for GATA3 and KLRG1, which are both known to be expressed in activated ILC2s (Figure 1B).16,22,34 The population of events identified as ILC2s (CD45+Lin1-GATA3+KLRG1+), appeared rather rare among all CD45+ cells, which is consistent with prior reports of ILC2s in nasal polyps.25 Similar to our findings for SCCs, nasal polyps show a higher frequency of ILC2 cells compared to matched non-polypoid turbinate tissue (median 2.40%, range 0.10-5.40% vs. 0.19%, range 0.010-1.02%, p = 0.008). Figure 1C demonstrates a summary of the flow gating for SCC and ILC2 population frequencies in all nasal polyps versus turbinate samples taken from the same patient. Taken together, these flow cytometry data demonstrate that both cell types, SCCs and ILC2s, are enriched in nasal polyps compared to non-inflamed turbinate mucosa. Moreover, epithelial IL-25, a cytokine known to act on ILC2 cells, appears to be specifically produced by SCCs.
IL-13-mediated Expansion of SCCs
ILC2s are known to secrete type-2 pro-inflammatory cytokines, among them IL-13.23,35 Previously, a feed-forward loop has been proposed in the mouse gut, in which IL-13 released by ILC2s is able to stimulate epithelial expansion of chemosensory cells.9–11 Therefore, we sought to determine if exogenous human IL-13 affects SCC frequency in sinonasal epithelium. We chose a human explant model to test this hypothesis. Explant models offer the advantage of simulating the in-situ mucosal environment, in which SCCs exist, while keeping the epithelium intact over the stroma, where ILC2s reside.
Explants were stimulated for 7 days in the presence of IL-13, with and without dexamethasone. The SCC subpopulation frequency increased in polyp explants exposed to IL-13 (median 28.9% untreated vs. 40.2% IL-13 condition, p = 0.012) (Figure 2A). Moreover, we sought to determine if IL-13 was capable of stimulating IL-25 production. Mean fluorescence intensity (MFI) measured by flow cytometry can serve as a proxy for the quantity of intracellular protein aggregate36, in our case IL-25. When examining MFI of IL-25 labelled with an anti-IL25-FITC conjugated antibody, we found that exposure to exogenous IL-13 induced more IL-25 production (Figure 2B). This IL-13 effect was also observed in turbinate tissue (2.42% untreated vs.14.3% IL-13 condition, p = 0.037) (Figure 2A). Given that we observed rare SCCs even in non-inflamed epithelium of turbinates as demonstrated in Figure 1 (median 5.6%, range 1.00-10.2%), it is sensible that these SCCs can also be induced by IL-13 in a similar fashion as polyp tissue, but still not show the robust changes seen in polyps originating from the ethmoid sinus mucosa.
Figure 2.
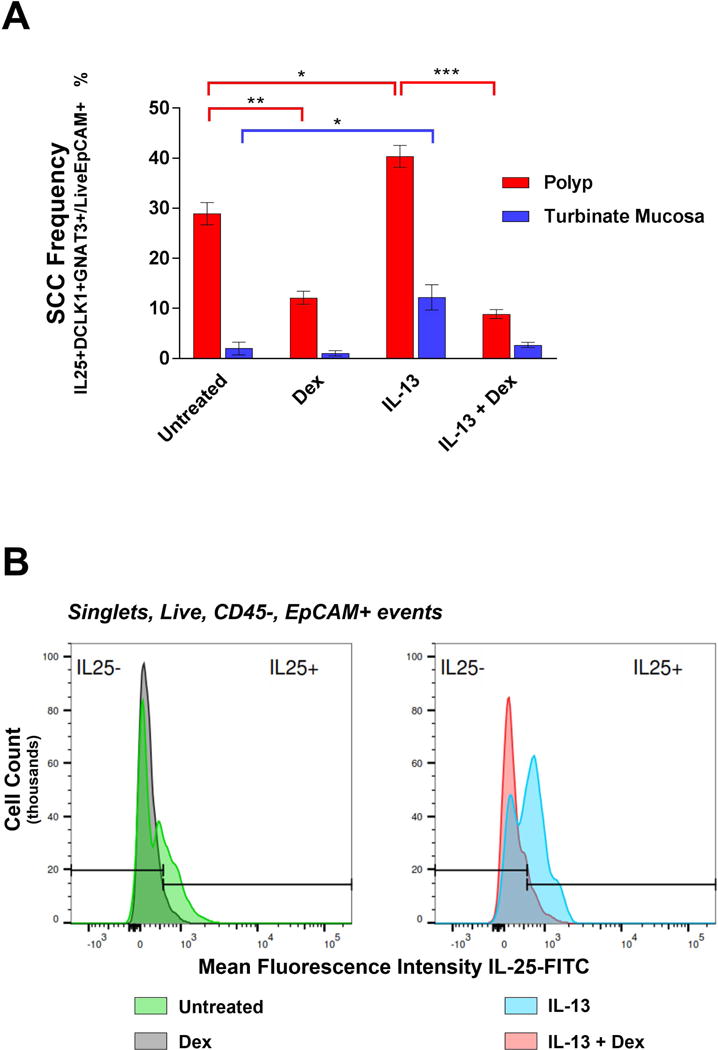
(A) Median and range of SCC population frequencies measured in tissue explants by flow cytometry (n=4 nasal polyps, n=3 turbinate mucosa in each of the 4 conditions, *p <0.05, **p <0.005, ***p <0.0005) (B) Mean fluorescence intensity of IL-25-FITC expression in respiratory epithelium (i.e., Live, CD45-, EpCAM+ subpopulation). Logarithmic histograms depict one representative explant under each of the four conditions.
