Abstract
A thermo-adapted strain of Zymomonas mobilis designated ZM AD41 that capable of growth and ethanol production at high temperature was obtained using the thermal stress adaptation technique. This thermo-adapted strain exhibited approximately 1.8- and 27-fold higher growth rate than the wild-type at 39 °C and 41 °C, respectively. It was more resistant to stress induced by acetic acid at 200 mM and hydrogen peroxide (H2O2) at 0.4 mM and produced approximately 1.8- and 38.6-fold higher ethanol concentrations than the wild-type at 39 °C and 41 °C, respectively. Moreover, it had better sedimentation performance during ethanol fermentation at high temperature than the wild-type. Based on the growth performance, heat, acetic acid and H2O2 stress treatments, sedimentation characteristics, and ethanol fermentation capability, Z. mobilis ZM AD41 was a good candidate for ethanol production at high temperature.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1493-7) contains supplementary material, which is available to authorized users.
Keywords: Ethanol production, High-temperature fermentation, Thermal stress adaptation, Thermotolerance, Zymomonas mobilis
Introduction
Bioethanol fermentation is generally achieved using mesophilic microorganisms, particularly ethanologenic yeasts and bacteria, and the optimum temperature for growth and ethanol production of these fermentative microbes is in the range of 25–35 °C. However, the large-scale production of industrial bioethanol is carried out in a closed fermenter, which generates much heat due to the metabolic activities of microorganisms during the fermentation process (Abdel-Banat et al. 2010). Thus, strict temperature control at the optimum growth range is usually required. Recently, global warming significantly has contributed to making temperature control more difficult to perform in the case of large-scale fermentation and high-temperature ethanol fermentation (HTEF). HTEF has several advantages including a reduced risk of contamination and reduced cooling costs because a chiller or cooler unit is not required during the fermentation process (Zhang et al. 2015). The benefits also include a high bioconversion rate, facilitated product recovery, and more efficient simultaneous saccharification and fermentation (SSF). However, high temperature during the fermentation process may inhibit cell growth and hamper cell viability, resulting in a reduction of ethanol yield (Thanonkeo et al. 2007; Sootsuwan et al. 2013; Techaparin et al. 2017). Therefore, highly thermotolerant microorganisms are essential for steady fermentation.
Zymomonas mobilis, a facultative anaerobic Gram-negative bacterium, has been considered as one of the most useful bacteria that may replace traditionally used yeast, Saccharomyces cerevisiae, for industrial ethanol production. Z. mobilis can produce 5–10% higher ethanol yield than S. cerevisiae. The maximum theoretical yield of ethanol production using Z. mobilis is approximately 97%, whereas S. cerevisiae can yield only 90–93% (Hayashi et al. 2012). Apart from this, Z. mobilis has a high tolerance to high levels of ethanol and high sugar concentrations. Similar to S. cerevisiae, the optimum temperature for growth and ethanol production by Z. mobilis is limited. A preliminary study in our laboratory demonstrated that Z. mobilis TISTR548 has a critical high temperature for growth and ethanol production at 38 °C. Therefore, the aim of this study was to develop a potentially useful strain of Z. mobilis that can grow and produce high ethanol concentrations and yield in high temperature fermentation using thermal stress adaptation technique. As demonstrated in this study, a thermo-adapted strain of Z. mobilis designated ZM AD41 exhibited faster growth and better ethanol production capabilities than the wild-type. Furthermore, it was more tolerant to heat, acetic acid and H2O2 stresses, and had better sedimentation characteristics during ethanol production at 41 °C.
Materials and methods
Bacterial strains and culture conditions
Zymomonas mobilis TISTR548 was obtained from the Thailand Institute of Scientific and Technological Research (TISTR), Bangkok, and was used as a parental strain for thermal stress adaptation. Z. mobilis wild-type and thermo-adapted strains were maintained at 4 °C on yeast extract-peptone glucose (YPG) agar (3.0 g/l yeast extract, 5.0 g/l peptone, 30.0 g/l glucose and 15.0 g/l agar) and subcultured monthly. For long-term storage, all strains were stored at − 80 °C in cryogenic vials containing 50% glycerol solution.
Thermal stress adaptation experiment
Thermal stress adaptation of Z. mobilis wild-type strain was performed using the method as described by Rudolph et al. (2010). The procedure of the thermal stress adaptation process is illustrated in Supplementary Fig. 1. A single colony of Z. mobilis TISTR548 was inoculated into 3 ml of YPG medium and incubated at 30 °C, 100 rpm until mid-exponential growth phase (optical density or OD at 550 nm reached 0.8–1.0). Thereafter, cells were transferred into 10 ml of YPG medium at an initial OD550 of 0.05. This step of the cultivation process was repeated several times by gradually increasing the incubation temperature from 38 to 41 °C. In this study, the cultivation was first carried out at 38 °C for 30 cycles and then shifted to 39 °C for 90 cycles, 39.5 °C for 30 cycles, 40 °C for 30 cycles, 40.5 °C for 25 cycles, and finally 41 °C for 25 cycles. In the last step of cultivation, cells were collected and spread on a YPG agar plate incubated at 40 °C. Thermo-adapted colonies growing on the agar plate were screened and selected for assessment of cell growth under heat stress conditions. A high potential thermo-adapted strain capable of growing at high temperature was selected for further analysis.
