Abstract
Mechanical ventilation (MV) is a life-saving intervention for many critically ill patients. Unfortunately, prolonged MV results in rapid diaphragmatic atrophy and contractile dysfunction, collectively termed ventilator-induced diaphragm dysfunction (VIDD). Recent evidence reveals that endurance exercise training, performed prior to MV, protects the diaphragm against VIDD. While the mechanism(s) responsible for this exercise-induced protection against VIDD remain unknown, increased diaphragm antioxidant expression may be required. To investigate the role that increased antioxidants play in this protection, we tested the hypothesis that elevated levels of the mitochondrial antioxidant enzyme superoxide dismutase 2 (SOD2) is required to achieve exercise-induced protection against VIDD. Cause and effect was investigated in two ways. First, we prevented the exercise-induced increase in diaphragmatic SOD2 via delivery of an antisense oligonucleotide targeted against SOD2 post-exercise. Second, using transgene overexpression of SOD2, we determined the effects of increased SOD2 in the diaphragm independent of exercise training. Results from these experiments revealed that prevention of the exercise-induced increases in diaphragmatic SOD2 results in a loss of exercise-mediated protection against MV-induced diaphragm atrophy and a partial loss of protection against MV-induced diaphragmatic contractile dysfunction. In contrast, transgenic overexpression of SOD2 in the diaphragm, independent of exercise, did not protect against MV-induced diaphragmatic atrophy and provided only partial protection against MV-induced diaphragmatic contractile dysfunction. Collectively, these results demonstrate that increased diaphragmatic levels of SOD2 are essential to achieve the full benefit of exercise-induced protection against VIDD.
Keywords: Diaphragm, ROS, Antioxidants, Muscle atrophy, SOD2, Exercise training
Graphical abstract
Highlights
-
•
Prolonged mechanical ventilation results in diaphragmatic weakness which is labeled as ventilator-induced diaphragm dysfunction (VIDD).
-
•
Endurance exercise training performed prior to mechanical ventilation protects the diaphragm against VIDD.
-
•
Preventing exercise-induced increases of superoxide dismutase 2 (SOD2) in the diaphragm partially abolishes exercise protection against VIDD.
-
•
Transgenic overexpression of SOD2 in the diaphragm provides only partial protection against VIDD.
-
•
We conclude that increases in SOD2 abundance in the diaphragm contributes to the exercise-induced protection against VIDD.
1. Introduction
Mechanical ventilation (MV) is a life-saving intervention for patients in respiratory failure [30]. Conditions that commonly require ventilator support include critical illnesses, drug overdose, and surgery [30]. Unfortunately, an unintended consequence of prolonged MV is the rapid development of diaphragmatic atrophy and contractile dysfunction which is collectively termed ventilator-induced diaphragm dysfunction (VIDD) [3], [16], [30], [31]. VIDD is predicted to be a major contributor to difficulties in weaning patients from the ventilator [7], [30]. Indeed, as many as one third of MV patients that are exposed to ≥ 48 h of ventilator support experience difficult weaning [5], [6], [30]. Failure to wean from MV is a significant clinical problem as increased time in the intensive care unit greatly increases the risk of morbidity and mortality. Currently, no standard clinical therapy exists to prevent VIDD and therefore, it is imperative to identify biological targets in the diaphragm that can be manipulated to prevent VIDD.
Endurance exercise training results in a diaphragmatic phenotype that is protected against VIDD [34]. Although exercise training is not a practical therapy to prevent VIDD in critically ill patients, identifying the mechanism(s) responsible for exercise-induced protection of the diaphragm provides a unique experimental tool in the search for molecular targets to prevent VIDD. In this regard, we discovered that exercise training increases the abundance of a key mitochondrial antioxidant enzyme, superoxide dismutase 2, also known as manganese superoxide dismutase (SOD2) [34]. This is important because we have demonstrated that MV-induced oxidative damage to diaphragmatic mitochondria is essential for the development of VIDD [26]. Indeed, increases in mitochondrial production of reactive oxygen species (ROS) drive diaphragmatic atrophy by promoting both a decrease in diaphragmatic protein synthesis and an increase in protein degradation [28].
SOD2 is located within the mitochondrial matrix and is responsible for the dismutation of superoxide into hydrogen peroxide. It is encoded in the nuclear genome and is transported into the mitochondrial matrix via the mitochondrial “translocase of the outer membrane” and “translocase of the inner membrane 23″ [8]. Importantly, exercise increases the abundance of SOD2 in both the diaphragm and heart [14], [34], [36]. Formally, in studies investigating ischemia reperfusion injury in the heart, we uncovered that SOD2 played a primary role in exercise-induced cardioprotection [14] and similarly, we predict that increased SOD2 in the diaphragm may also contribute to exercise-induced protection against VIDD. Therefore, these experiments tested two hypotheses: 1) endurance exercise-induced protection against VIDD occurs, in part, due to increases in SOD2 in the diaphragm; and 2) overexpression of SOD2 in the diaphragm, independent of exercise, provides protection against VIDD. Using a well-established pre-clinical model of MV, we determined cause and effect by using innovative molecular tools to prevent exercise-induced increases in diaphragmatic SOD2. Further, in separate experiments using viral gene transfection, we promoted overexpression of SOD2 in the diaphragm, independent of exercise.
2. Methods
2.1. Animal model justification
To test our experimental hypothesis, we used a well-established animal model of MV (i.e., adult female Sprague-Dawley (SD) rats). We selected the SD rat as the experimental model because the diaphragm of the SD rat and humans are anatomically similar and share similar muscle fiber type characteristics [30]. Moreover, female rats were selected because effects of prolonged MV on diaphragm fibers are identical in male and female rats [29], [38] and female body weights remain relatively stable from 3 to 8 months of age [24]. Animals were 3–5 months of age and ~ 250 g at the time of our experiments.
2.2. Animal housing and diet
All animals were housed at the University of Florida Animal Care Services Center according to guidelines set forth by the Institutional Animal Care and Use Committee. Additionally, the University of Florida Animal Care and Use Committee approved these experiments. The experimental animals were maintained on a 12:12 h light-dark cycle and provided food and water ad libitum throughout the experimental period.
2.3. Experimental design
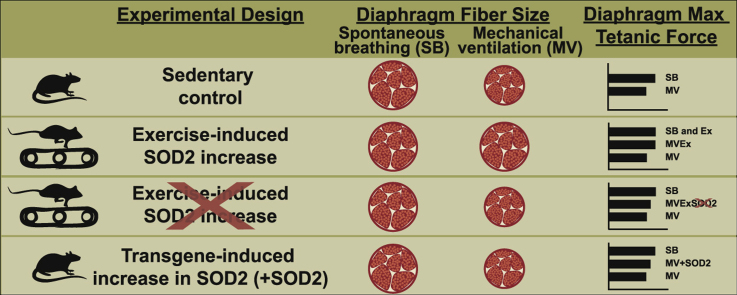
These experiments tested two hypotheses: 1) endurance exercise-induced protection against VIDD occurs, in part, due to increases in SOD2 in the diaphragm; and 2) overexpression of SOD2 in the diaphragm, independent of exercise, provides protection against VIDD. First, we determined if prevention of the exercise-induced increase in SOD2 in the diaphragm will avert the exercise-induced protection of VIDD (Experiments 1a and 1b). Second, we tested the hypothesis that transgenic overexpression of SOD2 in the diaphragm is sufficient to protect against VIDD (Experiment 2). A brief description of the experimental designs and general methods for these experiments follows.
2.3.1. Experiment 1a: Antisense prevention of exercise-induced increases in SOD2
Experiment 1a determined if preventing the exercise-induced increase in SOD2 using an antisense oligonucleotide against SOD2 results in diaphragmatic atrophy and contractile dysfunction. Adult female SD rats were randomly assigned to one of the following experimental groups (n = 10/group): 1) exercise trained animals with no experimental treatment (Ex) or 2) exercise trained animals injected with antisense oligonucleotide against SOD2 following each exercise session (Ex-AS). Animals underwent 10 days of endurance exercise training prior to sacrifice as described below [34]. Twenty-four hours after the last training session animals were acutely anesthetized and diaphragms were rapidly removed. Primary dependent variables measured in diaphragm muscle include contractile performance, fiber cross-sectional area (CSA), SOD2 protein abundance, and SOD2 activity.
2.3.2. Experiment 1b: SOD2 and exercise
To test the hypothesis that the exercise-induced increase in SOD2 is required for the exercise-induced protection against VIDD, adult female SD rats were randomly assigned to one of the following experimental groups (n = 10/group): 1) sedentary controls (CON), 2) sedentary animals exposed to 12 h of MV (MV), 3) exercise trained animals exposed to 12 hours of MV (MVEx), 4) exercise trained animals injected with antisense oligonucleotide against SOD2 following each exercise session and exposed to 12 h of MV (MVEx-AS).
2.3.3. Experiment 2: AAV9 overexpression of SOD2
These experiments tested the prediction that transgenic overexpression of SOD2 alone is sufficient to protect against VIDD. To test this hypothesis, adult female SD rats were randomly assigned to one of the following experimental groups (n = 10/group): 1) animals exposed to sham surgeries with no experimental intervention (CON), 2) animals exposed to sham surgery and 12 h of MV (MV), 3) animals exposed to surgical transduction of AAV9 to overexpress SOD2 in the diaphragm (without MV) (SOD2OvExp) or 4) animals exposed to surgical transduction of AAV9 to overexpress SOD2 in the diaphragm and 12 h of MV (MV-SOD2OvExp). Note, to avoid republication of data, CON and MV animals for experiment 2 are different animals from CON and MV animals in experiment 1b.