Steroid-sensitivity of SCC IL25 production
We proceeded to test if the IL-13 induced SCC expansion was sensitive to dexamethasone, as glucocorticoids are a mainstay of treatment for CRSwNP. Dexamethasone alone was able to reduce SCC frequency in polyp explants (28.9% untreated vs. 12.1% dexamethasone condition, p = 0.001) (Figure 2A). In explants with media containing IL-13 compared to both IL-13 and dexamethasone, the SCC frequency significantly declined with the addition of steroid (40.2% IL-13 condition vs. 8.9% IL-13 + dexamethasone, p < 0.0005). Moreover, when dexamethasone was present, MFI of IL-25-FITC measured by flow cytometry decreased in both the untreated polyp explants and IL-13 stimulated explants (Figure 2B). Therefore, it appears not only that steroid affects SCC frequency in epithelium, but also the amount of IL-25 produced within each SCC (Figure 2A-B). Although the same trend was observed in control mucosa grown in IL-13 versus IL-13 and dexamethasone, the SCC frequency was not statistically significantly different (12.2% vs. 2.5%, p = 0.060). Thus, the data demonstrate that IL-13 causes both increased epithelial solitary chemosensory cell frequency and IL-25 production, both of which appear to be steroid sensitive events.
Discussion
Research efforts are increasingly aiming to classify the endotypes of CRS, a truly heterogeneous disease.6,37,38 While studies have demonstrated that type-2 response is a major class of inflammation found in CRSwNP patients, the exact molecular and cellular mechanisms underlying nasal polyp formation have remained elusive.
Our data foremost corroborate recent studies demonstrating that IL-25 appears to be an epithelial-derived cytokine that is elevated in CRSwNP.39–42 In the present study, however, our data also demonstrate that DCLK1+/GNAT3+ cells, markers for solitary chemosensory cells, appear to be the exclusive cellular source of epithelial-derived IL-25. This finding supports a parallel model proposed in the murine intestine, where tuft cells, which are biochemically similar to SCCs, have been shown to produce IL-25 in the context of type-2 mucosal inflammation.9–11 An important difference in our findings compared to the helminth infection model is that frequencies of SCCs among the total epithelial population appear to be much higher than described in the murine intestine. However, unlike transient worm infection that occurs on the order of days to weeks, CRSwNP patients who are undergoing surgery for their disease have suffered from mucosal inflammation over the course of years, which may explain the much higher populations of chemosensory cells in the human inflamed tissues analyzed in this study compared to the mouse helminth infection models.