Cell morphology analysis
Zymomonas mobilis wild-type and thermo-adapted strains were grown in YPG broth and incubated at 30 °C and 37 °C, 100 rpm. After 18 h of cultivation, cells were collected, washed twice, and resuspended in 100 µl of 0.85% NaCl. The resulting cells were monitored under a microscope (Nikon E600, Japan), and the cell size was measured using PhotoRuler.
Stress treatment analysis
The effects of high temperature, acetic acid and H2O2 stress on the growth of Z. mobilis wild-type and thermo-adapted strains were studied. For high temperature stress, cells were grown in YPG medium and incubated at 30 °C, 100 rpm for 12 h. Then, bacterial cells were transferred to a fresh YPG medium at an initial OD550 of 0.05 and subsequently incubated at 30 °C, 37 °C and 40 °C for 24 h (Sootsuwan et al. 2013). For acetic acid stress, cells of the wild-type and thermo-adapted strains grown in YPG medium at 30 °C, 100 rpm for 12 h were transferred to a fresh YPG medium containing acetic acid (Sigma-Aldrich, USA) at a final concentration of 0 (control), 150 and 200 mM, and then statically incubated at 30 and 37 °C for 24 h (Liu et al. 2017). For H2O2 stress, cells were grown in the YPG medium supplemented with 100 mM H2O2 (Wako, Japan) to the final concentrations of 0.1–0.4 mM and then statically incubated at 30 °C and 37 °C for 24 h (Charoensuk et al. 2011). Cell growth was determined by measuring the OD at 550 nm using a spectrophotometer (Shimadzu, Japan).
Sedimentation performance analysis
Zymomonas mobilis wild-type and thermo-adapted strains were grown in the YPG medium at 30 °C, 100 rpm for 12 h and then the cultures were transferred to 15 ml of YPG medium in 20 × 150 mm test tubes at an initial OD550 of 0.05 and subsequently incubated at 30 °C, 37 °C and 40 °C. After 24-h incubation, cells were vigorously mixed using a vortex at a high speed for 30 s. Sedimentation of the cell suspension was immediately monitored, and a photograph was taken every 2 min.
Ethanol fermentation at high temperatures
Ethanol fermentation ability of the wild-type and thermo-adapted strains was evaluated at high temperatures. Cells were grown in the YPG medium at 30 °C, 100 rpm for 12 h, and then 10% (v/v) of the inoculum were transferred into 30 ml of the YPG medium containing 30 g/l glucose. The flasks were statically incubated at 30 °C, 37 °C, 39 °C and 41 °C. During ethanol fermentation, samples were withdrawn every 12 h, and ethanol concentrations in the fermentation broth were measured using gas chromatography (GC) as described by Nuanpeng et al. (2016).
Results and discussion
Selection of a thermo-adapted strain
Based on the thermal stress adaptation technique initially carried out repeatedly at 38 °C, the growth of the Z. mobilis wild-type strain was clearly improved after 30 cycles of cultivation. When the incubation temperature was shifted from 38 to 39 °C, the growth of the bacterial cells was remarkably decreased. A lag phase of growth (~ 72 h) was observed at the first stage after temperature increase; then, the growth of the bacteria was slightly increased to the OD550 of 0.9–1.0. It should be noted that after several cycles of repeated cultivation, the lag phase of bacterial growth became shorter, and the growth pattern was almost constantly. At 39.5 °C and 40 °C, the growth pattern of Z. mobilis was very similar with a final OD550 of 0.9–1.2. The maximum growth at 40 °C was reached after a stepwise increase in temperature for 172 generations. Based on this observation, an incubation temperature of the bacterial cells was increased to 40.5 °C. The thermo-adapted strains showed a good growth efficacy at 40.5 °C after 25 cycles of cultivation; however, they showed somewhat lower growth at 41 °C (Fig. 1). After 25 cycles of repeated cultivation at 41 °C, the bacterial cells were collected and spread on YPG agar plate. Random colonies on the agar medium were selected and their growth performance at high temperatures was evaluated. This selection yielded a thermo-adapted strain designated Z. mobilis AD41 (ZM AD41), which was chosen for further analysis due to good growth at high temperatures (data not shown).
Fig. 1.
