Abstract
Anorexia nervosa is a psychiatric disease characterized by a low-weight state due to self-induced starvation. This disorder, which predominantly affects women, is associated with hormonal adaptations that minimize energy expenditure in the setting of low nutrient intake. These adaptations include GH resistance, functional hypothalamic amenorrhea, and nonthyroidal illness syndrome. Although these adaptations may be beneficial to short-term survival, they contribute to the significant and often persistent morbidity associated with this disorder, including bone loss, which affects >85% of women. We review the hormonal adaptions to undernutrition, review hormonal treatments that have been studied for both the underlying disorder as well as for the associated decreased bone mass, and discuss the important challenges that remain, including the lack of long-term treatments for bone loss in this chronic disorder and the fact that despite recovery, many individuals who experience bone loss as adolescents have chronic deficits and an increased risk of fracture in adulthood.
Essential Points
Anorexia nervosa is a psychiatric disorder characterized by self-induced starvation and the inability to maintain a normal body weight
Nearly 90% of women with anorexia nervosa have low bone mineral density, and the disorder is associated with an increased risk of fracture
The bone loss in anorexia nervosa is primarily the result of hormonal adaptations to chronic caloric deprivation
Both hormonal and nonhormonal therapeutic strategies may be effective in reducing the bone loss associated with this disorder
Anorexia nervosa is a psychiatric disorder characterized by low body weight due to self-induced undernutrition. This disease predominantly affects women, with a lifetime prevalence approaching 2.2% (1). Typically presenting during adolescence (2), the long-term recovery rate for anorexia nervosa is low. Only 50% to 60% of women with anorexia nervosa have recovered even two decades after the initial diagnosis, and therefore the disease is also prevalent in older individuals (3, 4).
Anorexia nervosa is associated with multiple medical complications and comorbidities, the most common of which is significant loss of bone mass (5). Almost 90% of women with anorexia nervosa have bone mineral density (BMD) values >1 SD below the mean of comparably aged women (5, 6). Studies of bone microarchitecture and geometry in individuals with anorexia nervosa demonstrate that this disease affects both cortical and trabecular bone compartments; adolescent girls with anorexia nervosa have decreased cortical area and thickness and increased cortical porosity and trabecular separation, as well as reduced estimates of bone strength compared with normal-weight controls (7, 8), and these differences in microarchitectural parameters have been shown to be independent of BMD (9). Similarly, adult women with anorexia nervosa have worsened trabecular and cortical parameters compared with normal-weight controls, including decreased trabecular number and thickness, increased trabecular separation, and decreased cortical thickness as well as lower estimates of bone strength (10–13). Importantly, the low bone mass and impaired microarchitecture are associated with an increased risk of fracture in both adults and adolescents with this disease (14–17).
In anorexia nervosa, loss of bone mass is the result of hormonal adaptations to the state of starvation. Our evolutionary past was marked by periods of famine, and therefore several hormonal adaptations have evolved that minimize the utilization of energy on processes not necessary for survival, including reproduction and growth. Both starvation and anorexia nervosa, a form of chronic undernutrition, are characterized by hypogonadotropic hypogonadism, GH resistance, and hypercortisolemia, all of which confer a survival advantage in the short term in the setting of extreme nutrient deficiency. However, these factors also contribute to low bone mass and an increased risk of fracture in the long term. Nearly 30% of adolescent and adult women report a past history of fracture (5, 16), and a prospective study of young women with anorexia nervosa demonstrated a sevenfold increased risk of fracture when compared with similarly aged normal-weight women (14). Because anorexia nervosa presents most commonly during adolescence, a critical time for bone accrual, and because of the chronicity of the disease, the risk of fracture persists many years after diagnosis, as demonstrated by a population-based cohort study that found a 57% cumulative incidence of fracture in individuals with anorexia nervosa (18). Therefore, understanding the hormonal adaptations to undernutrition and how they contribute to the prevalent bone loss and fracture risk will be critical to determining strategies to prevent these complications. In this review, we discuss the hormonal perturbations that occur during periods of nutrient deficiency, their effect on bone mass, and potential hormonal and nonhormonal therapies for the treatment of the associated bone loss and/or failure to accrue peak bone mass.
Deviations From Normal Bone Modeling and Remodeling in Anorexia Nervosa
Anorexia nervosa has a lifetime prevalence in women of ∼2.2% (1), with a peak age of onset of 13 to 18 years of age (19). Adolescence is a time of bone modeling and accrual, with peak calcium accretion occurring at a mean of 12.5 years of age (±0.9 SD) in girls (20); therefore, alterations in the rate of bone formation during adolescence may have lifelong impact with respect to bone structure and fragility. Although histomorphometric data in adolescents with anorexia nervosa do not exist, markers of bone formation have been used as a surrogate for bone formation in this population, as bone formation markers have been shown to be correlated with histomorphometrically derived mineral apposition rates in adults (21). As compared with normal-weight adolescents, girls with anorexia nervosa have lower levels of bone formation markers and similar levels of surrogate markers of bone resorption (22–24), suggestive of overall decreased bone accrual and a low remodeling state. A study by Seeman et al. (25) exploited differences in rates of bone growth and accrual in the axial vs appendicular skeleton (26) before as compared with after puberty to demonstrate differential sites of deficits in individuals with anorexia nervosa, depending on when anorexia nervosa developed. Girls who developed anorexia nervosa before 15 years of age were found to have similar deficits in bone width in the vertebral body and femoral neck, whereas those who developed anorexia nervosa during puberty (15.1 to 19 years of age) had a greater reduction in vertebral body width as compared with femoral neck width likely due to the fact that there is an axial growth spurt during puberty and appendicular growth occurs more rapidly before puberty (25, 26). These reductions in bone size, coupled with deficits in bone mineral accrual, which the authors observed correlated with duration of disease, have important implications for site of future fracture risk in women with a history of anorexia nervosa (25) and may explain persistently low BMD in adults who were diagnosed with anorexia nervosa during adolescence but have recovered from the disease (27).
In contrast to adolescents with anorexia nervosa who have low levels of bone formation markers and similar levels of bone resorption markers as compared with healthy controls, adult women with anorexia nervosa have suppressed markers of bone formation and elevated levels of bone resorption markers when compared with healthy controls (28–31). This difference may be due to the fact that prepubertal adolescents all have low estrogen levels, whereas most adult women with anorexia nervosa are hypoestrogenemic relative to healthy, normal-weight women (see “Hypoestrogenemia” below), and estrogen deficiency is associated with accelerated bone remodeling and increased osteoclast longevity (32–35). Therefore, in contrast to the likely low remodeling state in adolescents with anorexia nervosa, in adult women hypoestrogenemia may result in greater remodeling activity but with resultant bone loss due to decreased bone formation within each basic multicellular unit—a unit of space within bone consisting of osteoblasts and osteoclasts where remodeling occurs (32). Because histomorphometric data in women with anorexia nervosa are scant—there are a total of six reports in the literature of tetracycline-labeled biopsies in women with anorexia nervosa (36–38)—bone formation and resorption markers are a surrogate means of understanding bone remodeling in this disease. The first report of a tetracycline-labeled biopsy in anorexia nervosa was of a single adult patient who sustained a fracture of the proximal femur (36); histomorphometry demonstrated minimal osteoblastic activity and increased resorptive surfaces and a slight elevation in osteoclast number (36), consistent with the bone turnover marker data in adults.
Although markers of bone formation and resorption are simply surrogate markers of osteoblast and osteoclast activity, the differences observed in adults as compared with adolescents may explain differences observed in response to treatments for low BMD in adults with anorexia nervosa as compared with adolescents. For example, although 12 months of treatment with bisphosphonates significantly increased spine and hip BMD in adult women with anorexia nervosa as compared with placebo-treated patients (39) (see “Treatment of Low BMD in Anorexia Nervosa” below), in adolescent girls, there was no significant difference in change in spine or femoral neck BMD in those treated with 12 months of bisphosphonates as compared with placebo (40). This difference may be due to the fact that bisphosphonates are antiresorptive agents and act by decreasing rates of bone remodeling (41). Therefore, bisphophonates may have more of an effect in hypoestrogenemic adults with anorexia nervosa who likely have a higher rate of remodeling as compared with adolescents with anorexia nervosa and their overall low remodeling state.
Hormonal Adaptations to Nutrient Deprivation
For hormonal adaptations to starvation potentially contributing to the low-bone-mass state of anorexia nervosa, see Table 1 (42–61).
Table 1.
