Abstract
Context
Progestin-based therapy is the first-line treatment for managing endometriosis-associated pain. However, response to progestins is currently variable and unpredictable. Predictive markers for response to progestin-based therapy would allow for a personalized approach to endometriosis treatment.
Objective
We hypothesize that progesterone receptor (PR) levels in endometriotic lesions determine response to progestin-based therapy.
Design
Retrospective cohort study.
Setting
Academic center.
Patients
Fifty-two subjects with histologically confirmed endometriosis and a previous documented response to hormonal therapy were included.
Interventions
Immunohistochemistry was performed on sections of endometriotic lesions using a rabbit polyclonal IgG for detection of PR-A/B.
Main Outcome Measures
The Histo (H)-score was used for quantifying PR status. Response to progestin-based therapies was determined from review of the electronic medical record.
Results
H-score was higher in responders compared with nonresponders. Subjects were categorized into three groups: high (H-score > 80, n = 7), medium (H-score 6 to 80, n = 28), and low (H-score ≤ 5, n = 17) PR status. The threshold of PR > 80 was associated with a 100% positive predictive value. The threshold of PR < 5 was associated with a 94% negative predictive value.
Conclusion
PR status is strongly associated with response to progestin-based therapy. Receptor status in endometriosis could be used to tailor hormonal-based regimens after surgery, and negate trialing progestin-based therapy to determine resistance. Ascertainment of PR status may allow for a novel, targeted, precision-based approach to treating endometriosis.
Progesterone receptor levels are strongly associated with response to progestin-based therapy and may allow for a novel, targeted, precision-based approach to treating endometriosis.
Endometriosis is a chronic gynecologic disease affecting approximately one in 10 reproductive-aged women and up to 50% to 60% of women with pelvic pain or unexplained infertility (1). It is characterized by endometrial-like tissue outside of the uterus, most frequently on the pelvic viscera and peritoneum (1). Endometriosis varies in appearance, from few minimal lesions on otherwise intact pelvic organs to large ovarian endometriotic cysts and deep infiltrating nodules (2). Women with endometriosis suffer from pelvic pain, dysmenorrhea, dyspareunia, and infertility (1). Endometriotic lesions undergo cycles of growth and bleeding in tandem with the menstrual cycle, explaining the typical cyclic exacerbation of symptoms. These debilitating symptoms severely impact the quality of life of women with endometriosis (3).
Although the etiology of endometriosis remains largely unknown, estrogen’s role in promoting the growth and progression of the disease is well characterized and demonstrated by several clinical observations (4). Endometriosis predominantly affects women during the reproductive phase of life and regresses after menopause, and the administration of estrogen-containing replacement therapy may cause relapse of the disease (2). In premenopausal women, the suppression of estradiol levels causes regression of endometriotic lesions and improvement of pain symptoms (5, 6). The recovery of estradiol levels after discontinuation of therapy is associated with relapse of the disease, underscoring the estrogen-dependent nature of endometriosis (5, 7).
Progesterone acts by regulating endometrial decidualization and inhibition of estrogen-driven endometrial proliferation (8, 9). Although serum levels of progesterone in women with endometriosis are similar to those of women without the disease, endometriotic lesions (ectopic endometrium) do not respond appropriately to progesterone (10, 11). The inappropriate response to progesterone (i.e., progesterone resistance) in endometriotic lesions explains the impaired efficacy of progestin-based therapies for endometriosis management (8, 12). Endometriotic lesions have altered expression of the progesterone receptor (PR) (12, 13). Specifically, it has been postulated that progesterone resistance is mediated by lower levels of PR (8, 14). Low PR may explain why progestin-containing agents [including combined oral contraceptives (OCs)] are associated with treatment failure in some patients (8, 14).
Developing an individualized approach to not only treat endometriosis, but also predict response, is needed. As such, the aim of our study was to first characterize PR status in endometriotic lesions. Secondly, we aimed to develop a scoring system based on PR levels for prediction of response to progestin-based therapy. We hypothesized that expression levels of PR in endometriotic lesions can be used to predict response to progestin-based agents. The development of a scoring system based on PR levels for prediction of response to progestin-based therapy could allow for an individualized, precision-based approach to the treatment of endometriosis.
