Abstract
Insurers and employers are increasingly offering lifestyle and weight-loss coaching programs; however, few evaluations have examined their effectiveness. Our objectives were to determine whether level of program engagement was associated with differences in healthcare utilization and weight pre/post coaching. We conducted a retrospective evaluation of enrollees in an insurer-based telephonic health coaching program in Maryland (2013–2014). Our independent variables were program engagement benchmarks (≥3 and ≥6 sessions). Our dependent variables included change in outpatient and emergency department (ED) visits (more visits post program, fewer visits post, or no change pre-post) and associated costs (difference pre-post) using claims data. We calculated mean percent weight change from baseline. We used multivariate-adjusted linear and multinomial logistic regression, as appropriate, to examine the association between outcomes and engagement benchmarks. We included 225 enrollees with mean age 50.7 years, 81.3% women, and mean body mass index of 35.0 kg/m2. Most participants focused on weight management (75.6%) and improving general health (57.8%). Few individuals had outpatient or ED visits, and no significant changes in healthcare utilization were associated with program engagement. Among the weight management subgroup (n = 170), mean weight change was −2.1% (SD 5.1). Participants achieved significantly greater weight loss if they met the 6-session engagement benchmark (β −3.5%, p < 0.01). Weight management is a popular focus for health coaching participants, and these programs can achieve modest weight loss. Programs should consider designing and testing strategies that promote engagement, given that weight-loss success was improved if participants completed at least 6 coaching sessions.
Keywords: Risk reduction behavior, Health services research, Health insurance
1. Introduction
Chronic conditions such as cardiovascular disease, type 2 diabetes, and obesity are the leading causes of death and disability in the U.S. (Anon., n.d.-a). Approximately half of all U.S. adults have at least one chronic condition (Ward et al., 2014), and having multiple chronic conditions accounts for approximately 71% of U.S. healthcare spending (Gerteis et al., 2014). Modifying lifestyle behaviors like diet, exercise, and smoking can prevent the development or better manage these conditions in individuals already with disease.
U.S. insurers and employers are increasingly offering telephone-based health coaching programs to promote behavior change with the aim to prevent or better manage chronic disease (Murphy et al., 2010; Society for Human Resource Management, 2016). While “health coaching” has been inconsistently defined in the literature, a recent systematic review has determined that health coaching refers to a patient-centered process based upon behavior change theories, including Social Cognitive Theory, Theory of Planned Behavior, Transtheoretical Model, Self-Determination Theory, Self-Perception Theory, and Motivational Enhancement, that is delivered by health professionals with diverse backgrounds (Wolever et al., 2013). The actual coaching process includes patient-determined goals, incorporates self-discovery and active learning processes, encourages accountability for behaviors, and provides education within the context of a consistent, ongoing relationship (Wolever et al., 2013). Coaches in these studies were typically trained in behavior change and communication skills. Prior systematic reviews have found that telephone-based coaching interventions can lead to positive behavior changes in study participants' physical activity, diet and smoking cessation (Eakin et al., 2007; Goode et al., 2012; Stead et al., 2013). Some trials have used telephone coaching to successfully promote weight loss (Appel et al., 2011; Eakin et al., 2014).
Little is known about how coaching programs perform when translated outside a clinical trial. One evaluation studied a government-sponsored health coaching program in Australia, which led to improvements in weight, exercise, and diet at 6 months (O'Hara et al., 2012). U.S. insurer-sponsored wellness coaching programs showed coaching's positive impacts on body mass index (BMI) and smoking cessation (Adams et al., 2013; Schmittdiel et al., 2017; Boccio et al., 2017). A wellness coaching program in an insured population produced small weight losses (Tao et al., 2014). Finally, an internet/telephone-based smoking cessation program showed 30-day quit rates of 21% (Zbikowski et al., 2008). Given health coaching's popularity among insurers and employers (Murphy et al., 2010; Society for Human Resource Management, 2016), more evaluations need to examine these programs' benefits and determine factors that promote participants' success.
Our first objective was to characterize participants' experience with an insurer-based telephonic health coaching program, particularly the area of focus for their coaching experience (e.g., weight loss). Given that patient engagement has been essential in improving health outcomes and reducing costs in healthcare settings (Krist et al., 2017), our second objective was to examine whether individuals' program engagement influenced outcomes, specifically outpatient and emergency department (ED) utilization and costs, as well as weight change among those participants focusing on weight management. We hypothesized that participants that completed at least 6 coach calls would have reduced healthcare utilization and greater weight loss as compared to participants who did not meet this benchmark.
