Abstract
Introduction
Frailty is a complex condition that results from the loss of physiological reserve across multiple systems. Its presence should be considered in the aging heart failure population, since it is an important predictor of death and institutionalization in the elderly.
Methods and results
In a prospective, observational and analytical single-center study of 100 elderly patients hospitalized for acute heart failure, we assessed the characteristics associated with an increased hospital and 1-year mortality. Frailty was evaluated with the Clinical Frailty Scale, and there was a significant association between its presence and 1-year mortality (RR = 2.03; 95% CI = 1.18–3.48; p = 0.014), although not with in-hospital mortality. After adjusting for probable confounders, it remained independently associated with 1-year mortality.
Conclusion
Frailty can be assessed with a simple bed-side scale and provides significant prognostic information in acute heart failure patients.
Keywords: Frailty, Heart failure, Acute heart failure, 1-year mortality, Elderly, CFS
1. Introduction
Heart failure (HF) is a global pandemic: according to some estimations, it affects around 26 million people worldwide [1]. Also, its prevalence grows continuously. For instance, in the US a 46% increase in its prevalence is expected by 2030 [2]. On the other hand, HF incidence is reported to be stable or even decreasing in several studies [3]. This could be explained by more accessible and effective treatment, and an extended life span of the population. We may anticipate, with some confidence, a drastic change in the demographic characteristics of HF patients in the future. They will be older and have more comorbidities as well as being frailer.
Frailty is a complex condition that results from the loss of physiological reserve across multiple systems. It manifests clinically as an increased vulnerability to adverse outcomes when faced to disease or even mild stress [4]. Frailty acts as a marker of biological age and is more closely associated with prognosis than chronological age [5]. In the Canadian Study of Health and Aging, it was the most important predictor of death and institutionalization with an odds ratio (OR) of 7.28 and 95% confidence interval (CI) of 5.01–10.58 among mildly frail people and 8.64 (95% CI = 4.92–15.17) among severely frail people [6].
Unfortunately, there is no universal method for measuring frailty. Two approaches summarize the multiple scales and definitions used across clinical studies: the frailty phenotype and the geriatric assessment. The former was developed and validated by Fried and colleagues in the Cardiovascular Health Study and describes a patient as being frail when three or more features are present from five specific measures: unintentional weight loss, exhaustion, slow walking speed, weakness and low physical activity [7]. The latter is a more holistic approach that involves the inclusion of four validated geriatric tools to assess domains of functional autonomy, cognitive deterioration, emotional disturbances and social risk [8]. Both are time-consuming and require special knowledge or instruments and would encounter resistance for application in every-day practice. There have been attempts to simplify frailty determination, such as the Clinical Frailly Scale (CFS), which correlates well with the Frailty Index [9].
The presence of frailty should be considered in the aging HF population, and it was investigated to some extent. It was shown to occur frequently in patients with HF, with a prevalence ranging from 15 to 74%, depending on the studied population and the method of assessment [10]. Also, it is associated with adverse outcomes during hospitalization, as well as events in the short and long term [[11], [12], [13], [14]]. Despite its importance, it is not accounted for in any prognostic score for HF as the MAGGIC score or the Seattle Heart Failure Model [15,16], and no frailty scales have been specifically validated for HF.
We proposed in this study to determine the performance of a simple and well-validated bed-side frailty scale to predict 1-year mortality in elderly patients admitted for acute HF in a tertiary hospital.
2. Methods
We conducted a prospective, observational and analytical single-center study in a University Hospital in Buenos Aires, Argentina. We screened all hospitalizations with a primary diagnosis of acute heart failure from June 1, 2016 to May 31, 2017, both in the general ward or in the Coronary Care Unit. All patients who gave their consent to participate were included in this study, following the principles of the Declaration of Helsinki and local regulation for protection of medical data. The study protocol was approved by the Bioethics Committee of the Hospital.