Methods
We conducted a retrospective cohort study utilizing endometriotic lesions obtained from women undergoing surgical evaluation for endometriosis at Yale New Haven Hospital. The study was approved by Yale University’s Institutional Review Board. Inclusion criteria were: women with histologically confirmed endometriosis, available information regarding endometriosis-associated pain symptoms, and information regarding response to progestin-based therapy. Subjects were excluded if data regarding response to progestin-based therapy were missing or insufficient. Data regarding subject characteristics and response to progestin-based therapies were determined from the electronic medical record. Fifty-seven subjects met the inclusion criteria. Five subjects were excluded due to insufficient data on response to progestin-based therapy. As such, 52 subjects were included in the analysis. Of the 52 subjects, 21 had more than one lesion collected at the time of surgery. In 17 subjects, eutopic endometrium was also collected at the time of surgery. Each subject was considered the biologic variable using the lesion with the highest Histo (H)-score for further analysis.
Immunohistochemistry
Tissue was embedded in paraffin, cut into 5-μm sections, and mounted onto slides. Immunohistochemical analysis of PR expression was performed as previously described (15–17). Briefly, slides were deparaffinized and dehydrated through a series of xylene and ethanol washes. After a 5-minute rinse in distilled water, slides were steamed in 0.01 M sodium citrate buffer for 15 minutes and cooled for 45 minutes. Slides were rinsed for 5 minutes in PBS with 0.1% Tween 20 (PBST), and sections were circumscribed with a hydrophobic pen. Endogenous peroxidase was quenched with 3% hydrogen peroxide for 5 minutes followed by a 5-minute PBST wash. Nonspecific binding was blocked with 5% normal goat serum in PBST for 1 hour at room temperature. The primary antibody used was PR H-190 (sc _7208; 1:800) and was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Slides were incubated with the primary antibody overnight at 4°C. Normal goat IgG (Santa Cruz Biotechnology) was used as a negative control; normal day-14 endometrium was used as a positive control. Goat antirabbit biotinylated secondary antibody was used for PR (Vector Laboratories, Burlingame, CA) and applied for 1 hour at room temperature. Slides were washed in 1× PBS, incubated in ABC Elite (Vector Laboratories) for 30 minutes at room temperature, washed in 1× PBS, and incubated for 41 seconds with diaminobenzidine (Vector Laboratories). A 30-second exposure to hematoxylin was used as a counterstain. Slides were rehydrated through 5-minute ethanol and xylene washes and mounted with Permount (Thermo Fisher Scientific, Waltham, MA).
An H-score for immunohistochemical staining was determined for each slide. Each slide was scored independently by two investigators blinded to subject response, and H-scores were averaged. The H-score was calculated using a modified H-score: the percentage of negative (score 0), weakly positive (score 1), positive (score 2), and strongly positive (score 3) cells were estimated, and the percentages were multiplied with the scores and summed (18).
Statistical analysis
Subject characteristics from the response and no response group were compared with an unpaired Student t test (continuous variables), Fisher exact test, or χ2 test (categorical variables). H-scores were compared using an unpaired Student t test. R Programming version 3.5.1 and prebuilt irr (Various Coefficients of Interrater Reliability and Agreement) package (https://cran.r-project.org/web/packages/irr/index.html) were used to calculate the intraclass correlation coefficient between the two blinded investigators. Thresholds for categorization of PR status were based on receiver operating characteristic (ROC) curve analysis. A χ2 test was used for analysis of the final contingency table for prediction of response to progestin therapy. Spearman rank correlation coefficient was used for analysis of matched eutopic and ectopic lesions. All analyses were two-sided, and a P value of ≤ 0.05 was considered significant.
Results
We identified 57 subjects who fulfilled the inclusion criteria of our study (histological confirmation of endometriosis, minimum one lesion available for immunohistochemistry, previous use of medical treatment of endometriosis). The electronic medical record of subjects was analyzed for information on the response to medical therapy. Five subjects were excluded because of missing or insufficient data. Out of 52 subjects included for analysis, 14 had responded to a progestin-based medical therapy, most commonly an OC, in the past, and 38 had inadequate or no response to therapy. No subject had used dienogest, as this study was conducted entirely in the United States, where dienogest is not available. Of the 52 included subjects, 21 had more than one lesion collected at the time of surgery, and 17 had eutopic endometrium collected at the time of laparoscopy. Demographic and clinical data of the 52 subjects included for analysis are shown in Table 1. The demographics, medical history, and characteristics of endometriosis were similar for the response and no response group except for current use of medication (Table 1).
Table 1.