2. Methods
2.1. Description of health coaching program
In contrast to disease management that typically focuses on individuals with poorly controlled, high cost condition(s), the insurer, Johns Hopkins HealthCare (JHHC), offers health coaching to promote health behavior change among members who are at-risk for developing or with preexisting, well-managed chronic conditions (e.g., hypertension, prediabetes). The program is promoted to all beneficiaries through websites and newsletters. Members may self-refer or be referred by their clinician, community health worker, case manager, or health educator. The program is open to members and their dependents enrolled in the health plan aged 18 and older.
Coaches typically have monthly calls with members. The program's core involves monthly goal setting and an individualized “Action Plan.” Motivational interviewing and behavior change strategies (e.g., goal setting, problem solving) are used to assist in the process of change. Motivational interviewing aims to activate patients' capacity for change, and promotes the following principles: express empathy through reflective listening; develop discrepancy between participants' goals and their current behavior; adjust to participant resistance rather than opposing it directly; and support self-efficacy and optimism (Miller, 2002). Throughout the program, coaches use various assessments to evaluate a member's progress and health status. Coaches hold degrees in health-related fields, and all are certified wellness/health coaches through accredited organizations.
2.2. Study design & data sources
We conducted a retrospective evaluation of enrollees in JHHC's health coaching program during 2013–2014, which examined all health-coaching participants enrolled in the program during this two-year period. The Johns Hopkins University School of Medicine Institutional Review Board approved this study.
We used data from three sources: health insurance enrollment and claims data, a participant database, and participants' Action Plans. The enrollment/claims data provided information on demographics and healthcare utilization/costs. The participant database contains information entered by coaches, which they use for documentation and tracking purposes. From this source, we obtained baseline weight and height, and final program weight (if available). An Action Plan (AP) document was generated by the coach after each session for the participant, where the plan discussed and goals set were recorded. We accessed an electronic repository where APs for participants were stored. We performed a content analysis of all available APs (n = 1127), which is a method to abstract information from text to create a dataset amenable for statistical analysis and research (Greenberg et al., 2003; Heuer et al., 2011; Gollust et al., 2012; Bloom et al., 2016). We developed a coding scheme to abstract the following content: program goal(s), smoking status, and amount of desired weight loss. Data abstractors were trained on how to use the abstraction form (a nursing student and JHHC employee). A study team member (NR) reviewed their initial abstractions for accuracy and met with abstractors regularly to address questions and provide clarifications. If this study team member could not resolve an issue, it was brought to the entire study team for discussion and decision.
2.3. Independent variables
For our first objective of characterizing area of focus in the health coaching program, we used the AP data to determine the type and number of program goals. Program goals were categorized as weight management, nutrition (without a weight management focus), fitness (without weight management focus), smoking cessation, stress management, general health, or other.
For our second objective, the independent variables were program engagement benchmarks at ≥3 and ≥ 6 sessions. We determined the number of coaching encounters completed by the number of APs recorded for each beneficiary, which we dichotomized at two thresholds – ≥3 sessions versus <3 and ≥6 sessions versus <6 – to explore whether different thresholds of engagement influenced outcomes. Typically, sessions occurred once a month, so these variables approximate ≥3 and ≥6 months, respectively.
2.4. Dependent variables
From the health insurance enrollment/claims data, we determined the dependent variables of change in outpatient and ED visits and their associated costs. For each participant, we examined outpatient and ED claims data from two periods: 6 months preceding coaching (“pre-coaching”) and 6 months following coaching (“post-coaching”). We focused on outpatient and ED services, as we hypothesized that coaching may have an effect on service use in these settings – coaching might increase awareness of health issues and improve health that prompts increased outpatient and decreased ED service use. Most individuals did not use outpatient or ED services (median visits 0 (IQR 0-0) for both settings in all time periods). Therefore, rather than examining difference in visits pre-post coaching as a continuous variable, which would have an overabundance of zeros and both negative and positive values, we categorized participants as having 1) more visits post, 2) fewer visits post, or 3) no change pre-post. After determining the allowed costs the insurer paid, we calculated the difference in pre-post coaching costs for both outpatient and ED services.
