Abstract
Objective: To assess impact of a 52-week elosulfase alfa enzyme replacement therapy (ERT) on exercise capacity in Morquio A patients and analyze cardiorespiratory and metabolic function during exercise to uncover exercise limitations beyond skeletal abnormalities.
Methods: Morquio A patients aged ≥7 years, able to walk >200 m in the 6-minute walk test (6MWT), received elosulfase alfa 2.0 mg/kg/week (N = 15) or 4.0 mg/kg/week (N = 10) for 52 weeks in the randomized, double-blind MOR-008 study (ClinicalTrials.gov NCT01609062) and its extension. Exercise capacity was assessed by 6MWT, 3-minute stair climb test (3MSCT), and cardiopulmonary exercise test (CPET; N = 15 dosage groups combined).
Results: Changes over 52 weeks in 6MWT and 3MSCT were minimal. Baseline CPET results showed impaired weight-adjusted peak oxygen uptake (VO2), partly attributable to inability to increase tidal volume during exercise. CPET measures of exercise function showed significant improvement at 25 and/or 52 weeks in exercise duration, peak workload, O2 pulse, and peak tidal volume (% increases in duration, 16.9 (P = 0.0045) and 9.4 (P = 0.0807); peak workload, 26.5 (P = 0.0026) and 21.2 (P = 0.0132); O2 pulse, 10.7 (P = 0.0187) and 2.3 (P = 0.643); peak tidal volume, 11.7 (P = 0.1117) and 29.1 (P = 0.0142)). In addition, decreased VO2/work ratio was noted (% decrease −7.6 [−11.9, 1.3] and −9.2 [−25.7, 5.1]), indicating performance of work at reduced oxygen cost.
Conclusions: CPET uncovers limitation in exercise capacity in Morquio A related to reduced lung function. ERT improves exercise capacity and efficiency of oxygen utilization, not attributable to changes in cardiac or pulmonary function. Further study of the long-term impact of ERT on exercise capacity and the clinical relevance of the observed changes is warranted.
Electronic supplementary material
The online version of this article (10.1007/8904_2017_70) contains supplementary material, which is available to authorized users.
Keywords: Cardiopulmonary exercise test, Elosulfase alfa, Endurance, Enzyme replacement therapy, Exercise capacity, Mucopolysaccharidosis IVA
Introduction
Morquio A syndrome (mucopolysaccharidosis IVA; OMIM 253000) is a rare autosomal recessive disease (1 per 71,000 to 1 per 500,000 live births) caused by a deficiency of the enzyme N-acetylgalactosamine-6-sulfatase (GALNS; EC 3.1.6.4); it is characterized by systemic accumulation of keratan sulfate (KS) and chondroitin-6-sulfate and disruption of cellular processes (Muenzer 2011; Yasuda et al. 2013; Leadley et al. 2014). The disease is genotypically and phenotypically very heterogeneous. Characteristic features include bone and joint abnormalities, short stature, obstructive airway/restrictive pulmonary disease, cardiac disease characterized by valve abnormalities and reduced stroke volume, spinal cord compression and/or atlantoaxial instability, hearing loss, impaired vision, and hepatomegaly (Montaño et al. 2007; Harmatz et al. 2013; Hendriksz et al. 2013, 2015).
Elosulfase alfa 2.0 mg/kg/week has been approved as enzyme replacement therapy (ERT) for Morquio A (Sanford and Lo 2014). In the pivotal phase 3 study (N = 176), treatment with 2.0 mg/kg/week was associated with a significant impact on endurance in the 6-minute walk test (6MWT) and an acceptable safety profile (Hendriksz et al. 2014). In parallel, a phase 2, randomized, double-blind, pilot study (MOR-008, ClinicalTrials.gov identifier NCT01609062) assessed the safety and efficacy of elosulfase alfa 2.0 and 4.0 mg/kg/week in 25 Morquio A patients. The primary outcome was safety over 27 weeks. Endurance in the 6MWT and 3-minute stair climb test (3MSCT) and exercise capacity in a cardiopulmonary exercise test (CPET) were secondary efficacy measures assessed at 24 and 25 weeks, respectively. Baseline CPET data showed reduced exercise capacity relative to the general population (Ten Harkel et al. 2011; Burton et al. 2015). At 25 weeks, a positive change in exercise capacity (increase in exercise duration, peak workload, and O2 pulse) and a decrease in oxygen uptake relative to work (VO2/watt) were observed (Burton et al. 2015). 6MWT and 3MSCT outcomes remained essentially unchanged during the primary treatment phase.
