Abstract
Context
Adrenarche refers to the rise of dehydroepiandrosterone sulfate (DHEA-S) associated with the development of a functional adrenal zona reticularis. Clinical features of adrenarche include onset of body odor, axillary hair, and pubic hair, which reflect increased androgen action. An early rise in adrenal androgens, or premature adrenarche (PremA), is a risk factor for adverse metabolic profiles in adolescence and adulthood. The bioactive androgens associated with adrenarche and PremA remain poorly understood. The adrenal gland is a potential source of testosterone (T) and the 11-oxygenated derivatives 11β-hydroxytestosterone (11OHT) and 11-ketotestosterone (11KT).
Objective
The objective of this study was to characterize the adrenal androgen biome contributing to adrenarche and PremA.
Participants and Methods
With the use of mass spectrometry, 19 steroids including the 11-oxygenated derivatives of T were measured in sera obtained from girls with PremA (n = 37; 4 to 7 years) and age-matched girls (n = 83; 4 to 10 years).
Results
In reference population girls, dehydroepiandrosterone, DHEA-S, androstenediol-3-sulfate, T, and 11KT all increased at the onset of adrenarche (6 to 8 years) and beyond (9 to 10 years) (P < 0.05 vs younger subjects 4 to 5 years). T, 11OHT, and 11KT were further elevated in PremA vs age-matched girls (P < 0.001). Circulating concentrations of 11KT during adrenarche and PremA exceeded those of T and 11OHT (11KT > T ≥ 11OHT). Androgen receptor activity and nuclear translocation studies demonstrated that 11KT is a potent androgen similar to T.
Conclusions
Our findings suggest that 11KT is the dominant bioactive androgen in children during adrenarche and PremA. Its androgenic capacity suggests that it may be responsible for the phenotypic changes seen in these phenomena.
The goal of this study was to characterize the adrenal androgen biome contributing to adrenarche and premature adrenarche.
Adrenarche is an endocrine process that results from the expansion of the adrenal zona reticularis (ZR) and its increased synthesis of 19-carbon (C19) steroids, particularly dehydroepiandrosterone (DHEA) and its sulfate (DHEA-S) (1–3). In children, DHEA and DHEA-S secretion begins to increase around age 6 to 8 years (4–7). Clinical manifestations of adrenarche include the initial stages of axillary and/or pubic hair growth (pubarche), adult body odor, and mild acne, all of which point to increased androgen activity (1, 8, 9). Pubarche as a result of adrenarche normally occurs after age 8 years in girls and 9 years in boys. Although DHEA and DHEA-S are very weak androgens, it has been assumed that these steroids act as precursors for the production of testosterone (T) in hair follicles and genital skin that exhibit the phenotypic effects associated with adrenarche (10–12).
Adrenarche was first recognized by Fuller Albright in the 1940s (13), and more recent studies have shown that dysregulation of adrenarche can have significant health consequences. Premature adrenarche (PremA) can be defined as the precocious commencement of adrenarche leading to pubarche before 8 years in girls and 9 years in boys, without the appearance of other secondary sex characteristics (14–17). Both normal adrenarche and PremA manifest with signs of increased androgen activity that result from direct secretion of adrenal bioactive androgens and peripheral metabolism of adrenal androgen precursors. PremA is distinguished from precocious puberty by a lack of progressive breast development in girls or testicular enlargement in boys. Although in the past PremA was thought to be a benign variant of maturation, recent studies suggest that this may not be the case. A subset of girls with PremA has been shown to be more susceptible to the development of polycystic ovary syndrome and insulin resistance as adults (18–22).
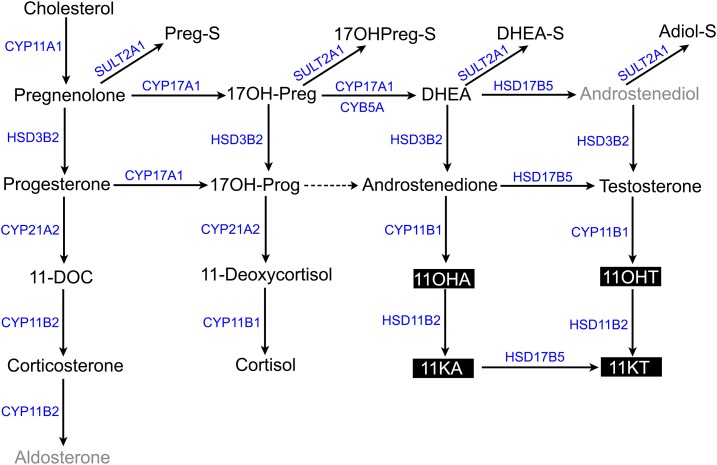
The age-related patterns of secretion of other weak and potent androgens have been investigated during adrenarche and PremA with varying results. Although some immunoassay studies suggested no adrenarchal rise in androstenedione (A4) (5, 23), recent liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis showed a slight adrenarche-related rise in this steroid (24). Increases in circulating T concentrations during adrenarche have also been an area of disagreement (5, 9, 24). Most of the early studies used immunoassays, and the differences in T levels during adrenarche might have resulted from steroid cross-reactivity, insufficient sensitivity, and high interassay variability when low T values were measured. LC-MS/MS is rapidly emerging as the preferred methodology for detection of multiple steroids using a small volume of serum or urine sample owing to its specificity and sensitivity. Although DHEA-S is the steroid historically associated with adrenarche, understanding of adrenal production of other active androgens could be of broad relevance to several diseases of androgen excess. Recent LC-MS/MS studies have demonstrated human adrenal production of 11-oxygenated C19 steroids and their potential as biomarkers in hyperandrogenic disorders (25, 26). The goal of the current study was to provide a detailed characterization of the steroid biome, including 11-oxygenated C19 androgens such as 11β-hydroxyandrostenedione (11OHA4), 11-ketoandrostenedione (11KA4), 11β-hydroxytestosterone (11OHT), and 11-ketotestosterone (11KT) (Fig. 1) in prepubertal girls with PremA and a reference population of girls without the confounding contribution of gonadal androgens.
Figure 1.
Steroidogenic pathways depicting adrenal mineralocorticoid, glucocorticoid, and androgen biosyntheses. The steroidogenic enzymes and cofactor proteins are marked in blue. The 11-oxygenated androgens are highlighted in black. Steroids not quantified are marked in gray. Dashed arrow denotes minor conversion to product. 11KA, 11-ketoandrostenedione; 11OHA, 11β-hydroxyandrostenedione.