Also in the same vein as the murine gut model, our data support that IL-13, which is produced by ILC2s, is capable of skewing the epithelium toward a higher proportion of SCCs. Hence, we propose a model demonstrated in Figure 3 as a component in the pathogenesis of CRSwNP. In this model, injury to the sinonasal epithelium (e.g., pathogenic infection, mechanical injury, toxin exposure, etc.) would cause SCCs to release IL-25. In turn, IL-25 stimulates ILC2 cells, a known effector cell of this cytokine, to produce canonical type-2 cytokines, IL-4, IL-5, and IL-13. This pro-Th2 milieu is known to cause eosinophilic infiltration, activation of B cell class switching to IgE, and airway remodeling.43 Of note, IL-13 is also known to cause goblet cell hyperplasia44,45 and ciliated cell apoptosis46. Our data support that IL-13 increases SCC frequency in sinonasal epithelium, which may result from SCC expansion, ciliated cell apoptosis, or a combination of these events. Indeed, population frequency data as quantified by flow cytometry is not intended to measure absolute population expansion; however, the relative expansion of SCCs observed among all EpCAM+ respiratory epithelial cells demonstrates that inflamed mucosa in the presence of IL-13 may have a relatively higher density of IL-25 producing SCCs in patients with CRSwNP.
Figure 3.
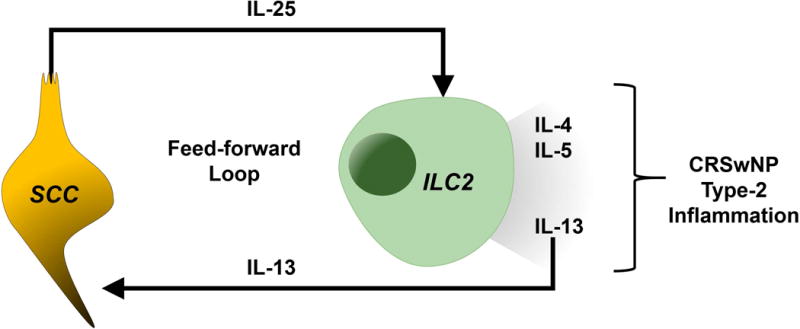
Proposed model of SCC and ILC2 interaction in CRSwNP.
Studies have demonstrated that elevated IL-25 portends more severe polyp disease.40,41,47 Hong et al recently reported that elevated IL-25 measured in serum and inflamed nasal tissue predicted corticosteroid responsiveness in CRSwNP patients.48 The finding that dexamethasone is capable of decreasing SCC frequency and IL-25 production in our explant model corroborates this finding. Given that steroids are heavily utilized in the armamentarium against CRSwNP, future studies should consider examining if steroids are acting preferentially on IL-25 production in SCCs or at the level of ILC2s as they upregulate production and secretion of IL-13.
It is important to consider that the proposed mechanism herein represents a partial depiction of what is likely at play. For instance, other epithelial cytokines, such as IL-33 and TSLP, have also been implicated in type-2 airway inflammation.49 It is very likely that these, and other pro-inflammatory signals, work concomitantly on SCCs, ILC2s, and Th2 cells to illicit the clinical features of CRSwNP. Given that this study represents a translation of a mechanism discovered in mouse to human, experimental challenges arise. While murine models afford the elegance of knock-out models, human studies are limited in our selection of controls. Here, we selected non-inflamed turbinate mucosa as controls for nasal polyps. Non-polypoid middle turbinates were used for the control group when measuring SCC and ILC2 population frequencies due to the benefit of having a nasal polyp and a piece of non-inflamed sinonasal mucosa from the same patient, thereby eliminating confounders such as differing host genetics, environmental exposures, and co-morbid conditions. Moreover, since SCC and ILC populations can vary from patient-to-patient, showing an enrichment of a specialized cell type through pair-wise samples offers more meaningful interpretation.
Another limitation of our study is the determination of how ILC2s are affected by SCC stimulation. Given their extreme rarity among immune cells, identification of ILC2 by flow cytometry can be challenging. Future studies will need to address ILC2-specific markers that can more efficiently identify the population of interest and understand how upstream factors influence their role in eliciting a Th2 response.
