Introduction
Merkel cell carcinoma (MCC) is an aggressive, neuroendocrine carcinoma of the skin with an incidence of 2,000 cases per year, and a 5-year survival rate of 30% to 64%.1 Risk factors for MCC include advanced age, fair skin, and immunosuppression, including history of organ transplantation, HIV, and lymphoproliferative disorders.1, 2, 3 MCC presents clinically as a rapidly growing cutaneous nodule with frequent locoregional involvement. Although surgery and radiation are mainstays of treatment, management of distant disease is more challenging.3 Cytotoxic chemotherapy has modest efficacy, but responses are short lived and relapses are common.
Checkpoint inhibitors provide an important therapeutic option for MCC. The Merkel cell polyomavirus is involved in as many as 80% of MCC cases, resulting in expression of viral oncoproteins.4 Meanwhile, noninfected tumors display characteristic ultraviolet signature mutations. As such, there is strong rationale for targeting MCC with immunotherapeutic approaches.
Programmed cell death protein-1 (PD-1) and programmed death ligand-1 (PD-L1) are checkpoint molecules that interact to exhaust effector T cells and promote formation of regulatory T cells, leading to immune escape. Antibodies blocking this PD-1/PD-L1 interaction result in an antitumor immune response.5 Pembrolizumab is a US Food and Drug Administration (FDA)-approved PD-1 inhibitor for the treatment of melanoma and other malignancies. In a phase II trial, 26 MCC patients were treated with pembrolizumab; the objective response rate was 56% with 4 complete responses (CR) and ten partial responses (PR), and the progression-free survival rate was 67% at 6 months.5
Talimogene laherparepvec (TVEC) is a first-in-class oncolytic viral immunotherapy FDA approved for melanoma patients with cutaneous, subcutaneous, or nodal disease.6 Given its tumor agnostic mechanism of action, there is interest in studying TVEC in other immune-sensitive malignancies. We report on an MCC patient who progressed after multiple lines of therapy but then experienced a CR after treatment using TVEC combined with pembrolizumab.
Case report
A 51-year-old white man presented in October 2009 with an oval-shaped 2.5-cm subcutaneous nodule on the distal anterolateral aspect of the right lower leg. Incisional biopsy of the nodule found a small round blue cell malignancy with immunohistochemical staining positive for CAM5.2, CK20 with punctate peri-nuclear pattern, chromogranin, and synaptophysin, consistent with MCC. Computed tomography (CT) scans showed no metastatic disease. He underwent wide local re-excision with sentinel biopsy, which found a residual 2.7-cm primary MCC and a 0.3-cm focus of MCC involving a right inguinal node. Subsequent inguinal dissection found a total of 2 of 12 involved lymph nodes—stage IIIA disease, pT2N1aMx. The patient received adjuvant radiation to the primary tumor site and nodal basin.
In March 2011, surveillance scans found a 1.2-cm right pelvic node lateral to the bladder (Fig 1); a biopsy found MCC. The patient was treated with 4 cycles of cisplatin/etoposide after which repeat imaging showed CR. A right iliac lymph node dissection showed no residual malignancy in 0 of 9 lymph nodes. Two additional cycles of cisplatin/etoposide were planned, but a wound dehiscence developed, and the remaining cycles were abandoned.
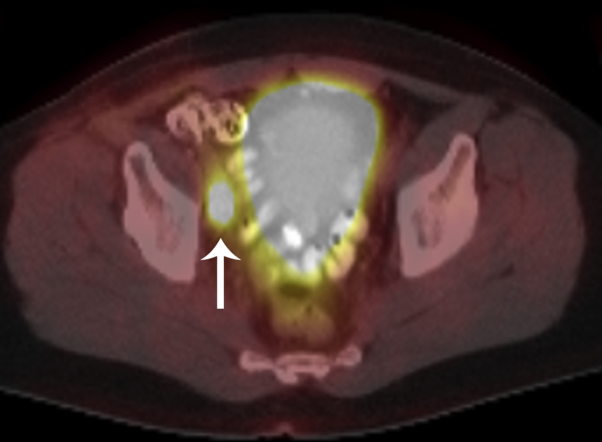
Fig 1.
18F-fluorodeoxyglucose positron emission tomography/CT fusion image shows a hypermetabolic right pelvic lymph node (arrow) lateral to the bladder, confirmed to be an MCC lymph node metastasis at subsequent CT-guided biopsy.
In July 2014, a 1.4-cm left para-aortic lymph node (Fig 2) developed as well as a well-circumscribed 1-cm cutaneous papule on his right anterior tibia. Biopsies of both found MCC. The patient received 5 cycles of cisplatin/etoposide, achieving CR. Approximately 1 year later, his disease recurred with 3 cutaneous papules on the lateral tibia measuring up to 1 cm in diameter. Wide local excision of all 3 papules found MCC (Fig 3). Unfortunately, only weeks after surgery, a rapidly growing subcutaneous nodule developed in the right popliteal fossa.