Hormonal Adaptations to Starvation Potentially Contributing to the Low-Bone-Mass State of Anorexia Nervosa
| Levels in Anorexia Nervosa | Association With Bone | References | |
|---|---|---|---|
| IGF-1 | Low due to GH resistance | Low IGF-1 levels are associated with decreased BMD in anorexia nervosa | (42, 43) |
| Estrogen | Low due to hypogonadotropic hypogonadism | Duration of amenorrhea is associated with decreased BMD in anorexia nervosa | (44) |
| Testosterone | Low | Low testosterone levels are associated with low BMD and worsened parameters of bone microarchitecture in anorexia nervosa | (11, 45, 46) |
| Cortisol | Elevated | High cortisol levels are associated with decreased BMD and decreased markers of bone formation in anorexia nervosa | (47–50) |
| DHEA | Low | Low DHEA levels are associated with low BMD in anorexia nervosa and increased markers of bone resorption | (46, 51–53) |
| FGF21 | Low or similar to normal-weight controls | FGF21 levels are associated with worsened parameters of bone microarchitecture | (54–57) |
| Leptin | Low | Low leptin levels are associated with decreased BMD in anorexia nervosa and worsened microarchitectural parameters | (11, 58–61) |
Functional hypothalamic amenorrhea
Hypoestrogenemia
Most postpubertal girls and women with anorexia nervosa are amenorrheic, and this was previously part of the diagnostic criteria for the disease prior to the current version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (62, 63). Amenorrhea in anorexia nervosa is a result of disruption of the GnRH secretory pattern, which results in low-amplitude LH pulsatility (64–68). Although the resulting hypogonadotropic hypogonadism diverts energy away from the costly process of reproduction during periods of nutrient deficiency, the hypoestrogenemic state is an important factor contributing to the loss of bone mass in anorexia nervosa. Estrogen deficiency is characterized by higher rates of bone remodeling, an increase in surrogate markers of bone resorption, and bone loss (32, 34, 35, 69), and in anorexia nervosa, duration of amenorrhea is correlated with decreased spine and femoral neck BMD (44, 70). Importantly, although there is normalization of the GnRH secretory pattern with weight recovery (68), because this disorder affects girls and women during the time of critical bone mass accrual, the impact of the amenorrhea can be long term (18, 27). A study comparing women with anorexia nervosa who developed amenorrhea before as compared with after the age of 18 years found that those who developed amenorrhea during adolescence had significantly lower spine BMD as compared with those who developed amenorrhea after the age of 18 years, despite both groups having similar durations of amenorrhea (44). Therefore, the time of onset of amenorrhea, and specifically its relationship to puberty, is also an important predictor of future BMD (25).
Although estrogen deficiency is an important contributor to the loss of bone mass in anorexia nervosa, treatment with oral estrogen does not improve BMD in women with this disease. Although retrospective studies suggested beneficial effects of oral estrogen in the form of oral contraceptive pills (70), randomized and/or prospective studies of oral estrogen have shown no difference in BMD in those receiving oral estrogen as compared with placebo (71–73), except in the subset of very low–weight patients (<70% of ideal body weight) (71). Importantly, compliance with estrogen use was monitored and confirmed in at least one of the studies by vaginal smears for evaluation of epithelial estrogenization (71). In contrast, in a study investigating the effects of predominantly transdermal estrogen replacement in adolescents with anorexia nervosa, we demonstrated a 2.6% increase in spine BMD after 18 months compared with the untreated group whose spine BMD increased <0.5% (74); importantly, however, the BMD in the treated group still remained lower than in normal controls (74). It is not known why there is a difference in BMD response to oral as compared with transdermal estrogen in anorexia nervosa, but the difference may be due to the route of estrogen administration and/or the doses of estrogen used. For example, in postmenopausal women, oral estrogen has been shown to suppress IGF-1—a bone anabolic hormone that is already decreased in states of undernutrition (42) and levels of which have been positively associated with BMD in women with anorexia nervosa (43)—whereas transdermal estrogen does not have the same IGF-1–suppressing effect (75, 76). Furthermore, higher doses of oral estrogen, such as those found in oral contraceptive pills, suppress IGF-1 more than do lower doses of oral estrogen (76). Studies are currently underway investigating the role of physiologic, transdermal estrogen replacement in the treatment of low bone mass in adult women with anorexia nervosa. It is important to note that the subset of women with anorexia nervosa who are eumenorrheic and do not develop hypogonadism also have low BMD (77), underscoring the fact that hypoestrogenemia is not the only factor contributing to loss of bone mass in this population.
Low testosterone
Levels of testosterone, an androgen produced in part by the ovary, adrenal glands, and peripheral conversion of androgen precursors, are also low in women with anorexia nervosa (45, 46) and have been associated with low BMD and worsened parameters of bone microarchitecture (11, 46). However, a randomized, double-blind, study in women with anorexia nervosa found no effect of transdermal testosterone on BMD at a dose targeted to keep testosterone levels in the normal range (39). Therefore, low levels of testosterone are not likely a significant contributor to loss of bone mass in this population.
GH resistance
Acquired GH resistance is another important adaptation that decreases energy expenditure in states of undernutrition (78–81), but it is also an important contributor to the loss of bone mass in anorexia nervosa. GH resistance is characterized by elevated levels of GH coincident with low levels of IGF-1, a hormone secreted by the liver in response to GH stimulation. Elevated levels of GH are critical during periods of starvation, as they help maintain a state of euglycemia (82) in part through fat mobilization (83), but the growth-promoting effects of GH, mediated predominantly by IGF-1, would be maladaptive during periods of nutrient deficiency. Therefore, GH resistance is a hormonal adaptation that allows for an uncoupling of the GH–IGF-1 axis during starvation.
Ghrelin as a mediator of GH resistance
In the setting of undernutrition, GH levels rise in part through a positive feedback response to the low IGF-1 levels (84) and via stimulation by ghrelin, an orexigenic hormone secreted by the fundal cells of the stomach. Ghrelin levels drop in response to food intake, and both girls and women with anorexia nervosa have higher ghrelin levels as compared with normal-weight controls (85–87). By attaching to its receptor, the GH secretagogue receptor (GHSR) 1a, the active form of ghrelin, acylated ghrelin, stimulates GH release and thereby is an important mediator of increased GH levels.
Despite having higher total ghrelin levels as compared with normal-weight controls, women with anorexia nervosa appear to be resistant to its effects. Ghrelin is an appetite-stimulating hormone and a potent stimulator of gastric motility, and even though their levels of ghrelin are higher, women with anorexia nervosa report experiencing less hunger compared with controls, as measured by a visual analog scale (88, 89). Similarly, delayed gastric emptying is a common finding in anorexia nervosa with rates reported to approach 50% to 80% (90–92). It is not known whether this observed resistance to ghrelin is due to differences in the ratio of the active form of ghrelin (acylated ghrelin) to total ghrelin or resistance at the level of, or downstream of, GHSR1a (93, 94). Recently, in a double-blind, randomized placebo-controlled study, we have shown that 4 weeks of treatment with a GHSR1a agonist in women with anorexia nervosa significantly decreases gastric emptying time and leads to a trend in weight gain as compared with women randomized to placebo (95). Importantly, although we were not able to quantitatively detect changes in hunger between subjects randomized to the GHSR1a agonist and those randomized to placebo, three subjects dropped out of the study or stopped using the treatment due to symptoms of increased hunger and all three were randomized to the GHSR1a agonist (95). Therefore, this study suggests that treatment with a GHSR1a agonist can potentially overcome the observed resistance to the effects of endogenously secreted ghrelin and that the resistance does not occur downstream from the receptor. Further studies will be necessary to determine whether this will be a safe, effective, long-term treatment for individuals with anorexia nervosa.
Women with anorexia nervosa may also be resistant to other effects of ghrelin, including potential bone anabolic effects. In rodent models, GHSR1a has been identified on osteoblast-like cells and ghrelin treatment has been shown to increase cell proliferation (96). In vivo administration of ghrelin in rat models also leads to increases in BMD (96). In humans, ghrelin levels are positively associated with BMD in normal-weight girls, but not in adolescent girls with anorexia nervosa (97, 98). In fact, in adolescent girls with anorexia nervosa there is an inverse association between baseline ghrelin level and longitudinal change in BMD (98). Whether treatment with a ghrelin agonist in women with anorexia nervosa overcomes the resistance to the potential bone anabolic effects of ghrelin is not known.
“Weight recovery, coupled with a resumption of menses, remains the most effective long-term treatment for bone loss in anorexia nervosa.”