Subject Characteristics
| Response | No Response | P Value | |
|---|---|---|---|
| Demographics | |||
| Age (mean ± SD) | 34 ± 6 | 30 ± 8 | 0.05 |
| BMI (mean ± SD) | 24 ± 4 | 26 ± 7 | 0.24 |
| Smoking | 0/14 (0%) | 2/38 (5%) | 0.99 |
| Family history of endometriosis | 4/14 (29%) | 9/38 (24%) | 0.73 |
| Ethnicity | 0.94 | ||
| White | 12/14 (86%) | 31/38 (82%) | |
| Hispanic | 1/14 (7%) | 3/38 (8%) | |
| African-American | 1/14 (7%) | 3/38 (8%) | |
| Asian | 0/14 (0%) | 1/38 (3%) | |
| Medical history | |||
| Nulligravids | 9/14 (64%) | 25/38 (66%) | 0.99 |
| Caesarean section | 1/14 (7%) | 5/38 (13%) | 0.99 |
| Previous surgery for endometriosis | 4/14 (29%) | 19/38 (50%) | 0.22 |
| Comorbidities | |||
| Depression | 3/14 (21%) | 7/38 (18%) | 0.99 |
| Anxiety | 1/14 (7%) | 8/38 (21%) | 0.42 |
| Adenomyosis | 1/14 (7%) | 4/38 (11%) | 0.99 |
| PCOS | 0/14 (0%) | 4/38 (11%) | 0.56 |
| Fibroids | 2/14 (14%) | 3/38 (8%) | 0.60 |
| IBS | 3/14 (21%) | 2/38 (5%) | 0.11 |
| Endometriosis | |||
| Pain | 14/14 (100%) | 38/38 (100%) | 0.99 |
| Currently on medication | 4/14 (29%) | 35/38 (92%) | 0.0001 |
| Progestins | 0/4 (0%) | 10/35 (29%) | |
| COC | 3/4 (75%) | 22/35 (63%) | |
| GnRHa + add back | 1/4 (25%) | 2/35 (6%) | |
| AI | 0/4 (0%) | 1/35 (3%) | |
| ASRM stagea | 0.25 | ||
| Stage 1 | 5/14 (36%) | 20/38 (53%) | |
| Stage 2 | 1/14 (7%) | 6/38 (16%) | |
| Stage 3 | 7/14 (50%) | 8/38 (21%) | |
| Stage 4 | 1/14 (7%) | 3/38 (8%) | |
| Phenotype | |||
| Peritoneal | 14/14 (100%) | 36/38 (95%) | 0.99 |
| Endometrioma | 7/14 (54%) | 9/38 (24%) | 0.09 |
| Deep endometriosis | 1/14 (8%) | 6/38 (15%) | 0.66 |
Abbreviations: AI, aromatase inhibitor; ASRM, American Society for Reproductive Medicine; COC, combined oral contraceptive; GnRHa + add back, leuprolide acetate plus norethindrone or conjugated estrogens/medroxyprogesterone; IBS, irritable bowel syndrome; PCOS, polycystic ovary syndrome.
One subject could not be staged as she did not have laparoscopy at time of cesarean section scar removal.
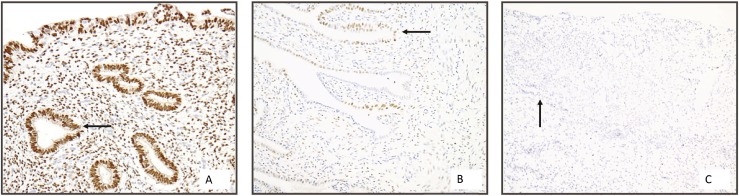
Representative images of immunohistochemical staining for PR expression, as calculated by H-score, are shown in Fig. 1. For all H-scores, the intraclass correlation coefficient score between the two blinded investigators was 0.985 (P < 0.0001; 95% CI: 0.978 < intraclass correlation coefficient < 0.99).
Figure 1.
PR immunohistochemistry. A to C, Representative images of PR expression in endometriotic lesions. PR expression quantified using H-score: A, high PR staining; B, medium PR staining; C, low PR staining. Arrows denote glandular epithelium (magnification ×20).