2.4. General methods
2.4.1. Mechanical ventilation
Aseptic techniques were adopted for all surgical procedures. Prior to initiation of MV, animals were injected with an IP injection of sodium pentobarbital (60 mg/kg body weight). Upon reaching a stage 3 plane of anesthesia (i.e, surgical plane of anesthesia), animals underwent a tracheostomy and were mechanically ventilated with a pressure-controlled ventilator (Servo Ventilator 300; Siemens, Munich, Germany) utilizing the “controlled MV” mode. In this mode, the ventilator performs the full work of breathing and the animal cannot trigger the ventilator. Animals were ventilated for 12 h at the following ventilator settings: upper airway pressure limit: 20 cmH2O; respiratory rate: 80 bpm; positive end-expiratory pressure: 1 cmH2O.
Post tracheostomy, both the carotid artery and jugular vein were cannulated for measurement of arterial blood pressure and continuous infusion of anesthesia (sodium pentobarbital, ~ 10 mg kg−1 h−1) respectively. In addition, arterial blood samples were obtained periodically to monitor arterial blood gas values (i.e., PaCO2 PaO2, and pH) (GEM Premier3000; Instrumentation Laboratory, Lexington, MA). When necessary, adjustments to both alveolar ventilation and inspired fractions of oxygen were performed to maintain blood gas and pH homeostasis during the 12 h of MV. Importantly, we have previously shown that in spontaneously breathing animals, anesthesia treatment does not adversely affect either diaphragm fiber CSA or contractile function [29].
During prolonged MV, continuous care was provided to the animals in the form of eye lubrication, expressing the bladder, removing airway mucus, and rotating the animals to combat blood pooling. A recirculating warming blanket was used to maintain body temperature at ~ 37 °C and heart rate was measured by lead II of an electrocardiograph. Finally, animals received glycopyrrolate (0.04–0.08 mg/kg) intramuscularly every 2 h throughout MV to reduce airway secretions. Following 12 h of MV, the diaphragm was rapidly removed for subsequent analysis.
2.4.2. Survival surgery for AAV9-SOD2 administration
Four weeks prior to MV, animals were injected with AAV9-SOD2 directly into the diaphragm. This time point was selected based on preliminary experiments indicating that four weeks following transfection is sufficient time to result in a stable expression of the transfected gene; complete details of our diaphragm gene transfection protocol can be found elsewhere [33], [35]. Briefly, animals were initially anesthetized using 2–4% inhaled isoflurane followed by continuous anesthesia (with 0.5–2.5% inhaled isoflurane) administered via nosecone throughout the surgical procedure. Excess isoflurane was scavenged with two Omnicon f/air canisters. All surgical procedures were performed under aseptic conditions by an experienced rodent surgeon. After reaching a surgical plane of anesthesia, a midline incision was made through the skin extending from the xiphoid process to the suprapubic region, the liver was retracted, and the diaphragm was injected as described previously [33]. Prior to awakening from the anesthetic, the animals received 6–8 mL subcutaneous fluids (0.9% Sodium Chloride) that had been warmed to 37 °C and Buprenorphine (0.05–0.1 mg/kg) for post-surgical relief of pain.
2.4.3. Exercise training protocol
Animals in the exercise group performed 5 consecutive days (d) of progressive habituation to treadmill running. Following 2 d of rest, each animal performed 10 d of treadmill running for 60 min/day at 30 m/min, 0% grade (estimated work rate of 70% VO2 max) with 2 d rest after day 5 of training. We chose this protocol based on previous experiments demonstrating its ability to promote exercise-induced protection of the diaphragm from VIDD [34]. Animals were ventilated ~ 24 h following the final exercise bout.
2.4.4. Preventing the exercise-induced increase of SOD2
Immediately following each 60 min exercise bout, animals received an IP injection with an antisense oligonucleotide phosphorothioate (5′-CACGCCGCCCGACACAACATTG-3′) at a dose of (10 mg/kg) targeted against SOD2 to inhibit protein translation. Previous reports have confirmed the effectiveness of this dosing protocol to prevent exercise-induced increases in SOD2 in skeletal muscle and heart [14].
2.4.5. AAV9 overexpression of SOD2
AAV9-CMV-SOD2 (AAV-290654) was purchased from Vector Biolabs (Malvern, PA). Four weeks prior to MV, animals designated to overexpress SOD2 underwent abdominal surgery for eight evenly spaced direct diaphragm injections of AAV9 at a concentration of 1 × 1011 viral genomes as previously described [33]. The dose of 1 × 1011 viral genomes was selected based on our prior experience with diaphragm protein overexpression utilizing an AAV9 serotype with CMV promotor driven expression; importantly, this approach results in overexpression of the transgene in ~ 80% of diaphragm fibers (independent of fiber type) [33].
2.5. Biochemical measurements
2.5.1. SOD2 protein abundance
Diaphragm samples were homogenized 1:10 (mg wt/μL buffer) in 5 mM Tris (pH 7.5) and centrifuged at 1500g for 5 min at 4 °C. Upon collection of the supernatant, protein content was assessed via Bradford method (Sigma-Aldrich) [37]. A rat SOD2 enzyme-linked immunosorbent assay kit (ELISA) (Life Span BioSciences, Inc.; LS-F6964) was used to determine total protein abundance of SOD2 in the diaphragm [23].
2.5.2. SOD2 enzyme activity
SOD2 activity in the diaphragm was measured with a commercially available enzyme activity kit according to the manufacturer's instructions (Cayman Chemical, Ann Arbor, MI Superoxide Dismutase Assay Kit; 706002), with the addition of 2 mM KCN to inhibit copper and zinc SOD. Briefly, tissue samples were homogenized 1:10 (mg wt/μL buffer) in 20 mM Hepes, 1 mM EGTA, 210 mM mannitol, and 70 mM sucrose (pH 7.2) and centrifuged at 1500 g for 5 min at 4 °C. SOD2 activity was normalized to protein concentration in the homogenate.
2.5.3. Glutathione reductase activity
Diaphragmatic glutathione reductase activity was measured with an enzyme activity kit (Cayman Chemical, Glutathione Reductase Assay Kit; 703202). Briefly, tissue samples were homogenized 1:10 (mg wt/μL buffer) in 20 mM Hepes, 1 mM EGTA, 210 mM mannitol, and 70 mM sucrose (pH 7.2) and centrifuged at 1500g for 5 min at 4 °C. After measuring the total glutathione reductase activity, the activities were normalized by the protein concentrations.
2.5.4. Measurement of protein carbonyls
Protein carbonylation in diaphragm samples was measured by a commercially available OxyBlot kit (Millipore, S7150) in the soluble fraction for experiment 1b while measured in the insoluble fraction in experiment 2.
2.5.5. Real-time polymerase chain reaction (PCR)
Total RNA was isolated from ~ 25 mg of diaphragm muscle tissue using Trizol reagent (Life Technologies, Carlsbad, CA). RNA was reverse transcribed using Superscript VILO (Invitrogen). PCR was performed as described previously [35]. mRNA transcripts were assayed using commercially available rat primer and probe sequences from Applied Biosystems: Fbxo32 for atrogin-1/MAFbx (Rn00591730_m1), Trim63 for MuRF1 (Rn00590197_m1), GUSB for β-glucronidase (Rn00566655_m1) was used as a reference gene.
2.5.6. Western blot analysis
Western blots were conducted on diaphragm protein as described previously with minor modifications [19]. Briefly, supernatant was drawn off and protein was assessed via Bradford method (Sigma), followed by normalizing the protein concentrations in Laemmli sample buffer (1610747, Bio Rad Hercules, CA) containing 5% dithiothreitol. Proteins were loaded on 4–20% gradient Criterion TGX gels (Bio-Rad) and transferred to LF-PVDF membrane (Millipore Burlington, MA). Following transfer, membranes were blocked in 5% milk solution for 2 h at room temperature; followed by incubation with primary antibodies. Membranes were exposed to either Alexa Fluor 680 IgG or 800 IgG (Odyssey Li-Cor) secondary for 30 min at 25 °C. Membranes were scanned and analyzed with the Li-Cor Odyssey Infrared Imager (Li-Cor Biosciences) using Odyssey 2.1 software. Primary antibodies of interest were superoxide dismutase 1 (SOD1) (Santa Cruz, sc-11407, 1:1000 incubated overnight at 4 °C secondary concentration 1:20,000), Glutathione peroxidase (Gpx1), (Santa Cruz, sc22145, 1:200 incubated 1.5 h at 25 °C secondary concentration 1:5000), Catalase (Gene Tex, GTX110704, 1:1000 incubated overnight at 4 °C secondary concentration 1:20,000), αII-spectrin (Santa Cruz, sc48382, 1:250 incubated overnight at 4 °C then 3 h at 25 °C secondary concentration 1:5000), calpastatin (Santa Cruz, sc20779, 1:500 incubated 3 days at 4 °C secondary concentration 1:5000), LC3 II (Cell Signaling, 2775, 1:500 incubated overnight at 4 °C secondary concentration 1:5000), and citrate synthase (Abcam, ab66600, 1:1000 incubated overnight at 4 °C secondary concentration 1:20,000). All westerns were normalized using the total protein in each lane labeled with Revert protein stain (Li-Cor), like Coomassie Blue. The 40 kDa band for total protein is shown as a representative image of the protein loading as reported previously [2].