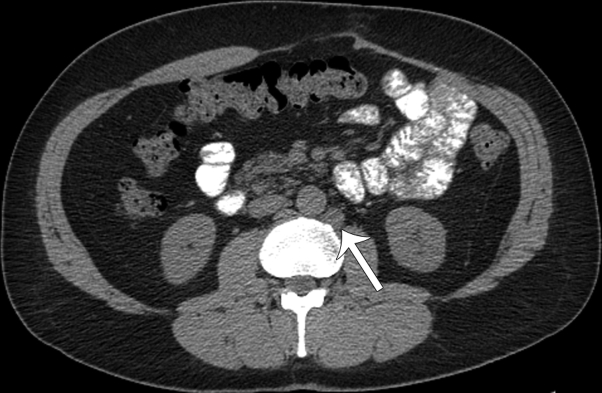
Fig 2.
CT of the abdomen/pelvis shows an enlarged left para-aortic lymph node (arrow), confirmed to be an MCC lymph node metastasis at subsequent CT-guided biopsy.
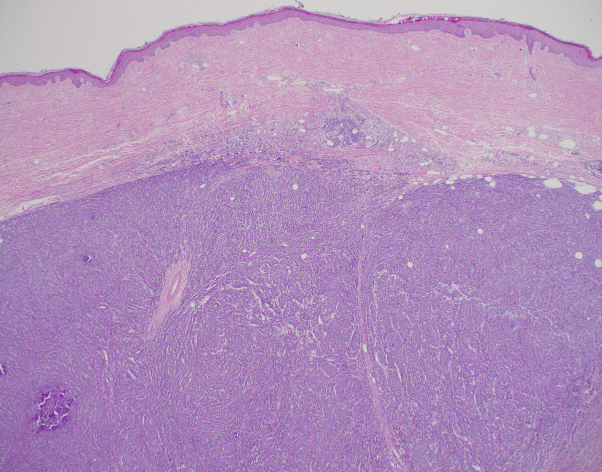
Fig 3.
Hematoxylin-eosin section shows a histologically normal epidermis and an intradermal tumor growing in a vaguely nodular pattern.
Salvage PD-1 therapy with nivolumab was started, but after 2 cycles he developed autoimmune diabetes, and treatment was discontinued. After just 2 cycles, he did achieve a PR, but the popliteal nodule eventually progressed while off therapy. After management of his diabetes, he resumed PD-1 therapy with pembrolizumab for 4 cycles, but disease radiographically progressed (Fig 4). TVEC was added to the fifth cycle of pembrolizumab. After 6 cycles of TVEC, the patient had a clinical response with resolution of the popliteal nodule. Because there was no remaining nodule to inject, he continued pembrolizumab alone. After 16 cycles of pembrolizumab, autoimmune colitis developed, and therapy was discontinued. As of June 2018, 8 months after the last cycle of pembrolizumab, the patient remains in CR with no clinical or radiographic evidence of disease (Fig 5).
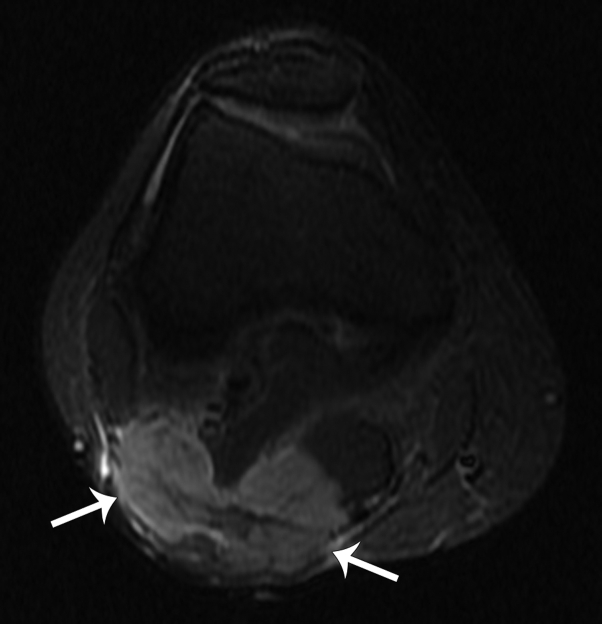
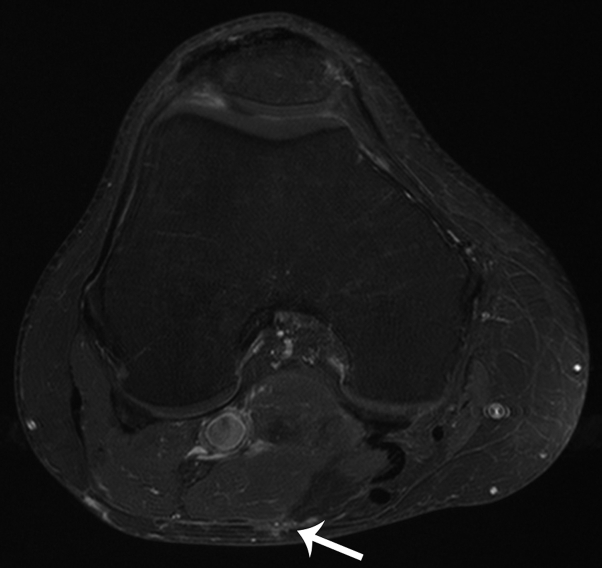
Fig 4.