From the participant database, we determined our dependent variables of mean weight change and mean percent weight change among those with a stated weight management goal. If final program weight was missing, we used a baseline-observation-carried-forward (BOCF) approach to address the missing data. We also identified individuals as achieving a clinically significant weight loss if they achieved ≥3% and ≥5% losses using the BOCF approach (Jensen et al., 2014). Finally, we also employed an approach limited to individuals who had a weight available at program end (“completers”) to calculate weight change and percent weight change, as well as determine whether this change met their specified goal at baseline. The weight-loss goal was abstracted from APs, if available, by capturing the desired pounds to lose/target weight.
2.5. Covariates
From the enrollment file, we obtained demographics including gender and age at coaching start. From the claims, the participants' resource utilization band (RUB) (range 0 to 5) from the year of coaching start was produced using the Adjusted Clinical Groups software (Anon., n.d.-b). RUB is a previously validated measure that reflects degree of morbidity (low [0] to high [5]) by using 12 months of complete claims data. We determined baseline smoking status from the AP data.
2.6. Analyses
We limited analyses among participants who had baseline age, gender and RUB available (225 out of 231) (97.4% of all coaching participants), as we considered these attributes potential confounders that needed to be included in the adjusted analyses described below. We performed descriptive analyses of all variables. We determined the proportion of participants with each program goal and calculated the median number of program goals set. We determined the median number of coaching sessions completed, and proportion achieving the program engagement benchmarks of ≥3 sessions and ≥6 sessions completed.
We calculated the proportion of participants in each visit pre-post group (i.e., more visits post, fewer visits post, or no change pre-post). We used multivariate multinomial logistic regression to examine the association between visit pre-post groups and the program engagement benchmarks, adjusted for age, gender, RUB, and health plan. We calculated the mean difference in pre-post costs, then used linear regression to examine their association with the program engagement benchmarks, adjusted for age, gender, RUB, and health plan.
Among weight-management participants, we calculated mean weight change and percent weight change using both the BOCF and completers' approaches described previously. We determined the proportion of weight-management participants achieving a clinically significant weight loss and meeting their stated weight-loss goal. We used multivariate linear and logistic regression, as appropriate, to examine the association between the BOCF weight outcomes and program engagement benchmarks, adjusted for age, gender, RUB, and health plan. We did not conduct regression analyses with the completers' sample given its small size (n = 61), and instead used t-tests to compare weight change and percent weight change by program engagement benchmarks.
3. Results
Our analytic sample included 225 individuals participating in JHHC health coaching during 2013–2014–50.2% started in 2013 and 49.8% started in 2014. Table 1 provides demographic information for these individuals, who were predominantly middle-aged women.
Table 1.
Characteristics of 225 health coaching participants overall and by engagement benchmarks.
| Overall |
3-Session benchmark |
6-Session benchmark |
|||||
|---|---|---|---|---|---|---|---|
| <3 sessions |
≥3 sessions |
p-Value | <6 sessions |
≥6 sessions |
p-Value | ||
| (N = 225) | (n = 50) | (n = 175) | (n = 143) | (n = 82) | |||
| Mean age in years (SD) | 50.7 (11.9) | 49.4 (10.4) | 51.1 (12.3) | 0.28 | 48.6 (11.6) | 54.3 (11.7) | <0.01 |
| Women | 81.3% | 80.0% | 81.7% | 0.78 | 81.1% | 81.7% | 0.91 |
| Resource utilization band | |||||||
| 0–1 (non-users/healthy users) | 4.9% | 6.0% | 4.6% | 0.90 | 7.0% | 1.2% | 0.15 |
| 2–3 (low/moderate morbidity) | 60.0% | 58.0% | 60.6% | 59.4% | 61.0% | ||
| 4–5 (high/very high morbidity) | 35.1% | 36.0% | 34.9% | 33.6% | 37.8% | ||
| Current smokersa | 6.4% | 8.2% | 5.9% | 0.56 | 7.1% | 5.0% | 0.53 |
| Weight in kg (SD)b | 96.3 (25.6) | 102.6 (29.9) | 94.7 (24.3) | 0.14 | 95.4 (26.6) | 97.6 (24.1) | 0.55 |
| BMI in kg/m2 (SD)b | 35.0 (9.0) | 36.1 (9.6) | 34.6 (8.8) | 0.40 | 34.3 (9.3) | 35.9 (8.4) | 0.24 |
Health coaching occurred during 2013–2014 among beneficiaries of a Maryland-based health insurer. Abbreviations: BMI – body mass index.