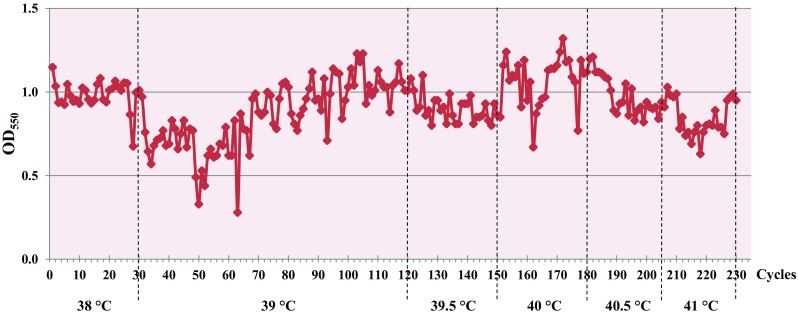
Growth of the Z. mobilis wild-type strain (TISTR 548) after adaptation in 10 ml YPG broth at high temperatures from 38 to 41 °C
To confirm thermotolerance of the selected thermo-adapted strain, a comparative study of the growth of the wild-type and ZM AD41 was carried out using YPG medium. The results showed that growth of the wild-type was approximately 2.4-fold higher than that of ZM AD41 at 30 °C. At 37 °C, the growth of ZM AD41 was similar to the wild-type. However, at 39 °C, the growth of ZM AD41 was significantly higher than the wild-type. When the incubation temperature was increased to 41 °C, only ZM AD41 could grow. No growth was detected in the wild-type culture at this temperature (Fig. 2).
Fig. 2.
Comparison of the growth of the Z. mobilis wild-type (TISTR 548) (filled diamonds) and thermo-adapted (ZM AD41) strains (filled triangles) at various temperatures
Cell morphology of the wild-type and thermo-adapted strains
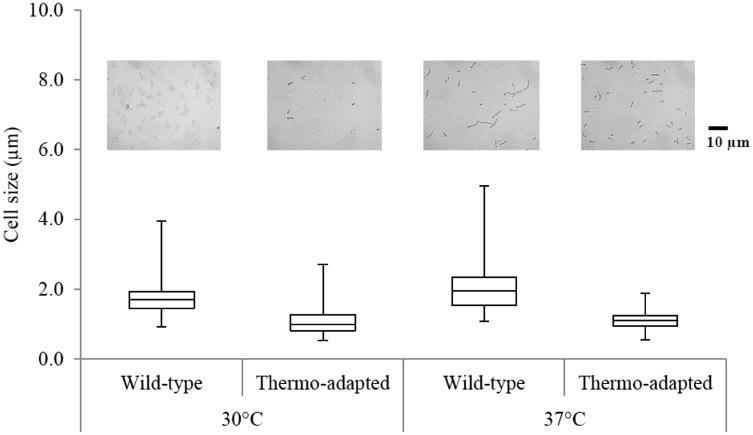
Wild-type and ZM AD41 cells were grown in a YPG medium under a static condition at various temperatures for 18 h, and the cell morphology was observed and analyzed using the PhotoRuler. The results revealed that cell sizes of the wild-type and ZM AD41 were almost similar at 30 °C. However, at 37 °C, the wild-type cells became elongated compared to the ZM AD41 cells. The average cell length of the wild-type strain was approximately 2.02 ± 0.35 µm, while the cell length of the ZM AD41 strain was approximately 1.12 ± 0.28 µm (Fig. 3). The elongation of the wild-type cells observed in this study may be due to a negative effect of high temperature on bacterial DNA molecule. Furthermore, high temperature may inhibit cell division as described by Charoensuk et al. (2011) and Hayashi et al. (2012). Other mechanisms such as reduced intracellular oxidative stress should be considered for the short cell lengths of the thermo-adapted ZM AD41 strain.
Fig. 3.
Cell size of the Z. mobilis wild-type (TISTR 548) and thermo-adapted (ZM AD41) strains after incubation at 30 °C and 37 °C. The length of 100 cells per strain was measured using PhotoRuler. The top and the bottom of a bar show the maximum and minimum cell length, respectively. The line in the box corresponds to the average cell size. Scale bar: 10 µm
Growth characterization of the wild-type and thermo-adapted strains under stress conditions
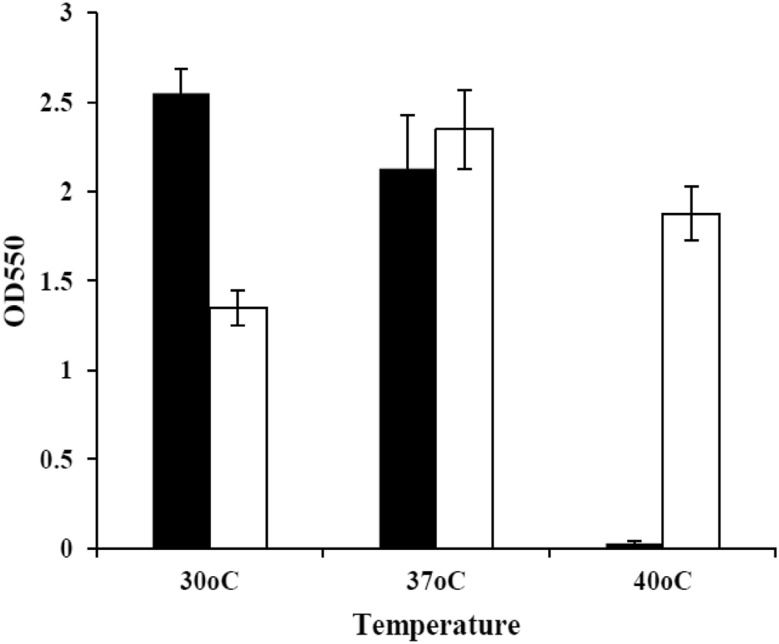
Growth rates of the wild-type and ZM AD41 were compared on YPG medium at various temperatures and the results are summarized in Fig. 4. The growth of ZM AD41 at 30 °C was remarkably slower than the wild-type. In contrast, ZM AD41 growth was approximately 1.1- and 62.5-fold higher than the wild-type at 37 °C and 40 °C, respectively. These results indicated that the thermo-adapted strain acquired thermotolerance independent of the fermentation capability over the course of thermal adaptation. The present study also confirms that thermal adaptation technique works well in Z. mobilis improving thermotolerance of cells and can be used as a powerful tool for strain development as described by Shui et al. (2015).