Nutrient regulation of IGF-1
Therefore, in part through the effects of ghrelin, levels of GH are elevated in anorexia nervosa but levels of IGF-1—the hormone secreted by the liver in response to GH stimulation and that mediates most of the growth-promoting effects of GH—are low. In addition to being regulated by GH, IGF-1 is also exquisitely sensitive to nutritional cues. Levels of IGF-1 decrease by 40% after only 4 days of fasting in normal women (99) and they are 50% lower in women with anorexia nervosa as compared with normal-weight controls (42). Of note, the decrease in IGF-1 levels in healthy, fasting women was accompanied by a significant decrease in markers of bone formation (99). Despite a continued fast for an additional 6 days, administration of recombinant human IGF-1 led to markedly increased surrogate markers of bone formation (99), demonstrating the relationship between IGF-1 and bone loss during starvation. IGF-1 levels also rise dramatically (by 50%) after 3 days of refeeding therapy in women with anorexia nervosa (28), again demonstrating the exquisite regulation of IGF-1 by nutrient availability. Given the fact that IGF-1 levels are low in chronic starvation in the setting of normal or elevated GH levels, the effects of nutritional status supersede those of GH in the regulation of IGF-1. This is supported by the fact that 12 weeks of treatment with supraphysiologic doses of recombinant human GH do not increase IGF-1 levels in women with anorexia nervosa as compared with those treated with placebo, despite the fact that fat mass significantly decreases in those treated with GH (100). These data suggest that the effects of GH that are critical for survival during starvation are maintained, whereas the growth-promoting effects of GH are minimized in anorexia nervosa.
Low levels of IGF-1, a bone anabolic hormone, are also an important contributor to the loss of bone mass in individuals with anorexia nervosa. IGF-1 has been shown to be a chemotactic factor, inducing osteoblast recruitment (101), and in an in vitro rodent model, IGF-1 increases bone collagen content (102). In women with anorexia nervosa, IGF-1 levels are positively associated with BMD (43) and treatment with recombinant human IGF-1 increases surrogate markers of bone formation in anorexia nervosa (103). Nine months of treatment with recombinant human IGF-1 in combination with oral estrogen increases lumbar spine BMD by 1.8% in women with anorexia nervosa (73), further demonstrating the important contribution of low IGF-1 levels to low bone mass in this disease. The impact on BMD of coadministration of IGF-1 with estrogen to adolescents with anorexia nervosa is currently under investigation.
Fibroblast growth factor 21
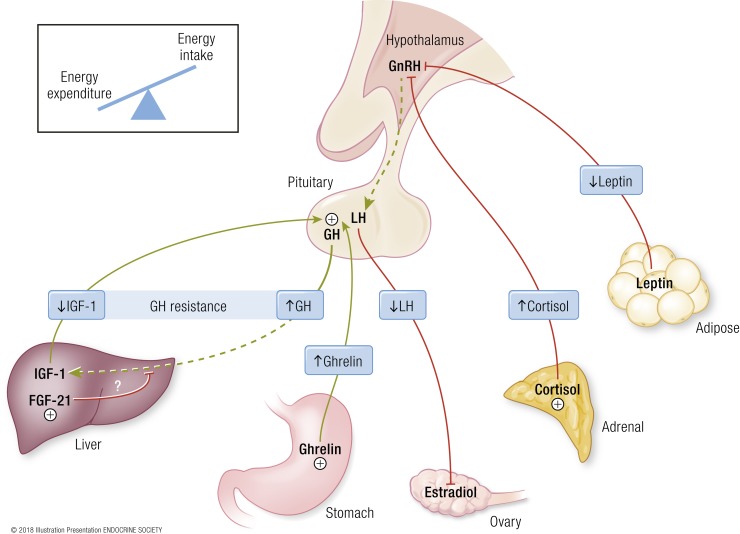
Although the mechanism by which IGF-1 secretion is regulated by nutrient status in humans is not known, fibroblast growth factor (FGF)21 may be an important factor (Fig. 1). FGF21 is a hormone secreted by the liver in states of starvation in both mice (104–106) and humans (107). FGF21 transgenic mice are phenotypically similar to individuals with anorexia nervosa; that is, their core body temperature and body weight are lower than those of wild-type littermates (106, 108) and they manifest GH resistance (109). The failure of GH to induce IGF-1 secretion in FGF21 transgenic mice is secondary to a reduction in STAT5, a key transcription factor in GH signaling (109). In anorexia nervosa, FGF21 may also be a key mediator of GH resistance; FGF21 levels are inversely associated with IGF-1 and positively associated with GH area under the curve (55) in adolescent girls with anorexia nervosa. In murine models and the human model of chronic starvation, that is, anorexia nervosa, FGF21 has also been associated with decreased bone mass, worsened bone microarchitecture parameters, and decreased bone strength (56, 110). Whether these effects are independent of or due to FGF21’s effects on IGF-1 is not known.
Figure 1.
Neuroendocrine adaptations to starvation contributing to loss of bone mass in anorexia nervosa. GH resistance—normal or elevated GH levels in the setting of low IGF-1 levels—may be mediated by FGF21 in states of starvation (55, 109). Low estradiol levels, secondary to hypothalamic amenorrhea, are likely a consequence of low leptin levels and elevated cortisol levels. Low IGF-1 levels, low estradiol levels, and elevated cortisol levels all contribute to the loss of bone mass in anorexia nervosa. Dashed lines represent disruption of the normal, physiologic pathway. [© 2018 Illustration Presentation ENDOCRINE SOCIETY]
Adrenal steroids
Hypercortisolemia
States of physiologic stress, including starvation, can lead to activation of the hypothalamic–pituitary–adrenal axis and an increase in levels of cortisol, an important counterregulatory hormone (111). In anorexia nervosa, cortisol levels are higher than those in normal-weight controls and may be frankly elevated in a subset of patients (47–50). The hypercortisolemia, which is the result in part of a decrease in cortisol clearance and an increase in the half-life of cortisol in the setting of a normal circadian pattern of cortisol secretion (48), has been shown to normalize with weight recovery (111, 112). Importantly, elevated cortisol levels are likely a significant contributor to the loss of bone mass in states of undernutrition. The mechanisms by which elevated cortisol levels contribute to the loss of bone mass include (1) a decrease in calcium absorption in the intestine and likely an increase in urinary calcium excretion (113–115), (2) a decrease in bone formation due to both a reduction in osteoblast proliferation (116) as well as a decrease in IGF-1 synthesis in bone-specific cells (117), and (3) increased bone resorption through inhibition of GnRH-induced gonadotropin secretion (118) and potentially via increased PTH receptor expression in osteoblasts (119). In clinical studies of individuals with anorexia nervosa, adolescent girls have been shown to have a relative decrease in calcium absorption and increase in urinary calcium excretion concomitant with increased urinary free cortisol levels (120). Various measures of cortisol, including urinary cortisol and pooled overnight cortisol levels, have also been negatively associated with both markers of bone formation and BMD in girls and women with anorexia nervosa (44, 49, 50). Therefore, increased cortisol levels are observed in states of undernutrition, including anorexia nervosa, and are likely an important mediator of the loss of bone mass.
Dehydroepiandrosterone
In contrast to cortisol, women with anorexia nervosa have low levels of an adrenal androgen precursor, dehydroepiandrosterone (DHEA), and its sulfated form, DHEA-S (51, 52), which have been associated with increased bone resorption (53) and decreased BMD (46). There have been several studies investigating the effects of treatment with DHEA on BMD in anorexia nervosa. Treatment with DHEA for 6 months or 1 year did not improve BMD in girls and women randomized to DHEA as compared with placebo (121, 122), whereas 18 months of treatment in conjunction with oral contraceptives led to maintenance of both BMD and hip structural geometry compared with the placebo-treated group in whom a loss of these parameters was observed (123, 124). Therefore, low levels of DHEA may be a factor in the loss of bone mass in anorexia nervosa.
Nonthyroidal illness syndrome
Individuals with anorexia nervosa have low levels of thyroid hormone levels (T3 and T4) with normal levels of TSH and elevated levels of reverse T3, which are hallmarks of the nonthyroidal illness syndrome (125–128). Low T3 and T4 levels without an expected increase in TSH suggests a central source of suppression and is likely an adaptive response to decrease energy expenditure in the setting of poor nutrient availability. T3 levels increase with weight gain in anorexia nervosa (129, 130) and are positively associated with changes in resting energy expenditure (131), demonstrating the reversibility of this adaptive response.
Although loss of bone mass is characteristic of states of hyperthyroidism (132), low levels of thyroid hormone and possibly TSH may also be associated with abnormal skeletal development and homeostasis (133). Thyroid hormone receptors have been identified on human osteoblasts (134), and in murine models, mice lacking thyroid hormone receptors have decreased trabecular BMD and increased marrow adiposity (135), a characteristic finding in girls and women with anorexia nervosa that has been inversely associated with BMD (136, 137). IGF-1, a bone anabolic hormone, also increases with normalization of thyroid hormone levels in individuals with hypothyroidism and levels of IGF-1 are positively associated with free T3 and T4 levels (138), suggesting another mechanism by which low levels of thyroid hormone may affect BMD. Despite this possible contribution of low thyroid hormone levels to the low-bone-mass state in anorexia nervosa, nonthyroidal illness syndrome is an adaptive mechanism that likely prevents unnecessary energy expenditure during nutrient deprivation and therefore should not be treated with thyroid hormone replacement.