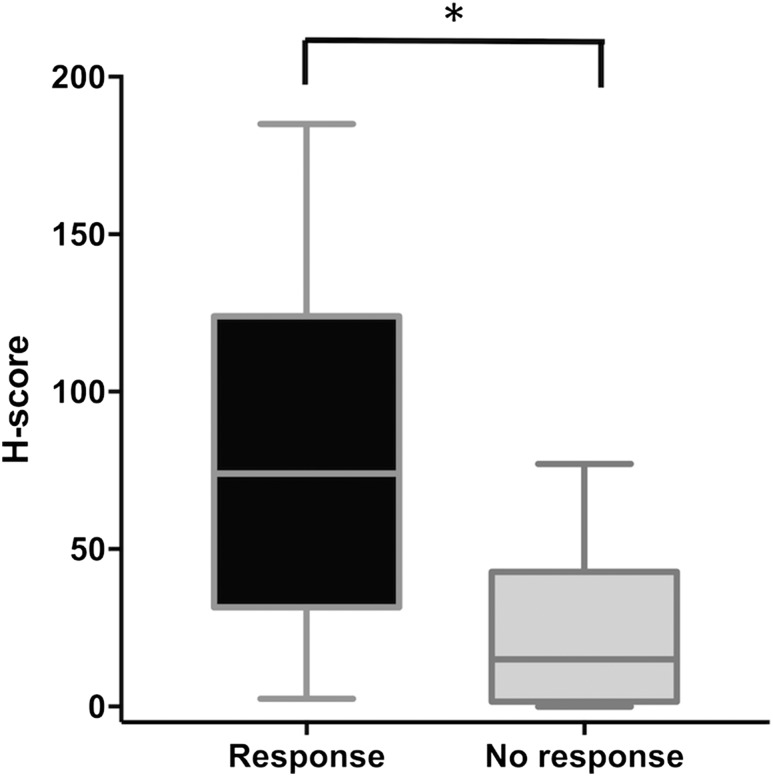
The H-score was significantly higher in responders compared with nonresponders (P < 0.0001, Fig. 2). The ROC curve for prediction of response based on H-score showed an area under the curve of 84% (95% CI: 71% to 96%; P = 0.002).
Figure 2.
H-scores in the response and no response subjects. Comparison of H-scores in responders and nonresponders. Data are shown as box plots (median and interquartile range 25th to 75th percentiles) and whisker plots (minimum to maximum). H-scores were higher in the response group (*P < 0.0001).
Based on ROC curve analysis with a two-threshold strategy, subjects were categorized into three groups: high (H-score ≥ 80, n = 7), medium (H-score 6 to 80, n = 28), and low (H-score ≤ 5, n = 17) PR status (Fig. 1). The threshold of H-score > 5 was selected because of its high sensitivity (93%, 95% CI: 69% to 100%) and negative predictive value (94%, 95% CI: 73% to 100%). The threshold of H-score ≥ 80 was selected because of its high specificity (100%, 95% CI: 91% to 100%) and positive predictive value (100%, 95% CI: 65% to 100%).
Contingency table analysis showed that PR status was significantly associated with response to progestin therapy (P < 0.0001; Table 2). All subjects with high PR responded to progestin therapy (100%). Ninety-four percent of subjects in the low PR group did not respond to progestin therapy. The medium PR group had a response rate of 21%, in line with the overall response rate of 27%.
Table 2.
Prediction of Response Using PR Status
| High PR | Medium PR | Low PR | |
|---|---|---|---|
| No Response | 0 | 22 | 16 |
| Response | 7 | 6 | 1 |
| Response Rate | 100% | 21% | 6% |
In subjects with more than one lesion collected at the time of surgery, the number of additional lesions ranged from 1 to 6, with a mean of 2.8 lesions per subject. There was intrasubject variation between lesions, however with only two subjects in the responder group having a lesion with both a high and low H-score value.
Of the 17 subjects with eutopic endometrium collected at time of laparoscopy, 5 subjects were responders, while 12 were nonresponders. The eutopic endometrium in all 5 responders had H- scores > 80. In 5 of 12 nonresponders, the H-scores in the eutopic endometrium were also >80, compared with H-scores < 80 in the ectopic lesions (P = 0.17, r = 0.4). High PR expression in eutopic endometrium does not assure high PR in the ectopic lesion or response to therapy.