Materials and Methods
Study design and participants
PremA patients
After approval from the institutional review boards at the University of Michigan and University of Texas Southwestern (UTSW) Medical Center, we enrolled 37 girls with PremA between ages 4 and 7 years. Peripheral serum was obtained during routine visits to the pediatric endocrinology clinics at both institutions. The inclusion criteria for the PremA group were Tanner stage II pubic hair before the age of 8 years and the absence of true puberty (Supplemental Table 1). We included patients with no evidence of adrenal pathologies, including enzyme deficiencies such as congenital adrenal hyperplasia, or an adrenocortical tumor. We excluded patients with chronic disorders (including thyroid dysfunction or diabetes mellitus) or who were taking medications known to affect adrenal function, gonadal function, or energy metabolism.
Reference population of girls
Serum samples from 83 girls between 4 and 10 years of age were collected during visits to the University of Michigan and UTSW pediatric clinics for minor health conditions and routine visits. Of the total 83 subjects, 54 age-matched girls between 4 and 7 years were used for comparison with the PremA group. Exclusion criteria were congenital adrenal hyperplasia, major comorbidities, acute or debilitating chronic illnesses, and corticosteroid therapy. This population included 13 girls diagnosed with hypothyroidism at UTSW, who were assessed clinically and did not have any pubic and axillary hair growth. The others were not specifically examined for clinical signs of adrenarche; consequently, the axillary and pubic hair status of the remaining 70 girls was not assessed.
Quantitation of serum steroids by LC-MS/MS
C19 and 21-carbon (C21) serum steroids were measured using LC-MS/MS (refer to Supplemental Materials and Methods and Supplemental Tables 1–3).
Androgen receptor functional studies
Responses of C19 steroids to a cell-based androgen receptor bioassay
To characterize C19 steroid androgenic activity, the CV1-ARLuc cell line was treated with the indicated unconjugated and sulfated C19 steroids. Although the unconjugated steroids were tested at 100 nM, the sulfated steroids were assessed at 1000 nM. Higher concentrations for sulfated steroids were used in order to be in the vicinity of their physiological concentrations. As previously described in detail, CV1-ARLuc cells are a selective androgen-responsive reporter model derived from African green monkey kidney CV1 cells that were engineered to express androgen receptor (AR) and an AR-driven Gaussia luciferase reporter (27). For experiments, the cells were plated at a density of 25,000 cells per well in a 48-well dish in DMEM/F12 (Life Technologies, Carlsbad, CA) growth medium containing 10% fetal bovine serum (FBS; GE Healthcare Life Sciences, Pittsburgh, PA) and 1% penicillin-streptomycin (Thermo Fisher, Waltham, MA) for 24 hours and then treated with 100 nM of T or 11KT (Steraloids, Newport, RI) in 10% charcoal-stripped FBS (GE Healthcare Life Sciences) for 18 hours (27). The medium was collected after the incubation period. For the luminescence bioassay, 25 μL of medium was mixed with 50 μL of 0.01 mg/mL coelenterazine (GoldBio, St. Louis, MO). The coelenterazine solution was prepared in an assay buffer comprising of 50 mM TrisHCl (pH 7.5) and 150 mM of NaCl. Luminescence was then measured by the FLUOstar OPTIMA Microplate Reader (BMG Labtech, Durham, NC). The cells were frozen at −80°C for RNA isolation for RNA sequencing (RNA-Seq) analysis as well as subsequent cDNA generation and quantitative PCR (qPCR) analysis, as described later.
RNA isolation and RNA-Seq Analysis
Total RNA was extracted from the cells using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA). The purity and integrity of the RNA were checked spectroscopically using a NanoDrop spectrometer (NanoDrop Technologies, Wilmington, DE), and 100 ng of RNA was used to generate 50-bp single-end reads in an Illumina HiSEQ 4000 sequencer (Illumina, San Diego, CA) at the University of Michigan sequencing core. FastQC was used to assess the quality of the FASTQ files (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Reads were aligned to the latest assembly (version 1.1) of the green monkey (Chlorocebus sabaeus) genome downloaded from www.ensembl.org using STAR (28). Qualimap was used to assess the total number of aligned reads, intronic rates, and exonic rates of the resulting BAM files (29). The number of counts per gene were assessed for each condition (basal was quantified by featureCounts) (30). The R/Bioconductor packages edgeR (31) and limma (32) were then used to calculate the reads per kilobase of transcript per million (RPKM) and the log2-transformed counts-per-million values for each gene. Fold-change of gene expression between treatment conditions (T and 11KT) and basal in the CV1-ARLuc cells was estimated by the ratio of the RPKM values of each condition.
cDNA generation and quantitative real-time RT-PCR
The High-Capacity cDNA Kit (Applied Biosystems, Foster City, CA) was used to reverse transcribe 200 ng of total RNA. Quantitative real-time RT-PCR was performed in the ABI StepOnePlus Real-Time PCR systems (Applied Biosystems). Genes previously shown to be androgen responsive (33–36) and with an RPKM value >1 at basal levels as observed with the RNA-Seq data were selected for qPCR analysis. Primer pairs (Integrated DNA Technologies, Coralville, IA) for the following genes were designed using the green monkey (Chlorocebus sabaeus) genome (Supplemental Table 6): protein C inhibitor (SERPINA5), FK506 binding protein 5 (FKBP5), serum/glucocorticoid regulated kinase 1 (SGK1), folate receptor α (FOLR1), and ras homolog family member U (RHOU). The HPRT1 (Hypoxanthine Phosphoribosyltransferase 1) gene was used as a reference gene for sample normalization in the CV1-ARLuc cells (37).
AR nuclear translocation study
CV1-ARLuc cells were grown on microscope slides (Globe Scientific, Paramus, NJ), previously treated with 50 μg/mL of Poly-d-lysine (Sigma-Aldrich, St. Louis, MO) at room temperature for 1 hour in 100-mm plates, in growth medium as previously described (27). After 24 hours, the cells were rinsed with PBS (Life Technologies) followed by treatment with 100 nM of T or 11KT (Steraloids) for 18 hours in 10% charcoal-stripped FBS. Cells were subsequently fixed with methanol at −20°C for 20 minutes and washed three times with PBS (Life Technologies). Slides were incubated overnight with a mouse monoclonal anti-human AR antibody (1:500 dilution; Santa Cruz, Dallas, TX) and then with a secondary Alexa Fluor 594-conjugated AffiniPure goat anti-mouse antibody (1:100 dilution; Jackson Immunoresearch, West Grove, PA) for 1 hour at room temperature. The slides were then incubated with Prolong Gold mounting medium with 4′,6-diamidino-2-phenylindole (Thermo Fisher) for 24 hours at 4°C with coverslips on.
Statistical analysis
Steroid levels across age groups for the reference population of girls were compared by Kruskal-Wallis tests followed by the Dunn post hoc test using the SigmaPlot 12.5 software package (Systat Software, Inc., San Jose, CA). Steroid levels between girls with PremA and age-matched girls were compared by the nonparametric Mann-Whitney U test. A P value <0.05 was considered statistically significant.