The present study evaluates the effect of ERT for 52 weeks on exercise capacity, as assessed by CPET and measures of endurance. In addition, given the multisystem involvement characteristic of Morquio A disease, detailed analysis of cardiorespiratory function was performed from the CPET data to uncover limitations to exercise beyond skeletal abnormalities.
Methods
Study Design
MOR-008 is a multinational, multicenter, phase 2, two-arm, randomized, double-blind, pilot study. Inclusion and exclusion criteria and study design of the primary treatment phase have been described previously (Burton et al. 2015). Briefly, after a 3-week screening period, 25 patients aged ≥7 years able to walk >200 m in the 6MWT and to perform an exercise test were randomized in a double-blind fashion to elosulfase alfa 2.0 mg/kg/week (N = 15) or 4.0 mg/kg/week (N = 10) for 27 weeks. Randomization was stratified by cohort: CPET (N = 15) and no CPET (N = 10). The primary endpoint was safety and tolerability of elosulfase alfa over 27 weeks. Secondary endpoints were effect on endurance, exercise capacity, respiratory function, muscle strength, cardiac function, pain, and urinary KS level. Patients who completed the primary treatment phase were enrolled in the extension, during which all patients continued on the same dose of elosulfase alfa up to 52 weeks. Each participant, or his/her legally authorized representative, provided written informed consent before entering the study.
Evaluation of Endurance and Exercise Capacity
Endurance was measured by the 6MWT (American Thoracic Society 2002) and the 3MSCT at screening, week 12, week 24 (primary treatment phase), and week 52 (extension) as previously described (Burton et al. 2015). Each test was performed twice at each time point within a 7-day window, with only one test allowed per day.
Exercise capacity during CPET was assessed in the first 15 patients enrolled in the study by cycle ergometry using an incremental workload protocol at baseline, week 25, and week 52. The workload was increased every minute to determine each patient’s peak exercise capacity. Expired O2 and CO2 concentrations were analyzed using a metabolic cart; heart rate was monitored by continuous 3- or 12-lead ECG, and O2 saturation was measured via pulse oximetry. Breath-by-breath calculations, including exhaled minute ventilation (VE), O2 uptake (VO2), CO2 production (VCO2), respiratory exchange ratio (RER), and peak and rest tidal volume (volume of air displaced between normal inhalation and exhalation), were made from conventional equations. The O2 pulse was derived as the oxygen uptake per heart beat (VO2/HR). VO2 at the ventilatory threshold (VT; the level of oxygen consumption above which aerobic energy production is supplemented by anaerobic mechanisms) was calculated by the V-slope method (Albouaini et al. 2007).
Statistical Methods
As the sample size of the study was not determined by statistical power considerations, the primary analysis plan was to perform descriptive statistics at baseline, 24 weeks (endurance) or 25 weeks (CPET; primary treatment phase), and 52 weeks (extension). Correlations between peak VO2, determined at each patient’s maximal workload, and 6MWT and 3MSCT results were estimated using the Pearson correlation coefficient (r). Post hoc, paired t-test analysis was performed on the CPET variables to evaluate for significant changes at week 25 and week 52 compared with baseline. In addition, linear regression analysis was performed to determine if the change in peak workload from baseline to either week 25 or 52 was related to an improvement in an individual patient’s ability to increase tidal volume during exercise. Results are presented for the modified intent-to-treat (MITT) population consisting of all patients who were randomized to study treatment, received at least one dose of study drug, and had at least one posttreatment observation.
Results
Patient Characteristics
All 25 patients (median age 12 years; range 8–21 years) completed the primary treatment phase and were enrolled in and completed 52 weeks of the extension study. One patient stopped ERT at 24 weeks (after having missed four previous infusions) when she moved further away from the study site but remained on the study. Another patient had a last infusion at 39 weeks. Demographics and baseline characteristics for all patients have been presented in a previous publication (Burton et al. 2015). Table 1 shows baseline characteristics for the 15 patients included in the CPET analysis. Baseline characteristics were reasonably well balanced between treatment groups. 6MWT and 3MSCT results were better than in the phase 3 study (Table 2) (Hendriksz et al. 2014). Lung function was impaired, as evidenced by a low median forced vital capacity (FVC; 1.17 L) and forced expiratory volume in 1 s (FEV1; 0.93 L).
Table 1.