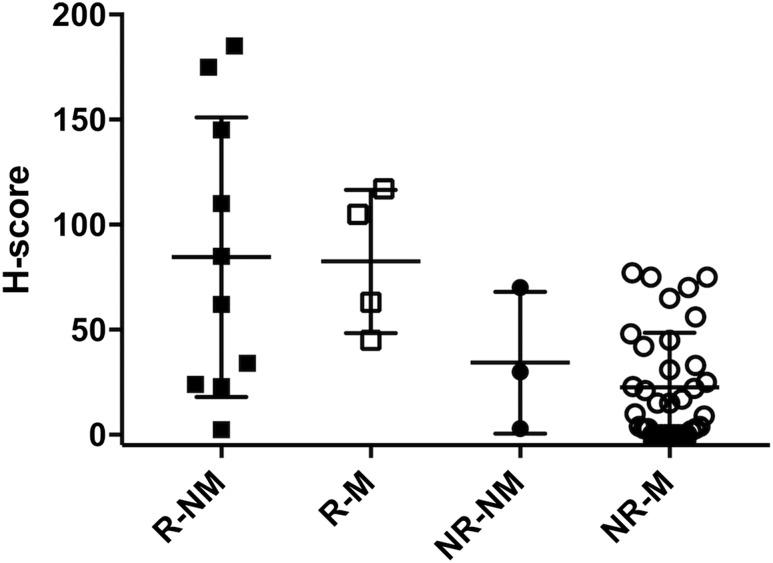
The proportion of subjects on hormonal medication at the time of surgery was different in the response vs the no response group (Table 1). In the no response group, more subjects were using hormonal medication at the time of surgery, while in the response group, more subjects were not using medication at the time of surgery. Therefore, we performed a subgroup analysis for this possible confounder using a Mann-Whitney U test. In both the response and no response group, there was no significant difference in H-scores between subjects using or not using hormonal medication (respective P values: 0.84 and 0.45; Fig. 3).
Figure 3.
H-scores in responders and nonresponders by medication use. Subgroup analysis of current medication use at time of surgery, represented as a scatter plot. Error bars represent the group mean ± SD. There was no significant difference in H-scores in the response and no response group with respect to medication use at time of surgery. NR-M, no response on medication; NR-NM, no response not on medication; R-M, response on medication; R-NM, response not on medication.
Discussion
In this study, we have shown that PR status is strongly associated with response of endometriosis to progestin-based therapies (including combined OCs). Subjects who did not respond to progestin-based therapies had significantly lower PR levels than subjects who did respond. These data support the notion that PR levels are an important modulator of progesterone resistance in endometriosis.
Our data confirm previous findings that PR levels are altered in endometriotic lesions (19–21). Attia et al. (12) were the first to report lower expression of PR in ectopic endometrium compared with paired eutopic endometrium. When comparing ectopic endometrium of women with endometriosis to the eutopic endometrium of controls, PR has also been found to be lower in endometriotic lesions (14, 22). Utilizing endometriosis tissue microarray and immunohistochemistry, Colon-Caraballo et al. (23) found that there were differences in PR expression levels in endometriotic lesions across different subjects’ samples. Similarly, PR levels in deep infiltrating endometriosis is variable among different subjects’ samples (24). In our subject cohort, we found important intersubject and intrasubject variation in the PR levels of endometriotic lesions. Given the role of PR levels in progesterone resistance, and variation of PR levels in endometriotic lesions, this raises the possibility of using PR levels for individual tailoring of hormonal therapy.
Unlike our study, prior work has not assessed the ability of PR status to predict response to progestin-based therapy. We previously demonstrated that PR levels in the eutopic endometrium of endometriosis subjects differ and predicted that PR status would also be variable in ectopic lesions (25). We have further built upon our previous work by using ectopic endometrium and comparing PR status in lesions to subjects’ response to progestin-containing agents.
A major strength of our study is that, for a subset of subjects (n = 21), we included multiple endometriotic lesions in the development of the classification thresholds. In subjects with multiple lesions, we observed variation in PR levels, with values in the high, medium, and low range. In responders with H-scores > 80, only two had an additional lesion with an H-score < 6, which would have resulted in misclassification of these two subjects had analysis been limited to a single sample. As we had a binary outcome for response/no response, in responders we would expect improvement in symptoms in the presence of some PR-expressing lesions. Because all subjects with at least one lesion in the high PR group responded to progestin-based therapies, we found the H-score threshold of ≥80 to be a very strong positive predictor of response to progestin-based therapy. Thus, we chose to use the highest H-score in our model, to avoid the potential for misclassification. We did find that one subject in the clinical response group was classified as a nonresponder by H-score. As this patient had only one lesion removed at the time of laparoscopy, there may have been an unbiopsied lesion with high PR, explaining her prior response to progestin-based therapy despite an H-score < 6. Given this variability and at times discrepancy in H-score and response, we recommend sampling several lesions per patient, as having multiple lesions available for analysis will help prevent inappropriate categorization of patients. Sampling multiple lesions and utilizing the highest H-score avoids any subject being incorrectly classified.