Logistic regression was performed using R software (Free Software Foundation, Boston, MA) to nominate steroids with high discriminatory power in distinguishing PremA from age-matched girls. We next used a stepwise approach based on linear discriminant analysis implemented in the stepclass function from klaR (38) to identify the combination of steroid hormones that would allow the best discrimination between the two groups. Once the combination of steroids was obtained, the performance of the classifier was evaluated by the correctness rate (the percentage of cases with identical a priori and a posteriori group allocations). Lastly, receiver operating characteristic curve analysis (MedCalc Statistical Software version 17.9.7) was performed to calculate the specificity and sensitivity of each steroid in the combination to diagnose PremA. Youden index J was used to estimate the cutoff values. Multiple linear regression analysis was performed to test 11KT as a function of age and BMI z score (Statistical Applied Software, SAS Institute Inc., Cary, NC).
Results
Changes in the circulating steroid biome in prepubertal girls
Subjects were grouped by chronological age, and their steroid concentrations were assessed (Table 1). Ages 6 to 8 years represent the expected window of the initiation of adrenarche, wherein the adrenal ZR starts to develop and produce C19 steroids. Of the unconjugated C21 steroids that included mineralocorticoids, glucocorticoids, and their precursors, only 11-deoxycorticosterone exhibited a significant decrease at the commencement of adrenarche (P < 0.05) (Table 1). However, the initiation of adrenarche was marked by significant increases in unconjugated and sulfated C19 steroids compared with C21 steroids. DHEA-S demonstrated the first increment between ages 6 and 8 years in girls (P < 0.001) (Table 1). DHEA and Adiol-S demonstrated parallel increases with DHEA-S during the 6- to 10-year window (all P < 0.001) (Table 1). Serum levels of the classic bioactive androgen T showed age-dependent increases after the first 5 years of life (P < 0.005) (Table 1). In addition, two of the 11-oxygenated C19 steroids (11KA4 and 11KT) had an adrenarche-related increase (P < 0.05 for 11KA4; P < 0.005 for 11KT) (Table 1). In all, seven steroids out of the 19 examined significantly changed around the expected time of onset of adrenarche in the reference population of girls.
Table 1.
Serum Concentrations of Steroids in a Reference Population of Girls Between Ages 4 and 10 Years
| Steroid, ng/dL | Aged 4–5 y (n = 22) | Aged 6–8 y (n = 38) | Aged 9–10 y (n = 23) |
|---|---|---|---|
| Unconjugated 21-carbon steroids | |||
| Pregnenolone | 26.3 [14.9–38. 9] | 21.45 [14.5–31.7] | 25.9 [19.8–40.5] |
| 17OH-Pregnenolone | ND | ND | ND |
| Progesterone | 5.3 [5.0–7.7] | 5.1 [4.4–6.1] | 4.7 [4.2–6.6] |
| 17OH-Progesterone | 17.4 [10.9–28.2] | 15.4 [12.8–21.8] | 22.3 [13.5–34.2] |
| 11-Deoxycorticosterone | 17.3 [8.0–44.4] | 7.62 [5.03–19.77]a | 8.1 [4.3–18.0]a |
| Corticosterone | 145.4 [85.2–295.1] | 113.5 [66.0–232] | 84.2 [70.5–221] |
| 11-Deoxycortisol | 45.9 [26.6–62.7] | 35.5 [22.4–51.1] | 35.5 [26.3–52.4] |
| Cortisol | 5481 [3562–9561] | 4457 [3504–7100] | 4361 [3212–9106] |
| Unconjugated 19-carbon steroids | |||
| DHEA | 41.3 [23.0–54.7] | 64.2 [43.5–141.4]a | 134 [84.1–186]a,b |
| A4 | 10.4 [8.4 –15.2] | 15.5 [10.3–24.7] | 21.2 [19.1–28.6]a,b |
| T | 3.8 [2.4–4.9] | 4.8 [4.2–5.3]a | 6.3 [5.2–8.1]a,b |
| 11OHA4 | 17.6 [11.2–34.0] | 27.0 [20.0–39.7] | 26.1[17.4–44.8] |
| 11KA4 | 8.18 [6.77–11.23] | 11.3 [9.90–14.47]a | 12.8 [8.7–16.8]a |
| 11OHT | 3.0 [2.6–6.5] | 4.6 [3.2–6.7] | 5.5 [4.1–6.5]a |
| 11KT | 8.6 [7.3–10.9] | 13.4 [10.3–18.1]a | 17.6 [14.2–22.5]a,b |
| Sulfated steroids | |||
| Preg-S | 934 [555–1462 ] | 1029 [718–1338] | 1435 [975–2243] |
| 17OHPreg-S | 347 [257–614] | 445 [370–712] | 645 [507–1003]a |
| DHEA-S | 5902 [3545–10,231] | 13,750 [7672–34,899]a | 34,604 [27,308–50,856]a,b |
| Adiol-S | 527 [387–1228] | 1149 [663–2464]a | 2958 [1943–5540]a,b |
Data are expressed as median with corresponding interquartile range. Statistical significance was determined by Kruskal-Wallis one-way ANOVA followed by the Dunn post hoc test for multiple comparisons. Significance levels are indicated by the footnotes. Note: 17OH-Preg was detected (>172.6 ng/dL) in two subjects between ages 6–8 y and 9–10 y each.
Abbreviation: ND, not detected.
P < 0.05 vs age 4–5 y.
P < 0.05 vs age 6–8 y.
Changes in the serum steroid biome in PremA
Serum steroid levels were compared between girls with PremA and age-matched girls (aged 4 to 7 years). The targeted LC-MS/MS analysis of 19 serum steroids indicated broad-based changes in the serum steroid biome of the PremA cohort. Of the eight unconjugated C21 steroids measured, five showed significant alterations in concentration in PremA. Preg concentrations increased with PremA (P < 0.001), whereas Prog, 17OHProg, 11-deoxycorticosterone, and 11-deoxycortisol showed significant decreases (P < 0.05) (Table 2). LC-MS/MS analysis of serum androgens and their precursors in the patients with PremA revealed significantly increased levels of the classic androgen T (P < 0.001) and its precursor DHEA (P < 0.001) (Table 2). In addition, all sulfated steroids were elevated in PremA (all P < 0.001) (Table 2). Serum concentrations of the 11-oxygenated androgens 11OHA4, 11KA4, 11OHT, and 11KT were all significantly higher in the PremA cohort (P < 0.001 for 11OHA4, 11OHT, and 11KT; P = 0.012 for 11KA4) (Table 2). Specifically, 11KT was 3.5-fold higher than T in girls with PremA. These findings indicate broad alterations in adrenocortical steroids in children with PremA.
Table 2.