Patient's age, sex, and clinical data were obtained from the medical history. Comorbidities, previous medical treatment, and NYHA (New York Heart Association) class were recorded. The cause of decompensation and in-hospital medical treatment were evaluated, as well as left ventricle ejection fraction if an echocardiogram was performed. Patients were called by telephone 1 year after the hospitalization to determine their vital status or rehospitalization. When it was not possible to reach them or their next of kin, hospital records were reviewed.
Frailty was assessed be means of the CFS. This semi-quantitative instrument classifies patients into 9 categories, where 1 is the least frail and 9 is the frailest (Fig. 1). Patients were then divided into non-frail with a score of 4 or less, or frail with a score of 5 or more.
Fig. 1.
Clinical frailty scale. ©2007–2009 Version 1.2. All rights reserved. Geriatric Medicine Research, Dalhousie University, Halifax, Canada. Permission granted to copy the Clinical Frailty Scale for research and educational purposes only.
The primary end-point was 1-year mortality. In-hospital mortality and readmission rates were analyzed as secondary endpoints.
Sample-size calculations showed that 102 hospitalizations would be necessary to achieve an 80% power to detect a 25% difference in the primary end-point. Based on previous hospital statistics, we estimated that this amount of hospitalizations could take place within 1 year. The variables are presented as mean and standard deviation (±SD) or frequencies. A two-sided t-test was used to compare continuous variables if normally distributed, or a Wilcoxon test if not normally distributed. For categorical variables, chi-square test or Fisher's test were used, respectively. A logistic multivariate regression model was used to assess 1-year mortality in frail or non-frail patients adjusting for baseline clinical characteristics. All calculations were done with STATA v11.1. Statistical significance was considered if the p-value was <0.05.
3. Results
There were 101 hospitalizations with a primary diagnosis of heart failure from June 1, 2016 to May 31, 2017. Only 1 patient declined to participate in this study. The mean age was 77 ± 13.4 years, 56% were men and 86% were admitted to the general ward. 41% had an ejection fraction of the left ventricle of <40%. Most patients had some risk factor or comorbidity: 78% were hypertensive, 52% had chronic heart failure (60% in NYHA class II and 26% in class III), 23% had coronary disease, 36% were diabetic, 33% had atrial fibrillation, 24% had chronic renal disease and 24% had COPD. The most frequent causes for decompensation were disease progression (22%), infection (19%), atrial supraventricular tachycardia (15%), and non-adherence to diet (11%). All baseline characteristics and outcomes are presented in Table 1.
Table 1.
Baseline characteristics of the population.
| Variable | Mean or frequency |
|||
|---|---|---|---|---|
| Total (n = 100) | Frail (n = 26) | Non-frail (n = 66) | ||