Alterations in Adipokine Levels and Levels of Appetite-Regulating Hormones
For hormonal adaptations to starvation potentially contributing to the low-bone-mass state of anorexia nervosa and other potential mediators of low bone mass in anorexia nervosa, see Tables 1 and 2 (139–148), respectively.
Table 2.
Other Potential Mediators of Low Bone Mass in Anorexia Nervosa
| Levels in Anorexia Nervosa | Association With Bone | References | |
|---|---|---|---|
| Ghrelin | Elevated | Ghrelin positively associated with BMD in normal-weight adolescents and inversely associated with change in BMD in adolescent girls with anorexia nervosa | (85–87, 97, 98) |
| PYY | Elevated | Elevated PYY levels are associated with decreased BMD in girls and women with anorexia nervosa | (98, 139, 140) |
| Adiponectin | Elevated in most studies | Adiponectin is inversely associated with BMD in anorexia nervosa | (141–144) |
| Amylin | Low | Amylin is positively associated with BMD in anorexia nervosa | (145) |
| Oxytocin | Low | Low oxytocin levels are associated with decreased BMD | (146, 147) |
| Sclerostin | Elevated | No known association between sclerostin and spine or hip BMD in anorexia nervosa | (148) |
| DKK1 | Low | No known association between DKK1 and BMD in anorexia nervosa | (148) |
| Pref-1 | Elevated | Pref-1 is inversely associated with BMD in anorexia nervosa | (60) |
| Marrow adipose tissue | Elevated | Marrow adipose tissue is inversely associated with BMD | (136, 137) |
Low leptin levels
Levels of leptin, an anorexigenic hormone secreted predominantly by subcutaneous adipose tissue, are decreased in states of starvation, including anorexia nervosa (59, 149). Leptin is likely an important mediator between nutrient availability and hypogonadotropic hypogonadism. In women with functional hypothalamic amenorrhea, LH pulse frequency increases after only 2 weeks of treatment with recombinant human leptin (150), and in a randomized placebo-controlled study, more women with functional hypothalamic amenorrhea recovered menstruation in the group treated with recombinant human leptin as compared with placebo-treated patients (151). In women with functional hypothalamic amenorrhea, treatment with recombinant human leptin increases osteocalcin and bone-specific alkaline phosphatase levels, two markers of bone formation (150), and in anorexia nervosa, leptin is positively associated with BMD (60, 61) and microarchitectural parameters (11). However, an important potential side effect of treatment with recombinant human leptin is weight loss, which may be due to decreased appetite (150). In a randomized, placebo-controlled study of 20 women with hypothalamic amenorrhea (151), one participant had to be removed from the study due to significant weight loss (>8% of baseline body weight) and 4 additional participants out of the 11 subjects in the recombinant human leptin group required dose reductions due to weight loss (151). Although changes in appetite were not directly measured, self-reported caloric intake was similar in both groups during the course of the study (151). The treatment group also had a significant loss of fat mass (mean loss of 2.02 kg) during the 36-week study compared with participants in the placebo group, but whether this was due to leptin-induced lipid mobilization could not be determined (151). Therefore, until the mechanisms underlying the loss of weight and fat mass are better delineated so that these side effects can be prevented, recombinant human leptin is not a plausible treatment for bone loss in individuals with anorexia nervosa.
Elevated peptide YY levels
Girls and women with anorexia nervosa have significantly higher levels of peptide YY (PYY), an intestinally secreted hormone with anorexigenic properties (139, 140). Whether these inappropriately elevated levels contribute to the decreased sensation of hunger reported by individuals with anorexia nervosa (89) and the decreased nutrient intake is not known. Importantly, elevated levels of PYY may contribute to the loss of bone mass in anorexia nervosa. In animal models, the PYY receptor system inhibits bone formation; the Y1 receptor is located both in neuronal tissue as well as bone, and osteoblast-specific Y1 receptor knockout mice have increased trabecular thickness, number, and increased femoral trabecular bone volume due to increased osteoblast activity (152). Similarly, mice who lack the Y2 receptor have increased trabecular bone volume, trabecular number, and trabecular thickness (153). Additionally, whereas PYY knockout mice have increased BMD in the lumbar vertebrae and greater trabecular bone volume in the femur as compared with wild-type mice, PYY overexpressing female mice have decreased femoral BMD and decreased trabecular bone volume in the both the femur and lumbar vertebrae as compared with wild-type littermates (154). Elevated PYY has been associated with decreased BMD in both girls and women with anorexia nervosa (98, 140), suggesting that this hormone may also be a mediator of the low-bone-mass state characteristic of anorexia nervosa.
Elevated adiponectin levels
Paradoxically, normal-weight individuals have higher levels of adiponectin, an adipocyte-derived hormone, compared with those who are obese (155). In most studies, individuals with anorexia nervosa have higher total adiponectin levels as compared with normal-weight controls (141–144). These higher levels of adiponectin may be an important determinant of bone mass in anorexia nervosa. In vitro studies demonstrate that adiponectin negatively affects bone, predominantly by increasing RANK ligand, an osteoclast activator (156). In murine models, mice that transgenically overexpress adiponectin are observed to have decreased femoral bone mineral content and strength as compared with wild-type littermates of similar weight (157). Similarly, in anorexia nervosa, adiponectin is negatively associated with BMD (141), suggesting that high adiponectin levels may be a determinant of low bone mass in this disease.
Low oxytocin levels
Low oxytocin levels may also be a determinant of low bone mass in anorexia nervosa. Levels of oxytocin, a hormone secreted by the posterior pituitary, are lower in anorexia nervosa than in normal-weight controls (146). Oxytocin, which serves important functions during childbirth and lactation by promoting uterine contractions and mediating milk ejection, may also regulate appetite (158–160) and be a hormonal determinant of bone mass (161). In murine models, mice deficient in oxytocin or its receptor have decreased bone mass (162), and subcutaneous oxytocin treatment in osteoporotic mice improves parameters of trabecular bone microarchitecture (163) and decreases marrow adipocyte number, another possible mediator of low bone mass. In anorexia nervosa, an integrated measure of overnight oxytocin levels obtained by pooling overnight frequent samples, was shown to be positively associated with spine and hip BMD (146, 147).
Low amylin levels
Amylin, a hormone that is cosecreted with insulin by the β cells of the pancreas, is a regulator of bone resorption in mouse models (164). In amylin-deficient mice, cortical and trabecular thickness are decreased as compared with wild-type mice, despite similar body weight, fat pad weight, and food intake (164). Similarly, women with anorexia nervosa have lower amylin levels compared with normal-weight controls, and amylin is positively associated with BMD of the spine and hip (145). Therefore, low amylin levels may also be a significant contributor to low bone mass in anorexia nervosa.
Other Potential Mediators of Loss of Bone Mass
For other potential mediators of low bone mass in anorexia nervosa, see Table 2.
Decreased nutrient intake and electrolyte abnormalities
Calcium and vitamin D
Although women with anorexia nervosa are in a state of negative energy balance due to insufficient caloric intake, inadequate intake of calcium and vitamin D is likely not an important contributor to the low bone mass observed in this disease. Compared with normal-weight girls, adolescent girls with anorexia nervosa consume more calcium and vitamin D, predominantly through supplements (165). Adolescent girls with anorexia nervosa are also less likely to be vitamin D deficient compared with their normal-weight counterparts (165, 166). Therefore, calcium and vitamin D deficiency are not important mediators of loss of bone mass in anorexia nervosa, unless inadequate supplementation causes significant vitamin D deficiency.
Protein
Girls and women with anorexia nervosa are in an overall state of negative energy balance. Importantly, individuals who consume sufficient calories may still be at risk for bone loss in the setting of isolated nutrient deficiencies. For example, isolated protein deficiency has been associated with low IGF-1 levels (167) and decreased cortical bone thickness (168). Low protein intake in the setting of a vegetarian diet in normal-weight individuals has also been associated with low BMD (169). Multiple studies examining macronutritent intake in adolescent girls with anorexia nervosa have shown no difference in protein intake in the individuals with anorexia nervosa as compared with normal-weight controls (139, 165, 170), suggesting that low protein intake does not independently contribute to low BMD in individuals with anorexia nervosa.
Hyponatremia
Low solute intake is associated with a decreased ability to excrete free water and resultant hyponatremia. Therefore, girls and women with anorexia nervosa are at risk for hyponatremia, and 7% to 17% of women with anorexia nervosa have low plasma sodium levels (5, 171). In rodent models, hyponatremia is associated with loss of BMD (172), likely due, in part, to increased osteoclast formation and activity (173). Hyponatremia has also been associated with a 2.87 increased odds of femoral neck osteoporosis in a large population-based study (172), and in studies of both women (174) and men (175), hyponatremia is an independent predictor of fracture. We have shown that in women with anorexia nervosa, those with hyponatremia (plasma sodium <135 mmol/L) have lower BMD at the spine and hip as compared with those without hyponatremia, even after controlling for age, body mass index, and disease duration (176). Therefore, low plasma sodium levels may contribute to the low BMD in individuals with anorexia nervosa.