Smoking status available for 220 participants (98%).
Weight available for 185 participants (82%) and BMI available for 172 participants (76%).
3.1. Program goals
Overall, median number of program goals set was 2 (IQR 1–3). Most participants focused on weight management (75.6%), which typically included nutrition and fitness goals. Few individuals focused on nutrition (15.6%) or fitness (16.9%) without having a weight-management focus. Overall, 57.8% had a general health goal, 28.9% stress management, 7.6% smoking cessation, and 6.7% other (e.g., financial).
3.2. Program engagement
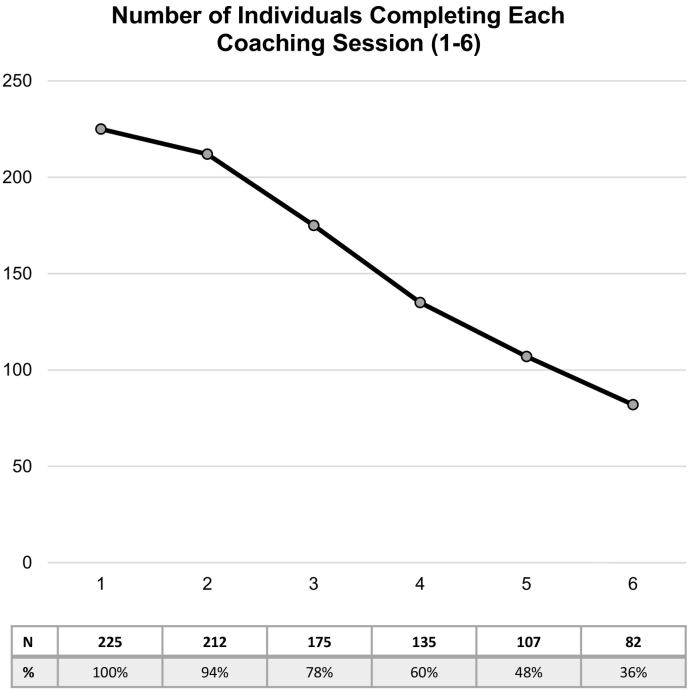
The median number of coaching sessions completed was 4.0 (IQR 3–6). Fig. 1 displays the number of individuals who completed sessions 1–6. Few individuals had >6 sessions – 56 had 7 sessions (25%), 41 had 8 sessions (18%), 25 had 9 sessions 9 (11%), and 5 had 10 sessions (2%). Overall, 77.8% completed ≥3 sessions and 36.4% completed ≥6 sessions. Table 1 compares the between-group baseline characteristics. There were no significant differences by the 3-session benchmark. However, individuals that completed ≥6 sessions were significantly older than those who did not (54.3 versus 48.6, respectively (p < 0.01)).
Fig. 1.
Number of individuals completing each coaching session (1–6). Coaching sessions occurred during 2013–2014 among beneficiaries of a Maryland-based health insurer. Displays the number of individuals who completed each coaching session, and percent of sample completing each session is located in the table below the graph.
3.3. Outpatient services utilization and costs
Overall, 12.0% had more outpatient visits post, 10.7% had fewer visits post, and 77.3% had no change pre-post. Mean difference in pre-post outpatient costs was +$27.46 (SD $790.01). In adjusted models, there were no significant differences in outpatient visit pre-post groups by the 3-session (reference group no change pre-post; more visits: RR 1.46, 95%CI 0.45–4.70, p = 0.53; fewer visits: RR 1.24, 95%CI 0.35–4.32, p = 0.74) or 6-session benchmarks (reference group no change pre-post; more visits: RR 0.49, 95%CI 0.16–1.45, p = 0.20; fewer visits: RR 0.74, 95%CI 0.23–2.42, p = 0.62). Similarly, no significant differences existed in pre-post outpatient costs by these benchmarks (3-session: β −$261.73, 95%CI −$594.42–$70.97, p = 0.12; 6-session: β + $33.00, 95%CI −$261.94–$327.94, p = 0.83).