Serum Concentrations of Steroids in Girls With PremA vs Age-Matched Girls Between Ages 4 and 7 Years
| Steroid, ng/dL | Age-Matched Girls (n = 54) | Girls With PremA (n = 37) | P Value |
|---|---|---|---|
| Unconjugated 21-carbon steroids | |||
| Pregnenolone | 25.6 [14.6–46.2] | 50.6 [32.8–83.4] | <0.001 |
| 17OH-Pregnenolone | ND | ND | — |
| Progesterone | 5.3 [4.7–7.1] | 2.7 [2.0–5.6] | <0.001 |
| 17OH-Progesterone | 16.2 [12.8–26.3] | 12.8 [9.5–22.1] | 0.030 |
| 11-Deoxycorticosterone | 9.7 [6.8–34.1] | 7.9 [4.2–14.4] | 0.008 |
| Corticosterone | 131 [82.8–220] | 92.2 [50.4–218] | 0.054 |
| 11-Deoxycortisol | 40.5 [29.4–55.0] | 30.4 [19.2–48.1] | 0.025 |
| Cortisol | 5487 [3784–7873] | 4911 [3352–6528] | 0.089 |
| Unconjugated 19-carbon steroids | |||
| DHEA | 52.3 [32.8–89.5] | 242 [162–429] | <0.001 |
| A4 | 13.1 [9.2–22.4] | 17.0 [11.4–22.8] | 0.238 |
| T | 4.6 [3.7–5.2] | 5.2 [4.6–5.8] | <0.001 |
| 11OHA4 | 26.1 [16.7–38.9] | 45.7 [29.3–67.5] | <0.001 |
| 11KA4 | 10.8 [8.2–13.8] | 15.0 [12.4–22.1] | <0.001 |
| 11OHT | 3.8 [2.6–6.1] | 6.2 [4.9–7.4] | <0.001 |
| 11KT | 11.2 [8.4–16.5 ] | 18.1 [12.3–22.9] | <0.001 |
| Sulfated steroids | |||
| Preg-S | 958 [582–1198] | 1722 [1162–2124] | <0.001 |
| 17OHPreg-S | 416 [270–605] | 803 [564–1198] | <0.001 |
| DHEA-S | 9321 [4333–17637] | 38,227 [23,035–57,073] | <0.001 |
| Adiol-S | 764 [450–1606 ] | 4102 [2323–6660] | <0.001 |
Data are expressed as median with corresponding interquartile range. Statistical significance was determined by nonparametric Mann–Whitney U test. P < 0.05 was considered statistically significant. Note: 17OH-Preg was detected (>172.6 ng/dL) in only one reference subject between ages 4 and 7 y and no subjects with PremA.
Abbreviation: ND, not detected.
Our logistic regression analysis indicated DHEA exhibited the most discriminatory power between PremA and age-matched girls, followed by Adiol-S and DHEA-S (Supplemental Table 7). Concordant to our logistic regression analysis, DHEA was again nominated as the top performing steroid, with a correctness rate of 0.8222. In addition, including additional steroids in the model did not improve the performance of the classifier. From a diagnostic perspective, DHEA, Adiol-S, and DHEA-S were the best steroids to discriminate girls with PremA from age-matched girls (Supplemental Fig. 1).
Correlation between BMI and serum 11KT levels
Girls with PremA had a significantly higher BMI z score than age-matched girls (P < 0.013) (Supplemental Table 2). Girls who had PremA and a BMI >85th percentile displayed higher 11KT levels than those with a BMI <85th percentile. In contrast, age-matched girls did not demonstrate this difference (Supplemental Fig. 2). Multivariate analysis revealed that 11KT was significantly affected by age when adjusted for BMI z score (β = 1.9; P < 0.001) but showed no dependence on BMI z score after adjustment for age (β = −0.5; P = 0.34).
Effect of unconjugated and sulfated C19 steroids on AR activation
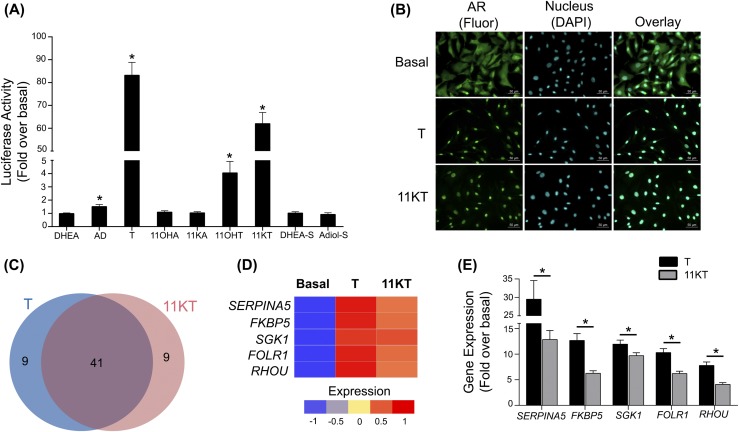
To better understand the physiologic function of the adrenal-derived C19 steroids, nine of the steroid products were tested for their ability to activate the AR in CV1-ARLuc cells (27) (Fig. 2). Luciferase activity was significantly increased on treatment with A4 (1.5-fold vs basal; P < 0.005), T (83-fold; P < 0.001), and its 11-oxygenated derivatives 11OHT (fourfold; P < 0.004) and 11KT (62-fold; P < 0.001) [Fig. 2(A)]. We further traced the activation of AR by monitoring its nuclear translocation in CV1-ARLuc cells after separate additions of 100 nM of T and 11KT [Fig. 2(B)]. In the absence of ligand, AR was predominantly located in the cytoplasm. Addition of either T or 11KT for 18 hours led to nuclear localization in 100% of the cells. RNA-Seq analysis performed on CV1-ARLuc cells treated with androgens demonstrated an overlap of 41 of the top 50 genes induced by T and 11KT [Fig. 2(C)]. Several of the genes on this list were previously shown to be AR targets. These include SERPINA5, FKBP5, SGK1, FOLR1, and RHOU, all of which were confirmed to increase using quantitative RT-PCR [Fig. 2(D) and 2(E)].
Figure 2.
Androgen studies using the CV1-ARLuc cell line. (A) Gaussia reporter gene regulation in response to unconjugated and sulfated C19 steroids. The data represent the mean ± SEM of three independent experiments, each performed in triplicate. *P < 0.05 vs basal. Basal denotes treatment with 0.1% methanol (vehicle). (B) T and 11KT binding causes AR nuclear translocation. Fluorescence microscopy of CV1-ARLuc cells stably transfected with a human AR and treated with or without 100 nM of T and 11KT, respectively, for 18 hours. Green fluorescence represents AR immunoreactivity, and blue fluorescence is DAPI (nucleus). (C) The 50 most highly upregulated genes in CV1-ARLuc cells after treatment with 100 nM of T and 11KT as identified by RNA-Seq. Both T and 11KT stimulated 41 of these 50 genes. (D) RNA-Seq and (E) quantitative RT-PCR validation of the expression of CV1-ARLuc genes stimulated by treatment with T and 11KT. These genes were previously shown to be androgen responsive. The data represent the mean ± SEM of three independent experiments, each performed in triplicate. *P < 0.05, T vs 11KT. AD, androstenedione; DAPI, 4′,6-diamidino-2-phenylindole.