Exercise
Weight-bearing exercise is an important determinant of BMD in normal populations (177). In a group of healthy, normal-weight and overweight adults randomized to either weight loss through caloric restriction or weight loss through increased physical activity, despite losing similar amounts of weight (a mean of 8.4% to 10.7% weight loss during 12 months), those randomized to caloric restriction lost a mean of 2.2% of BMD at their lumbar spine and hip, whereas those randomized to exercise did not exhibit significant changes in BMD during the 12-month study (178). Therefore, in healthy populations, physical activity in the setting of weight loss appears to have bone-protective effects.
Multiple studies have also investigated the effects of exercise and physical activity on bone in individuals with anorexia nervosa, but in this population, the results have been mixed. A number of studies in both adolescents and adults have shown a positive association between physical activity level and BMD in the hip, spine, and radius (53, 70, 179–181), whereas other studies have shown no correlation between BMD and degree of physical activity (24, 44, 182). Importantly, one study demonstrated a dose-dependent effect, such that individuals who were moderate exercisers, defined as 1 to 6 h/wk of physical activity, had the highest spine and femoral neck BMD compared with individuals who exercised <1 h/wk or >6 h/wk (183). Data collection in these studies was predominantly based on individual recall and self-report of activity, making interpretation of the results even more challenging, but given the fact that physical activity may be associated with delayed menarche and amenorrhea (184) and will result in increased caloric requirements, the totality of the data do not support encouraging exercise as a means of protecting BMD in this population, but moderate physical activity likely need not be discouraged either.
Depression and use of psychotropic medications
Depression is a common comorbidity in girls and women with anorexia nervosa, and depressive symptoms have been associated with low BMD in this population, potentially mediated in part by elevations in cortisol levels (50, 185). Both selective serotonin reuptake inhibitors (SSRIs) and antipsychotics are commonly prescribed medications in this population (186). Mouse models have demonstrated impairments in bone mineral accrual in predominantly weight-bearing sites with SSRI treatment (187). SSRIs have also been associated with decreased BMD in adolescents (188), bone loss in postmenopausal women (189), and an increased risk of fracture (190). Two studies in adolescents with anorexia nervosa have demonstrated that SSRI use is a predictor of low BMD in this population, although depressive symptoms were not controlled for in either study (180, 191). Therefore, whether SSRIs are an independent mediator of low BMD in this population is not known. Atypical antipsychotic medications, another psychotropic medication frequently prescribed in this population (186), have been associated with hyperprolactinemia (192), low BMD (193), and increased fracture risk (194). Thus, it is possible that use of antipsychotic medications also contributes to low BMD in this population.
Marrow fat and preadipocyte factor-1
Marrow adipose tissue may be an important determinant of BMD and fracture risk. We have reported that levels of marrow adipose tissue are elevated in anorexia nervosa, a disease state characterized by low levels of subcutaneous and visceral adipose tissue (136). Although the function of marrow adipose tissue is not currently known, the fact that this fat depot increases in size when lipid is actively being used as an energy source suggests that marrow fat serves an important role. Marrow adipose tissue has been associated with measures of decreased bone integrity (195), and in anorexia nervosa, levels of marrow adipose tissue are inversely associated with BMD (136) and with estimated bone strength (8). However, it is unknown whether marrow adipose tissue is a significant determinant of the increased fracture risk observed in anorexia nervosa. Understanding the determinants of marrow adipose tissue and its association with bone metabolism will be critical to determining the role and function of this fat depot.
One potential determinant of marrow adipose tissue is preadipocyte factor (Pref)-1, a member of the epidermal growth factor family of proteins. Circulating Pref-1 levels are higher in anorexia nervosa as compared with healthy controls, and although Pref-1 has been shown to inhibit both adipocyte and osteoblast differentiation (196), Pref-1 is positively associated with marrow adipose tissue in anorexia nervosa (60, 197). In women with anorexia nervosa, BMD is also inversely associated with Pref-1 (60, 197). Importantly, however, it is not known whether Pref-1’s association with BMD is independent of its effects on marrow adipose tissue. Further studies are necessary to better delineate the relationship between Pref-1, marrow adipose tissue, and BMD.
Sclerostin and dickkopf-related protein 1
Secreted by osteocytes, sclerostin is an inhibitor of bone formation through Wnt signaling inhibition. In rodent models, treatment with a sclerostin monoclonal antibody increases bone mass and strength (198). In postmenopausal women, treatment with a sclerostin monoclonal antibody for 12 months also increases spine and hip BMD (199, 200). It is not known whether sclerostin is a mediator of bone loss in anorexia nervosa. Although sclerostin levels are higher in individuals with anorexia nervosa as compared with normal-weight controls (148), sclerostin does not mediate the increases in BMD observed with physiologic estrogen replacement in girls with anorexia nervosa (201) or the increases in spine BMD observed in teriparatide-treated women with anorexia nervosa (202).
Dickkopf-related protein (DKK)1 is also a Wnt signaling pathway inhibitor. Unlike sclerostin, DKK1 levels are low in anorexia nervosa (148). Although DKK1 levels have been shown to be positively associated with bone formation markers (148) in individuals with anorexia nervosa, no associations between DKK1 and BMD have been observed in this disorder. Therefore, further studies are needed to better understand whether sclerostin and/or DKK1 are mediators of anorexia nervosa–associated bone loss.
Treatment of Low BMD in Anorexia Nervosa
For therapies that have shown efficacy in the treatment of low bone mass in anorexia nervosa, see Table 3 (203).
Table 3.
Therapies That Have Shown Efficacy in the Treatment of Low Bone Mass in Anorexia Nervosa
| Therapy | Change in BMD (Population Studied) | Duration of Treatment | References |
|---|---|---|---|
| Physiologic estrogen replacement | 2.6% increase in spine BMD (adolescents) | 18 mo | (74) |
| IGF-1 plus oral contraceptives | 1.8% increase in spine BMD (adults) | 9 mo | (73) |
| DHEA plus oral contraceptives | Maintenance of BMD and parameters of hip structural geometry as compared with loss of these parameters in placebo group (adolescents and adults) | 18 mo | (123, 124) |
| Bisphosphonates | 3% to 4% increase in spine BMD compared with placebo (adults; no significant increase in BMD in adolescents when compared with placebo) | 12 mo | (39, 40) |
| 2% increase in hip BMD compared with placebo (adults; no significant increase in BMD in adolescents when compared with placebo) | |||
| Teriparatide | 6% to 10% increase in spine BMD (adults) | 6 mo | (202) |
| Low-magnitude mechanical stimulation | No data on BMD. Marker of bone formation (bone-specific alkaline phosphatase) maintained vs a decrease in placebo group (adolescents) | 10 min/d for 5 d | (203) |
Amenorrheic women with anorexia nervosa have a rate of bone loss of 2.6% per year in the spine and 2.4% per year in the hip (204). Weight recovery, coupled with a resumption of menses, remains the most effective long-term treatment for bone loss in women with anorexia nervosa, as recovery results in a 3.1% per year increase in spine BMD and a 1.8% per year increase in hip BMD (204). However, as only 50% to 60% of women with anorexia nervosa recover even two decades after their diagnosis (3, 4), therapeutic treatment options must be considered in this population to reduce the chronic bone loss and the significantly increased fracture risk observed in individuals with anorexia nervosa.
Given the hypogonadotropic hypogonadism characteristic of this disease, hormonal treatments including estrogen, testosterone, and DHEA have been extensively investigated. Whereas oral estrogen, predominantly in the form of oral contraceptive pills, does not improve BMD in adult women (71, 73) or adolescents (72) with anorexia nervosa, 18 months of treatment with physiologic, predominantly transdermal estrogen replacement increases spine BMD by 2.6% in adolescent girls (74). Oral estrogen has been shown to be effective in maintaining BMD in a population of adolescent and adult women with anorexia nervosa when combined with the androgen precursor DHEA (123, 124), and oral estrogen increases spine BMD by 1.8% in adult women with anorexia nervosa when combined with recombinant human IGF-1 for 9 months, although treatment with recombinant human IGH-1 alone showed no effect (73). Therefore, although oral estrogen alone does not increase bone mass, or prevent its decline in anorexia nervosa, an estrogen-replete state appears necessary for other hormonal therapies to affect bone mass.