3.4. Emergency department services utilization and costs
In our sample, 4.9% had more ED visits post, 5.3% had fewer visits post, and 89.8% had no change pre-post. Overall, mean difference in pre-post ED costs was −$24.98 (SD $1224.43). In adjusted models, there were no significant differences in the ED visit pre-post groups by the 3-session (reference group no change pre-post; more visits: RR 4.46, 95%CI 0.49–40.60, p = 0.18; fewer visits: RR 1.60, 95%CI 0.30–8.61, p = 0.58) or 6-session benchmarks (reference group no change pre-post; more visits: RR 2.55, 95%CI 0.57–11.40, p = 0.22; fewer visits: RR 0.91, 95%CI 0.20–4.13, p = 0.90). Similarly, no significant differences existed in pre-post ED costs by the engagement benchmarks (3-session: β −$426.08, 95%CI −$946.53–$94.37, p = 0.11; 6-session: β −$218.44, 95%CI −$678.94–$242.06, p = 0.35).
3.5. Weight loss
Overall, 170 individuals stated a weight-management goal. Among this subgroup, mean age was 51.2 years (SD 11.7), 81.8% were women, and baseline BMI was 35.5 kg/m2 (SD 8.4). With respect to morbidity risk, 3.5% had a RUB of 0–1, 60.6% had a band of 2–3, and 35.9% had a band of 4–5. Overall, 102 individuals set a weight-loss goal (mean −14.1% (SD 8.8)).
Using the BOCF approach to address missing final weight (n = 109), the mean weight change was −4.1 kg (SD 6.8) and mean percent weight change was −2.1% (SD 5.1), while 22.4% achieved a ≥3% weight loss and 17.7% achieved a ≥5% loss. Table 2 displays the weight outcomes by program engagement benchmarks in adjusted models. Only participants that completed ≥6 sessions lost significantly more weight and were significantly more likely to achieve a clinically significant weight loss.
Table 2.
Adjusteda weight loss outcomes by engagement benchmarks among health coaching participants with a weight management goal (n = 170).
| 3-Session benchmarkb |
6-Session benchmarkb |
|||||
|---|---|---|---|---|---|---|
| Effect (β or OR) | 95%CI | p-Value | Effect (β or OR) | 95%CI | p-Value | |
| Mean % weight change, BOCF | −2.1% | −4.4–0.2% | 0.07 | −3.5% | −5.4–(−1.5)% | <0.01 |
| Met ≥3% weight loss, BOCF | 3.52 | 0.95–13.00 | 0.06 | 6.38 | 2.43–16.70 | <0.01 |
| Met ≥5% weight loss, BOCF | 4.10 | 0.88–19.08 | 0.07 | 4.65 | 1.68–12.88 | <0.01 |
Health coaching occurred during 2013–2014 among beneficiaries of a Maryland-based health insurer. Abbreviations: BOCF – baseline observation carried forward approach to handle missing weight data.
Multivariate linear and logistic regression models, as appropriate, adjusted for age, gender, baseline body mass index, resource utilization band, and health plan.
3-Session benchmark compares group that completed ≥3 sessions versus <3; 6-session benchmark compares group that completed ≥6 sessions versus <6.
In completers' analyses among the subgroup with reported final weight values (n = 61), mean weight change was −5.8 kg (SD 7.5), mean percent weight change was −5.9% (SD 7.1), and 60.6% achieved their initial stated weight-loss goal. In this group, 89% met the 3-session and 67% met the 6-session benchmarks. There were no significant differences in mean percent weight change by the engagement benchmarks in bivariate analyses (3-session: −6.3% for meeting benchmark versus −3.2% for not (p = 0.17); 6-session: −6.5% for meeting benchmark versus −4.7% for not (p = 0.31)).
4. Discussion
Given the popularity of health coaching programs among U.S. health insurers and employers (Murphy et al., 2010; Society for Human Resource Management, 2016), evaluations are critical to examine effectiveness and identify targets that might improve outcomes. Our results report the outcomes of an insurer-based coaching program, and are one of the few such studies that describe results outside the clinical trial setting. In contrast to telephone coaching in clinical trials which focus on one behavioral goal, coaching programs outside the trial setting may support multiple behaviors (O'Hara et al., 2012; Adams et al., 2013; Schmittdiel et al., 2017). In our program, we found that most participants' primary goal was weight loss, although improving general health was another common goal. Stress management and smoking cessation were less commonly identified as the goal. Weight management has been a common focus for other coaching programs outside the trial setting (O'Hara et al., 2012; Schmittdiel et al., 2017; Tao et al., 2014).