Discussion
Although DHEA and DHEA-S are the conventional biomarkers of both normal and PremA (4–7), these steroids are not active androgens and hence are unlikely to directly mediate the androgenic phenotype observed in normal and PremA. It is worth noting that DHEA and DHEA-S constitute a pool of precursors for potential peripheral conversion to T. In fact, the adrenal gland has long been regarded as a major contributor to the circulating T pool in women and prepubertal children (39, 40). Using LC-MS/MS analysis, we and others have defined a broader array of adrenal-derived steroids, including 11-oxygenated metabolites of T, that can activate the AR (25, 26, 41, 42). The role of 11-oxygenated C19 steroids in adrenarche and PremA has not been determined. The goal of this study was to characterize alterations in the circulating steroid biome, including the 11-oxygenated androgens in girls with PremA and a reference population of girls. This comprehensive report compared the steroid biome (including bioactive androgens) in girls, including those with PremA. In addition to DHEA and DHEA-S, we found that T and 11KT significantly increased during adrenarche compared with the preadrenarche window. Furthermore, we demonstrated that 11KT, 11OHT, and T levels were significantly higher in girls with PremA than in age-matched girls.
The adrenarchal rise in DHEA and DHEA-S has been attributed to the differential expression of 3β-hydroxysteroid dehydrogenase type 2 in the zona fasciculata and cytochrome b5 type A and sulfotransferase type 2A1 in the developing ZR (Fig. 1) (3, 6, 43, 44). Cytochrome b5 type A enhances the 17,20-lyase activity of the steroidogenic enzyme CYP17A1 (17α-hydroxylase/17,20-lyase), thereby allowing DHEA synthesis in the ZR. An exaggerated rise in androgen precursors in PremA, as shown here and in previous studies, is in part caused by increasing 17,20-lyase activity owing to a precociously developed ZR (45, 46). In the current study, although we observed an increase in DHEA in PremA, we were unable to accurately deduce the augmentation in the 17,20-lyase activity during adrenarche owing to low circulating 17OH-Preg levels that were often below the limit of detection of our LC-MS/MS assay. Our recent analysis that traced steroid sulfates in children confirmed that levels of DHEA-S but not Preg-S or 17OHPreg-S correlated directly with age during adrenarche (44). We also demonstrated that Adiol-S, another steroid sulfate of ZR origin, shows a concomitant age-related increase with DHEA-S and proposed that Adiol-S could represent a surrogate measure of the progression of adrenarche (44). Considering that Adiol-S is one enzymatic step closer to T than DHEA-S, this steroid provides another adrenarche-associated precursor for conversion to androgens in peripheral tissues. Using gas chromatography-mass spectrometry, Remer et al. (7) demonstrated an age-related logarithmic increase in the urinary excretion of DHEA and Adiol conjugates in both sexes, with the first significant increase occurring as early as 7 to 8 years of age. Only one report has compared Adiol-S levels in girls with PremA with those of age-matched controls, and it found increased circulating Adiol-S in girls with PremA (47). In addition to confirming that Adiol-S rises during adrenarche, we demonstrated the utility of all four steroid sulfates as biomarkers for PremA in the current study.
As opposed to testicular androgen production, which utilizes 17β-hydroxysteroid dehydrogenase type 3, 17β-hydroxysteroid dehydrogenase type 5 is a pivotal factor in the adrenal synthesis of bioactive androgens. This enzyme has the ability to convert A4 to T in the adrenal ZR (42, 48–50). Previous studies have shown that although the adrenal contributes only 1% of the total circulating T in postpubertal males, it provides ∼30% to 50% in women and children (39, 40). Although many studies have shown that T levels do not rise during the early stages of adrenarche (5, 9, 24), several other analyses have suggested that T does increase and therefore contributes to the progression of PremA (5, 51–56). Our data indicate that circulating T concentrations rose significantly with age in our reference population of girls and showed an early surge in PremA.
T and A4 are good substrates for the adrenal-specific 11β-hydroxylase enzyme leading to the synthesis of 11OHT and 11OHA4, respectively (57, 58) (Fig. 1). 11OHT and 11OHA4 can both be oxidized to their respective keto-metabolites, 11KT and 11KA4, and this process occurs predominantly in peripheral tissues expressing HSD11B2 (42, 58–60) (Fig. 1). Here, we demonstrated that two of the 11-oxygenated C19 steroids (11KT and 11KA4) increased considerably with age and that all four 11-oxygenated C19 steroids exhibited an early surge in girls with PremA compared with age-matched girls. The 11-oxygenated C19 steroids are elevated in two other hyperandrogenic disorders, primarily 21-hydroxylase deficiency and also polycystic ovary syndrome (25, 26).
Until now, T has been the conventional biomarker of androgen excess in females and prepubertal children. Using in vitro luciferase reporter assays, we and others have shown that 11OHT and 11KT activate the human AR similarly to T (27, 42, 60). Intriguingly, 11KT can potentially be converted to 11-keto-5α-dihydrotestosterone, a bioactive androgen as potent as DHT (60). Our current study also demonstrated an increase in the expression of known androgen-responsive genes (33–36) in the T- and 11KT-treated CV1-ARLuc cells. Pretorius et al. (61) also indicated that 11KT, T, and their 5α-reduced metabolites promoted AR-regulated gene expression in two androgen-dependent prostate cancer cell lines. These data collectively indicate that 11KT is a key androgen in the progression of both normal and PremA.
The androgen precursors DHEA, DHEA-S, and Adiol-S were all better clinical discriminators of PremA than all active androgens, including T, 11OHT, and 11KT (Supplemental Fig. 1; Supplemental Table 7). Therefore, the contribution of androgen precursors to the clinical manifestations of normal adrenarche and PremA cannot be neglected. It is likely that the phenotypic effects associated with these phenomena can be attributed to the combined action of direct adrenal secretion of active androgens and peripheral conversion of DHEA to T (62–68) and 11OHA4 to 11KT (59, 60) in target tissues such as hair follicles or genital skin (8, 10, 68). The lower discriminatory value of 11KT could also be associated with the presence of confounding factors such as BMI and race in the PremA and age-matched reference populations.