| Age | 77 ± 13.4 | 80 ± 11.3 | 71 ± 13.6 | |
| Males | 56% | 30.77% | 62.12% | |
| History | Hypertension | 78% | 75.76% | 88.46% |
| Diabetes | 36% | 34.62% | 40.91% | |
| Smokers | 27% | 23.08% | 28.79% | |
| Former smokers | 23% | 19.23% | 22.73% | |
| Dyslipidemia | 7% | 7.69% | 7.58% | |
| Obesity | 7% | 11.54% | 6.06% | |
| Coronary disease | 23% | 19.23% | 24.24% | |
| Chronic Heart failure | 52% | 69.23% | 43.94% | |
| Ejection fraction <40% | 51% | 35.29% | 55.36% | |
| Peripheral artery disease | 5% | 0% | 6.06% | |
| Renal disease | 24% | 19.23% | 22.73% | |
| Stroke | 4% | 7.69% | 3.03% | |
| Atrial fibrillation | 33% | 34.62% | 30.30% | |
| COPD | 10% | 11.54% | 9.09% | |
| Pacemaker | 8% | 3.85% | 9.09% | |
| Medication | ACE inhibitors | 36% | 38.46% | 36.36% |
| ARBs | 27% | 30.77% | 27.27% | |
| Beta blockers | 60% | 69.23% | 57.58% | |
| Mineralocorticoid receptor inhibitors | 24% | 26.92% | 24.24% | |
| Calcium channel blockers | 12% | 7.69% | 13.64% | |
| Aspirin | 32% | 23.08% | 36.36% | |
| Statins | 35% | 38.46% | 36.36% | |
| Furosemide | 49% | 42.31% | 48.48% | |
| Anticoagulants | 27% | 26.92% | 27.27% | |
| Amiodarone | 9% | 11.54% | 9.09% | |
| Digoxin | 3% | 3.85% | 3.03% | |
| Decompensation motive | Progression | 22% | 29.17% | 25.42% |
| Non-adherence to diet | 11% | 4.17% | 10.17% | |
| Lack of medication | 9% | 8.33% | 10.17% | |
| Supraventricular tachycardia | 15% | 4.17% | 22.03% | |
| Infection | 19% | 41.67% | 15.25% | |
| Renal failure | 5% | 8.33% | 3.39% | |
| Others | 9% | 4.17% | 13.56% | |
| Unknown | 10% | 13.56% | 10.61% | |
| Etiology | Ischemic | 37% | 38.89% | 32.65% |
| Chagas | 6% | 5.56% | 10.20% | |
| Valvular | 18% | 22.22% | 28.57% | |
| Idiopathic | 6% | 5.56% | 10.20% | |
| Others | 13% | 27.78% | 16.33% | |
| Unknown | 30% | 30.77% | 27.27% | |
| NYHA class | I | 9% | 5.00% | 9.52% |
| II | 60% | 30.00% | 68.25% | |
| III | 26% | 55.00% | 19.05% | |
| IV | 5% | 10.00% | 3.17% | |
| Hospitalization | Intensive care | 14% | 3.85% | 18.18% |
| General ward | 86% | 96.15% | 81.82% | |
| CFS | 1 | 7% | – | – |
| 2 | 14% | – | – | |
| 3 | 32% | – | – | |
| 4 | 18% | – | – | |
| 5 | 9% | – | – | |
| 6 | 10% | – | – | |
| 7 | 9% | – | – | |
| 8 | 1% | – | – | |
| 9 | 0% | – | – | |
| Mortality | In-hospital | 10.2% | 8.00% | 7.69% |
| 1-year | 40.96% | 59.09% | 29.09% | |
The total mortality rate was 10.20% for in-hospital deaths and 40.96% after 1 year of follow-up, with complete data for 83 patients.
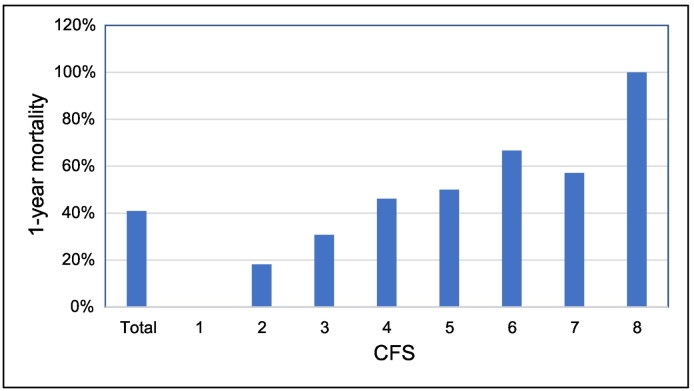
Frailty was present in 28% of the patients, according to the CSF. It was not associated with in-hospital mortality (RR = 1.04; 95% CI = 0.22–5.02; p = 0.96). There was a significant association between frailty and 1-year mortality (RR = 2.03; 95% CI = 1.18–3.48; p = 0.014). Frail patients had a 59.09% 1-year mortality, while for non-frail patients it was 29.09% (p = 0.014). 1-year mortality according to the CFS can be visualized in Fig. 2.
Fig. 2.
1-year mortality per CFS.