Other treatments that have been investigated in girls and women with anorexia nervosa include short-term treatments for hospitalized patients as well as more long-term antiresorptive and anabolic treatments. In hospitalized patients, standing on a platform that delivers low-magnitude mechanical stimulation for 10 minutes per day for 5 days prevented decreases in bone-specific alkaline phosphatase, a marker of bone formation (203), and therefore further studies will be necessary to determine whether this is an effective means of reducing loss of bone mass in anorexia nervosa. We have reported that oral bisphosphonate therapy and the anabolic agent teriparatide are very effective in increasing BMD in adults with anorexia nervosa. Treatment with 12 months of an oral bisphosphonate increased spine and hip BMD by 2% to 4% compared with placebo (39), and 6 months of treatment with teriparatide increased spine BMD by 6% to 10% (202) in adult women with anorexia nervosa. In contrast, in a population of adolescents treated with a bisphosphonate for 12 months, there was no significant difference in change in BMD in the treatment group as compared with the placebo group (40), likely due to differences in bone remodeling rates in the adolescent vs adult populations (see “Deviations From Normal Bone Modeling and Remodeling in Anorexia Nervosa” above) and underscoring the importance of evaluating treatments for bone loss in both adult and adolescent populations. Importantly, we do not know the effect of any of these treatments on fractures in individuals with anorexia nervosa and none of the described studies extended beyond 18 months. Additionally, there are no studies investigating how to treat individuals with persistently low BMD after recovery from anorexia nervosa. Therefore, future studies are necessary to better assess the long-term effects and safety of these promising treatments on bone mass in girls and women with anorexia nervosa and to determine the best treatment strategies for individuals with persistently low BMD after recovery.
Conclusions
Anorexia nervosa is a psychiatric illness in which individuals are unable to maintain a normal weight due to inappropriate caloric intake (62, 63). Only ∼50% to 60% of women with this disorder recover >20 years after their initial diagnosis and therefore the state of negative energy balance is chronic for many patients with anorexia nervosa (3, 4). The hormonal adaptations that confer a survival advantage during short periods of nutrient deficiency, including hypogonadotropic hypogonadism and GH resistance, are important mediators of the significant loss of bone mass (5, 6) and increased risk of fracture observed in individuals with anorexia nervosa (5, 14). Given the chronicity of this disorder, therapeutic treatment strategies are critical to prevent this long-term bone loss and increased fracture risk. Although there are no approved treatments for bone loss in this population, a number of therapies have been investigated in short-term studies and have demonstrated efficacy. However, a number of significant unmet challenges remain for the treatment of bone loss in anorexia nervosa. These include the lack of long-term treatment options for loss of bone mass, particularly for the 40% to 50% of patients that do not recover from anorexia nervosa even 20 years after diagnosis (3, 4). Although teriparatide and bisphosphonates have shown promise in increasing BMD in adult women with anorexia nervosa, neither of these treatments is a long-term option owing to the fact that teriparatide is only approved for 24 months of use and because of the increased risk of atypical femoral fractures with long-term bisphosphonate use (205). Additionally, more personalized treatment protocols that address the differential patterns of bone loss that may develop in individuals based on the timing of anorexia nervosa onset (25) may help address the difficult challenge of treating long-term loss of bone mass and increased fracture risk in individuals who developed the disease during adolescence but have since recovered (18, 27). Therefore, future studies are necessary to optimize treatment strategies for the loss of bone mass and increased fracture risk in anorexia nervosa.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health Grants R01 DK052625 (to A.K.), R01 DK062249 (to A.K.), K23 DK094820 (to P.K.F.), and R03 DK106410 (to P.K.F.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Summary: A.K. received grant support from Motus Therapeutics (formerly Rhythm Pharmaceuticals, Boston, MA) for an investigator-initiated study. P.K.F. has nothing to disclose.
Glossary
Abbreviations
- BMD
bone mineral density
- DHEA
dehydroepiandrosterone
- DKK
dickkopf-related protein
- FGF
fibroblast growth factor
- GHSR
GH secretagogue receptor
- Pref
preadipocyte factor
- PYY
peptide YY
- SSRI
selective serotonin reuptake inhibitor
References
- 1. Keski-Rahkonen A, Hoek HW, Susser ES, Linna MS, Sihvola E, Raevuori A, Bulik CM, Kaprio J, Rissanen A. Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry. 2007;164(8):1259–1265. [DOI] [PubMed] [Google Scholar]
- 2. Lucas AR, Crowson CS, O’Fallon WM, Melton LJ III. The ups and downs of anorexia nervosa. Int J Eat Disord. 1999;26(4):397–405. [DOI] [PubMed] [Google Scholar]
- 3. Löwe B, Zipfel S, Buchholz C, Dupont Y, Reas DL, Herzog W. Long-term outcome of anorexia nervosa in a prospective 21-year follow-up study. Psychol Med. 2001;31(5):881–890. [DOI] [PubMed] [Google Scholar]
- 4. Eddy KT, Tabri N, Thomas JJ, Murray HB, Keshaviah A, Hastings E, Edkins K, Krishna M, Herzog DB, Keel PK, Franko DL. Recovery from anorexia nervosa and bulimia nervosa at 22-year follow-up. J Clin Psychiatry. 2017;78(2):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller KK, Grinspoon SK, Ciampa J, Hier J, Herzog D, Klibanski A. Medical findings in outpatients with anorexia nervosa. Arch Intern Med. 2005;165(5):561–566. [DOI] [PubMed] [Google Scholar]
- 6. Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, Herzog D, Klibanski A. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med. 2000;133(10):790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faje AT, Karim L, Taylor A, Lee H, Miller KK, Mendes N, Meenaghan E, Goldstein MA, Bouxsein ML, Misra M, Klibanski A. Adolescent girls with anorexia nervosa have impaired cortical and trabecular microarchitecture and lower estimated bone strength at the distal radius. J Clin Endocrinol Metab. 2013;98(5):1923–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singhal V, Tulsiani S, Campoverde KJ, Mitchell DM, Slattery M, Schorr M, Miller KK, Bredella MA, Misra M, Klibanski A. Impaired bone strength estimates at the distal tibia and its determinants in adolescents with anorexia nervosa. Bone. 2018;106:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bredella MA, Misra M, Miller KK, Madisch I, Sarwar A, Cheung A, Klibanski A, Gupta R. Distal radius in adolescent girls with anorexia nervosa: trabecular structure analysis with high-resolution flat-panel volume CT. Radiology. 2008;249(3):938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Milos G, Spindler A, Rüegsegger P, Seifert B, Mühlebach S, Uebelhart D, Häuselmann HJ. Cortical and trabecular bone density and structure in anorexia nervosa. Osteoporos Int. 2005;16(7):783–790. [DOI] [PubMed] [Google Scholar]
- 11. Lawson EA, Miller KK, Bredella MA, Phan C, Misra M, Meenaghan E, Rosenblum L, Donoho D, Gupta R, Klibanski A. Hormone predictors of abnormal bone microarchitecture in women with anorexia nervosa. Bone. 2010;46(2):458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walsh CJ, Phan CM, Misra M, Bredella MA, Miller KK, Fazeli PK, Bayraktar HH, Klibanski A, Gupta R. Women with anorexia nervosa: finite element and trabecular structure analysis by using flat-panel volume CT. Radiology. 2010;257(1):167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frølich J, Hansen S, Winkler LA, Andresen AK, Hermann AP, Støving RK. The role of body weight on bone in anorexia nervosa: a HR-pQCT study. Calcif Tissue Int. 2017;101(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rigotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR. The clinical course of osteoporosis in anorexia nervosa. A longitudinal study of cortical bone mass. JAMA. 1991;265(9):1133–1138. [PubMed] [Google Scholar]
- 15. Vestergaard P, Emborg C, Støving RK, Hagen C, Mosekilde L, Brixen K. Fractures in patients with anorexia nervosa, bulimia nervosa, and other eating disorders--a nationwide register study. Int J Eat Disord. 2002;32(3):301–308. [DOI] [PubMed] [Google Scholar]