2.6. Histological measurements
2.6.1. Diaphragm myofiber CSA
Frozen diaphragm sections were cut at a thickness of 10 µm with a cryostat (HM 550 Cryostat, Thermo Scientific, Waltham, MA) and stained for dystrophin (Thermo Scientific #RB-9024-R7), myosin heavy chain I (Hybridoma Bank A4.840 s IgM 1:15), and myosin heavy chain IIa (Hybridoma Bank SC-71c IgG 1:50) for CSA analysis. CSA was analyzed with Scion Image software (National Institutes of Health) as previously described [19].
2.7. Functional measurements
2.7.1. Diaphragmatic contractile function
To assess diaphragm contractile function, a costal diaphragm muscle strip (~ 3 mm wide), including the tendinous attachments at the central tendon and rib cage was suspended vertically between two lightweight Plexiglas clamps with one end connected to an isometric force transducer (model FT-03, Grass Instruments, Quincy, MA) within a jacketed tissue bath containing 25 °C Krebs-Hensleit solution equilibrated with 95% O2–5% CO2 gas. Optimal muscle length (L0) was found and then the muscle was electrically stimulated using field stimulation to contract over a frequency range of 15–160Hz. The force output was recorded using LabView (National Instruments Corporation, Austin, TX) as previously described [27,55]. To control for differences in muscle strip size, force production was normalized to physiological CSA and specific force production was compared between the experimental groups.
2.7.2. Permeabilized muscle fiber preparation
Approximately 15 mg of costal diaphragm muscle was dissected and placed on a plastic Petri dish filled with ice-cold buffer X (60 mM K-Mes, 35 mM KCl, 7.23 mM K2EGTA, 2.77 mM CaK2EGTA, 20 mM imidazole, 0.5 mM dithiothreitol, 20 mM taurine, 5.7 mM ATP, 15 mM PCr, and 6.56 mM MgCl2, pH 7.1). The muscle was then cut into four ~ 3 mg pieces and gently separated to expose each fiber in ice-cold buffer X. Muscle fiber bundles were then placed on a rotator for 30 min at 4 °C in ice-cold buffer X containing 75 μg mL−1 saponin. Finally, the permeabilized fiber bundles were washed three times for 5 min in ice-cold buffer Z (110 mM K-Mes, 35 mM KCl, 1 mM EGTA, 5 mM K2HPO4, and 3 mM MgCl2, 0.005 mM glutamate, 0.02 mM malate, and 0.5 mg · mL−1 BSA, pH 7.1).
2.7.3. Diaphragm mitochondrial respiration in permeabilized fibers
Mitochondrial respiration was measured polarographically in a respiration chamber (Hansatech Instruments) maintained at 37 °C. After the respiration chamber was calibrated, permeabilized fiber bundles (~ 3 mg) were incubated with 1 mL of respiration buffer Z containing 20 mM creatine to saturate creatine kinases [56,57]. Flux through complex I was measured using 5 mM pyruvate and 2 mM malate. The ADP-stimulated respiration (state 3) was initiated by adding 0.25 mM ADP to the respiration chamber. Basal respiration (state 4) was determined in the presence of 10 μg/mL oligomycin to inhibit ATP synthesis. The respiratory control ratio (RCR) was calculated by dividing oxygen consumption during state 3 respiration by the oxygen consumption during state 4 respiration.
2.7.4. Diaphragm mitochondrial ROS production in permeabilized fibers
Mitochondrial H2O2 emission rate was measured continuously in permeabilized fiber bundles (~ 3 mg) via a spectrofluorometer (Fluorolog-3 HORIBA Jobin Yvon, Edison, NJ, USA) at 37 °C with Amplex™ UltraRed (Molecular Probes, Eugene, OR) (10 μM)/horseradish peroxidase (1 U/mL) system. The assay was performed at 37 °C in a magnetic stirring cuvette using succinate as the substrate. Specifically, this assay uses horseradish peroxidase to catalyze the H2O2-dependent oxidation of non-fluorescent Amplex™ UltraRed to a fluorescent resorufin, and it was used to measure H2O2 as an indicator of superoxide production. Excitation and emission was set at 565 and 600 respectively. Blebbistatin (25 μM) prevented myofiber contraction during the measurements. The levels of resorufin were recorded every 30 s for 10 min, and H2O2 production was calculated using a standard curve. Each fiber bundle was washed and dehydrated for normalization of the measurements. The rate of ROS production was expressed as H2O2 emission in pmol·min−1 mg−1 dry wt.
2.8. Statistical analysis
Group sample size was determined via a power analysis using preliminary data from our laboratory. Comparisons between groups were made by a one-way ANOVA, and when appropriate a Tukey HSD test was performed post hoc. Significance was established at p < 0.05.
3. Results
Animals used in these experiments were 3–5 months of age with no significant differences in body weight between groups (n = 10/group): Experiment 1a (Ex = 257 g ± 10.4, Ex-AS = 249 g ± 11.1). The following is a summary of our experimental findings.
3.1. Experiment 1a
3.1.1. Physiological response to SOD2 antisense oligonucleotide treatment
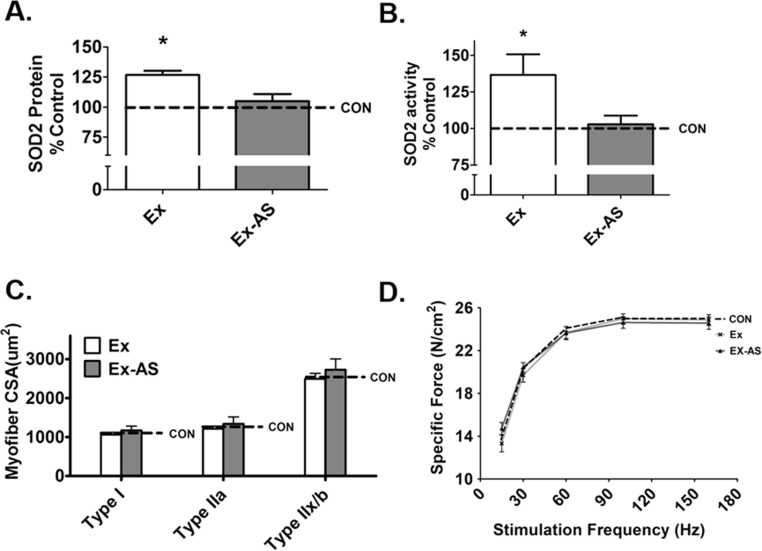
To determine if administration of an antisense oligonucleotide directed against SOD2 resulted in alterations in diaphragm muscle fiber CSA and contractile function, animals were trained for 10 days and administered either saline or antisense following each training session. Our results demonstrate that compared to Ex animals, antisense treatment is sufficient to prevent the exercise-induced increase in SOD2 levels but did not impact diaphragm fiber CSA and specific force production (Fig. 1C-D).
Fig. 1.
Antisense oligonucleotide prevents the exercise-induced increase in SOD2 activity and protein abundance without altering CSA and diaphragm muscle force. A) ELISA of diaphragm SOD2 protein abundance, n = 10, B) SOD2 activity, n = 8, C) diaphragm CSA, n = 10, and D) force frequency curve, n = 10. Values are mean percent change of control ± SE. CON, control (dotted line is representative control value); Ex, exercise; Ex-AS, exercise with antisense. * p < 0.05 Ex is different from Ex-AS.
3.2. Experiment 1b
3.2.1. Biological responses to mechanical ventilation
Importantly, no significant differences existed in heart rates, blood gases, and blood pressure between the experimental groups at the conclusion of the experimental protocols (Table 1). Even though we have demonstrated previously that VIDD is not caused by barotrauma [6], we visually examined the lungs for signs of damage and the peritoneal cavity for evidence of infection. Our examination revealed no visual evidence of barotrauma in the lungs and no evidence of infection existed following the 12-h MV protocol.
Table 1.