Conclusion
The downstream mechanisms of type-2 mucosal inflammation leading to eosinophilic infiltrate and nasal polyps has been studied over the past decade; however, the upstream triggers of the Th2 response in upper airway epithelium is a new frontier. Herein, we demonstrate that SCCs and ILC2 are two cell populations that are enriched in nasal polyps from CRSwNP patients. We additionally demonstrate that SCCs are the primary epithelial source of IL-25 production. IL-13 is capable of increasing SCC frequency, while dexamethasone induced reduction of the SCC population and decreased IL-25 production. Identifying the cellular and molecular components at play early in the type-2 cascade driving CRSwNP is critical to further understanding this disease process and identifying specific targets for future interventions.
Acknowledgments
Funding: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1R001880 (NNP), GM083204-08A1 UO1AI125940 R01AI095289 (DRH), R01DC013588 (NAC). The content is solely the responsibility of the authors and does not necessarily represent the offices views of the National Institutes of Health.
Footnotes
Podium Presentation at the American Rhinologic Society at the Combined Otolaryngology Spring Meeting (COSM), National Harbor, MD, April 20, 2018.
Disclosures: Authors have nothing to disclose
References
- 1.Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: National health interview survey, 2012. Vital Health Stat 10. 2014;10(260):1–171. doi:24819891. [PubMed] [Google Scholar]
- 2.Bhattacharyya N, Orlandi RR, Grebner J, Martinson M. Cost burden of chronic rhinosinusitis: a claims-based study. Otolaryngol Head Neck Surg. 2011;144(3):440–445. doi: 10.1177/0194599810391852. [DOI] [PubMed] [Google Scholar]
- 3.Toros SZ, Bolukbasi S, Naiboglu B, et al. Comparative outcomes of endoscopic sinus surgery in patients with chronic sinusitis and nasal polyps. Eur Arch Otorhinolaryngol. 2007;264(9):1003–1008. doi: 10.1007/s00405-007-0301-5. [DOI] [PubMed] [Google Scholar]
- 4.Banerji A, Piccirillo JF, Thawley SE, et al. Chronic rhinosinusitis patients with polyps or polypoid mucosa have a greater burden of illness. Am J Rhinol. 2007;21(1):19–26. doi: 10.2500/ajr.2007.21.2979. [DOI] [PubMed] [Google Scholar]
- 5.Cho S-W, Kim DW, Kim J-W, Lee CH, Rhee C-S. Classification of chronic rhinosinusitis according to a nasal polyp and tissue eosinophilia: limitation of current classification system for Asian population. Asia Pac Allergy. 2017;7(3):121. doi: 10.5415/apallergy.2017.7.3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennis SK, Lam K, Luong A. A Review of Classification Schemes for Chronic Rhinosinusitis with Nasal Polyposis Endotypes. Laryngoscope Investig Otolaryngol. 2016;1(5):130–134. doi: 10.1002/lio2.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akdis CA, Bachert C, Cingi C, et al. Endotypes and phenotypes of chronic rhinosinusitis: A PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131(6):1479–1490. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomassen P, Vandeplas G, Van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137(5):1449–1456.e4. doi: 10.1016/j.jaci.2015.12.1324. [DOI] [PubMed] [Google Scholar]
- 9.von Moltke J, Ji M, Liang H-E, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2–epithelial response circuit. Nature. 2015;529(7585):221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howitt MR, Lavoie S, Michaud M, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science (80-) 2016;351(6279):1329–1333. doi: 10.1126/science.aaf1648. doi:science.aaf1648 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerbe F, Sidot E, Smyth DJ, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529(7585):226–230. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen F, Hong H, Sun Y, et al. Nasal interleukin 25 as a novel biomarker for patients with chronic rhinosinusitis with nasal polyps and airway hypersensitiveness: A pilot study. Ann Allergy, Asthma Immunol. 2017;119(4):310–316.e2. doi: 10.1016/j.anai.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Hong H-Y, Chen F-H, Sun Y-Q, et al. Local IL-25 contributes to Th2-biased inflammatory profiles in nasal polyps. Allergy. 2017 Aug; doi: 10.1111/all.13267. [DOI] [PubMed] [Google Scholar]