- 16. Faje AT, Fazeli PK, Miller KK, Katzman DK, Ebrahimi S, Lee H, Mendes N, Snelgrove D, Meenaghan E, Misra M, Klibanski A. Fracture risk and areal bone mineral density in adolescent females with anorexia nervosa. Int J Eat Disord. 2014;47(5):458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagata JM, Golden NH, Leonard MB, Copelovitch L, Denburg MR. Assessment of sex differences in fracture risk among patients with anorexia nervosa: a population-based cohort study using the health improvement network. J Bone Miner Res. 2017;32(5):1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lucas AR, Melton LJ III, Crowson CS, O’Fallon WM. Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc. 1999;74(10):972–977. [DOI] [PubMed] [Google Scholar]
- 19. Weaver L, Liebman R. Assessment of anorexia nervosa in children and adolescents. Curr Psychiatry Rep. 2011;13(2):93–98. [DOI] [PubMed] [Google Scholar]
- 20. Bailey DA, Martin AD, McKay HA, Whiting S, Mirwald R. Calcium accretion in girls and boys during puberty: a longitudinal analysis. J Bone Miner Res. 2000;15(11):2245–2250. [DOI] [PubMed] [Google Scholar]
- 21. Eriksen EF, Charles P, Melsen F, Mosekilde L, Risteli L, Risteli J. Serum markers of type I collagen formation and degradation in metabolic bone disease: correlation with bone histomorphometry. J Bone Miner Res. 1993;8(2):127–132. [DOI] [PubMed] [Google Scholar]
- 22. Saggese G, Bertelloni S, Baroncelli GI, Di Nero G. Serum levels of carboxyterminal propeptide of type I procollagen in healthy children from 1st year of life to adulthood and in metabolic bone diseases. Eur J Pediatr. 1992;151(10):764–768. [DOI] [PubMed] [Google Scholar]
- 23. Heer M, Mika C, Grzella I, Heussen N, Herpertz-Dahlmann B. Bone turnover during inpatient nutritional therapy and outpatient follow-up in patients with anorexia nervosa compared with that in healthy control subjects. Am J Clin Nutr. 2004;80(3):774–781. [DOI] [PubMed] [Google Scholar]
- 24. Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab. 1999;84(12):4489–4496. [DOI] [PubMed] [Google Scholar]
- 25. Seeman E, Karlsson MK, Duan Y. On exposure to anorexia nervosa, the temporal variation in axial and appendicular skeletal development predisposes to site-specific deficits in bone size and density: a cross-sectional study. J Bone Miner Res. 2000;15(11):2259–2265. [DOI] [PubMed] [Google Scholar]
- 26. Bass S, Delmas PD, Pearce G, Hendrich E, Tabensky A, Seeman E. The differing tempo of growth in bone size, mass, and density in girls is region-specific. J Clin Invest. 1999;104(6):795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bachrach LK, Katzman DK, Litt IF, Guido D, Marcus R. Recovery from osteopenia in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 1991;72(3):602–606. [DOI] [PubMed] [Google Scholar]
- 28. Hotta M, Fukuda I, Sato K, Hizuka N, Shibasaki T, Takano K. The relationship between bone turnover and body weight, serum insulin-like growth factor (IGF) I, and serum IGF-binding protein levels in patients with anorexia nervosa. J Clin Endocrinol Metab. 2000;85(1):200–206. [DOI] [PubMed] [Google Scholar]
- 29. Weinbrenner T, Zittermann A, Gouni-Berthold I, Stehle P, Berthold HK. Body mass index and disease duration are predictors of disturbed bone turnover in anorexia nervosa. A case-control study. Eur J Clin Nutr. 2003;57(10):1262–1267. [DOI] [PubMed] [Google Scholar]
- 30. Bolton JG, Patel S, Lacey JH, White S. A prospective study of changes in bone turnover and bone density associated with regaining weight in women with anorexia nervosa. Osteoporos Int. 2005;16(12):1955–1962. [DOI] [PubMed] [Google Scholar]
- 31. Viapiana O, Gatti D, Dalle Grave R, Todesco T, Rossini M, Braga V, Idolazzi L, Fracassi E, Adami S. Marked increases in bone mineral density and biochemical markers of bone turnover in patients with anorexia nervosa gaining weight. Bone. 2007;40(4):1073–1077. [DOI] [PubMed] [Google Scholar]
- 32. Seeman E. Estrogen, androgen, and the pathogenesis of bone fragility in women and men. Curr Osteoporos Rep. 2004;2(3):90–96. [DOI] [PubMed] [Google Scholar]
- 33. Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-β. Nat Med. 1996;2(10):1132–1136. [DOI] [PubMed] [Google Scholar]
- 34. Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106(12):1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Finkelstein JS, Lee H, Leder BZ, Burnett-Bowie SA, Goldstein DW, Hahn CW, Hirsch SC, Linker A, Perros N, Servais AB, Taylor AP, Webb ML, Youngner JM, Yu EW. Gonadal steroid-dependent effects on bone turnover and bone mineral density in men. J Clin Invest. 2016;126(3):1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaplan FS, Pertschuk M, Fallon M, Haddad J. Osteoporosis and hip fracture in a young woman with anorexia nervosa. Clin Orthop Relat Res. 1986; (212):250–254. [PubMed]
- 37. Kreipe RE, Hicks DG, Rosier RN, Puzas JE. Preliminary findings on the effects of sex hormones on bone metabolism in anorexia nervosa. J Adolesc Health. 1993;14(4):319–324. [DOI] [PubMed] [Google Scholar]
- 38. Hiramatsu R, Ubara Y, Suwabe T, Hoshino J, Sumida K, Hasegawa E, Yamanouchi M, Hayami N, Sawa N, Takaichi K. Bone histomorphometric analysis in a patient with anorexia nervosa. Bone. 2013;56(1):77–82. [DOI] [PubMed] [Google Scholar]
- 39. Miller KK, Meenaghan E, Lawson EA, Misra M, Gleysteen S, Schoenfeld D, Herzog D, Klibanski A. Effects of risedronate and low-dose transdermal testosterone on bone mineral density in women with anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2011;96(7):2081–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Golden NH, Iglesias EA, Jacobson MS, Carey D, Meyer W, Schebendach J, Hertz S, Shenker IR. Alendronate for the treatment of osteopenia in anorexia nervosa: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2005;90(6):3179–3185. [DOI] [PubMed] [Google Scholar]
- 41. Seeman E. Reduced bone formation and increased bone resorption: rational targets for the treatment of osteoporosis. Osteoporos Int. 2003;14(Suppl 3):S2–S8. [DOI] [PubMed] [Google Scholar]
- 42. Counts DR, Gwirtsman H, Carlsson LM, Lesem M, Cutler GB Jr. The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol Metab. 1992;75(3):762–767. [DOI] [PubMed] [Google Scholar]
- 43. Grinspoon S, Miller K, Coyle C, Krempin J, Armstrong C, Pitts S, Herzog D, Klibanski A. Severity of osteopenia in estrogen-deficient women with anorexia nervosa and hypothalamic amenorrhea. J Clin Endocrinol Metab. 1999;84(6):2049–2055. [DOI] [PubMed] [Google Scholar]
- 44. Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab. 1989;68(3):548–554. [DOI] [PubMed] [Google Scholar]
- 45. van Binsbergen CJ, Coelingh Bennink HJ, Odink J, Haspels AA, Koppeschaar HP. A comparative and longitudinal study on endocrine changes related to ovarian function in patients with anorexia nervosa. J Clin Endocrinol Metab. 1990;71(3):705–711. [DOI] [PubMed] [Google Scholar]
- 46. Miller KK, Lawson EA, Mathur V, Wexler TL, Meenaghan E, Misra M, Herzog DB, Klibanski A. Androgens in women with anorexia nervosa and normal-weight women with hypothalamic amenorrhea. J Clin Endocrinol Metab. 2007;92(4):1334–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Walsh BT, Katz JL, Levin J, Kream J, Fukushima DK, Hellman LD, Weiner H, Zumoff B. Adrenal activity in anorexia nervosa. Psychosom Med. 1978;40(6):499–506. [DOI] [PubMed] [Google Scholar]
- 48. Boyar RM, Hellman LD, Roffwarg H, Katz J, Zumoff B, O’Connor J, Bradlow HL, Fukushima DK. Cortisol secretion and metabolism in anorexia nervosa. N Engl J Med. 1977;296(4):190–193. [DOI] [PubMed] [Google Scholar]
- 49. Misra M, Miller KK, Almazan C, Ramaswamy K, Lapcharoensap W, Worley M, Neubauer G, Herzog DB, Klibanski A. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2004;89(10):4972–4980. [DOI] [PubMed] [Google Scholar]
- 50. Lawson EA, Donoho D, Miller KK, Misra M, Meenaghan E, Lydecker J, Wexler T, Herzog DB, Klibanski A. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab. 2009;94(12):4710–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zumoff B, Walsh BT, Katz JL, Levin J, Rosenfeld RS, Kream J, Weiner H. Subnormal plasma dehydroisoandrosterone to cortisol ratio in anorexia nervosa: a second hormonal parameter of ontogenic regression. J Clin Endocrinol Metab. 1983;56(4):668–672. [DOI] [PubMed] [Google Scholar]