Axial short tau inversion recovery (STIR) magnetic resonance image shows a 5-cm bilobed nodule of the posterior aspect of the popliteal fossa (arrows), confirmed to be an MCC metastasis at subsequent ultrasound-guided biopsy.
Fig 5.
Axial T1 fat saturation postcontrast magnetic resonance image shows near-complete resolution of the previously seen popliteal nodule (arrow).
Discussion
MCC is an aggressive skin cancer with high morbidity and mortality, but novel immunotherapeutics may improve patient outcomes. In 2017, the PD-L1 inhibitor avelumab became the first FDA-approved treatment for MCC. In a phase II trial, 88 MCC patients treated with avelumab achieved an objective response rate of 33%, with 10 CR and 19 PR.7 However, additional treatments are needed for MCC patients who do not respond to PD-1/PD-L1 inhibition. TVEC is an oncolytic viral therapy, which may have a role in managing MCC in patients with injectable tumors. We report the case of a patient who had progression of disease after PD-1 inhibition but achieved a CR with the addition of TVEC.
Subset analyses from the OPTIM study found that melanoma patients without visceral disease derived the highest benefit from TVEC, including improved survival.6 Similarly, this case report suggests a potential role for TVEC in managing MCC in patients with cutaneous, subcutaneous, and nodal disease, including those who did not respond to prior PD-1/PD-L1 inhibition. Furthermore, because TVEC induces neoantigen production and induces an inflammatory state, there may be potential synergy when combined with PD-1/PD-L1 inhibition. Although attribution of this response could be caused by a delayed effect of pembrolizumab, in the Nghiem study, 1 of 16 responses to PD-1 inhibition occurred after 12 weeks, whereas in the Kaufman study, only 1 of 28 responses occurred after 14 weeks, with 79% responding at 7 weeks. Therefore, a late response to PD-1/PD-L1 at 28 weeks, as in this case, would be rare.5, 7
Although there are studies and reports of either PD-1/PD-L1 therapy or TVEC monotherapy in MCC, to our knowledge, this is the first report using concurrent PD-1 therapy and TVEC.4, 5 Ongoing trials are assessing the combination of TVEC with PD-1/PD-L1 inhibitors for treatment of MCC, melanoma, and other cutaneous malignances (NCT02978625, NCT02965716).8, 9 Combinations such as these are needed to improve outcomes in this challenging, yet highly immune-responsive malignancy.
Footnotes
Funding sources: This article is supported by patient donations.
Conflicts of interest: Dr Gino K. In has a relationship with Regeneron/Sanofi as described in the conflict of interest forms.
References
- 1.Paulson K.G., Park S.Y., Vandeven N.A. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78(3):457–463.e452. doi: 10.1016/j.jaad.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen P.J., Bowne W.B., Jaques D.P., Brennan M.F., Busam K., Coit D.G. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23(10):2300–2309. doi: 10.1200/JCO.2005.02.329. [DOI] [PubMed] [Google Scholar]
- 3.Bichakjian C.K., Lowe L., Lao C.D. Merkel cell carcinoma: critical review with guidelines for multidisciplinary management. Cancer. 2007;110(1):1–12. doi: 10.1002/cncr.22765. [DOI] [PubMed] [Google Scholar]
- 4.Blackmon J.T., Dhawan R., Viator T.M., Terry N.L., Conry R.M. Talimogene laherparepvec for regionally advanced Merkel cell carcinoma: a report of 2 cases. JAAD Case Rep. 2017;3(3):185–189. doi: 10.1016/j.jdcr.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nghiem P.T., Bhatia S., Lipson E.J. PD-1 Blockade with pembrolizumab in advanced Merkel-Cell carcinoma. N Engl J Med. 2016;374(26):2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andtbacka R.H., Kaufman H.L., Collichio F. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman H.L., Russell J., Hamid O. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talimogene laherparepvec and nivolumab in treating patients with refractory lymphomas or advanced or refractory non-melanoma skin cancers. 2016. https://clinicaltrials.gov/ct2/show/NCT02978625 Retrieved from: Accessed Identification No. NCT02978625.
- 9.Talimogene laherparepvec and pembrolizumab in treating patients with stage III-IV melanoma. 2016. https://clinicaltrials.gov/ct2/show/NCT02965716 Retrieved from: Accessed Identification No. NCT02965716.