The main limitation of our study is the exclusive inclusion of girls. PremA is underreported in boys, thereby making it difficult to obtain serum samples from boys with PremA (69). However, comparisons between patients with 21-hydroxylase deficiency and control groups have demonstrated that the 11-oxygenated androgens are similarly elevated in males and females across different ages (25). The other limitation of our study is that the age-matched reference girls were not examined for clinical assessment of pubarche; therefore, we could not exclude the possible inclusion of a few children with PremA in this reference population. Nevertheless, we found a significant difference in steroid biomarkers in the PremA group, which only strengthens the conclusions regarding the increase in 11-oxygenated steroids. Because adipose tissue has been shown to participate in the peripheral biotransformation of androgen precursors (68, 70), we examined the association between BMI z score and 11KT levels in both girls with PremA and age-matched reference girls. When adjusted for age, BMI z score did not demonstrate a linear correlation with circulating 11KT concentrations; however, girls with PremA and BMI >85th percentile showed the highest 11KT values of all groups. Further studies are needed to understand the association between elevated circulating 11KT concentration and increased body fat in PremA. Larger cohorts, including a well-characterized reference population of children with normal adrenarche and boys with PremA, will aid in comprehensively characterizing the biologic significance and clinical utility of these 11-oxygenated androgens in normal adrenarche and PremA.
In summary, we have profiled the steroid biome including the 11-oxygenated androgens in a reference population of girls and in PremA. We have demonstrated that increases in circulating levels of Adiol-S, T, and 11KT in addition to the traditional markers DHEA and DHEA-S occur in girls between the ages of 6 and 8 years. Our findings suggest that PremA can be characterized by broad changes in the steroid biome, including early increases in circulating 11-oxygenated androgens (11OHA4, 11KA4, 11OHT, and 11KT) as well as DHEA, T, and sulfated Δ5-steroids. Given that 11KT has an androgenic potency approaching that of T and is the dominant circulating androgen in girls during adrenarche and PremA, we suggest that 11KT may be partly responsible for the androgenic manifestations of both normal adrenarche and PremA. Further investigations are needed to better define the relative roles of direct adrenal secretion of androgens vs conversion of androgen precursors in peripheral and target tissues in the process of normal adrenarche and PremA.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by National Institutes of Health Grants R01DK069950 and R01DK43140 (to W.E.R.), R01GM086596 (to R.J.A.), and 1K08DK109116 (to A.F.T.) and by National Center for Advancing Translational Sciences Grant 2UL1TR000433 (to J.R.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 11KA4
11-ketoandrostenedione
- 11KT
11-ketotestosterone
- 11OHA4
11β-hydroxyandrostenedione
- 11OHT
11β-hydroxytestosterone
- A4
androstenedione
- AR
androgen receptor
- BMI
body mass index
- C19
19-carbon
- C21
21-carbon
- DHEA
dehydroepiandrosterone
- DHEA-S
dehydroepiandrosterone sulfate
- FBS
fetal bovine serum
- FKBP5
FK506 binding protein 5
- FOLR1
folate receptor α
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- PremA
premature adrenarche
- qPCR
quantitative PCR
- RHOU
ras homolog family member U
- RNA-Seq
RNA sequencing
- RPKM
reads per kilobase of transcript per million
- SERPINA5
protein C inhibitor
- SGK1
serum/glucocorticoid regulated kinase 1
- T
testosterone
- UTSW
University of Texas Southwestern
- ZR
zona reticularis
References
- 1. Cutler GB Jr, Loriaux DL. Andrenarche and its relationship to the onset of puberty. Fed Proc. 1980;39(7):2384–2390. [PubMed] [Google Scholar]
- 2. Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004;22(4):337–347. [DOI] [PubMed] [Google Scholar]
- 3. Hui XG, Akahira J, Suzuki T, Nio M, Nakamura Y, Suzuki H, Rainey WE, Sasano H. Development of the human adrenal zona reticularis: morphometric and immunohistochemical studies from birth to adolescence. J Endocrinol. 2009;203(2):241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Korth-Schutz S, Levine LS, New MI. Dehydroepiandrosterone sulfate (DS) levels, a rapid test for abnormal adrenal androgen secretion. J Clin Endocrinol Metab. 1976;42(6):1005–1013. [DOI] [PubMed] [Google Scholar]
- 5. Korth-Schutz S, Levine LS, New MI. Serum androgens in normal prepubertal and pubertal children and in children with precocious adrenarche. J Clin Endocrinol Metab. 1976;42(1):117–124. [DOI] [PubMed] [Google Scholar]
- 6. Guran T, Firat I, Yildiz F, Kaplan Bulut I, Dogru M, Bereket A. Reference values for serum dehydroepiandrosterone-sulphate in healthy children and adolescents with emphasis on the age of adrenarche and pubarche. Clin Endocrinol (Oxf). 2015;82(5):712–718. [DOI] [PubMed] [Google Scholar]
- 7. Remer T, Boye KR, Hartmann MF, Wudy SA. Urinary markers of adrenarche: reference values in healthy subjects, aged 3‒18 years. J Clin Endocrinol Metab. 2005;90(4):2015–2021. [DOI] [PubMed] [Google Scholar]
- 8. Rosenfield RL, Lucky AW. Acne, hirsutism, and alopecia in adolescent girls: clinical expressions of androgen excess. Endocrinol Metab Clin North Am. 1993;22(3):507–532. [PubMed] [Google Scholar]
- 9. Ducharme JR, Forest MG, De Peretti E, Sempé M, Collu R, Bertrand J. Plasma adrenal and gonadal sex steroids in human pubertal development. J Clin Endocrinol Metab. 1976;42(3):468–476. [DOI] [PubMed] [Google Scholar]
- 10. Kaufman FR, Stanczyk FZ, Matteri RK, Gentzschein E, Delgado C, Lobo RA. Dehydroepiandrosterone and dehydroepiandrosterone sulfate metabolism in human genital skin. Fertil Steril. 1990;54(2):251–254. [PubMed] [Google Scholar]
- 11. Rosenfield RL. Hirsutism and the variable response of the pilosebaceous unit to androgen. J Investig Dermatol Symp Proc. 2005;10(3):205–208. [DOI] [PubMed] [Google Scholar]
- 12. Pelletier G. Expression of steroidogenic enzymes and sex-steroid receptors in human prostate. Best Pract Res Clin Endocrinol Metab. 2008;22(2):223–228. [DOI] [PubMed] [Google Scholar]