In clinical trials, telephone-delivered programs typically achieve weight losses of 5 kg at 6 months (Jensen et al., 2014). A previous coaching program evaluation found that participants achieved a 4.5% loss at 6 months (O'Hara et al., 2012). A Kaiser Permanente coaching program among patients with diabetes found that the BMI trajectory was altered at 12 months after coaching as compared to a matched control group, which was consistent with weight loss (Schmittdiel et al., 2017). Another insurer-based coaching program produced only 0.4 kg loss (Tao et al., 2014). In our program, those individuals who focused on weight management achieved modest weight loss success (4.1 kg or 2.1% loss) using the conservative BOCF approach, and our completers' analysis results (5.8 kg or 5.9% loss) were similar to those achieved in trials and other programs. In contrast to these other coaching program evaluations, we explored whether program engagement alters weight-loss outcomes. We found that the mean percent weight loss and likelihood of achieving a clinically significant weight loss were significantly greater among individuals completing ≥6 sessions. There was no significant benefit with the lower engagement benchmark of ≥3 sessions. In summary, health coaching programs can help participants achieve weight-loss success outside the clinical trial setting; however, our results suggest that promoting continued engagement by completing ≥6 sessions may be an important target for programs to achieve to increase likelihood of participant success.
With respect to program engagement, individuals in our program typically completed between 3 and 6 sessions with their coach and over a third completed ≥6 sessions. In an Australian coaching program, 19.1% completed the 6-month program (O'Hara et al., 2012). In the Kaiser Permanente coaching program, mean number of sessions completed was 1.8 (Schmittdiel et al., 2017). Our results are consistent with others suggesting that retention and continued program engagement are challenges across health coaching programs. This challenge is not unique to health coaching, as popular commercial weight-loss programs also report difficulties with retention outside the clinical trial setting (Finley et al., 2006). Overall, different strategies or additional resources may need to be dedicated to retention in these programs, particularly if insurers or government agencies aim to achieve significant weight loss within their populations as described above.
Our study examined changes in utilization and cost outcomes among coaching participants. In general, use of outpatient and ED services was low in our sample. We saw no substantial changes in utilization or costs for outpatient or ED services pre-post coaching, and no effect of program engagement on these outcomes. These results may have occurred for several reasons. First, most individuals in our sample had a low morbidity burden, which likely decreases the need for care, particularly in the outpatient setting. Second, participants may have infrequently sought out or required healthcare services during the periods examined. Third, participants may have received care in other settings not captured in our analyses (e.g., urgent care; hospitalizations). Additional evaluations are needed to examine whether health coaching has no effect on healthcare utilization and costs to confirm our findings.
It is noteworthy to discuss the data challenges that we and other evaluations of coaching programs have experienced, which contrasts from clinical trials. First, outcomes in these evaluations often rely upon participants' continued program engagement, creating a biased sample. Few participants complete assessments after they are no longer working with the program, whether they drop out or complete. As a result, missing data was a challenge for our study and has been reported by others (O'Hara et al., 2012; Schmittdiel et al., 2017). We attempted to address this missingness with the conservative BOCF approach. Second, our evaluation and others have relied upon self-reported weight, which subject to bias (Rowland, 1990), to determine weight loss. Future evaluations might consider pursuing data linkages to relevant electronic health records (EHR), as such access would provide 1) a more complete record of values to reduce data missingness, 2) measured weights rather than self-reported, and 3) values at time points after program end (e.g., 12-month follow up). Based on recent recommendations (IOM (Institute of Medicine), 2014), EHRs may now begin to include measures of diet and exercise that could be used for monitoring of other behavioral outcomes.
This evaluation has several limitations. First, we examined the pre-post results of a health coaching program; therefore, unmeasured personal attributes, such motivation or self-efficacy, may confound our results as we cannot differentiate between their effects with that of the coaching program. We did not have access to the data to know the total number of individuals who were eligible for health coaching during this period, therefore, we could not determine what percentage of this population our sample represents. Comparing outcomes among health coaching participants and those who were eligible but did not participate (e.g., diagnosed obesity or cigarette smokers) would more clearly examine the benefits of health coaching. Second, most of our participants were women, which has also been reported in other evaluations of health coaching programs outside the trial setting (O'Hara et al., 2012; Schmittdiel et al., 2017). Our program relied upon self-referral and referral from healthcare providers, which attracted few men to participate. Health coaching programs may need to design and evaluate different strategies to improve recruitment of men. Third, we did not have access to data to characterize race or socioeconomic status of our participants, which may influence health coaching outcomes particularly in regards to weight loss (West et al., 2008; Kahn et al., 1991).