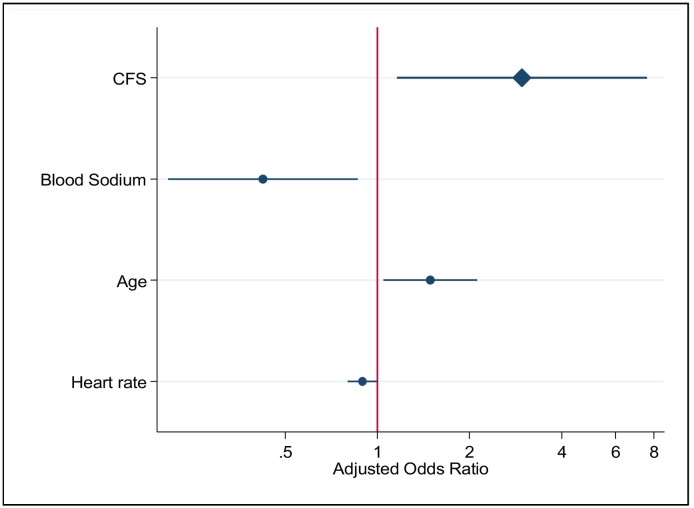
Finally, we constructed a logistic regression model with a forward-selection stepwise approach. The variables significantly associated with an increase in 1-year mortality in the final model were frailty, advanced age, increased heart rate at admission, history of dyslipidemia, COPD or peripheral artery disease, reduced blood sodium level, and treatment with furosemide. Treatment with aspirin, beta blockers, calcium channel blockers and aldosterone antagonists showed a protective effect. Frailty assessed by means of the CFS was therefore significantly and independently associated with 1-year mortality (OR 2.97; 95% CI = 1.16–7.59; p = 0.023, Fig. 3 [17]).
Fig. 3.
Adjusted mortality odds ratio.
4. Discussion
HF and frailty are closely related, both in their pathogenesis and impact on prognosis. They are systemic syndromes, in which inflammation has been well established as contributing to their development, adverse prognosis and progression. High levels of inflammatory markers in HF patients (IL-6, CRP and TNF alpha) have been associated with muscle loss, cardiac cachexia and declining physical function, factors known to play an important role in frailty [18]. Simultaneously, frail patients show elevated levels of inflammation markers: white blood cells, interleukin 6, C-reactive protein, factor VIII, fibrinogen and D-dimer [19]. Also, endothelial dysfunction can contribute to worse outcomes in HF, and the analysis of results from the Toledo Study for Healthy Aging revealed that endothelial function is impaired in frail patients [20]. Age alone is recognized to contribute to cardiovascular disease, since senescent cardiomyocytes are characterized by prolonged relaxation, diminished contraction velocity, a decrease in β-adrenergic response, and increased myocardial stiffness [21]. Cardiac changes with chronological aging are multiple (increased vasoconstriction and impaired vasodilatation, increased atherosclerosis, reduced vasodilatory capacity, impaired microvascular function, increased left ventricular mass and stiffness, increased prevalence of atrial fibrillation) [22], but they seem not to explain on their own the worst prognosis associated with biological aging or frailty.
In this study, we demonstrated that frailty is an independent prognostic factor for 1-year mortality, using a simple and fast scale for its assessment. These findings are similar to several others reported in the past. The FRAIL-HF study evaluated frailty in 450 patients admitted because of acute heart failure in Spain [23]. It used the Freid criteria to determine frailty status, and found it was significantly associated with 1-year all-cause mortality (HR = 2.13; 95% CI = 1.07–4.23). Another study performed in the United Kingdom in 265 hospitalized heart failure patients showed that the addition of the CFS to a predictive model enhanced its performance compared with a base model. It also showed that frailty is related to the nutritional status [24]. In Japan, the frailty of 181 patients was retrospectively assessed after discharge by means of grip strength and performance measures. During 2 years of follow up, subjects who met all the criteria had a 4 times greater risk of cardiac events compared with those with no frailty criteria [25]. Finally, in the USA, a cohort of 56 consecutive hospitalized HF patients showed a higher risk for 6-month readmission or mortality if they had a weak grip and cognitive impairment [26].