- 13. Albright F, Smith PH, Fraser R. A syndrome characterized by primary ovarian insufficiency and decreased stature: report of 11 cases with a digression on hormonal control of axillary and pubic hair. Am J Med Sci. 1942;204:625–648. [Google Scholar]
- 14. Talbot NB, Butler AM, Berman RA, Rodriguez PM, Maclachlan EA. Excretion of 17-keto steroids by normal and abnormal children. Am J Dis Child. 1943;65(3):364–375. [Google Scholar]
- 15. Voutilainen R, Jääskeläinen J. Premature adrenarche: etiology, clinical findings, and consequences. J Steroid Biochem Mol Biol. 2015;145:226–236. [DOI] [PubMed] [Google Scholar]
- 16. Ibañez L, Potau N, Virdis R, Zampolli M, Terzi C, Gussinyé M, Carrascosa A, Vicens-Calvet E. Postpubertal outcome in girls diagnosed of premature pubarche during childhood: increased frequency of functional ovarian hyperandrogenism. J Clin Endocrinol Metab. 1993;76(6):1599–1603. [DOI] [PubMed] [Google Scholar]
- 17. Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99(4):505–512. [DOI] [PubMed] [Google Scholar]
- 18. Ibáñez L, Potau N, Carrascosa A. Insulin resistance, premature adrenarche, and a risk of the polycystic ovary syndrome (PCOS). Trends Endocrinol Metab. 1998;9(2):72–77. [DOI] [PubMed] [Google Scholar]
- 19. Kousta E. Premature adrenarche leads to polycystic ovary syndrome? Long-term consequences. Ann N Y Acad Sci. 2006;1092(1):148–157. [DOI] [PubMed] [Google Scholar]
- 20. Legro RS. Detection of insulin resistance and its treatment in adolescents with polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2002;15(Suppl 5):1367–1378. [PubMed] [Google Scholar]
- 21. Rosenfield RL. Clinical review: identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(3):787–796. [DOI] [PubMed] [Google Scholar]
- 22. Şiklar Z, Öçal G, Adiyaman P, Ergur A, Berberoğlu M. Functional ovarian hyperandrogenism and polycystic ovary syndrome in prepubertal girls with obesity and/or premature pubarche. J Pediatr Endocrinol Metab. 2007;20(4):475–481. [DOI] [PubMed] [Google Scholar]
- 23. Parker LN, Sack J, Fisher DA, Odell WD. The adrenarche: prolactin, gonadotropins, adrenal androgens, and cortisol. J Clin Endocrinol Metab. 1978;46(3):396–401. [DOI] [PubMed] [Google Scholar]
- 24. Kulle AE, Riepe FG, Melchior D, Hiort O, Holterhus PM. A novel ultrapressure liquid chromatography tandem mass spectrometry method for the simultaneous determination of androstenedione, testosterone, and dihydrotestosterone in pediatric blood samples: age- and sex-specific reference data. J Clin Endocrinol Metab. 2010;95(5):2399–2409. [DOI] [PubMed] [Google Scholar]
- 25. Turcu AF, Nanba AT, Chomic R, Upadhyay SK, Giordano TJ, Shields JJ, Merke DP, Rainey WE, Auchus RJ. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. Eur J Endocrinol. 2016;174(5):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O’Reilly MW, Kempegowda P, Jenkinson C, Taylor AE, Quanson JL, Storbeck KH, Arlt W. 11-oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(3):840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Campana C, Rege J, Turcu AF, Pezzi V, Gomez-Sanchez CE, Robins DM, Rainey WE. Development of a novel cell based androgen screening model. J Steroid Biochem Mol Biol. 2016;156:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okonechnikov K, Conesa A, García-Alcalde F. Qualimap 2: advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics. 2016;32(2):292–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–930. [DOI] [PubMed] [Google Scholar]
- 31. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wakita T, Hayashi T, Yuasa H, Nishioka J, Kawamura J, Suzuki K. Molecular cloning, tissue distribution and androgen regulation of rat protein C inhibitor. FEBS Lett. 1998;429(3):263–268. [DOI] [PubMed] [Google Scholar]
- 34. Sivakumaran S, Zhang J, Kelley KM, Gonit M, Hao H, Ratnam M. Androgen activation of the folate receptor α gene through partial tethering of the androgen receptor by C/EBPα. J Steroid Biochem Mol Biol. 2010;122(5):333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Romanuik TL, Wang G, Holt RA, Jones SJ, Marra MA, Sadar MD. Identification of novel androgen-responsive genes by sequencing of LongSAGE libraries. BMC Genomics. 2009;10(1):476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, Yamamoto KR. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007;21(16):2005–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chey S, Claus C, Liebert UG. Validation and application of normalization factors for gene expression studies in rubella virus-infected cell lines with quantitative real-time PCR. J Cell Biochem. 2010;110(1):118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weihs C, Ligges U, Luebke K, Raabe N. klaR analyzing German business cycles. In: Baier D, Decker R, Schmidt-Thieme L, eds. Data Analysis and Decision Support. Berlin, Germany: Springer-Verlag; 2005:335‒434.
- 39. Kirschner MA, Bardin CW. Androgen production and metabolism in normal and virilized women. Metabolism. 1972;21(7):667–688. [DOI] [PubMed] [Google Scholar]
- 40. Turcu A, Smith JM, Auchus R, Rainey WE. Adrenal androgens and androgen precursors: definition, synthesis, regulation and physiologic actions. Compr Physiol. 2014;4(4):1369–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. du Toit T, Bloem LM, Quanson JL, Ehlers R, Serafin AM, Swart AC. Profiling adrenal 11β-hydroxyandrostenedione metabolites in prostate cancer cells, tissue and plasma: UPC2-MS/MS quantification of 11β-hydroxytestosterone, 11keto-testosterone and 11keto-dihydrotestosterone. J Steroid Biochem Mol Biol. 2017;166:54–67. [DOI] [PubMed] [Google Scholar]
- 42. Rege J, Nakamura Y, Satoh F, Morimoto R, Kennedy MR, Layman LC, Honma S, Sasano H, Rainey WE. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J Clin Endocrinol Metab. 2013;98(3):1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gell JS, Carr BR, Sasano H, Atkins B, Margraf L, Mason JI, Rainey WE. Adrenarche results from development of a 3beta-hydroxysteroid dehydrogenase-deficient adrenal reticularis. J Clin Endocrinol Metab. 1998;83(10):3695–3701. [DOI] [PubMed] [Google Scholar]
- 44. Rege J, Karashima S, Lerario AM, Smith JM, Auchus RJ, Kasa-Vubu JZ, Sasano H, Nakamura Y, White PC, Rainey WE. Age-dependent increases in adrenal cytochrome b5 and serum 5-androstenediol-3-sulfate. J Clin Endocrinol Metab. 2016;101(12):4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miller WL, Tee MK. The post-translational regulation of 17,20 lyase activity. Mol Cell Endocrinol. 2015;408:99–106. [DOI] [PubMed] [Google Scholar]