One novelty of our study lies in the double threshold approach to classify PR levels, allowing identification of subjects very likely to respond (high H-score group) and very unlikely to respond (low H-score group) to progestin based-therapy. As such, in nearly half of subjects, a clear prediction of response (negative or positive) to therapy can be made, and management can be adjusted appropriately. In addition, given an intraclass correlation coefficient of 0.985 between the two blinded investigators, the H-score holds potential as a reliable scoring system for determining PR expression levels. As this scoring system will be used exclusively after surgery, the existing clinical paradigm of trialing patients on OCs preoperatively remains. In patients not responding to progestins, options include undergoing surgical evaluation, or alternative hormonal therapy. Utilizing PR expression of excised lesions allows the provider to determine the reason for failed response to OCs. For example, lesions may be predominantly adhesions/fibrosis, which are unlikely to respond to progestin-based therapy. Conversely, lesions can have some PR expression, suggesting that lack of response may be due to insufficient progestin dose or noncompliance with therapy (i.e., inability to tolerate side effects). Postoperatively, this scoring system may have utility in that it would help identify patients in whom progestin therapy is unlikely to be successful. In patients who are found to be PR resistant (i.e., low H-score), it may be prudent to avoid progestin-containing agents in favor of alternative therapeutic options—GnRH analogs, danazol, or aromatase inhibitors (5, 26). In patients with medium PR expression, and/or multiple lesions with variable PR expression, OCs or high-dose progestins can be trialed, yet with a low threshold to adjust therapy if endometriosis-associated pain recurs.
It is plausible that the presence of varying levels of PR in lesions from the same subject may ultimately lead to selective growth of PR-resistant lesions while on progestin treatment. We will continue to follow these subjects to understand how the heterogeneity of PR expression in endometriotic lesions affects PR resistance. As it can take years for endometriosis to recur following surgery, this will be a long-term endeavor (27). Future studies will allow us to determine if the presence of PR heterogeneity may result in more PR-resistant disease after progestin treatment. If so, then patients with PR heterogeneity may benefit from treatment options other than long-term progestin-based regiments.
One of the limitations of our study is that the sample size of the response group is substantially lower than the no response group, with an overall response rate to medical therapy of 27%. This can be explained by our selection process. Only subjects with histologic confirmation of endometriosis and biopsies to assess were included. Hence the cohort was composed of women undergoing surgery for endometriosis. Subjects not responding to medical therapy are more likely to undergo surgery. As use of hormonal therapy could be a confounder, we performed a subgroup analysis for this using a Mann-Whitney U test. In both the response and no response group, there was no significant difference in H-scores between subjects using or not using hormonal medication.
Subjects who respond well to current medical treatment are less likely to undergo surgery. However, we did have a subset of subjects not on hormonal therapy at the time of surgery classified as responders. These subjects had responded to progestin-based therapy in the past, but stopped medication to conceive. Given recurrence of pain while off therapy, they underwent surgery. The majority of these subjects had H-scores placing them in the high or medium PR group. Although the study did not include subjects currently using and responding to progestin-based therapy, it would be interesting to study this population in the future.
We did have 17 subjects with eutopic endometrium collected at time of surgery, which was used to assess the correlation between H-scores in ectopic and eutopic endometrium. There was poor correlation between matched eutopic and ectopic lesions in responders or nonresponders. Thus H-scores in eutopic endometrium cannot be used to predict response to progestin-based therapy. Given the lack of correlation, ectopic lesions will be needed for prediction of response to progestin-based therapy.
Although hormonal therapy is aimed at reducing disease burden, it is important to recognize that endometriosis is a systemic disorder with effects on the brain and metabolic and inflammatory processes (28–30). Women with endometriosis have altered pain sensitization and an increased incidence of anxiety and depression (28). Endometriosis modulates gene expression in regions of the brain controlling pain response, as well as emotional and behavioral changes (28). Similarly, women with endometriosis have a lower body mass index (BMI), which may be explained by the effects of endometriosis on gene expression in the liver (29). In a murine model of endometriosis, a lower BMI was recapitulated, and it was demonstrated that the lower BMI was due to changes in hepatic gene expression in metabolic pathways (29). Endometriosis is also considered a proinflammatory state (31). Although inflammation in endometriosis has been well established, the mechanism by which endometriosis results in increased systemic inflammation is not clear. We have previously demonstrated that differential expression of miRNAs 125b-5p and let7b-5p can influence cytokine expression of macrophages, contributing to increased inflammation (30). As miRNAs are stable circulating markers in the blood stream, this provides further support of endometriosis having systemic effects distant from ectopic endometrium in the pelvis. Although surgical therapy may treat local disease in the pelvis, it is also important to identify appropriate treatments that will also affect the systemic aspects of this disease.