Demographics and baseline characteristics of patients included in the cardiopulmonary exercise test (CPET) analysis (modified intent-to-treat population)
| Elosulfase alfa 2 mg/kg/week N = 10 | Elosulfase alfa 4 mg/kg/week N = 5 | Total N = 15 | |
|---|---|---|---|
| Age at enrolment (years) Median (range) |
11 (8, 21) | 12 (8, 14) | 12 (8, 21) |
| Sex, N (%) Female |
7 (70) | 3 (60) | 10 (66) |
| Height (cm) Median (range) |
102.0 (85, 167) | 108.0 (96, 147) | 106.5 (85, 167) |
| Weight (kg) Median (range) |
26.5 (12, 54) | 22.0 (17, 49) | 26.4 (12, 54) |
| 6MWT (m) Median (range) |
327 (273, 466) | 338 (281, 453) | 331 (273, 466) |
| 3MSCT (stairs/min) Median (range) |
61 (28, 84) | 55 (30, 87) | 58 (28, 87) |
| FVC (L) Median (range) |
0.96 (0.68, 4.56) | 1.24 (0.90, 2.77) | 1.17 (0.68, 4.56) |
| FEV1 (L) Median (range) |
0.76 (0.57, 3.94) | 1.12 (0.84, 2.20) | 0.93 (0.57, 3.94) |
3MSCT 3-minute stair climb test, 6MWT 6-minute walk test, FEV 1 forced expiratory volume in 1 s, FVC forced vital capacity
Table 2.
Endurance outcomes at baseline and after follow-up at 24 and 52 weeks (modified intent-to-treat population)
| N | Baseline | Week 24 | Week 52 | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Median change (IQR) | Mean (SD) | Median change (IQR) | ||
| 6MWT (m) | 25a | 372.2 (80.6) | 364.5 (86.4) | 1.4 (−29.3, 6.9) | 395.6 (96.3) | 9.9 (−15.8, 36.5) |
| 3MSCT (stairs/min) | 25b | 65.0 (21.7) | 68.6 (24.4) | 4.8 (−3.6, 11.3) | 66.3 (24.5) | 0.4 (−8.2, 5.5) |
IQR interquartile range, 3MSCT 3-minute stair climb test, 6MWT 6-minute walk test
aN = 24 at week 52
bN = 24 at week 24
Endurance Outcomes
There were no meaningful changes from baseline in walk distance in the 6MWT in either dose group at 12 or 24 weeks (Table 2) (Burton et al. 2015). An improvement was seen after 52 weeks in the 4.0 mg/kg/week treatment group (N = 10); mean and median increases from baseline were 31.8 m (95% CI −7.2, 70.8) and 22.1 m, respectively (Supportive online material 1). Mean and median changes from baseline in the 2.0 mg/kg/week group were 9.0 m (95% CI −18.9, 37.0) and 1.9 m, respectively.
3MSCT results improved from baseline at both 24 and 52 weeks in the 4.0 mg/kg/week treatment group only (Supportive online material 1). The small sample size does not allow any conclusions regarding a dose-response effect of elosulfase alfa on endurance.
CPET Outcomes
Baseline and 25-week data that have been presented previously showed impaired weight-adjusted peak VO2 rates: mean baseline 30.7 (SD 7.5) mL/kg/min vs. ≈40 mL/kg/min in healthy individuals (Table 3) (Ten Harkel et al. 2011; Burton et al. 2015). Similarly, peak heart rate during CPET averaged 164 beats/min, which is below age-matched reference values of 199–212 beats/min (for age range 8–21 years) (Table 3). Although peak exercise capacity was reduced, the peak RER (VCO2/VO2) at baseline was >1 indicating that patients exercised to workload that was beyond VT, compatible with adequate patient effort during the study (Table 3).
Table 3.