Fig. 4.
Comparison of the growth of the Z. mobilis wild-type (TISTR 548) and thermo-adapted (ZM AD41) strains at various temperatures. The wild-type and thermo-adapted strains were precultured at 30 °C in YPG medium for 12 h, and then transferred to a fresh YPG medium and subsequently incubated at 30 °C, 37 °C and 40 °C for 24 h. Closed and open columns represent the optical density of the wild-type and thermo-adapted strains, respectively
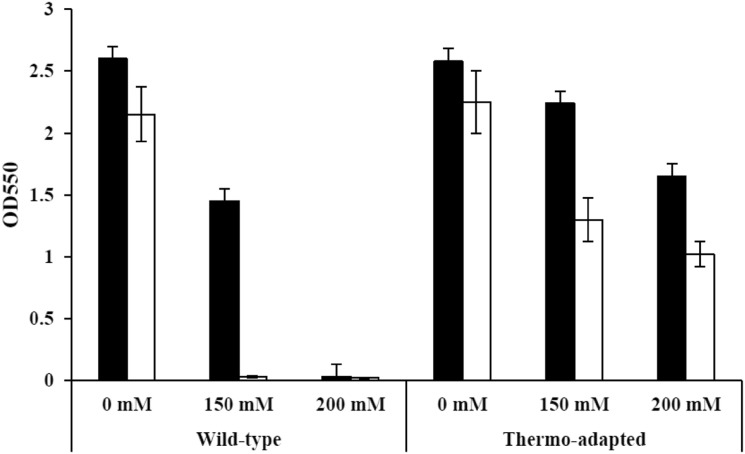
Acetic acid is one of the major inhibitors formed and released during pretreatment and hydrolysis process of the lignocellulosic material. It can inhibit growth and fermentation activity of ethanologenic organisms, such as S. cerevisiae and Z. mobilis (Kumari and Pramanik 2012; Franden et al. 2013). The amount of acetic acid in lignocellulosic hydrolysates is varied from 16.7 to 258 mM depended on the feedstocks. In this study, the effect of acetic acid on the growth of the wild-type and ZM AD41 was investigated and the results are summarized in Fig. 5. At 30 °C, the growth of the wild-type and ZM AD41 in the medium without acetic acid supplementation was almost similar. However, ZM AD41 displayed approximately 1.5- and 55.0-fold higher growth than the wild-type when acetic acid concentrations in the medium were increased to 150 and 200 mM, respectively. At 37 °C, the growth of the wild-type and ZM AD41 was insignificant in the medium without acetic acid supplementation. However, ZM AD41 exhibited significantly higher tolerance to high concentrations of acetic acid than the wild-type at this temperature. On the contrary, the growth of the wild-type was completely inhibited by acetic acid at a concentration of 150 and 200 mM at 37 °C. These results are similar to that reported in Z. mobilis ZM481 and sodium acetate-tolerant mutant strains ZMA-142 and ZMA-167 (Liu et al. 2017). The molecular mechanisms in response to acetic acid in Z. mobilis are complicated and required several genes related to membrane stabilization, transporter and regulator. Yang et al. (2010) demonstrated that a regulator hfq protein in Z. mobilis is involved in acetate-tolerance. Liu et al. (2017) reported that removal of terminator putative sequences of ZMO0117 results in high expression of hydroxylamine reductase, leading to a stress resistance of microbial cells. With respect to thermo-adapted strain ZM AD41, further studies to clarify the molecular responses to acetic acid are required.
Fig. 5.