Taken together, we have shown that PR status can be used to predict response to progestin-based therapy. Although endometriosis is not a malignant condition, it is a chronic, debilitating disorder with adverse effects on quality of life, including substantial cost burden (3, 32). Delays not only in diagnosis, but also finding the most effective treatment, further contribute to reduced quality of life (1). The goal of hormonal therapy is to induce atrophy of endometriotic lesions. Yet the ability to predict which medication each individual patient will respond to has not been established. Similarly, as existing medical therapies allow for suppression (as opposed to regression) of endometriotic lesions, patients require long-term treatment (33). Hormonal therapy and surgery are the two cornerstones of endometriosis management. Recurrence rates are high even following surgery. Yet it is not known if surgery itself was incomplete (i.e., microscopic disease) or if other factors, such as aberrant PR expression, influence recurrence. Although this is a retrospective study, we were able to propose a scoring system/clinical algorithm that can be used in practice to help guide hormonal therapy choice following surgical management of endometriosis. Our proposed algorithm can be used postoperatively to provide insight into the reason for lack of response to preoperative hormonal therapy and determine the ideal postsurgical hormonal therapy. Following surgery, it is routine to place patients on hormonal therapy in an effort to reduce risk of recurrence, and including our model may further reduce this risk by choosing therapy based on PR expression/H- scores. PR status in endometriosis could be used in a manner analogous to the use of estrogen receptor/PR status in breast cancer for tailoring hormonal-based regimens after obtaining tissue. In summary, although our scoring system/clinical algorithm requires validation in prospective clinical trials, we anticipate that utilization of patients’ PR status will allow for a novel, targeted approach to treating endometriosis.
Acknowledgments
We thank Dr. Harvey Kliman for generously providing normal endometrium for use in immunohistochemistry experiments.
Financial Support: This work was supported by National Institutes of Health Grant HD076422 (to H.S.T.).
Current Affiliation: A. Vanhie’s current affiliation is the Department of Development and Regeneration, Catholic University Leuven, 3000 Leuven, Belgium.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- H
Histo
- OC
oral contraceptive
- PBST
PBS with 0.1% Tween 20
- PR
progesterone receptor
- ROC
receiver operating characteristic
References
- 1. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am. 2012;39(4):535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T, Falcone T, Graham B, Halis G, Horne A, Kanj O, Kjer JJ, Kristensen J, Lebovic D, Mueller M, Vigano P, Wullschleger M, D’Hooghe T. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299. [DOI] [PubMed] [Google Scholar]
- 4. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 5. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261–275. [DOI] [PubMed] [Google Scholar]
- 6. Vanhie A, Tomassetti C, Peeraer K, Meuleman C, D’Hooghe T. Challenges in the development of novel therapeutic strategies for treatment of endometriosis. Expert Opin Ther Targets. 2016;20(5):593–600. [DOI] [PubMed] [Google Scholar]
- 7. Vercellini P, Somigliana E, Viganò P, Abbiati A, Barbara G, Crosignani PG. Endometriosis: current therapies and new pharmacological developments. Drugs. 2009;69(6):649–675. [DOI] [PubMed] [Google Scholar]
- 8. Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96(6):623–632. [DOI] [PubMed] [Google Scholar]
- 9. Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010;28(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lessey BA, Metzger DA, Haney AF, McCarty KS Jr. Immunohistochemical analysis of estrogen and progesterone receptors in endometriosis: comparison with normal endometrium during the menstrual cycle and the effect of medical therapy. Fertil Steril. 1989;51(3):409–415. [DOI] [PubMed] [Google Scholar]
- 11. Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248(1-2):94–103. [DOI] [PubMed] [Google Scholar]
- 12. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85(8):2897–2902. [DOI] [PubMed] [Google Scholar]