Cardiopulmonary exercise test (CPET) outcomes at baseline and after follow-up at 25 and 52 weeks (two dose groups combined; modified intent-to-treat population)
| N | Baseline | Week 25 | P-value | Week 52 | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Median % change (IQR) | Mean (SD) | Median % change (IQR) | ||||
| Exercise duration (min) | 15 | 7.7 (2.3) | 8.6 (2.5) | 16.9 (−0.5, 23.1) | 0.0045 | 8.4 (2.4) | 9.4 (−5.7, 20.2) | 0.0807 |
| Peak VO2 (mL/min) | 15 | 807.1 (412.7) | 890.1 (469.67) | 5.3 (−6.3, 31.7) | 0.0665 | 856.7 (486.9) | 9.8 (−21.3, 25.9) | 0.3597 |
| Peak VO2 (mL/kg/min) | 15 | 30.7 (7.5) | 32.5 (10.3) | 3.1 (−14.2, 20.5) | 0.3449 | 29.4 (8.8) | −0.8 (−27.8, 16.9) | 0.4588 |
| O2 pulse (mL/beat) | 15a | 4.9 (2.2) | 5.3 (2.5) | 10.7 (1.3, 19.8) | 0.0187 | 5.2 (2.4) | 2.3 (−21.2, 26.8) | 0.6343 |
| Peak workload (watts) | 15 | 40.9 (25.9) | 50.3 (29.9) | 26.5 (5.1, 42.4) | 0.0026 | 48.7 (31.0) | 21.2 (0.0, 47.5) | 0.0132 |
| VO2/work ratio (mL/watt) | 14 | 13.3 (3.2) | 12.2 (3.2) | −7.6 (−11.9, 1.3) | 0.0173 | 11.6 (4.1) | −9.2 (−25.7, 5.1) | 0.2476 |
| Peak RER | 15 | 1.1 (0.1) | 1.1 (0.2) | 4.0 (−3.9, 10.8) | 0.2899 | 1.0 (0.2) | 0.0 (−8.1, 7.5) | 0.4915 |
| VO2 at VT (mL/min) | 15b | 492.8 (217.0) | 533.0 (250.4) | 9.2 (−0.9, 19.5) | 0.0650 | 592.3 (306.7) | 18.3 (4.9, 30.7) | 0.0472 |
| Peak heart rate (beats/min) | 15 | 164.3 (16.3) | 167.3 (21.0) | 4.0 (−9.6, 11.0) | 0.6174 | 164.4 (19.6) | −0.7 (−3.5, 6.8) | 0.9815 |
| Rest RR (breaths/min) | 15c | 24.5 (5.8) | 23.6 (8.0) | −6.3 (−18.2, 0.0) | 0.7171 | 23.3 (6.6) | −6.1 (−27.6, 22.7) | 0.2361 |
| Peak RR (breaths/min) | 15c | 47.4 (10.9) | 47.8 (13.5) | 6.1 (−15.6, 16.7) | 0.5501 | 45.6 (10.3) | −2.3 (−12.9, 1.8) | 0.1000 |
| Rest tidal volume (mL) | 15d | 324.3 (119.8) | 331.6 (148.4) | −2.8 (−6.5, 7.9) | 0.9493 | 383.2 (177.3) | 23.9 (0.9, 38.2) | 0.0879 |
| Peak tidal volume (mL) | 15d | 578.9 (400.5) | 663.0 (429.1) | 11.7 (−0.0, 26.4) | 0.1117 | 762.4 (523.6) | 29.1 (3.3, 46.2) | 0.0142 |
| Δ tidal volume (mL) | 15d | 254.6 (300.5) | 331.4 (297.2) | 56.2 (17.1, 98.1) | 0.1385 | 379.2 (379.6) | 38.3 (−6.4, 237.1) | 0.0457 |
Δ tidal volume (calculated as peak minus rest)
IQR interquartile range, RER respiratory exchange ratio, RR respiratory rate, VT ventilatory threshold, VO 2 oxygen uptake
aN = 14 at week 52
bN = 13 at week 52
cN = 14 at week 25 and 13 at week 52
dN = 14 at week 25
Further exploration of baseline data showed a resting respiratory rate (mean 24.5 breaths/min) that was relatively high for children and above the upper limit of normal for adults (Charbek 2015). Although the peak respiratory rate (mean 47.4 breaths/min) remained below the upper limit of normal of 60 breaths/min (ATS and ACCP 2003), there was evidence of an abnormal ventilatory response to exercise. Specifically, a minimal <2-fold increase in tidal volume was noted in most patients (vs. ≈2–3 times increase in healthy individuals, Santuz et al. 1997) (Supportive online material 2). The tidal volume response to exercise was particularly low in patients with a low FVC and low FEV1 but comparable with healthy individuals in those with higher FVC and FEV1 (Supportive online material 3). There was no evidence of dynamic cardiac impairment based on appropriate increase in stroke volume as assessed by O2 pulse during exercise.