Comparison of the growth of the Z. mobilis wild-type (TISTR 548) and thermo-adapted (ZM AD41) strains under acetic acid stress. The wild-type and thermo-adapted strains were precultured at 30 °C in YPG medium for 12 h, and then transferred to a fresh YPG medium containing acetic acid at a final concentration of 0, 150 and 200 mM. Cells were statically incubated at 30 °C and 37 °C for 24 h. Closed and open columns represent the optical density at 30 °C and 37 °C, respectively
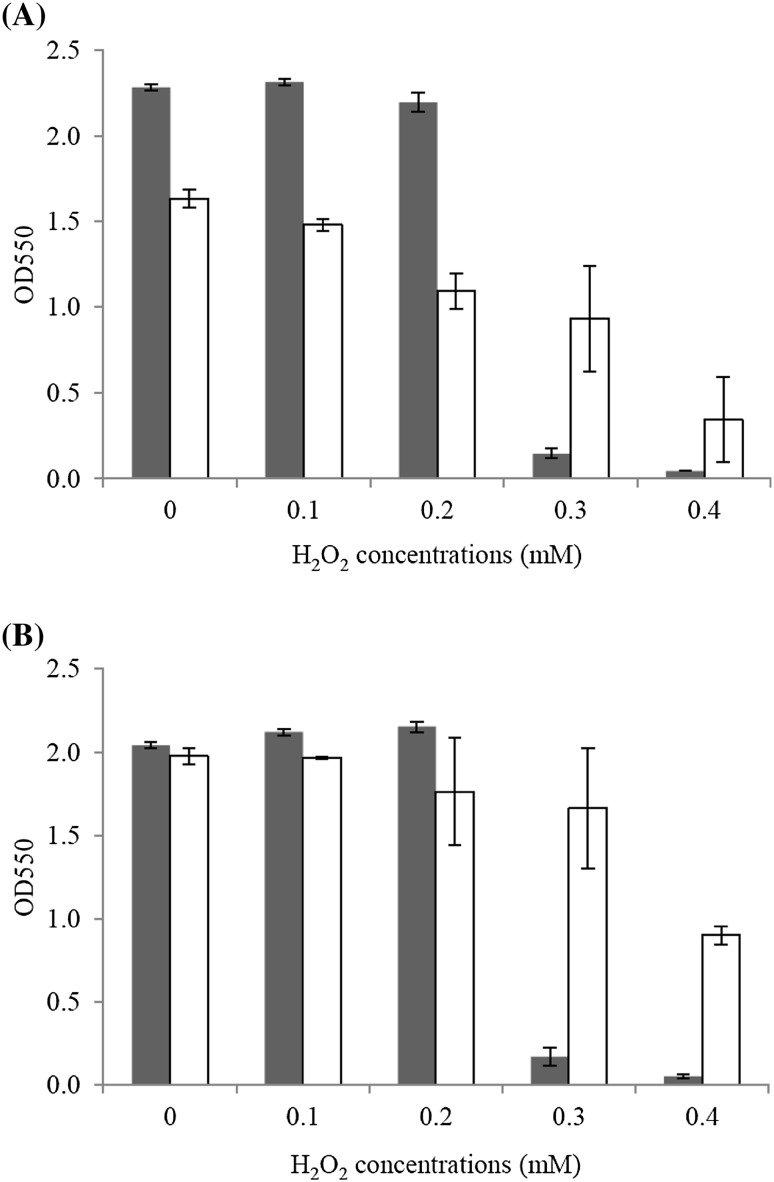
Sensitivity to H2O2 of the wild-type and ZM AD41 strains was examined using YPG medium containing H2O2 at the final concentrations of 0.1, 0.2, 0.3 and 0.4 mM. The results are summarized in Fig. 6. At 30 °C, the growth of wild-type was approximately 1.4- to 2.0-time higher than ZM AD41 when H2O2 concentrations in the medium were in the range of 0.0–0.2 mM. When H2O2 concentrations in the medium were increased up to 0.3 or 0.4 mM, a pronounced decrease in growth of the wild-type was observed compared to ZM AD41. Similar results were observed at 37 °C; the growth of the wild-type was slightly higher than ZM AD41 when growing in a medium containing H2O2 concentrations of 0.0, 0.1 and 0.2 mM. However, the thermo-adapted strain displayed 9.7- and 16.6-fold higher tolerance to H2O2 at 0.3 and 0.4 mM, respectively, at 37 °C. It has been reported that oxidative stress products induced by endogenous reactive oxygen species (ROS) including H2O2 are accumulated at a high temperature. H2O2 causes disruption of membrane lipids, cellular proteins, and DNA molecules and this disruption influences cell morphology and cell survival at high temperatures (Cabiscol et al. 2000; Dixon and Stockwell 2014). H2O2 also oxidizes solvent-exposed iron resulting in the formation of hydroxyl radicals which severely damage DNA molecules. Hence, cells stop dividing and become filamentous. High growth performance of the ZM AD41 strain at high concentrations of H2O2 may be related to the expression of genes involved in the synthesis of antioxidant enzymes. It has been reported that expression levels of Zmsod, ZmahpC and ZMO1573 encoding for antioxidant enzymes in Z. mobilis are increased at high temperature (Charoensuk et al. 2011). Additional study of the expression of these genes in ZM AD41 may be needed to clarify the mechanism of this phenomenon.