5. Conclusion
Weight management is the most popular focus for participants in an insurer-based health coaching program, and participating in these programs can achieve modest weight loss. In this study, weight-loss success was improved if participants completed at least 6 coaching sessions. This program did not appear to influence healthcare utilization or costs.
Funding
This project was conducted as part of the Johns Hopkins HealthCare (JHHC) Population Health – Welch Center for Prevention, Epidemiology, and Clinical Research (Welch Center) Collaboration, funded by Johns Hopkins HealthCare LLC. KAG was supported by a career development award from the National Heart, Lung, and Blood Institute (K23HL116601).
Disclosure
JT and SK are employees of Johns Hopkins HealthCare, LLC, which is the health insurer that provided data on the health coaching program evaluated.
Acknowledgements
We thank the staff at Johns Hopkins HealthCare, LLC who contributed to data abstraction and data procurement (Shannon Murphy and Robyn Mazur), as well as Linda Dunbar, PhD, Lawrence Appel, MD, MPH, and Felicia Hill-Briggs, PhD, ABPP, the leadership team of the JHHC Population Health – Welch Center Collaboration.
References
- Adams S.R., Goler N.C., Sanna R.S. Patient satisfaction and perceived success with a telephonic health coaching program: the natural experiments for translation in diabetes (NEXT-D) study, northern California, 2011. Prev. Chronic Dis. 2013;10 doi: 10.5888/pcd10.130116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anon. Chronic disease prevention and health promotion. Chronic diseases. http://www.cdc.gov/chronicdisease/overview/ Accessed on February 23, 2016 at.
- Anon. The Johns Hopkins ACG system. https://www.hopkinsacg.org Accessed on August 24, 2017 at.
- Appel L.J., Clark J.M., Yeh H.C. Comparative effectiveness of weight-loss interventions in clinical practice. N. Engl. J. Med. 2011;365:1959–1968. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B.B., Mehta A.K., Clark J.M., Gudzune K.A. Guideline-concordant weight-loss programs in an urban area are uncommon and difficult to identify through the internet. Obesity (Silver Spring) 2016;24:583–588. doi: 10.1002/oby.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccio M., Sanna R.S., Adams S.R. Telephone-based coaching: a comparison of tobacco cessation programs in an integrated health system. Am. J. Health Promot. 2017;31:136–142. doi: 10.4278/ajhp.140821-QUAN-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakin E.G., Lawler S.P., Vandelanotte C., Owen N. Telephone interventions for physical activity and dietary behavior change. Am. J. Prev. Med. 2007;32:419–434. doi: 10.1016/j.amepre.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Eakin E.G., Winkler E.A., Dunstan D.W. Living well with diabetes: 24-month outcomes from a randomized trial of a telephone-delivered weight loss and physical activity intervention to improve glycemic control. Diabetes Care. 2014;37:2177–2185. doi: 10.2337/dc13-2427. [DOI] [PubMed] [Google Scholar]
- Finley C.E., Barlow C.E., Greenway F.L., Rock C.L., Rolls B.J., Blair S.N. Retention rates and weight loss in a commercial weight loss program. Int. J. Obes. 2006;31:292–298. doi: 10.1038/sj.ijo.0803395. [DOI] [PubMed] [Google Scholar]
- Gerteis J., Izrael D., Deitz D. Agency for Healthcare Research and Quality; Rockville, MD: 2014. Multiple Chronic Conditions Chartbook. AHRQ Publications No, Q14-0038.https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/prevention-chronic-care/decision/mcc/mccchartbook.pdf [Google Scholar]