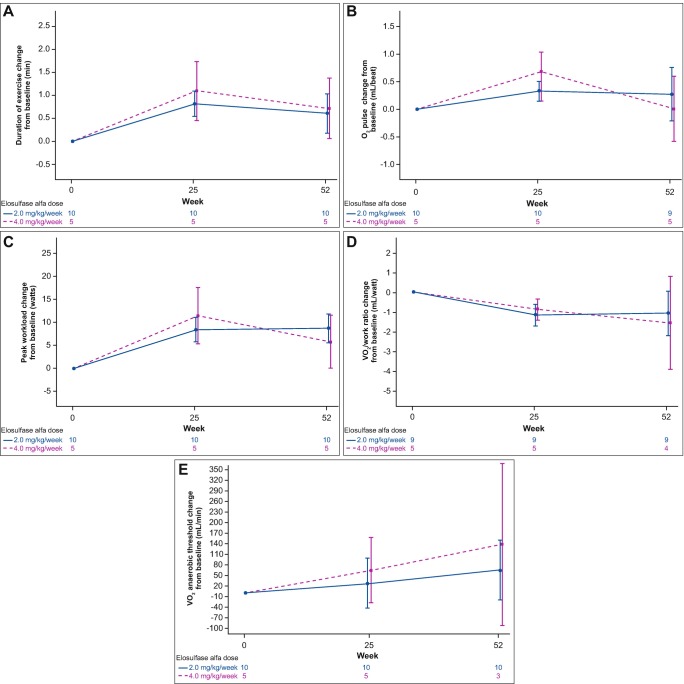
CPET data at 25 and 52 weeks showed a positive change in exercise capacity (Table 3, Fig. 1a–e). Exercise duration, peak workload, VO2 at VT, peak tidal volume, and Δ tidal volume all increased with treatment. A small increase from baseline in O2 pulse was seen after 25 weeks, but not sustained at 52 weeks. Work efficiency improved as defined by a decrease in the required O2 uptake per unit of work (VO2/watt) (not significant at week 52). There were numerical differences between the 2.0 mg/kg dose and the 4.0 mg/kg dose at week 25 and week 52 (Fig. 1a–e), but the small sample size did not allow statistical comparison between doses. Peak RER remained >1 after 25 and 52 weeks of treatment with values that were similar to baseline. Peak heart rate and respiratory rates remained stable.
Fig. 1.
Mean (standard error) change in cardiopulmonary exercise test (CPET) outcomes from baseline to week 52 with elosulfase alfa 2 mg/kg and 4 mg/kg per week (modified intent-to-treat population). VO 2 oxygen uptake
The main reasons for discontinuing CPET were generalized fatigue or leg fatigue (N = 13 at baseline). Other reasons were shortness of breath or labored breathing (N = 2) and pain (N = 1). These reasons did not change markedly over time.
Linear regression analysis demonstrated that patients who were able to increase their tidal volume during exercise more at week 25 compared with baseline demonstrated the greatest improvement in peak exercise workload (r 2 = 0.37, P = 0.02). This relationship was less obvious at week 52 (r 2 = 0.1622, P = 0.1622) (Supportive online material 4).
Correlations Between Endurance Measures and Peak VO2 (Supportive online material 5)
Correlation analysis showed strong positive relationships between peak VO2 and endurance measures at baseline (6MWT, r = 0.61, and 3MSCT, r = 0.55). Changes from baseline to week 25 or 52 in peak VO2 were not correlated with changes in 6MWT over the same period (r = −0.10 and r = 0.05, respectively); there was a moderately positive correlation between changes in peak VO2 and 3MSCT at 25 and 52 weeks (r = 0.35 and r = 0.38, respectively). Positive correlations were not observed between the changes in VO2 at VT with either 6MWT or 3MSCT at any time point.
Discussion
MOR-008 is the first study to use an incremental workload CPET in addition to volitional endurance tests (6MWT and 3MSCT) to evaluate exercise capacity in Morquio A patients. This study provided new insights into the pathophysiology and symptomatology of patients with Morquio A and the impact of ERT.
MOR-008 was specifically designed to recruit a patient population healthy enough to perform CPET and endurance tests. Therefore, the patients had better endurance results than those from the phase 3 study, i.e., a median of 331 m in the 6MWT versus approximately 220 m in the phase 3 study and a median of 58 stairs/min in the 3MSCT versus approximately 30 stairs/min in the phase 3 study (Hendriksz et al. 2014). Nevertheless, all patients showed reduced maximal exercise capacity, and the majority had an abnormal ventilatory response to exercise compared with healthy individuals (Ten Harkel et al. 2011). Baseline mean peak VO2 and peak heart rate during CPET were considerably below age-/weight-matched reference values (ATS and ACCP 2003). Baseline tidal volume response was limited, related to the patients’ reduced lung function and height. Resting respiratory rate was correspondingly elevated.