- 14.Iinuma T, Okamoto Y, Yamamoto H, et al. Interleukin-25 and mucosal T cells in noneosinophilic and eosinophilic chronic rhinosinusitis. Ann Allergy, Asthma Immunol. 2015;114(4):289–298. doi: 10.1016/j.anai.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Ballantyne SJ, Barlow JL, Jolin HE, et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007;120(6):1324–1331. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 16.Salimi M, Barlow JL, Saunders SP, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210(13):2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mjösberg JM, Trifari S, Crellin NK, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12(11):1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 18.Hams E, Armstrong ME, Barlow JL, et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci U S A. 2014;111(1):367–372. doi: 10.1073/pnas.1315854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue L, Salimi M, Panse I, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazenberg MD, Spits H. Human innate lymphoid cells. Blood. 2014 doi: 10.1182/blood-2013-11-427781. [DOI] [PubMed] [Google Scholar]
- 21.Walker JA, McKenzie ANJ. Development and function of group 2 innate lymphoid cells. Curr Opin Immunol. 2013 doi: 10.1016/j.coi.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mjösberg J, Bernink J, Golebski K, et al. The Transcription Factor GATA3 Is Essential for the Function of Human Type 2 Innate Lymphoid Cells. Immunity. 2012;37(4):649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Zhu J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine. 2015;75(1):14–24. doi: 10.1016/j.cyto.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karta MR, Broide DH, Doherty TA. Insights into Group 2 Innate Lymphoid Cells in Human Airway Disease. Curr Allergy Asthma Rep. 2016;16(1):8. doi: 10.1007/s11882-015-0581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poposki JA, Klingler AI, Tan BK, et al. Group 2 innate lymphoid cells are elevated and activated in chronic rhinosinusitis with nasal polyps. Immunity, Inflamm Dis. 2017;(Ilc):233–243. doi: 10.1002/iid3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee RJ, Kofonow JM, Rosen PL, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. 2014;124(3):1393–1405. doi: 10.1172/JCI72094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barham HP, Cooper SE, Anderson CB, et al. Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int Forum Allergy Rhinol. 2013;3(6):450–457. doi: 10.1002/alr.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tizzano M, Gulbransen BD, Vandenbeuch A, et al. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci. 2010;107(7):3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.OHMOTO M, YAMAGUCHI T, YAMASHITA J, BACHMANOV AA, HIROTA J, MATSUMOTO I. Pou2f3/Skn-1a Is Necessary for the Generation or Differentiation of Solitary Chemosensory Cells in the Anterior Nasal Cavity. Biosci Biotechnol Biochem. 2013;77(10):2154–2156. doi: 10.1271/bbb.130454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fokkens W, Lund V, Mullol J. European Position Paper on Rhinosinusitis and Nasal Polyps. Rhinology. 2012;(20):1–136. doi: 10.4193/Rhino50E2. [DOI] [PubMed] [Google Scholar]
- 31.Orlandi RR, Kingdom TT, Hwang PH. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis Executive Summary. Int Forum Allergy Rhinol. 2016;6:S3–S21. doi: 10.1002/alr.21694. [DOI] [PubMed] [Google Scholar]
- 32.Bolger WE, Kuhn FA, Kennedy DW. Middle turbinate stabilization after functional endoscopic sinus surgery: The controlled synechiae technique. Laryngoscope. 1999;109(11):1852–1853. doi: 10.1097/00005537-199911000-00025. [DOI] [PubMed] [Google Scholar]