At the conclusion of the experimental protocols in experiment 1B, no significant differences existed between experimental groups in body weight, heart rate (HR), arterial partial pressure of carbon dioxide (PaCO2), arterial pressure of oxygen (PaO2), arterial pH, and systolic blood pressure (SBP). Data presented as mean ± standard deviation.
| MV | MVEx | MVEx-AS | |
|---|---|---|---|
| Weight (g) | 250 ± 15 | 260 ± 15 | 260 ± 17 |
| HR | 354 ± 30 | 351 ± 20 | 337 ± 18 |
| PaCO2(mmHg) | 37 ± 7.7 | 33 ± 3.8 | 34 ± 6.8 |
| PaO2(mmHg) | 70 ± 11 | 73 ± 8 | 76 ± 16 |
| pH | 7.46 ± 0.05 | 7.47 ± 0.03 | 7.50 ± 0.05 |
| SBP (mmHg) | 122 ± 16 | 117 ± 10 | 110 ± 10 |
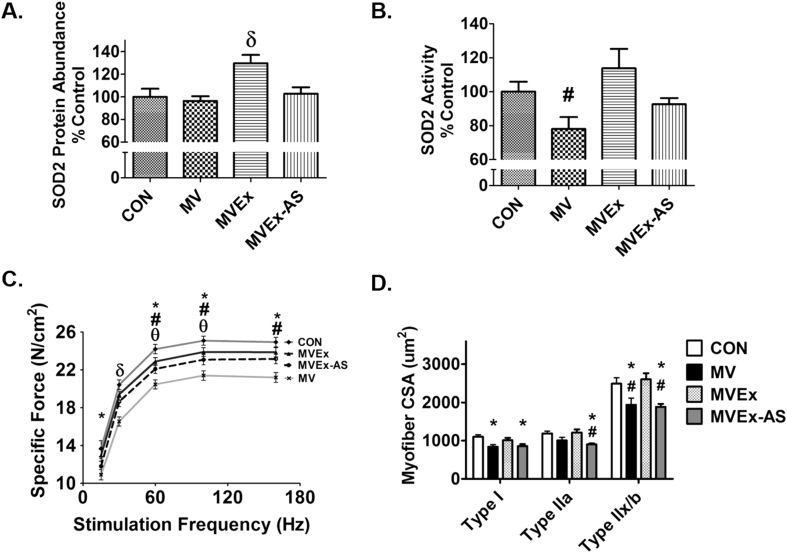
3.2.2. Antisense treatment prevents the exercise-induced increase of SOD2 protein abundance and activity in the diaphragm of mechanically ventilated animals
Post-exercise treatment with an antisense oligonucleotide directed against SOD2 prevented the exercise-induced increase in protein abundance of SOD2 in the diaphragm of mechanically ventilated animals (Fig. 2 A). Importantly, antisense treatment did not reduce SOD2 levels in the diaphragm below control levels.
Fig. 2.
Treatment of animals with an antisense oligonucleotide against SOD2 prevents the exercise-induced increase in SOD2 activity and protein abundance in the diaphragm while attenuating exercise protection against VIDD and abolishing exercise protection against atrophy. A) ELISA of SOD2 protein abundance in diaphragm, n = 9/group. B) SOD2 activity in diaphragm, n = 8/group. Values are mean percent change of control ± SE. # p < 0.05 different from MVEx; δ p < 0.05 different from all groups. C) Diaphragm specific force, n = 10/group. Values are mean ± SE. * p < 0.05 MV different from CON, # p < 0.05 MV different from MVEx; θ p < 0.05 MVEx-AS different from CON; δ p < 0.05 MV different from all groups. D) Diaphragm cross-sectional area. Values are mean ± SE. * p < 0.05 different from CON, # p < 0.05 different from MVEx.
3.2.3. Elevated SOD2 contributes to exercise-induced protection against MV-induced contractile dysfunction in the diaphragm
To determine if increased SOD2 in the diaphragm is essential for exercise-induced protection against MV-induced diaphragm contractile dysfunction, we measured contractile properties in strips of costal diaphragm muscle in vitro. Consistent with previous studies [1], [3], [10], [15], [17], prolonged MV resulted in a significant decrease in the diaphragm force-frequency curve. Importantly, animals exposed to endurance exercise training prior to MV were protected against this MV-induced diaphragmatic contractile dysfunction. However, when the exercise-induced increase in SOD2 in the diaphragm was prevented, exercise-induced protection against MV-induced diaphragmatic dysfunction was partially attenuated (Fig. 2C).
3.2.4. Increased expression of SOD2 in the diaphragm contributes to exercise training-induced protection against MV-induced diaphragmatic atrophy
Similar to previous findings, exercise training preconditioned the diaphragm and protected against MV-induced fiber atrophy [34]. Importantly, treatment of animals with the antisense oligonucleotide against SOD2 abolished the exercise-induced protection against MV-induced atrophy in all diaphragm fiber types (Fig. 2D).
3.2.5. Increased SOD2 is not required to rescue mitochondria from MV-induced uncoupling
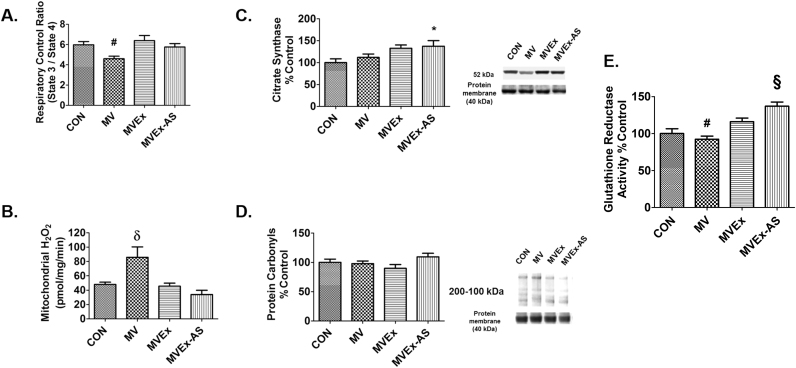
To determine if the exercise-induced increase in SOD2 in the diaphragm is required for protection against MV-induced mitochondrial dysfunction in diaphragm fibers, mitochondrial respiration was measured during both state 3 and state 4, mitochondrial coupling was assessed by RCR. Prolonged MV resulted in mitochondrial uncoupling as evidenced by a significantly lower RCR; however, exercise training performed prior to MV resulted in protection against MV-induced mitochondrial uncoupling (Fig. 3A). Moreover, prevention of the exercise-induced increase in SOD2 in diaphragm mitochondria did not decrease this exercise-induced protection against MV-induced mitochondrial uncoupling.
Fig. 3.
Impact of MV on mitochondrial contents, respiration, hydrogen peroxide emission, glutathione reductase, and protein carbonyls in diaphragm. A) Respiratory control ratio (RCR) calculated by mitochondrial state 3 respiration/state 4 respiration, n = 9/group. B) Mitochondrial hydrogen peroxide emission n = 10/group. Values are mean ± SE. # p < 0.05 different from MVEx, δ p < 0.05 different from all groups. C) Diaphragm citrate synthase levels in diaphragm muscle used as a marker of mitochondrial volume, n = 8/group. D) Protein carbonyls, n = 10/group. E) Glutathione Reductase activity determined colorimetrically by measuring the rate of NADPH oxidation in diaphragm samples n = 7/group. Values are mean percent change of control ± SE. * p < 0.05 different from CON, # p < 0.05 different from MVEx, δ p < 0.05 different from all groups.
We next measured citrate synthase protein content in diaphragm fibers as a surrogate biomarker of mitochondrial volume. Importantly, treatment of animals with the antisense oligonucleotide against SOD2 did not decrease citrate synthase activity in the diaphragm (Fig. 3C).
3.2.6. Exercise training protects against MV-induced increases in mitochondrial ROS emission
Consistent with previous findings, prolonged MV resulted in a significant increase in diaphragmatic mitochondrial ROS emission and 10 days of exercise training prevented the increased ROS in the diaphragm [34]. Interestingly, preventing the exercise-induced increase in SOD2 did not result in increased H2O2 production from the mitochondria (Fig. 3B). Protein carbonyls in the diaphragm were measured as a biomarker of oxidative stress; paradoxically, despite an increase in ROS emission from mitochondria during MV, no differences existed between experimental groups in diaphragm levels of protein carbonylation (Fig. 3D).
3.2.7. Reduced SOD2 in exercise-trained diaphragms results in increased glutathione reductase activity during MV
Glutathione reductase is an antioxidant enzyme that maintains the pool of reduced glutathione by recycling glutathione disulfide back to its reduced form [9]. In studies where SOD2 is in a lower abundance in cells, reduced glutathione is also lower suggesting that there is a compensatory interplay between SOD2 and glutathione [4]. Therefore, we measured the activity of glutathione reductase in the diaphragm of all experimental groups. Compared to both groups of exercise trained animals exposed to MV, glutathione reductase activity was significantly lower in the diaphragm of sedentary animals exposed to prolonged MV. Surprisingly, preventing the exercise-induced increase in SOD2 resulted in elevated glutathione reductase activity in the diaphragm following MV compared to all other groups (Fig. 3E).