Maximal exercise capacity numerically increased after 25 weeks of elosulfase alfa treatment and remained relatively stable thereafter up to week 52 (Table 3). The greatest increases were seen in the tidal volume at peak workload and in the VO2 at VT, indicating that patients were able to breathe more efficiently and to exercise to a higher workload before reaching their VT. Of note, the VO2 at VT is not dependent on patient effort indicating that the changes seen in the CPET variables reflected physiological improvement. The VO2 at VT continued to increase beyond 25 weeks (week 25, +9%; week 52, +18%) indicating progressive improvement in exercise capacity throughout the study period. Analysis of the remaining CPET parameters provided additional objective support that the increase in exercise capacity is not attributable to volitional factors. At baseline, mean peak RER was >1 indicating that patients exercised to a workload beyond VT, in accordance with adequate patient effort at study entry. While this does not preclude small test-to-test differences in volitional effort between subjects, the peak RER remained unchanged on subsequent CPET evaluations at weeks 25 and 52, indicating that patient performance did not change during the study. Moreover, patients showed minimal differences in peak heart rate and respiratory rate between baseline and week 52 (which remained below age-adjusted norms), indicating that exercise was terminated at similar cardiorespiratory stress levels at each study visit.
These results illustrate how CPET provides an assessment of exercise capacity that is more comprehensive than the 6MWT and 3MSCT, which are submaximal and volitional tests that may depend on self-motivational factors (ATS and ACCP 2003; Guazzi et al. 2009; Crescimanno et al. 2015). The 6MWT and 3MSCT do not require special equipment or training to implement, but only provide indirect assessments of endurance and functionality (Fleming and Powers 2012) with no information on the factors that limit exertion. Although more complicated, CPET provides an integrated assessment of exercise responses from the cardiovascular, pulmonary, and skeletal muscle systems, thereby allowing evaluation of both submaximal and peak exercise responses (ATS and ACCP 2003; Albouaini et al. 2007). The fundamental differences between these tests might (partly) explain the apparent lack of correlation between CPET and endurance outcomes. While positive correlations between peak VO2 and endurance measures were seen at baseline, changes in both measures over 25 or 52 weeks were not (6MWT) or only moderately (3MSCT) correlated. Changes in VO2 at VT were also not positively correlated with endurance outcomes.
Despite the improvements observed in CPET measures of exercise capacity and work efficiency, improvements in endurance measures were small. These findings suggest that patients continued to self-regulate their performance to a similar degree on the volitional tests despite improvement in maximal exercise capacity. Alternatively, the relatively good endurance of the study population at baseline left little room for further improvement in the 6MWT or 3MSCT, particularly given the orthopedic abnormalities in these patients (50% with knee deformity, 40% with joint pain, and 27% with hip dysplasia at baseline) (Burton et al. 2015). Because of this ceiling effect, the 6MWT and 3MSCT test may be less suitable to assess treatment effects in patients with relatively good baseline endurance.
It is unlikely that a cardiac effect contributes to the effect of elosulfase alfa on exercise capacity, as no impact was seen on ejection fraction over 52 weeks (data on file, BioMarin). This was in accord with expectations based on data from the phase 3 study after 120 weeks of treatment (data on file, BioMarin). In the absence of a change in cardiac function, the increase in VO2 at VT is compatible with improvements in either peripheral O2 extraction or mitochondrial function. The rapid increases seen in work efficiency and VO2 at VT are consistent with reports of improved sense of well-being and decreased fatigue in patients on elosulfase alfa in the phase 3 study, even before changes in height and lung function are seen (data on file, BioMarin). Overall, these findings suggest that ERT improves aerobic efficiency as evidenced by performance of work at reduced metabolic demand during CPET. Patients receiving ERT seem to better extract and/or utilize O2 when exercising, despite their inability to increase tidal volume or further maximize heart or respiratory rate, which were already at maximal capacity prior to ERT. The effect of growth on exercise capacity was not assessed.
Limitations of the study design that should be considered when interpreting these results include the small sample size, which does not allow for statistical comparison between treatment groups, the lack of a control arm, and a possible training effect of some CPET variables (e.g., exercise duration) with repeated testing.
Conclusions
Overall, use of CPET in the evaluation of exercise capacity in patients with Morquio A uncovered a limitation in exercise performance related to reduced lung function (i.e., restrictive respiratory disease). In addition, the 52-week CPET outcomes of the MOR-008 pilot study provide evidence for a positive effect of elosulfase alfa on exercise capacity and efficiency of oxygen utilization that was not attributable to changes in either cardiac or respiratory function. As orthopedic challenges may limit the impact of treatment on endurance test results in these patients, analysis of data obtained during CPET may be a valuable addition to the 6MWT and 3MSCT to monitor treatment effects on cardiorespiratory capacity. Further study of the impact of ERT on exercise capacity in larger patient groups and with longer follow-up is warranted to establish the clinical relevance of the observed changes and the usefulness of CPET in the evaluation of Morquio A patients in clinical practice.