- 33.Lee MR, Marple BF. Middle turbinate medialization for improved access during endoscopic sinus surgery. Int Forum Allergy Rhinol. 2011;1(3):187–190. doi: 10.1002/alr.20013. [DOI] [PubMed] [Google Scholar]
- 34.Krug N, Hohlfeld JM, Kirsten A-M, et al. Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. N Engl J Med. 2015;372(21):1987–1995. doi: 10.1056/NEJMoa1411776. [DOI] [PubMed] [Google Scholar]
- 35.Jia Y, Fang X, Zhu X, et al. IL-13+ Type 2 Innate Lymphoid Cells Correlate with Asthma Control Status and Treatment Response. Am J Respir Cell Mol Biol. 2016;55(5):675–683. doi: 10.1165/rcmb.2016-0099OC. [DOI] [PubMed] [Google Scholar]
- 36.Shiber A, Breuer W, Ravid T. Flow cytometric quantification and characterization of intracellular protein aggregates in yeast. Prion. 2014;8(3):276–284. doi: 10.4161/19336896.2014.968445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim DW, Cho SH. Emerging endotypes of chronic rhinosinusitis and its application to precision medicine. Allergy, Asthma Immunol Res. 2017;9(4):299–306. doi: 10.4168/aair.2017.9.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lam M, Hull L, Mclachlan R, et al. Clinical severity and epithelial endotypes in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013;3(2):121–128. doi: 10.1002/alr.21082. [DOI] [PubMed] [Google Scholar]
- 39.Hong H, Chen F, Sun Y, et al. Nasal IL-25 predicts the response to oral-corticosteroid in chronic rhinosinusitis with nasal polyps (CRSwNP) J Allergy Clin Immunol. 2018;0(0):7–9. doi: 10.1016/j.jaci.2017.10.050. [DOI] [PubMed] [Google Scholar]
- 40.Shin H-W, Kim D-K, Park M-H, et al. IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2015;135(6):1476–85.e7. doi: 10.1016/j.jaci.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Hong H-Y, Chen F-H, Sun Y-Q, et al. Local IL-25 contributes to Th2-biased inflammatory profiles in nasal polyps. Allergy. 2017;(58):0–2. doi: 10.1111/all.13267. [DOI] [PubMed] [Google Scholar]
- 42.Lam EPS, Kariyawasam HH, Rana BMJ, et al. IL-25/IL-33–responsive T H 2 cells characterize nasal polyps with a default T H 17 signature in nasal mucosa. J Allergy Clin Immunol. 2016;137(5):1514–1524. doi: 10.1016/j.jaci.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kato A. Immunopathology of chronic rhinosinusitis. Allergol Int. 2015;64(2):121–130. doi: 10.1016/j.alit.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanoh S, Tanabe T, Rubin BK. IL-13-induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells. Clin Exp Allergy. 2011;41(12):1747–1756. doi: 10.1111/j.1365-2222.2011.03852.x. [DOI] [PubMed] [Google Scholar]
- 45.Baba S, Kondo K, Kanaya K, et al. Expression of IL-33 and its receptor ST2 in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2014;124(4):115–122. doi: 10.1002/lary.24462. [DOI] [PubMed] [Google Scholar]
- 46.Laoukili J, Perret E, Willems T, et al. IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest. 2001;108(12):1817–1824. doi: 10.1172/JCI200113557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ozturan A, Eyigor H, Eyigor M, et al. The role of IL-25 and IL-33 in chronic rhinosinusitis with or without nasal polyps. Eur Arch Oto-Rhino-Laryngology. 2017;274(1):283–288. doi: 10.1007/s00405-016-4260-6. [DOI] [PubMed] [Google Scholar]
- 48.Hong H, Chen F, Sun Y, et al. Nasal IL-25 predicts the response to oral-corticosteroid in chronic rhinosinusitis with nasal polyps (CRSwNP) J Allergy Clin Immunol. 2018;0(0) doi: 10.1016/j.jaci.2017.10.050. [DOI] [PubMed] [Google Scholar]
- 49.Liao B, Cao PP, Zeng M, et al. Interaction of thymic stromal lymphopoietin, IL-33, and their receptors in epithelial cells in eosinophilic chronic rhinosinusitis with nasal polyps. Allergy Eur J Allergy Clin Immunol. 2015;70(9):1169–1180. doi: 10.1111/all.12667. [DOI] [PubMed] [Google Scholar]