3.2.8. Exercise training and diaphragm antioxidant enzymes
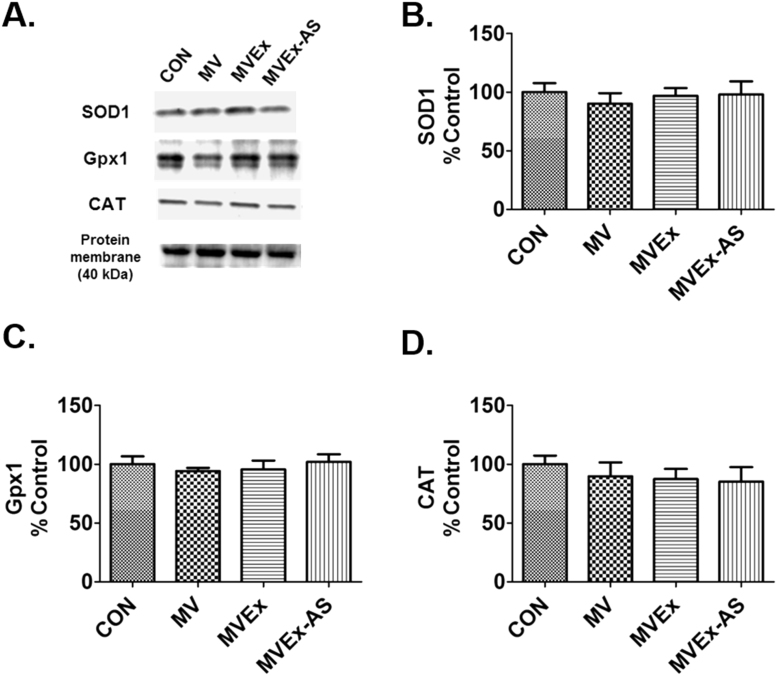
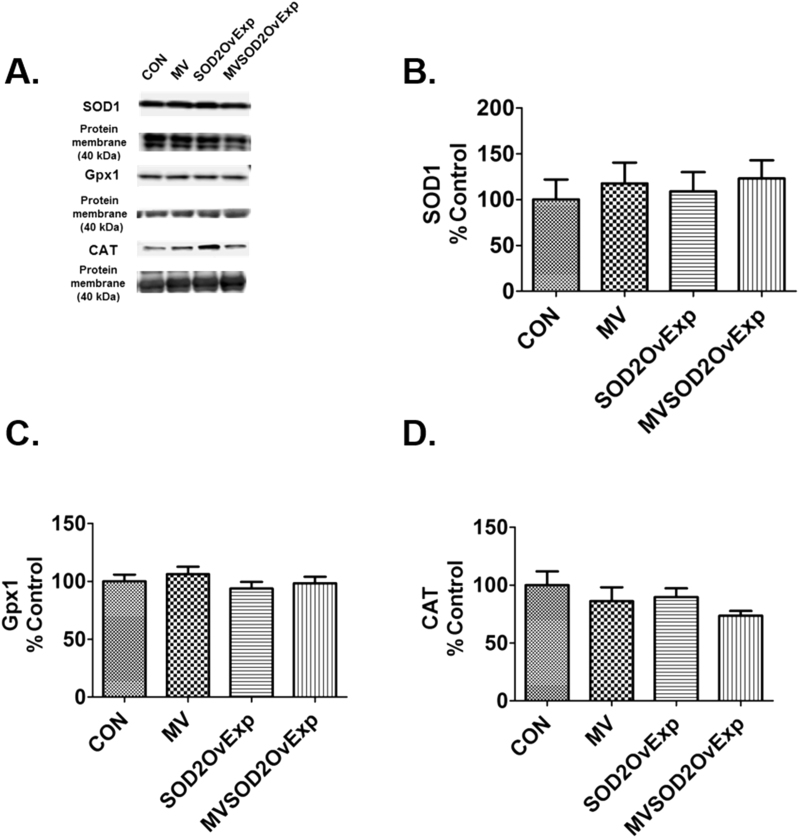
A complex network of antioxidant enzymes exist in skeletal muscle fibers [27]. To determine if exercise training increases the abundance of key antioxidant enzymes in the diaphragm, we measured the protein abundance of SOD1, Gpx1, and CAT. Although exercise training significantly increased diaphragmatic levels of SOD2 in mechanically ventilated animals (Fig. 2A), exercise did not alter diaphragmatic levels of SOD1, Gpx1 and CAT (Fig. 4A-D). Additionally, prevention of exercise-induced increases in diaphragmatic SOD2 did not impact diaphragmatic levels of any of these antioxidant enzymes (Fig. 4A-D).
Fig. 4.
Diaphragm antioxidant enzymes after 12 h of MV. A) representative protein blots, B) superoxide dismutase 1 (SOD1), C) glutathione peroxidase 1 (Gpx1), D) Catalase (CAT). n = 8/group for all graphs. Values are mean percent change of control ± SE.
3.2.9. Exercise training protects against MV-induced proteolysis in the diaphragm
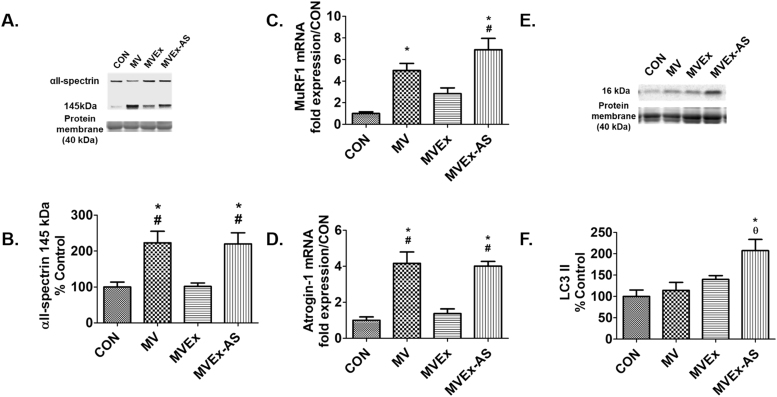
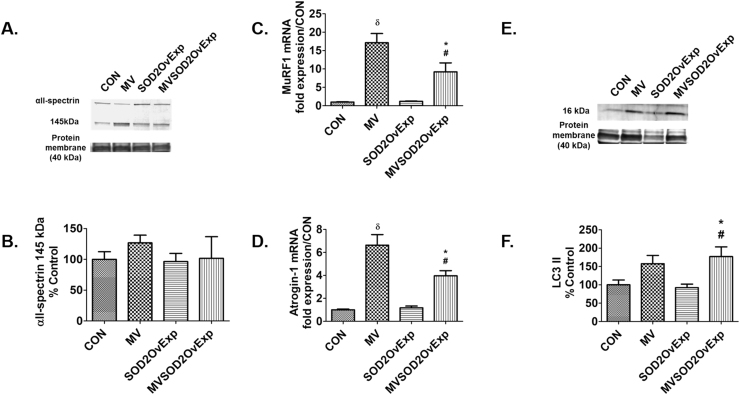
Prolonged MV results in calpain activation and increased ubiquitination in the diaphragm; importantly, active calpain promotes diaphragmatic atrophy and contractile dysfunction [12], [21]. The current study confirms that endurance exercise training preconditions the diaphragm and protects against MV-induced increases in proteolysis. Specifically, compared to both CON and MVEx animals, ubiquitin ligase mRNA expression (MuRF1 and Atrogin-1) was increased in the diaphragms of both MV and MVEx-AS (Figs. 5C and 5D). Calpain activation in the diaphragm was measured by the accumulation of the calpain-specific cleavage product of αII-spectrin and calpain activity in the diaphragm was higher in both MV and MVEx-AS animals compared to MVEx and CON (Fig. 5B). As reported previously, LC3 II was used as a marker of autophagosome content [18]. Preventing the exercise induced increase of SOD2 resulted in a significant increase in diaphragmatic levels of LC3 II following prolonged MV (Fig. 5F).
Fig. 5.
Diaphragm proteolytic pathways are increased in animals lacking the exercise-induced increase in SOD2. A) Diaphragm αII-spectrin degradation was used as an assessment of calpain activity from experiment 2. Representative image of αII-spectrin and total protein, n = 8/group, B) αII-spectrin protein degradation from experiment 2, n = 8/group, C) MuRF1 mRNA expression from experiment 2, n = 6/group, D) Atrogin-1 mRNA expression, n = 6/group, E) Diaphragm LC3 II was used as a marker of autophagosome formation in experiment 2. Representative image of LC3 II and total protein, F) LC3 II protein from experiment 2, n = 8. Values are mean percent change of control ± SE. * p < 0.05 different from CON, # p < 0.05 different from MVEx, θ p < 0.05 different from MV.
3.3. Experiment 2
3.3.1. Biological responses to mechanical ventilation
No significant differences existed in heart rates, blood gases, and blood pressure between the experimental groups at the conclusion of the experimental protocols (Table 2). Even though we have demonstrated previously that VIDD is not caused by barotrauma [6], we visually examined the lungs for signs of damage and the peritoneal cavity for evidence of infection. Our examination revealed no visual evidence of barotrauma in the lungs and no evidence of infection existed following the 12-h MV protocol.
Table 2.