Electronic Supplementary Material
Mean (standard error) change in 6-minute walk test (6MWT) and 3-minute stair climb test (3MSCT) outcomes from baseline to week 52 with elosulfase alfa 2 mg/kg and 4 mg/kg per week (modified intent-to-treat population) (DOCX 122 kb)
Change from resting to peak tidal volume (mL) at baseline in (A) the elosulfase alfa 2.0 mg/kg/week and (B) the elosulfase alfa 4.0 mg/kg/week treatment group (modified intent-to-treat population). Different lines represent different patients (DOCX 151 kb)
Scatter plot of baseline change from resting to peak tidal volume (mL) by forced expiratory volume in 1 s (FEV1) revealing that the abnormal ventilatory response to exercise was correlated with FEV1 at baseline (modified intent-to-treat population) (DOCX 94 kb)
Changes from baseline in peak workload versus Δ tidal volume during exercise at (A) week 25 and (B) week 52 (DOCX 72 kb)
Correlations between endurance measures and peak oxygen uptake (VO2) (modified intent-to-treat population) (DOCX 13 kb)
Acknowledgments
The authors are grateful to Ismar Healthcare NV for their assistance in the writing of the manuscript, which was funded by BioMarin Pharmaceutical Inc. Fred Genter is acknowledged for his work as a statistician for the study.
Take-Home Message
Morquio A patients have a limitation in exercise performance, which is related to reduced lung function; elosulfase alfa enzyme replacement therapy for 52 weeks has a positive effect on exercise capacity and efficiency of oxygen utilization, not attributable to changes in either cardiac or pulmonary function.
Compliance with Ethics Guidelines
Conflict of Interest
Kenneth I. Berger has worked as consultant and study investigator for BioMarin Pharmaceutical Inc. and has received an honorarium.
Barbara K. Burton has worked as consultant and study investigator for BioMarin Pharmaceutical Inc. and has received an honorarium.
Gregory D. Lewis has worked as consultant and study investigator for BioMarin Pharmaceutical Inc. and has received an honorarium.
Mark Tarnopolsky has worked as consultant and study investigator for BioMarin Pharmaceutical Inc. and has received an honorarium.
Paul R. Harmatz has worked as consultant and study investigator for BioMarin Pharmaceutical Inc. and has received an honorarium.
John J. Mitchell has worked as consultant and study investigator for BioMarin Pharmaceutical Inc. and has received an honorarium.
Nicole Muschol has worked as consultant and study investigator for BioMarin Pharmaceutical Inc. and has received an honorarium.
Simon A. Jones has worked as consultant and study investigator for BioMarin Pharmaceutical Inc. and has received an honorarium.
Vernon R. Sutton has worked as consultant and study investigator for BioMarin Pharmaceutical Inc. and has received an honorarium.
Gregory M. Pastores has worked as consultant and study investigator for BioMarin Pharmaceutical Inc. and has received an honorarium.
Heather Lau has worked as consultant and study investigator for BioMarin Pharmaceutical Inc. and has received an honorarium.
Rebecca Sparkes has worked as consultant and study investigator for BioMarin Pharmaceutical Inc. and has received an honorarium.
Adam J. Shaywitz is an employee of BioMarin Pharmaceutical Inc.
This study was sponsored by BioMarin Pharmaceutical Inc. and supported, in part, by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through UCSF-CTSI Grant Number UL1 TR000004 (Dr. Harmatz). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Support in the process of manuscript development was also funded by BioMarin Pharmaceutical Inc.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Details of the Contributions of Individual Authors
Barbara K. Burton, Kenneth I. Berger, Gregory D. Lewis, Mark Tarnopolsky, Paul R. Harmatz, John J. Mitchell, Nicole Muschol, Simon A. Jones, Vernon R. Sutton, Gregory M. Pastores, Heather Lau, and Rebecca Sparkes were all members of the steering committee, contributed to the planning of the study, and were involved in the clinical examinations and collection of patient data and in the preparation and critical review of the manuscript.
Adam J. Shaywitz assisted with conduction of the study, data analyses, and development of the manuscript.