Unfortunately, there is no validated tool to assess frailty in HF patients. A systematic review showed that, in 20 studies published in 24 articles, 7 different instruments were used [27]. The most frequently used was Fried's Frailty Phenotype, followed by the Comprehensive Geriatric Assessment. Only 5 of the 20 identified studies evaluated hospitalized patients due to acute HF, and most investigated chronic or advanced heart failure patients.
Since the most common used instruments to assess frailty are time-consuming and require special knowledge or instruments, we intended to determine the performance of a simple and fast instrument for the risk assessment of hospitalized HF patients. The CFS can be calculated by the attending physician in a matter of seconds and has some advantages over simply “eye-balling” the patient; it establishes a numerical value (however subjective), and it compels the physician to thoroughly consider his patient's frailty status. No HF risk assessment tool considers frailty as a prognostic factor, but there is enough evidence that it is strongly and independently related to hard outcomes. It should be accounted for in future risk models, and in the meantime implemented in everyday clinical practice.
The principal limitations of this study are the small sample size that did not allow the evaluation of multiple outcomes, and the lack of comparison with other frailty assessment tools. Also, because it was done in a single center with high-risk patients, its results should be considered with caution before extrapolating to the general population.
5. Conclusion
Frailty was associated with a 2-fold increase in the in 1-year mortality risk after a hospitalization due to acute heart failure. It was assessed with the CFS, which can be implemented easily in clinical practice. It should be considered in future risk models, since it is independently linked to worse outcomes.
Conflicts of interest
None declared.
Acknowledgements
We thank Dr. Martín Garré and Dr. Alejo A. Pérez de la Hoz for their assistance during data collection, as well as all the attending physicians and participating patients.
References
- 1.Ponikowski P., Anker S.D., Al Habib K.F. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1:4–25. doi: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D., Benjamin E.J., Go A.S., American Heart Association Statistics Committee; Stroke Statistics Subcommittee Heart disease and stroke Statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Levy D., Kenchaiah S., Larson M.G. Long-term trends in the incidence of and survival with heart failure. N. Engl. J. Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 4.Jha S.R., Ha H.S., Hickman L.D. Frailty in advanced heart failure: a systematic review. Heart Fail. Rev. 2015;20:553–560. doi: 10.1007/s10741-015-9493-8. [DOI] [PubMed] [Google Scholar]
- 5.Kulmala J., Nykanen I., Hartikainen S. Frailty as a predic- tor of all-cause mortality in older men and women. Geriatr Gerontol Int. 2014;14:899–905. doi: 10.1111/ggi.12190. [DOI] [PubMed] [Google Scholar]
- 6.Rockwood K., Howlett S.E., MacKnight C. Prevalence, attributes, and outcomes of fitness and frailty in community- dwelling older adults: report from the Canadian study of health and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2004;59:1310–1317. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 7.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J. Frailty in older adults evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 8.Altimir S., Lupon J., Gonzalez B., Prats M., Parajon T., Urrutia A. Sex and age differences in fragility in a heart failure population. Eur. J. Heart Fail. 2005;7(5):798–802. doi: 10.1016/j.ejheart.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Rockwood K. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchmanowicz I., Łoboz-Rudnicka M. Szela̧g P, Jankowska-Polańska B, Łoboz-Grudzień K. Frailty in heart failure. Curr. Heart Fail. Rep. 2014;11(3):266–273. doi: 10.1007/s11897-014-0198-4. [DOI] [PubMed] [Google Scholar]