At the conclusion of the experimental protocols in experiment 2, no significant differences existed between experimental groups in body weight, heart rate (HR), arterial partial pressure of carbon dioxide (PaCO2), arterial pressure of oxygen (PaO2), arterial pH, and systolic blood pressure (SBP). Data presented as mean ± standard deviation.
| CON | MV | SOD2 OvExp | MVSOD2 OvExp | |
|---|---|---|---|---|
| Weight (g) | 250 ± 20 | 255 ± 18 | 255 ± 18 | 258 ± 9 |
| HR | 346 ± 22 | 358 ± 28 | ||
| PaCO2(mmHg) | 36 ± 7.9 | 39 ± 3.8 | ||
| PaO2(mmHg) | 86 ± 19 | 78 ± 8.4 | ||
| pH | 7.47 ± 0.09 | 7.44 ± 0.03 | ||
| SBP (mmHg) | 134 ± 29 | 138 ± 20 |
3.3.2. AAV9-mediated overexpression of SOD2 protein abundance and activity in the diaphragm
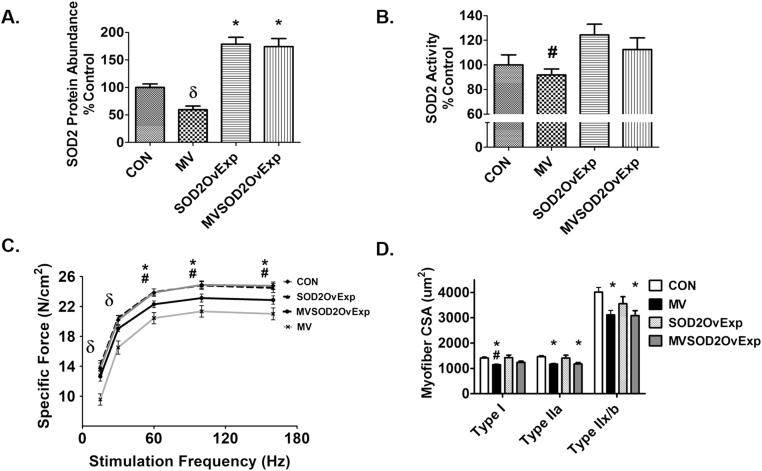
Four weeks prior to MV, diaphragms underwent viral transduction with an AAV9 to overexpress SOD2. Fig. 6 demonstrates that diaphragm transduction with AAV9-SOD2 increased SOD2 protein abundance compared to control and mechanically ventilated sham treated animals (Fig. 6A). In addition, SOD2 activity was significantly increased in control animals overexpressing SOD2 compared to MV animals (Fig. 6B).
Fig. 6.
Protein abundance of successful SOD2 overexpression in the diaphragm as well as activity, diaphragm specific force, and cross-sectional area. A) ELISA of SOD2 protein abundance, n = 9/group. B) SOD2 activity, n = 7/group. Values are mean percent change of control ± SE. * p < 0.05 different from CON, # p < 0.05 different from SOD2OvExp, δ p < 0.05 different from all groups. C) Diaphragm specific force n = 10/group. Values are mean ± SE. * p < 0.05 MV different from CON, # p < 0.05 MV different from SOD2OvExp, δ p < 0.05 MV different from all groups. D) Diaphragm cross-sectional area, n = 10/group. Values are mean ± SE. * p < 0.05 different from CON, # p < 0.05 different from SOD2OvExp.
3.3.3. SOD2 overexpression in the diaphragm partially prevents MV-induced contractile dysfunction
To determine if an increase in SOD2 alone is sufficient to protect against MV-induced diaphragm contractile dysfunction, we measured diaphragm force-frequency response in vitro from strips of costal diaphragm muscle. Importantly, overexpression of SOD2 in the diaphragm does not affect contractile function compared to control animals. In agreement with Experiment 1b, MV resulted in a significant reduction in diaphragm muscle specific force production. Finally, transgenic overexpression of SOD2 in the diaphragm partially attenuated the MV-induced decrease in specific force (Fig. 6C).
3.3.4. Overexpression of SOD2 does not protect the diaphragm against MV-induced fiber atrophy
To determine if SOD2 overexpression in the diaphragm is protective against MV-induced myofiber atrophy, we measured costal diaphragm myofiber CSA. Although transgenic overexpression of SOD2 alone in the diaphragm did protect against MV-induced atrophy of type I muscle fibers, overexpression of SOD2 did not protect against MV-induced diaphragmatic atrophy in either type IIa or type IIb/x fibers (Fig. 6D).
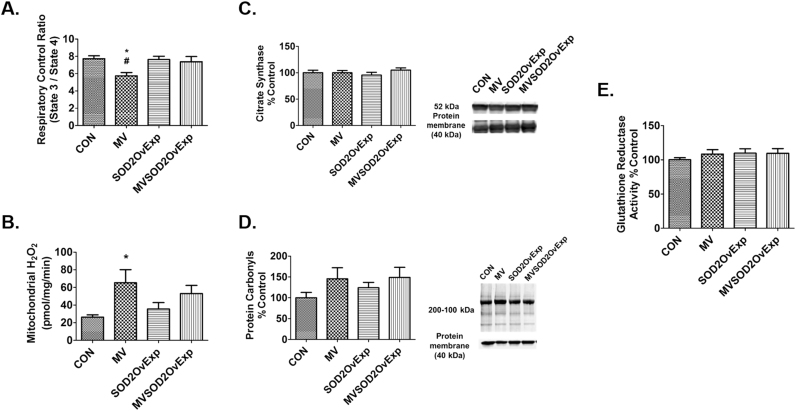
3.3.5. SOD2 overexpression in the diaphragm provides partial protection against MV-induced mitochondrial uncoupling
To determine if SOD2 overexpression in the diaphragm is protective against MV-induced mitochonodrial dysfunction, mitochondrial respiration was measured in conditions of both state 3 and state 4. The MV-induced decrease in RCR was partially protected in MV animals overexpressing SOD2 (Fig. 7A).
Fig. 7.
Impact of MV on mitochondrial volume, respiration, hydrogen peroxide emission, glutathione reductase, and protein carbonyls in diaphragm. A) Respiratory control ratio (RCR) calculated by mitochondrial state 3 respiration/state 4 respiration, n = 9/group. B) Mitochondrial hydrogen peroxide emission, n = 7/group. Values are mean ± SE. * p < 0.05 different from CON, # p < 0.05 different from SOD2OvExp. C) Diaphragm citrate synthase levels in diaphragm muscle used as a marker of mitochondrial volume, n = 10/group. D) Protein carbonyls, n = 10/group. E) Glutathione reductase activity determined colorimetrically by measuring the rate of NADPH oxidation in diaphragm samples, n = 7/group. Values are mean percent change of control ± SE.
3.3.6. Overexpression of SOD2 in the diaphragm does not protect against MV-induced increases in mitochondrial ROS emission
Consistent with previous findings, prolonged MV results in a marked increase in mitochondrial ROS production in diaphragm fibers [26]. Transgenic overexpression of SOD2 in the diaphragm did not alter mitochondrial ROS emission in either control animals or animals exposed to prolonged MV (Fig. 7B).
3.3.7. Overexpression of SOD2 in the diaphragm does not impact glutathione reductase activity
Glutathione reductase plays a key role in maintaining adequate levels of reduced glutathione in the cell by converting oxidized glutathione to reduced glutathione. Importantly, overexpression of SOD2 in the diaphragm did not alter glutathione reductase activity in the diaphragm (Fig. 7E).
3.3.8. Overexpression of SOD2 does not impact diaphragmatic abundance of key antioxidant enzymes
To determine if transgenic overexpression of SOD2 influenced the abundance of key antioxidant enzymes in the diaphragm, we measured the protein abundance of three primary antioxidant enzymes. Our results reveal that overexpression of SOD2 in the diaphragm did not impact diaphragm levels of SOD1, Gpx1, and CAT (Fig. 8A-D).
Fig. 8.
Impact of MV on diaphragm antioxidant enzymes, probed for on separate membranes. A) Representative images of diaphragm antioxidant proteins. B) Superoxide dismutase 1 (SOD1). C) Glutathione peroxidase 1 (Gpx1). D) Catalase (CAT). n = 10/group in all graphs. Values are mean percent change of control ± SE.
3.3.9. Overexpression of SOD2 in the diaphragm partially prevents increased proteolysis
Prolonged MV results in increased ubiquitin ligase mRNA in the diaphragm; importantly, the current study demonstrates that overexpression of SOD2 in the diaphragm partially protects against MV-induced increases in mRNA expression of the E3 ubiquitin ligases MuRF1 and Atrogin-1 (Fig. 9C-D). Also, overexpression of SOD2 in the diaphragm prior to MV did not prevent a rise in LC3 II protein content in the diaphragm (Fig. 9E-F). Taken collectively, these data suggest that SOD2 plays a role in MuRF1 and Atrogin-1 mRNA.
Fig. 9.
Transgenic overexpression of SOD2 in the diaphragm alters proteolytic pathways. A) Diaphragm αII-spectrin degradation was used as an assessment of calpain activity from experiment 2, Representative image of αII-spectrin and total protein, n = 8/group. B) αII-spectrin protein degradation from experiment 2, n = 8/group. C) MuRF1 mRNA expression from experiment 2, n = 6/group. D) Atrogin-1 mRNA expression, n = 6/group. E) Diaphragm LC3 II was used as a marker of autophagosome formation in experiment 2. Representative image of LC3 II and total protein. F) LC3 II protein from experiment 2 n = 8/group. Values are mean percent change of control ± SE. *p < 0.05 different from CON, # p < 0.05 different from SOD2OvExp, δ p < 0.05 different from all groups.
4. Discussion
4.1. Overview of principal findings
The experiments tested two related hypotheses: 1) endurance exercise-induced protection against VIDD occurs, in part, due to increases in SOD2 in the diaphragm; and 2) overexpression of SOD2 in the diaphragm, independent of exercise, provides protection against VIDD. Our findings support the hypothesis that increases in diaphragmatic SOD2 play a key role in exercise-induced protection against VIDD. In contrast, transgenic overexpression of SOD2 alone in the diaphragm provides only partial protection against VIDD. These novel findings provide new insight on the mechanisms mediating exercise-protection against VIDD. A critique of our experimental approach and a detailed discussion of our findings follows.