Fig. 6.
Comparison of growth of the Z. mobilis wild-type and thermo-adapted strains at 30 °C (a) and 37 °C (b) in YPG medium containing various concentrations of H2O2. Cells were grown in YPG medium supplemented with 100 mM H2O2 at final concentrations from 0.1 to 0.4 mM and then incubated at 30 °C and 37 °C for 24 h. Closed and open columns represent the optical density of the wild-type and thermo-adapted strains, respectively
Sedimentation performance of the wild-type and thermo-adapted strains
Sedimentation is the most economically competitive engineering decision compared to centrifugation that requires heavy capital investment and intensive energy consumption for the operation. The flocculating organisms not only facilitate biomass recovery by cost-effective sedimentation instead of centrifugation, but also enhance tolerance to environmental stresses including toxic byproducts that are released during pretreatment of lignocellulosic biomass (Weber et al. 2010; Zhao et al. 2014). In this study, sedimentation performance of the wild-type and ZM AD41 at various temperatures was qualitatively characterized, and the results are summarized in Fig. 7. It was clear that ZM AD41 could flocculate better than the wild-type strain at all tested temperatures. The thermo-adapted strain could have evolved a mechanism of flocculent behavior to protect the cells from high-temperature stress. This flocculent behavior is complex and involves several genes encoding for proteins related to the flocculation process (Zhao et al. 2012). On the other hand, the results of this experiment indicate that ZM AD41 may be a promising organism for high-gravity ethanol fermentation.
Fig. 7.
Sedimentation performance of the Z. mobilis wild-type and thermo-adapted strains at 30 °C, 37 °C and 40 °C
Ethanol production at high temperatures
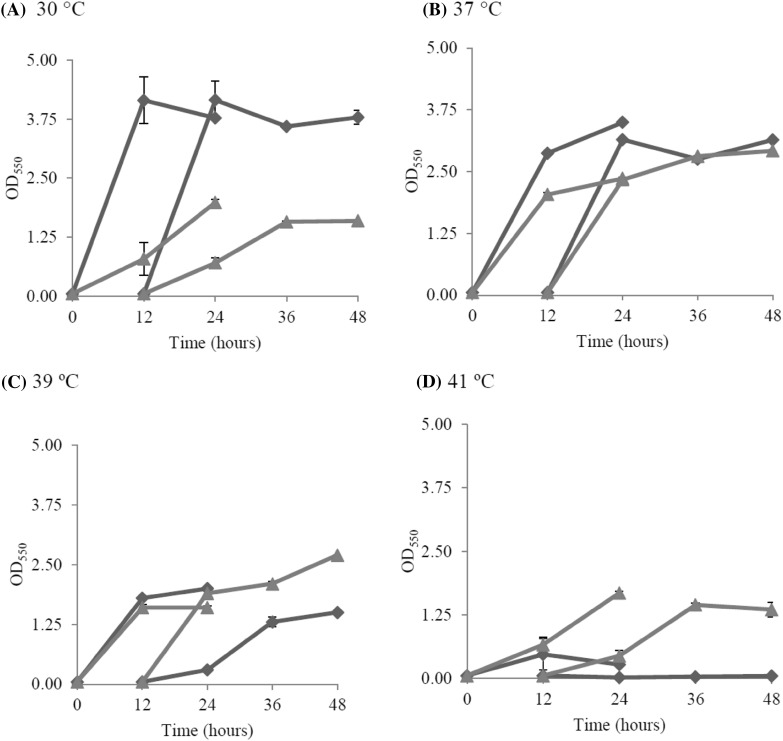
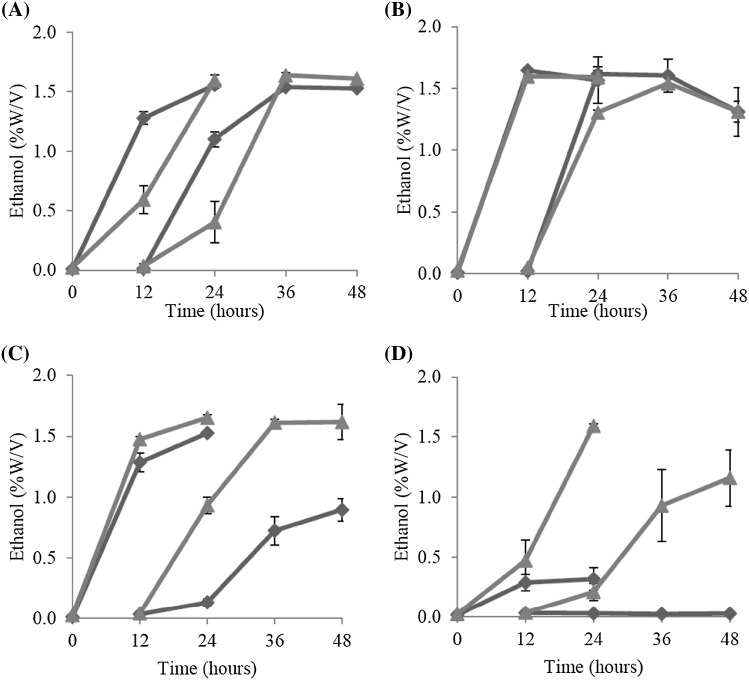
Ethanol production by the wild-type and thermo-adapted strains at high temperatures was evaluated using YPG medium containing 30 g/l glucose. The results showed that ethanol production between the wild-type and ZM AD41 was not significantly different at 30 °C and 37 °C. However, ZM AD41 produced approximately 1.8- and 38.6-fold higher ethanol concentrations than the wild-type at 39 °C and 41 °C, respectively (Fig. 8). A relatively high level of ethanol was detected at 41 °C in the ZM AD41 culture, whereas the amount of ethanol detected at this temperature in the wild-type culture was very low. These findings clearly demonstrate that thermo-adapted strain ZM AD41 has better ethanol fermentation activity than the wild-type, particularly at high temperature, suggesting that ZM AD41 is a good candidate for HTEF. The results also clearly indicate that the thermal stress adaptation process is an effective strategy for overcoming the disadvantage of the Z. mobilis wild-type strain in tolerating heat stress.