- 11.Volpato S., Cavalieri M., Sioulis F. Predictive value of the short physical performance battery following hospitali- zation in older patients. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:89–96. doi: 10.1093/gerona/glq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lupon J., Gonzalez B., Santaeugenia S., Altimir S., Urrutia A., Mas D. Prognostic implication of frailty and depressive symptoms in an outpatient population with heart failure. Rev. Esp. Cardiol. 2008;61(8):835–842. [PubMed] [Google Scholar]
- 13.Cacciatore F., Abete P., Mazzella F., Viati L., Della Morte D., D'Ambrosio D. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur. J. Clin. Investig. 2005;35(12):723–730. doi: 10.1111/j.1365-2362.2005.01572.x. ([Internet], Available from:) [DOI] [PubMed] [Google Scholar]
- 14.McNallan S.M., Chamberlain A.M., Gerber Y., Singh M., Kane R.L., Weston S.A. Measuring frailty in heart failure: a community perspective. Am. Heart J. 2013;166(4):768–774. doi: 10.1016/j.ahj.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pocock S.J., Ariti C.A., McMurray J.J., Maggioni A., Kober L., Squire I.B., Swedberg K., Dobson J., Poppe K.K., Whalley G.A., Doughty R.N. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur. Heart J. 2013;34:1404–1413. doi: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 16.Levy W.C., Mozaffarian D., Linker D.T., Sutradhar S.C., Anker S.D., Cropp A.B., Anand I., Maggioni A., Burton P., Sullivan M.D., Pitt B., Poole-Wilson P.A., Mann D.L., Packer M. The Seattle heart failure model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 17.Jann Ben. Plotting regression coefficients and other estimates. Stata J. 2014;14(4):708–737. [Google Scholar]
- 18.Anker S.D., von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart. 2004;90(4):464–470. doi: 10.1136/hrt.2002.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walston J., McBurnie M.A., Newman A., Tracy R.P., Kop W.J., Hirsch C.H. Frailty and activation of the inflammation and coagula- tion systems with and without clinical comorbidities: results from the cardiovascular health study. Arch. Intern. Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 20.Alonso-Bouzon C., Carcaillon L., Garcia-Garcia F.J., Amor-Andres M.S., El Assar M., Rodriguez-Manas L. Association between endo- thelial dysfunction and frailty: the Toledo study for healthy aging. Age. 2013;20:20. doi: 10.1007/s11357-013-9576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakatta E.G., Levy D. Arterial and cardiac aging: major sharehold- ers in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 22.Nanayakkara S., Marwick T.H., Kaye D.M. The ageing heart: the systemic and coronary circulation. Heart. 2018;104(5):370–376. doi: 10.1136/heartjnl-2017-312114. [DOI] [PubMed] [Google Scholar]
- 23.Vidan M.T., Blaya-Novakova V., Sanchez E. Prevalence and prognostic impact of frailty and its components in non- dependent elderly patients with heart failure. Eur. J. Heart Fail. 2016;18:869–875. doi: 10.1002/ejhf.518. [DOI] [PubMed] [Google Scholar]
- 24.Sze S., Zhang J., Pellicori P., Morgan D., Hoye A., Clark A.L. Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin. Res. Cardiol. 2017;106(7):533–541. doi: 10.1007/s00392-017-1082-5. [DOI] [PubMed] [Google Scholar]
- 25.Yamada S., Kamiya K., Kono Y. Frailty may be a risk marker for adverse outcome in patients with congestive heart failure. ESC Heart Fail. 2015;2(3):168–170. doi: 10.1002/ehf2.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joyce E., Howell E.H., Senapati A., Starling R.C., Gorodeski E.Z. Prospective assessment of mini-cog and grip strength identifies hospitalized heart failure patients at increased. Circulation. 2016;134 doi: 10.1002/ehf2.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonagh J., Martin L., Ferguson C., Jha S.R., Macdonald P.S., Davidson P.M. Frailty assessment instruments in heart failure: a systematic review. Eur. J. Cardiovasc. Nurs. 2018;17(1):23–35. doi: 10.1177/1474515117708888. [DOI] [PubMed] [Google Scholar]