4.2. Critique of experimental model
Due to the invasive nature of these studies, our experiments were performed using a preclinical animal model of MV. The rat was selected as the experimental model because the rat diaphragm shares both anatomic and muscle fiber type similarities with human diaphragm [22], [25]. Importantly, prolonged MV results in similar rates of diaphragmatic atrophy and contractile dysfunction in rats and humans [30].
The current experiments investigated mitochondrial function and ROS in a permeabilized fiber preparation. While this preparation possesses the advantage of studying mitochondrial function within their normal reticulum formation within muscle fibers, there are also limitations with this experimental approach. ROS generated from succinate-induced conditions is a supraphysiological consequence of electron backflow from high complex II activity with low complex I activity in the absence of ADP, which does not happen in vivo [20]. However, measurement of hydrogen peroxide in the exogenous buffer does not reveal whether the primary site of hydrogen peroxide production is the subsarcolemma or intermyofibrillar mitochondria. Further, our permeabilized fiber preparation cannot determine if ROS measured in the exogenous buffer is exclusively from mitochondria or other sources (e.g., NADPH oxidase). Nevertheless, previous experiments from our group have demonstrated that the mitochondria are the major sources of hydrogen peroxide production during MV [26].
4.3. Exercise-induced increases in SOD2 in the diaphragm are required to achieve the full benefit of exercise-induced protection against VIDD
Our results provide the first evidence that an exercise-induced increase in SOD2 is an essential link in the chain of exercise-induced changes in diaphragm fibers that provide protection against MV-induced diaphragm contractile dysfunction, fiber atrophy, and calpain activation. Hence, these findings support our prediction that SOD2 is a critical player in the exercise-induced protection against VIDD. This hypothesis evolved from our previous work revealing that MV-induced oxidative stress in the diaphragm is essential for the development of VIDD (reviewed in 30 [30]). More specifically, increased mitochondrial ROS emission in the diaphragm is a required upstream trigger to promote both accelerated proteolysis and contractile dysfunction in the diaphragm during prolonged MV [26]. Therefore, we postulated that exercise-induced increases in mitochondrial antioxidant defenses (i.e., SOD2) would lower MV-induced mitochondrial ROS emission and protect against MV-induced diaphragmatic atrophy and contractile dysfunction. Our results support this prediction and demonstrate that increased SOD2 abundance in diaphragm fibers is essential to achieve the full benefit of exercise-induced protection against MV-induced contractile dysfunction [34]. Specifically, prevention of the exercise-induced increase in SOD2 abundance in the diaphragm attenuated exercise-mediated protection against MV-induced diaphragm contractile dysfunction. We interpret this finding as evidence that while an increase in diaphragmatic SOD2 contributes to exercise-induced protection against MV-induced contractile dysfunction, the increase in SOD2 abundance functions in concert with other cytoprotective molecules in mediating exercise-induced protection against MV-induced diaphragm contractile dysfunction.
In regard to other potential cytoprotective molecules, exercise training also increases diaphragmatic levels of the chaperone protein heat shock protein 72 (HSP72) [34]. This is potentially important because previous experiments have demonstrated that HSP72 overexpression alone prevents disuse muscle atrophy in locomotor skeletal muscles [32]. Therefore, it is feasible that exercise-induced protection against MV-induced diaphragm contractile dysfunction occurs via a combination of exercise-induced increases in cytoprotective molecules. Hence, additional research on this topic is required to determine if increases in SOD2 act in concert with increases in HSP72 to provide the exercise-induced protection against MV-induced impairments in diaphragm contractile properties.
To investigate the mechanisms responsible for the exercise-induced protection against MV-induced proteolysis and diaphragm atrophy conferred by increased SOD2, we examined numerous biomarkers of proteolysis in the diaphragm (calpain activity, ubiquitin ligases MuRF1 and Atrogin-1) and diaphragm fiber CSA. Based on our previous research in exercise-induced cardioprotection [11], [14], we hypothesized that exercise-induced increases in SOD2 in the diaphragm are required to provide protection against MV-induced proteolysis and diaphragm fiber atrophy.
Our results support the conclusion that increases in diaphragmatic SOD2 are important for the prevention of MV-induced calpain activation and increased E3 ubiquitin ligase mRNA expression in the diaphragm. This is significant because previous experiments reveal that pharmacological inhibition of calpain activation protects the diaphragm against MV-induced atrophy [21]. In the current study, we demonstrate that exercise training prevents MV-induced calpain activation in the diaphragm and that this protection against calpain activation is abolished without an exercise-induced increase in diaphragmatic levels of SOD2. Additionally, prevention of the exercise-induced increase in SOD2 abolished the exercise-induced protection against elevated MuRF1 and Atrogin-1 mRNA expression in diaphragms from animals exposed to prolonged MV. Together, these results indicate that increased SOD2 is required for the exercise-induced protection against MV-induced increases in proteolysis in the diaphragm. A logical link between increased SOD2 in the diaphragm fibers and a reduction in MV-induced calpain activation is that oxidative stress promotes increased levels of cytosolic free calcium and therefore, increased calpain activation [13]. It follows that an increase in diaphragmatic levels of SOD2 likely protects against oxidative stress, calcium dysregulation, and calpain activation in the diaphragm during prolonged MV.
4.4. Transgenic overexpression of SOD2 alone in the diaphragm provides limited protection against VIDD
Although increases in diaphragmatic SOD2 contribute to exercise-induced protection against VIDD, our findings indicate that overexpression of SOD2 does not provide the level of protection against VIDD afforded by endurance exercise training. Indeed, overexpression of SOD2 alone in the diaphragm provides only partial protection against MV-induced diaphragm contractile dysfunction. This finding is consistent with the observation that prevention of the exercise-induced increase of SOD2 in the diaphragm attenuates the exercise protection against MV-induced diaphragm contractile dysfunction. Although the mechanisms responsible for this SOD2-mediated protection are not clear, it is likely that increases in diaphragmatic SOD2 defend against MV-induced oxidative modification of contractile proteins in diaphragm fibers and the resultant contractile dysfunction.
Moreover, our findings reveal that transgenic overexpression of SOD2 alone does not prevent MV-induced diaphragm proteolysis and atrophy. Indeed, microtubule-associated protein light chain 3 II (LC3 II) is a marker of autophagosome content [18] and is markedly increased in the diaphragm of animals exposed to MV; nonetheless, transgenic overexpression of SOD2 did not prevent this MV-induced increase in LC3 II. Further, muscle specific E3 ubiquitin ligases MuRF1 and Atrogin-1 expression was significantly increased in diaphragms of MV animals and transgenic overexpression of SOD2 alone provided only partial protection against MV-induced increases. Together, these results indicate that while increases in diaphragmatic SOD2 contribute to diaphragm protection against VIDD, additional exercise-induced changes in diaphragm phenotype are required to achieve the full exercise-induced protection against MV-induced proteolysis in the diaphragm.
5. Conclusions
These experiments reveal for the first time that the exercise-induced increase in SOD2 is required to achieve the full benefit of exercise-induced protection against VIDD. Additionally, overexpression of SOD2 alone in the diaphragm prior to MV only provides partial protection against VIDD. These findings reveal that although increased expression of SOD2 plays a role in exercise-induced protection against VIDD, an increase in diaphragmatic SOD2 likely works in concert with other cytoprotective molecules to provide the full exercise-induced protection against VIDD.
Acknowledgments
This study was supported by National Institutes of Health Grant R01-AR-064189 (to S. Powers). Aaron B. Morton was supported by a National Institutes of Health Training Grant T32-HD043730.
The authors thank Kelli Morton for her preparation of the graphical abstract.
Acknowledgments
Conflicts of interest
None.
Funding
This work was supported by National Institutes of Health, USA Grant R01-AR-064189 (to S. Powers). Aaron B. Morton was supported by a National Institutes of Health, USA Training Grant T32-HD043730.