Fig. 8.
Ethanol production at 30 °C, 37 °C, 39 °C and 41 °C by the Z. mobilis wild-type and thermo-adapted strains. Wild-type (filled diamonds) and thermo-adapted (filled triangles) strains were grown in YPG medium containing 30 g/l glucose at 30 °C (a), 37 °C (b), 39 °C (c) and 41 °C (d) under static conditions
The molecular responses of Z. mobilis to a high-temperature or heat stress are complicated and are regulated by many genes related to general metabolism, membrane stabilization or membrane formation, transporter, DNA repair, tRNA/rRNA modification, protein quality control, translation control, cell division, and transcriptional regulation. Moreover, the mechanisms of thermotolerance in this organism are also overlapped with that of ethanol and acetic acid tolerance (Charoensuk et al. 2017; Liu et al. 2017). An acquisition of thermotolerance in the thermo-adapted strain ZM AD41 may correlate to the regulation of those genes as mentioned above. An additional experiment such as the genome sequencing of the ZM AD41 and the wild-type strains is needed to clarify this assumption, and it is now under our investigation.
Conclusions
This is the first report demonstrating that a thermotolerance strain of Z. mobilis can grow and produce ethanol at a high temperature of 41 °C. The thermotolerance strain designated ZM AD41 displayed a high resistant not only to high concentrations of acetic acid, but also high concentrations of H2O2, which is very useful for high-temperature ethanol production using lignocellulosic hydrolysates as feedstock. A good sedimentation performance of the thermotolerance strain also facilitated an off-cost process for cell separation from fermentation products which does not require energy input. Therefore, ZM AD41 is a good candidate for high-temperature ethanol production particularly in tropical countries where average daytime temperatures are usually high throughout the year.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was financially supported by the Thailand Research Fund (TRF), Thailand through the Royal Golden Jubilee Ph.D. Program (Grant no. PHD/0198/2553). The authors are very grateful to the Fermentation Research Center for Value Added Agricultural Products, Khon Kaen University, and the New Core to Core Program (CCP) A. Advanced Research Networks on “Establishment of an International Research Core for New Bio-research Fields with Microbes from Tropical Areas World Class Research Hub of Tropical Microbial Resources and Their Utilization” for use of the facilities and technical support.