- Gollust S.E., Eboh I., Barry C.L. Picturing obesity: analyzing the social epidemiology of obesity conveyed through US news media images. Soc. Sci. Med. 2012;74:1544–1551. doi: 10.1016/j.socscimed.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Goode A.D., Reeves M.M., Eakin E.G. Telephone-delivered interventions for physical activity and dietary behavior change: an updated systematic review. Am. J. Prev. Med. 2012;42:81–88. doi: 10.1016/j.amepre.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Greenberg B.S., Eastin M., Hofschire L., Lachlan K., Brownell K. Portrayals of overweight and obese individuals on commercial television. Am. J. Public Health. 2003;93:1342–1348. doi: 10.2105/ajph.93.8.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer C.A., McClure K.J., Puhl R.M. Obesity stigma in online news: a visual content analysis. J. Health Commun. 2011;16:976–987. doi: 10.1080/10810730.2011.561915. [DOI] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) The National Academies Press; Washington, DC: 2014. Capturing Social and Behavioral Domains and Measures in Electronic Health Records: Phase 2. [PubMed] [Google Scholar]
- Jensen M.D., Ryan D.H., Apovian C.M. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society. Circulation. 2014;129:S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn H.S., Williamson D.F., Stevens J.A. Race and weight change in US women: the roles of socioeconomic and marital status. Am. J. Public Health. 1991;3:319–323. doi: 10.2105/ajph.81.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krist A.H., Tong S.T., Aycock R.A., Longo D.R. Engaging patients in decision-making and behavior change to promote prevention. Stud. Health Technol. Inform. 2017;240:284–302. [PMC free article] [PubMed] [Google Scholar]
- Miller W. Facilitating change. In: Miller W., Rollnick S., editors. Motivational Interviewing: Preparing People for Change. Guilford Press; New York, NY: 2002. pp. 20–29. [Google Scholar]
- Murphy B.M., Schoenman J.A., Pirani H. Health insurers promoting employee wellness: strategies, program components and results. Am. J. Health Promot. 2010;24:e1–10. doi: 10.4278/ajhp.080702-QUAL-113. [DOI] [PubMed] [Google Scholar]
- O'Hara B.J., Phonsavan P., Venugopal K. Effectiveness of Australia's Get Health Information and Coaching Service: translational research with population wide effect. Prev. Med. 2012;55:292–298. doi: 10.1016/j.ypmed.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Rowland M.L. Self-reported weight and height. Am. J. Clin. Nutr. 1990;52:1125–1133. doi: 10.1093/ajcn/52.6.1125. [DOI] [PubMed] [Google Scholar]
- Schmittdiel J.A., Adams S.R., Goler N. The impact of telephonic wellness coaching on weight loss: a “natural experiments in translation in diabetes (NEXT-D)” study. Obesity (Silver Spring) 2017;25:352–356. doi: 10.1002/oby.21723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Society for Human Resource Management SHRM Research: Health and Wellness Benefits. 2016. https://www.shrm.org/hr-today/trends-and-forecasting/special-reports-and-expert-views/Documents/Health%20and%20Wellness%20Benefits.pdf Accessed on August 24, 2017 at.
- Stead L.F., Hartmann-Boyce J., Perera R., Lancaster T. Telephone counseling for smoking cessation. Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD002850.pub3. [DOI] [PubMed] [Google Scholar]
- Tao M., Rangarajan K., Paustian M.L., Wasilevich E.A., El Reda D.K. Dialing in: effect of telephonic wellness coaching on weight loss. Am. J. Manag. Care. 2014;20:e35–e42. [PubMed] [Google Scholar]
- Ward B.W., Schiller J.S., Goodman R.A. Multiple chronic conditions among US adults: a 2012 update. Prev. Chronic Dis. 2014;11 doi: 10.5888/pcd11.130389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West D.S., Elaine Prewitt T., Bursae Z., Felix H.C. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity (Silver Spring) 2008;16:1413–1420. doi: 10.1038/oby.2008.224. [DOI] [PubMed] [Google Scholar]
- Wolever R.Q., Simmons L.A., Sforzo G.A. A systematic review of the literature on health and wellness coaching: defining a key behavioral intervention in healthcare. Glob. Adv. Health Med. 2013;2:38–57. doi: 10.7453/gahmj.2013.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbikowski S.M., Hapgood J., Smucker Barnwell S., McAfee T. Phone and web-based tobacco cessation treatment: real-world utilization patterns and outcomes for 11,000 tobacco users. J. Med. Internet Res. 2008;10 doi: 10.2196/jmir.999. [DOI] [PMC free article] [PubMed] [Google Scholar]