References
- 1.Agten A., Maes K., Smuder A., Powers S.K., Decramer M., Gayan-Ramirez G. N-Acetylcysteine protects the rat diaphragm from the decreased contractility associated with controlled mechanical ventilation. Crit. Care Med. 2011;39:777–782. doi: 10.1097/CCM.0b013e318206cca9. [DOI] [PubMed] [Google Scholar]
- 2.Ahn B., Beharry A.W., Frye G.S., Judge A.R., Ferreira L.F. NAD(P)H oxidase subunit p47phox is elevated, and p47phox knockout prevents diaphragm contractile dysfunction in heart failure. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;309:L497–L505. doi: 10.1152/ajplung.00176.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betters J.L., Criswell D.S., Shanely R.A., Van Gammeren D., Falk D., Deruisseau K.C., Deering M., Yimlamai T., Powers S.K. Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. Am. J. Respir. Crit. Care Med. 2004;170:1179–1184. doi: 10.1164/rccm.200407-939OC. [DOI] [PubMed] [Google Scholar]
- 4.Bresciani G., González-Gallego J., da Cruz I.B., de Paz J.A., Cuevas M.J. The Ala16Val MnSOD gene polymorphism modulates oxidative response to exercise. Clin. Biochem. 2013;46:335–340. doi: 10.1016/j.clinbiochem.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Bruells C.S., Marx G., Rossaint R. Ventilator-induced diaphragm dysfunction: clinically relevant problem. Anaesthesiologist. 2014;63:47–53. doi: 10.1007/s00101-013-2248-9. [DOI] [PubMed] [Google Scholar]
- 6.Bruells C.S., Smuder A.J., Reiss L.K., Hudson M.B., Nelson W.B., Wiggs M.P., Sollanek K.J., Rossaint R., Uhlig S., Powers S.K. Negative pressure ventilation and positive pressure ventilation promote comparable levels of ventilator-induced diaphragmatic dysfunction in rats. Anesthesiology. 2013;119:652–662. doi: 10.1097/ALN.0b013e31829b3692. [DOI] [PubMed] [Google Scholar]
- 7.Cader S.A., de Souza Vale R.G., Zamora V.E., Costa C.H., Dantas E.H. Extubation process in bed-ridden elderly intensive care patients receiving inspiratory muscle training: a randomized clinical trial. Clin. Interv. Aging. 2012;7:437–443. doi: 10.2147/CIA.S36937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Candas D., Li J.J. MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxid. Redox Signal. 2014;20:1599–1617. doi: 10.1089/ars.2013.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couto N., Wood J., Barber J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016;95:27–42. doi: 10.1016/j.freeradbiomed.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Falk D.J., Deruisseau K.C., Van Gammeren D.L., Deering M.A., Kavazis A.N., Powers S.K. Mechanical ventilation promotes redox status alterations in the diaphragm. J. Appl. Physiol. 2006;101:1017–1024. doi: 10.1152/japplphysiol.00104.2006. [DOI] [PubMed] [Google Scholar]
- 11.French J.P., Hamilton K.L., Quindry J.C., Lee Y., Upchurch P.A., Powers S.K. Exercise-induced protection against myocardial apoptosis and necrosis: MnSOD, calcium-handling proteins, and calpain. FASEB J. 2008;22:2862–2871. doi: 10.1096/fj.07-102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goll D.E., Neti G., Mares S.W., Thompson V.F. Myofibrillar protein turnover: the proteasome and the calpains. J. Anim. Sci. 2008;86:E19–E35. doi: 10.2527/jas.2007-0395. [DOI] [PubMed] [Google Scholar]
- 13.Goll D.E., Thompson V.F., Taylor R.G., Zalewska T. Is calpain activity regulated by membranes and autolysis or by calcium and calpastatin? Bioessays. 1992;14:549–556. doi: 10.1002/bies.950140810. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton K.L., Quindry J.C., French J.P., Staib J., Hughes J., Mehta J.L., Powers S.K. MnSOD antisense treatment and exercise-induced protection against arrhythmias. Free Radic. Biol. Med. 2004;37:1360–1368. doi: 10.1016/j.freeradbiomed.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 15.Hudson M.B., Smuder A.J., Nelson W.B., Bruells C.S., Levine S., Powers S.K. Both high level pressure support ventilation and controlled mechanical ventilation induce diaphragm dysfunction and atrophy. Crit. Care Med. 2012;40:1254–1260. doi: 10.1097/CCM.0b013e31823c8cc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabitz H.J., Windisch W., Schönhofer B. Understanding ventilator-induced diaphragmatic dysfunction (VIDD): progress and advances. Pneumologie. 2013;67:435–441. doi: 10.1055/s-0033-1344241. [DOI] [PubMed] [Google Scholar]
- 17.Kwon O.S., Smuder A.J., Wiggs M.P., Hall S.E., Sollanek K.J., Morton A.B., Talbert E.E., Toklu H.Z., Tumer N., Powers S.K. AT1 receptor blocker losartan protects against mechanical ventilation-induced diaphragmatic dysfunction. J. Appl. Physiol. 2015;119:1033–1041. doi: 10.1152/japplphysiol.00237.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizushima N., Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 19.Morton A.B., Mor Huertas A., Hinkley J.M., Ichinoseki-Sekine N., Christou D.D., Smuder A.J. Mitochondrial accumulation of doxorubicin in cardiac and diaphragm muscle following exercise preconditioning. Mitochondrion. 2018 doi: 10.1016/j.mito.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller F.L., Liu Y., Abdul-Ghani M.A., Lustgarten M.S., Bhattacharya A., Jang Y.C., Van Remmen H. High rates of superoxide production in skeletal-muscle mitochondria respiring on both complex I- and complex II-linked substrates. Biochem. J. 2008;409:491–499. doi: 10.1042/BJ20071162. [DOI] [PubMed] [Google Scholar]
- 21.Nelson W.B., Smuder A.J., Hudson M.B., Talbert E.E., Powers S.K. Cross-talk between the calpain and caspase-3 proteolytic systems in the diaphragm during prolonged mechanical ventilation. Crit. Care Med. 2012;40:1857–1863. doi: 10.1097/CCM.0b013e318246bb5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen T., Shrager J., Kaiser L., Mei L., Daood M., Watchko J., Rubinstein N., Levine S. Developmental myosin heavy chains in the adult human diaphragm: coexpression patterns and effect of COPD. J. Appl. Physiol. 2000;88:1446–1456. doi: 10.1152/jappl.2000.88.4.1446. [DOI] [PubMed] [Google Scholar]
- 23.Oh S.H., Choi Y.B., Kim J.H., Weihl C.C., Ju J.S. Comparisons of ELISA and Western blot assays for detection of autophagy flux. Data Brief. 2017;13:696–699. doi: 10.1016/j.dib.2017.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pahl P.J. Growth curves for body weight of the laboratory rat. Aust. J. Biol. Sci. 1969;22:1077–1080. doi: 10.1071/bi9691077. [DOI] [PubMed] [Google Scholar]
- 25.Powers S.K., Demirel H.A., Coombes J.S., Fletcher L., Calliaud C., Vrabas I., Prezant D. Myosin phenotype and bioenergetic characteristics of rat respiratory muscles. Med. Sci. Sports Exerc. 1997;29:1573–1579. doi: 10.1097/00005768-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Powers S.K., Hudson M.B., Nelson W.B., Talbert E.E., Min K., Szeto H.H., Kavazis A.N., Smuder A.J. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit. Care Med. 2011;39:1749–1759. doi: 10.1097/CCM.0b013e3182190b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powers S.K., Jackson M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powers S.K., Morton A.B., Ahn B., Smuder A.J. Redox control of skeletal muscle atrophy. Free Radic. Biol. Med. 2016;98:208–217. doi: 10.1016/j.freeradbiomed.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powers S.K., Shanely R.A., Coombes J.S., Koesterer T.J., McKenzie M., Van Gammeren D., Cicale M., Dodd S.L. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J. Appl. Physiol. 2002;92:1851–1858. doi: 10.1152/japplphysiol.00881.2001. [DOI] [PubMed] [Google Scholar]
- 30.Powers S.K., Wiggs M.P., Sollanek K.J., Smuder A.J. Ventilator-induced diaphragm dysfunction: cause and effect. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R464–R477. doi: 10.1152/ajpregu.00231.2013. [DOI] [PubMed] [Google Scholar]
- 31.Schild K., Neusch C., Schönhofer B. Ventilator-induced diaphragmatic dysfunction (VIDD) Pneumologie. 2008;62:33–39. doi: 10.1055/s-2007-980137. [DOI] [PubMed] [Google Scholar]
- 32.Senf S.M., Dodd S.L., McClung J.M., Judge A.R. Hsp70 overexpression inhibits NF-kappaB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J. 2008;22:3836–3845. doi: 10.1096/fj.08-110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smuder A.J., Falk D.J., Sollanek K.J., Nelson W.B., Powers S.K. Delivery of recombinant adeno-associated virus vectors to rat diaphragm muscle via direct intramuscular injection. Hum. Gene Ther. Methods. 2013;24:364–371. doi: 10.1089/hgtb.2013.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smuder A.J., Min K., Hudson M.B., Kavazis A.N., Kwon O.S., Nelson W.B., Powers S.K. Endurance exercise attenuates ventilator-induced diaphragm dysfunction. J. Appl. Physiol. 2012;112:501–510. doi: 10.1152/japplphysiol.01086.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smuder A.J., Sollanek K.J., Nelson W.B., Min K., Talbert E.E., Kavazis A.N., Hudson M.B., Sandri M., Szeto H.H., Powers S.K. Crosstalk between autophagy and oxidative stress regulates proteolysis in the diaphragm during mechanical ventilation. Free Radic. Biol. Med. 2018;115:179–190. doi: 10.1016/j.freeradbiomed.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sollanek K.J., Burniston J.G., Kavazis A.N., Morton A.B., Wiggs M.P., Ahn B., Smuder A.J., Powers S.K. Global proteome changes in the rat diaphragm induced by endurance exercise training. PLoS One. 2017;12:e0171007. doi: 10.1371/journal.pone.0171007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sollanek K.J., Smuder A.J., Wiggs M.P., Morton A.B., Koch L.G., Britton S.L., Powers S.K. Role of intrinsic aerobic capacity and ventilator-induced diaphragm dysfunction. J. Appl. Physiol. 2015;118:849–857. doi: 10.1152/japplphysiol.00797.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas D., Maes K., Agten A., Heunks L., Dekhuijzen R., Decramer M., Van Hees H., Gayan-Ramirez G. Time course of diaphragm function recovery after controlled mechanical ventilation in rats. J. Appl. Physiol. 2013;115:775–784. doi: 10.1152/japplphysiol.00302.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]