- 13. Cakmak H, Taylor HS. Molecular mechanisms of treatment resistance in endometriosis: the role of progesterone-hox gene interactions. Semin Reprod Med. 2010;28(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bulun SE, Cheng YH, Pavone ME, Xue Q, Attar E, Trukhacheva E, Tokunaga H, Utsunomiya H, Yin P, Luo X, Lin Z, Imir G, Thung S, Su EJ, Kim JJ. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93(6):2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee B, Du H, Taylor HS. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod. 2009;80(1):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor HS, Fei X. Emx2 regulates mammalian reproduction by altering endometrial cell proliferation. Mol Endocrinol. 2005;19(11):2839–2846. [DOI] [PubMed] [Google Scholar]
- 18. van Diest PJ, van Dam P, Henzen-Logmans SC, Berns E, van der Burg ME, Green J, Vergote I; European Organization for Research and Treatment of Cancer-Gynaecological Cancer Cooperative Group . A scoring system for immunohistochemical staining: consensus report of the task force for basic research of the EORTC-GCCG. J Clin Pathol. 1997;50(10):801–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prentice A, Randall BJ, Weddell A, McGill A, Henry L, Horne CH, Thomas EJ. Ovarian steroid receptor expression in endometriosis and in two potential parent epithelia: endometrium and peritoneal mesothelium. Hum Reprod. 1992;7(9):1318–1325. [DOI] [PubMed] [Google Scholar]
- 20. Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–3826. [DOI] [PubMed] [Google Scholar]
- 21. Bruner-Tran KL, Herington JL, Duleba AJ, Taylor HS, Osteen KG. Medical management of endometriosis: emerging evidence linking inflammation to disease pathophysiology. Minerva Ginecol. 2013;65(2):199–213. [PMC free article] [PubMed] [Google Scholar]
- 22. Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, Bulun SE. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007;77(4):681–687. [DOI] [PubMed] [Google Scholar]
- 23. Colon-Caraballo M, Garcia M, Mendoza A, Flores I.. Human endometriosis tissue microarray reveals site-specific expression of estrogen receptors, progesterone receptor, and Ki67 [published online ahead of print 7 April 2018]. Appl Immunohistochem Mol Morphol. doi: 10.1097/PAI.0000000000000663. [DOI] [PMC free article] [PubMed]
- 24. Brichant G, Nervo P, Albert A, Munaut C, Foidart JM, Nisolle M. Heterogeneity of estrogen receptor α and progesterone receptor distribution in lesions of deep infiltrating endometriosis of untreated women or during exposure to various hormonal treatments. Gynecol Endocrinol. 2018;34(8):651–655. [DOI] [PubMed] [Google Scholar]
- 25. Hou Z, Mamillapalli R, Taylor HS. Predictive biomarkers may allow precision therapy of endometriosis. J Endometr Pelvic Pain Disord. 2017;9(4):279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor HS, Giudice LC, Lessey BA, Abrao MS, Kotarski J, Archer DF, Diamond MP, Surrey E, Johnson NP, Watts NB, Gallagher JC, Simon JA, Carr BR, Dmowski WP, Leyland N, Rowan JP, Duan WR, Ng J, Schwefel B, Thomas JW, Jain RI, Chwalisz K. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40. [DOI] [PubMed] [Google Scholar]
- 27. Guo SW. Recurrence of endometriosis and its control. Hum Reprod Update. 2009;15(4):441–461. [DOI] [PubMed] [Google Scholar]
- 28. Li T, Mamillapalli R, Ding S, Chang H, Liu ZW, Gao XB, Taylor HS. Endometriosis alters brain electro-physiology, gene expression and increased pain sensitization, anxiety, and depression in female mice. Biol Reprod. 2018;99(2):349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goetz LG, Mamillapalli R, Taylor HS. Low body mass index in endometriosis is promoted by hepatic metabolic gene dysregulation in mice. Biol Reprod. 2016;95(6):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nematian SE, Mamillapalli R, Kadakia TS, Majidi Zolbin M, Moustafa S, Taylor HS. Systemic inflammation induced by microRNAs: endometriosis-derived alterations in circulating microRNA 125b-5p and Let-7b-5p regulate macrophage cytokine production. J Clin Endocrinol Metab. 2018;103(1):64–74. [DOI] [PubMed] [Google Scholar]
- 31. Reis FM, Petraglia F, Taylor RN. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum Reprod Update. 2013;19(4):406–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Soliman AM, Taylor HS, Bonafede M, Nelson JK, Castelli-Haley J. Incremental direct and indirect cost burden attributed to endometriosis surgeries in the United States. Fertil Steril. 2017;107(5):1181–1190.e2. [DOI] [PubMed] [Google Scholar]
- 33. Somigliana E, Vercellini P, Vigano P, Benaglia L, Busnelli A, Fedele L. Postoperative medical therapy after surgical treatment of endometriosis: from adjuvant therapy to tertiary prevention. J Minim Invasive Gynecol. 2014;21(3):328–334. [DOI] [PubMed] [Google Scholar]