- 46. Auchus RJ, Geller DH, Lee TC, Miller WL. The regulation of human P450c17 activity: relationship to premature adrenarche, insulin resistance and the polycystic ovary syndrome. Trends Endocrinol Metab. 1998;9(2):47–50. [DOI] [PubMed] [Google Scholar]
- 47. Montalto J, Yong AB, Funder JW, Connelly JF. Serum 5-androstene-3β,17β-diol sulphate, sex hormone binding globulin and free androgen index in girls with premature adrenarche. J Steroid Biochem. 1989;33(6):1149–1154. [DOI] [PubMed] [Google Scholar]
- 48. Stahl NL, Teeslink CR, Beauchamps G, Greenblatt RB. Serum testosterone levels in hirsute women: a comparison of adrenal, ovarian and peripheral vein values. Obstet Gynecol. 1973;41(5):650–654. [PubMed] [Google Scholar]
- 49. Greenblatt RB, Colle ML, Mahesh VB. Ovarian and adrenal steroid production in the postmenopausal woman. Obstet Gynecol. 1976;47(4):383–387. [PubMed] [Google Scholar]
- 50. Nakamura Y, Hornsby PJ, Casson P, Morimoto R, Satoh F, Xing Y, Kennedy MR, Sasano H, Rainey WE. Type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J Clin Endocrinol Metab. 2009;94(6):2192–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Doberne Y, Levine LS, New MI. Elevated urinary testosterone and androstanediol in precocious adrenarche. Pediatr Res. 1975;9(10):794–797. [DOI] [PubMed] [Google Scholar]
- 52. Korth-Schutz S, Levine LS, New MI. Evidence for the adrenal source of androgens in precocious adrenarche. Acta Endocrinol (Copenh). 1976;82(2):342–352. [DOI] [PubMed] [Google Scholar]
- 53. Voutilainen R, Perheentupa J, Apter D. Benign premature adrenarche: clinical features and serum steroid levels. Acta Paediatr Scand. 1983;72(5):707–711. [DOI] [PubMed] [Google Scholar]
- 54. Dorn LD, Rose SR, Rotenstein D, Susman EJ, Huang B, Loucks TL, Berga SL. Differences in endocrine parameters and psychopathology in girls with premature adrenarche versus on-time adrenarche. J Pediatr Endocrinol Metab. 2008;21(5):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Llkltmaskul S, Cowell CT, Donaghue K, Kreutzmann DJ, Howard NJ, Blades B, Silink M. ‘Exaggerated adrenarche’ in children presenting with premature adrenarche. Clin Endocrinol (Oxf). 1995;42(3):265–272. [DOI] [PubMed] [Google Scholar]
- 56. Liimatta J, Laakso S, Utriainen P, Voutilainen R, Palvimo JJ, Jääskeläinen T, Jääskeläinen J. Serum androgen bioactivity is low in children with premature adrenarche. Pediatr Res. 2014;75(5):645–650. [DOI] [PubMed] [Google Scholar]
- 57. Strushkevich N, Gilep AA, Shen L, Arrowsmith CH, Edwards AM, Usanov SA, Park HW. Structural insights into aldosterone synthase substrate specificity and targeted inhibition. Mol Endocrinol. 2013;27(2):315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Swart AC, Schloms L, Storbeck KH, Bloem LM, Toit T, Quanson JL, Rainey WE, Swart P. 11β-hydroxyandrostenedione, the product of androstenedione metabolism in the adrenal, is metabolized in LNCaP cells by 5α-reductase yielding 11β-hydroxy-5α-androstanedione. J Steroid Biochem Mol Biol. 2013;138:132–142. [DOI] [PubMed] [Google Scholar]
- 59. Swart AC, Storbeck KH. 11β-Hydroxyandrostenedione: downstream metabolism by 11βHSD, 17βHSD and SRD5A produces novel substrates in familiar pathways. Mol Cell Endocrinol. 2015;408:114–123. [DOI] [PubMed] [Google Scholar]
- 60. Storbeck KH, Bloem LM, Africander D, Schloms L, Swart P, Swart AC. 11β-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer? Mol Cell Endocrinol. 2013;377(1-2):135–146. [DOI] [PubMed] [Google Scholar]
- 61. Pretorius E, Africander DJ, Vlok M, Perkins MS, Quanson J, Storbeck KH. 11-Ketotestosterone and 11-ketodihydrotestosterone in castration resistant prostate cancer: potent androgens which can no longer be ignored. PLoS One. 2016;11(7):e0159867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Arlt W, Callies F, van Vlijmen JC, Koehler I, Reincke M, Bidlingmaier M, Huebler D, Oettel M, Ernst M, Schulte HM, Allolio B. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Engl J Med. 1999;341(14):1013–1020. [DOI] [PubMed] [Google Scholar]
- 63. Arlt W, Justl HG, Callies F, Reincke M, Hübler D, Oettel M, Ernst M, Schulte HM, Allolio B. Oral dehydroepiandrosterone for adrenal androgen replacement: pharmacokinetics and peripheral conversion to androgens and estrogens in young healthy females after dexamethasone suppression. J Clin Endocrinol Metab. 1998;83(6):1928–1934. [DOI] [PubMed] [Google Scholar]
- 64. Ke Y, Labrie F, Gonthier R, Simard JN, Bergeron D, Martel C, Vaillancourt M, Montesino M, Lavoie L, Archer DF, Balser J, Moyneur E; other participating members of the Prasterone Clinical Research Group . Serum levels of sex steroids and metabolites following 12 weeks of intravaginal 0.50% DHEA administration. J Steroid Biochem Mol Biol. 2015;154:186–196. [DOI] [PubMed] [Google Scholar]
- 65. Labrie F, Cusan L, Gomez JL, Côté I, Bérubé R, Bélanger P, Martel C, Labrie C. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol. 2008;111(3-5):178–194. [DOI] [PubMed] [Google Scholar]
- 66. Martel C, Labrie F, Archer DF, Ke Y, Gonthier R, Simard JN, Lavoie L, Vaillancourt M, Montesino M, Balser J, Moyneur É; other participating members of the Prasterone Clinical Research Group . Serum steroid concentrations remain within normal postmenopausal values in women receiving daily 6.5mg intravaginal prasterone for 12 weeks. J Steroid Biochem Mol Biol. 2016;159:142–153. [DOI] [PubMed] [Google Scholar]
- 67. Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SS. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf). 1998;49(4):421–432. [DOI] [PubMed] [Google Scholar]
- 68. O’Reilly MW, Kempegowda P, Walsh M, Taylor AE, Manolopoulos KN, Allwood JW, Semple RK, Hebenstreit D, Dunn WB, Tomlinson JW, Arlt W. AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(9):3327–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Idkowiak J, Lavery GG, Dhir V, Barrett TG, Stewart PM, Krone N, Arlt W. Premature adrenarche: novel lessons from early onset androgen excess. Eur J Endocrinol. 2011;165(2):189–207. [DOI] [PubMed] [Google Scholar]
- 70. Kim SH, Moon JY, Sasano H, Choi MH, Park MJ. Body fat mass is associated with ratio of steroid metabolites reflecting 17,20-lyase activity in prepubertal girls. J Clin Endocrinol Metab. 2016;101(12):4653–4660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.