Contributor Information
Kenneth I. Berger, Email: Kenneth.Berger@nyumc.org
Collaborators: Matthias Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Albouaini K, Egred M, Alahmar A, Wright DJ. Cardiopulmonary exercise testing and its application. Postgrad Med J. 2007;83:675–682. doi: 10.1136/hrt.2007.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Thoracic Society ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- ATS and ACCP ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- Burton BK, Berger KI, Lewis GD, et al. Safety and physiological effects of two different doses of elosulfase alfa in patients with morquio a syndrome: a randomized, double-blind, pilot study. Am J Med Genet A. 2015;167A:2272–2281. doi: 10.1002/ajmg.a.37172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbek E (2015) Normal vital signs. Medscape. http://emedicine.medscape.com/article/2172054-overview
- Crescimanno G, Modica R, Lo Mauro R, Musumeci O, Toscano A, Marrone O. Role of the cardio-pulmonary exercise test and six-minute walking test in the evaluation of exercise performance in patients with late-onset Pompe disease. Neuromuscul Disord. 2015;25:542–547. doi: 10.1016/j.nmd.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Fleming TR, Powers JH. Biomarkers and surrogate endpoints in clinical trials. Stat Med. 2012;31:2973–2984. doi: 10.1002/sim.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guazzi M, Dickstein K, Vicenzi M, Arena R. Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: a comparative analysis on clinical and prognostic insights. Circ Heart Fail. 2009;2:549–555. doi: 10.1161/CIRCHEARTFAILURE.109.881326. [DOI] [PubMed] [Google Scholar]
- Harmatz P, Mengel KE, Giugliani R, et al. The Morquio A Clinical Assessment Program: baseline results illustrating progressive, multisystemic clinical impairments in Morquio A subjects. Mol Genet Metab. 2013;109:54–61. doi: 10.1016/j.ymgme.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Hendriksz CJ, Al-Jawad M, Berger KI, et al. Clinical overview and treatment options for non-skeletal manifestations of mucopolysaccharidosis type IVA. J Inherit Metab Dis. 2013;36:309–322. doi: 10.1007/s10545-012-9459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksz CJ, Burton B, Fleming TR, et al. Efficacy and safety of enzyme replacement therapy with BMN 110 (elosulfase alfa) for Morquio A syndrome (mucopolysaccharidosis IVA): a phase 3 randomised placebo-controlled study. J Inherit Metab Dis. 2014;37:979–990. doi: 10.1007/s10545-014-9715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksz CJ, Berger KI, Giugliani R, et al. International guidelines for the management and treatment of Morquio A syndrome. Am J Med Genet A. 2015;167A:11–25. doi: 10.1002/ajmg.a.36833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadley RM, Lang S, Misso K, et al. A systematic review of the prevalence of Morquio A syndrome: challenges for study reporting in rare diseases. Orphanet J Rare Dis. 2014;9:173. doi: 10.1186/s13023-014-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaño AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007;30:165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- Muenzer J. Overview of the mucopolysaccharidoses. Rheumatology (Oxford) 2011;50(Suppl 5):v4–v12. doi: 10.1093/rheumatology/ker394. [DOI] [PubMed] [Google Scholar]
- Sanford M, Lo JH. Elosulfase alfa: first global approval. Drugs. 2014;74:713–718. doi: 10.1007/s40265-014-0210-z. [DOI] [PubMed] [Google Scholar]
- Santuz P, Baraldi E, Filippone M, Zacchello F. Exercise performance in children with asthma: is it different from that of healthy controls? Eur Respir J. 1997;10:1254–1260. doi: 10.1183/09031936.97.10061254. [DOI] [PubMed] [Google Scholar]
- Ten Harkel ADJ, Takken T, Van Osch-Gevers M, Helbing WA. Normal values for cardiopulmonary exercise testing in children. Eur J Cardiovasc Prev Rehabil. 2011;18:48–54. doi: 10.1097/HJR.0b013e32833cca4d. [DOI] [PubMed] [Google Scholar]
- Yasuda E, Fushimi K, Suzuki Y, et al. Pathogenesis of Morquio A syndrome: an autopsied case reveals systemic storage disorder. Mol Genet Metab. 2013;109:301–311. doi: 10.1016/j.ymgme.2013.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean (standard error) change in 6-minute walk test (6MWT) and 3-minute stair climb test (3MSCT) outcomes from baseline to week 52 with elosulfase alfa 2 mg/kg and 4 mg/kg per week (modified intent-to-treat population) (DOCX 122 kb)
Change from resting to peak tidal volume (mL) at baseline in (A) the elosulfase alfa 2.0 mg/kg/week and (B) the elosulfase alfa 4.0 mg/kg/week treatment group (modified intent-to-treat population). Different lines represent different patients (DOCX 151 kb)
Scatter plot of baseline change from resting to peak tidal volume (mL) by forced expiratory volume in 1 s (FEV1) revealing that the abnormal ventilatory response to exercise was correlated with FEV1 at baseline (modified intent-to-treat population) (DOCX 94 kb)
Changes from baseline in peak workload versus Δ tidal volume during exercise at (A) week 25 and (B) week 52 (DOCX 72 kb)
Correlations between endurance measures and peak oxygen uptake (VO2) (modified intent-to-treat population) (DOCX 13 kb)