Author contributions
JS performed the experiments and collected the data. MY participated in the data analysis of stress treatments. PK participated in the design of the experiments and drafting the manuscript. PT contributed to the design of the experiments, conducted and analyzed the experimental data, and writing the manuscript. All of the authors read, corrected and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abdel-Banat BMA, Hoshida H, Ano A, Nonklang S, Akada R. High-temperature fermentation: how can processes for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast? Appl Microbiol Biotechnol. 2010;85:861–867. doi: 10.1007/s00253-009-2248-5. [DOI] [PubMed] [Google Scholar]
- Cabiscol E, Tamarit J, Ros J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol. 2000;3:3–8. [PubMed] [Google Scholar]
- Charoensuk K, Irie A, Lertwattanasakul N, Sootsuwan K, Thanonkeo P, Yamada M. Physiological importance of cytochrome c peroxidase in ethanologenic thermotolerant Zymomonas mobilis. J Mol Microbiol Biotechnol. 2011;20:70–82. doi: 10.1159/000324675. [DOI] [PubMed] [Google Scholar]
- Charoensuk K, Sakurada T, Tokiyama A, Murata M, Kosaka T, Thanonkeo P, Yamada M. Thermotolerant genes essential for survival at a critical high temperature in thermotolerant ethanologenic Zymomonas mobilis TISTR548. Biotechnol Biofuels. 2017;10:204. doi: 10.1186/s13068-017-0891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- Franden MA, Pilath HM, Mohagheghi A, Pienkos PT, Zhang M. Inhibition of growth of Zymomonas mobilis by model compounds found in lignocellulosic hydrolysates. Biotechnol Biofuels. 2013;6:99. doi: 10.1186/1754-6834-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Kato T, Furukawa K. Respiratory chain analysis of Zymomonas mobilis mutants producing high levels of ethanol. Appl Environ Microbiol. 2012;78:5622–5629. doi: 10.1128/AEM.00733-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari R, Pramanik K. Improvement of multiple stress tolerance in yeast strain by sequential mutagenesis for enhanced bioethanol production. J Biosci Bioeng. 2012;114:622–629. doi: 10.1016/j.jbiosc.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Liu YF, Hsieh CW, Chang YS, Wung BS. Effect of acetic acid on ethanol production by Zymomonas mobilis mutant strains through continuous adaptation. BMC Biotechnol. 2017;17:63. doi: 10.1186/s12896-017-0385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuanpeng S, Thanonkeo S, Yamada M, Thanonkeo P. Ethanol production from sweet sorghum juice at high temperatures using a newly isolated thermotolerant yeast Saccharomyces cerevisiae DBKKU Y-53. Energies. 2016;9:253. doi: 10.3390/en9040253. [DOI] [Google Scholar]
- Rudolph B, Gebendorfer KM, Buchner J, Winter J. Evolution of Escherichia coli for growth at high temperatures. J Biol Chem. 2010;285:19029–19034. doi: 10.1074/jbc.M110.103374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shui ZX, Qin H, Wu B, Ruan ZY, Wang LS, Tan FR, Wang JL, Tang XY, Dai LC, Hu GQ, He MX. Adaptive laboratory evolution of ethanologenic Zymomonas mobilis strain tolerant to furfural and acetic acid inhibitors. Appl Microbiol Biotechnol. 2015;99:5739–5748. doi: 10.1007/s00253-015-6616-z. [DOI] [PubMed] [Google Scholar]
- Sootsuwan K, Thanonkeo P, Keeratirakha N, Thanonkeo S, Jaisil P, Yamada M. Sorbitol required for cell growth and ethanol production by Zymomonas mobilis under heat, ethanol, and osmotic stresses. Biotechnol Biofuels. 2013;6:180–193. doi: 10.1186/1754-6834-6-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Techaparin A, Thanonkeo P, Klanrit P. Gene expression profiles of the thermotolerant yeast Saccharomyces cerevisiae strain KKU-VN8 during high-temperature ethanol fermentation using sweet sorghum juice. Biotechnol Lett. 2017;39:1521–1527. doi: 10.1007/s10529-017-2398-y. [DOI] [PubMed] [Google Scholar]
- Thanonkeo P, Laopaiboon P, Sootsuwan K, Yamada M. Magnesium ions improve growth and ethanol production of Zymomonas mobilis under heat or ethanol stress. Biotechnology. 2007;6:112–119. doi: 10.3923/biotech.2007.112.119. [DOI] [Google Scholar]
- Weber C, Farwick A, Benisch F, Brat D, Dietz H, Subtil T, Boles E. Trends and challenges in the microbial production of lignocellulosic bioalcohol fuels. Appl Microbiol Biotechnol. 2010;87:1303–1315. doi: 10.1007/s00253-010-2707-z. [DOI] [PubMed] [Google Scholar]
- Yang S, Pelletier DA, Lu TY, Brown SD. The Zymomonas mobilis regulator hfq contributes to tolerance against multiple lignocellulosic pretreatment inhibitors. BMC Microbiol. 2010;10:135. doi: 10.1186/1471-2180-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Shi J, Jiang L. Modulation of mitochondrial membrane integrity and ROS formation by high temperature in Saccharomyces cerevisiae. Electron J Biotechnol. 2015;18:202–209. doi: 10.1016/j.ejbt.2015.03.008. [DOI] [Google Scholar]
- Zhao N, Bai Y, Zhao XQ, Yang ZY, Bai FW. Draft genome sequence of the flocculating Zymomonas mobilis strain ZM401 (ATCC 31822) J Bacteriol. 2012;194:7008–7009. doi: 10.1128/JB.01947-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Bai Y, Liu CG, Zhao XQ, Xu JF, Bai FW. Flocculating Zymomonas mobilis is a promising host to be engineered for fuel ethanol production from lignocellulosic biomass. Biotechnol J. 2014;9:362–371. doi: 10.1002/biot.201300367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.