- 52. Winterer J, Gwirtsman HE, George DT, Kaye WH, Loriaux DL, Cutler GB Jr. Adrenocorticotropin-stimulated adrenal androgen secretion in anorexia nervosa: impaired secretion at low weight with normalization after long-term weight recovery. J Clin Endocrinol Metab. 1985;61(4):693–697. [DOI] [PubMed] [Google Scholar]
- 53. Gordon CM, Goodman E, Emans SJ, Grace E, Becker KA, Rosen CJ, Gundberg CM, Leboff MS. Physiologic regulators of bone turnover in young women with anorexia nervosa. J Pediatr. 2002;141(1):64–70. [DOI] [PubMed] [Google Scholar]
- 54. Dostálová I, Kaválková P, Haluzíková D, Lacinová Z, Mráz M, Papezová H, Haluzík M. Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J Clin Endocrinol Metab. 2008;93(9):3627–3632. [DOI] [PubMed] [Google Scholar]
- 55. Fazeli PK, Misra M, Goldstein M, Miller KK, Klibanski A. Fibroblast growth factor-21 may mediate growth hormone resistance in anorexia nervosa. J Clin Endocrinol Metab. 2010;95(1):369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fazeli PK, Faje AT, Cross EJ, Lee H, Rosen CJ, Bouxsein ML, Klibanski A. Serum FGF-21 levels are associated with worsened radial trabecular bone microarchitecture and decreased radial bone strength in women with anorexia nervosa. Bone. 2015;77:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mikolajczak A, Oswiecimska JM, Swietochowska E, Roczniak W, Ziora KT. Serum FGF21 in girls with anorexia nervosa - comparison to normal weight and obese female adolescents. Neuroendocrinol Lett. 2017;38(3):173–181. [PubMed] [Google Scholar]
- 58. Tolle V, Kadem M, Bluet-Pajot MT, Frere D, Foulon C, Bossu C, Dardennes R, Mounier C, Zizzari P, Lang F, Epelbaum J, Estour B. Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J Clin Endocrinol Metab. 2003;88(1):109–116. [DOI] [PubMed] [Google Scholar]
- 59. Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, Herzog DB, Klibanski A. Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289(3):E373–E381. [DOI] [PubMed] [Google Scholar]
- 60. Fazeli PK, Bredella MA, Misra M, Meenaghan E, Rosen CJ, Clemmons DR, Breggia A, Miller KK, Klibanski A. Preadipocyte factor-1 is associated with marrow adiposity and bone mineral density in women with anorexia nervosa. J Clin Endocrinol Metab. 2010;95(1):407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Legroux-Gérot I, Vignau J, Biver E, Pigny P, Collier F, Marchandise X, Duquesnoy B, Cortet B. Anorexia nervosa, osteoporosis and circulating leptin: the missing link. Osteoporos Int. 2010;21(10):1715–1722. [DOI] [PubMed] [Google Scholar]
- 62. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), 4th ed.Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 63. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 64. Wiegelmann W, Solbach HG. Effects of LH-RH on plasma levels of LH and FSH in anorexia nervosa. Horm Metab Res. 1972;4(5):404. [DOI] [PubMed] [Google Scholar]
- 65. Mecklenburg RS, Loriaux DL, Thompson RH, Andersen AE, Lipsett MB. Hypothalamic dysfunction in patients with anorexia nervosa. Medicine (Baltimore). 1974;53(2):147–159. [DOI] [PubMed] [Google Scholar]
- 66. Travaglini P, Beck-Peccoz P, Ferrari C, Ambrosi B, Paracchi A, Severgnini A, Spada A, Faglia G. Some aspects of hypothalamic-pituitary function in patients with anorexia nervosa. Acta Endocrinol (Copenh). 1976;81(2):252–262. [DOI] [PubMed] [Google Scholar]
- 67. Nillius SJ, Fries H, Wide L. Successful induction of follicular maturation and ovulation by prolonged treatment with LH-releasing hormone in women with anorexia nervosa. Am J Obstet Gynecol. 1975;122(8):921–928. [DOI] [PubMed] [Google Scholar]
- 68. Boyar RM, Katz J, Finkelstein JW, Kapen S, Weiner H, Weitzman ED, Hellman L. Anorexia nervosa. Immaturity of the 24-hour luteinizing hormone secretory pattern. N Engl J Med. 1974;291(17):861–865. [DOI] [PubMed] [Google Scholar]
- 69. Riis BJ, Rødbro P, Christiansen C. The role of serum concentrations of sex steroids and bone turnover in the development and occurrence of postmenopausal osteoporosis. Calcif Tissue Int. 1986;38(6):318–322. [DOI] [PubMed] [Google Scholar]
- 70. Seeman E, Szmukler GI, Formica C, Tsalamandris C, Mestrovic R. Osteoporosis in anorexia nervosa: the influence of peak bone density, bone loss, oral contraceptive use, and exercise. J Bone Miner Res. 1992;7(12):1467–1474. [DOI] [PubMed] [Google Scholar]
- 71. Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995;80(3):898–904. [DOI] [PubMed] [Google Scholar]
- 72. Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol. 2002;15(3):135–143. [DOI] [PubMed] [Google Scholar]
- 73. Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab. 2002;87(6):2883–2891. [DOI] [PubMed] [Google Scholar]
- 74. Misra M, Katzman D, Miller KK, Mendes N, Snelgrove D, Russell M, Goldstein MA, Ebrahimi S, Clauss L, Weigel T, Mickley D, Schoenfeld DA, Herzog DB, Klibanski A. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res. 2011;26(10):2430–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Weissberger AJ, Ho KK, Lazarus L. Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women. J Clin Endocrinol Metab. 1991;72(2):374–381. [DOI] [PubMed] [Google Scholar]
- 76. Kam GY, Leung KC, Baxter RC, Ho KK. Estrogens exert route- and dose-dependent effects on insulin-like growth factor (IGF)-binding protein-3 and the acid-labile subunit of the IGF ternary complex. J Clin Endocrinol Metab. 2000;85(5):1918–1922. [DOI] [PubMed] [Google Scholar]
- 77. Miller KK, Grinspoon S, Gleysteen S, Grieco KA, Ciampa J, Breu J, Herzog DB, Klibanski A. Preservation of neuroendocrine control of reproductive function despite severe undernutrition. J Clin Endocrinol Metab. 2004;89(9):4434–4438. [DOI] [PubMed] [Google Scholar]
- 78. Garfinkel PE, Brown GM, Stancer HC, Moldofsky H. Hypothalamic-pituitary function in anorexia nervosa. Arch Gen Psychiatry. 1975;32(6):739–744. [DOI] [PubMed] [Google Scholar]
- 79. Scacchi M, Pincelli AI, Caumo A, Tomasi P, Delitala G, Baldi G, Cavagnini F. Spontaneous nocturnal growth hormone secretion in anorexia nervosa. J Clin Endocrinol Metab. 1997;82(10):3225–3229. [DOI] [PubMed] [Google Scholar]
- 80. Støving RK, Veldhuis JD, Flyvbjerg A, Vinten J, Hangaard J, Koldkjaer OG, Kristiansen J, Hagen C. Jointly amplified basal and pulsatile growth hormone (GH) secretion and increased process irregularity in women with anorexia nervosa: indirect evidence for disruption of feedback regulation within the GH-insulin-like growth factor I axis. J Clin Endocrinol Metab. 1999;84(6):2056–2063. [DOI] [PubMed] [Google Scholar]
- 81. Misra M, Miller KK, Bjornson J, Hackman A, Aggarwal A, Chung J, Ott M, Herzog DB, Johnson ML, Klibanski A. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2003;88(12):5615–5623. [DOI] [PubMed] [Google Scholar]
- 82. Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA. 2010;107(16):7467–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gahete MD, Córdoba-Chacón J, Luque RM, Kineman RD. The rise in growth hormone during starvation does not serve to maintain glucose levels or lean mass but is required for appropriate adipose tissue response in female mice. Endocrinology. 2013;154(1):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gianotti L, Pincelli AI, Scacchi M, Rolla M, Bellitti D, Arvat E, Lanfranco F, Torsello A, Ghigo E, Cavagnini F, Müller EE. Effects of recombinant human insulin-like growth factor I administration on spontaneous and growth hormone (GH)-releasing hormone-stimulated GH secretion in anorexia nervosa. J Clin Endocrinol Metab. 2000;85(8):2805–2809. [DOI] [PubMed] [Google Scholar]
- 85. Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Riepl RL, Heiman ML, Lehnert P, Fichter M, Tschöp M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001;145(5):669–673. [PubMed] [Google Scholar]
- 86. Misra M, Miller KK, Herzog DB, Ramaswamy K, Aggarwal A, Almazan C, Neubauer G, Breu J, Klibanski A. Growth hormone and ghrelin responses to an oral glucose load in adolescent girls with anorexia nervosa and controls. J Clin Endocrinol Metab. 2004;89(4):1605–1612. [DOI] [PubMed] [Google Scholar]
- 87. Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, Herzog DB, Klibanski A. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289(2):E347–E356. [DOI] [PubMed] [Google Scholar]
- 88. Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. [DOI] [PubMed] [Google Scholar]
- 89. Halmi KA, Sunday S, Puglisi A, Marchi P. Hunger and satiety in anorexia and bulimia nervosa. Ann N Y Acad Sci. 1989;575:431–444, discussion 444–445. [DOI] [PubMed] [Google Scholar]
- 90. McCallum RW, Grill BB, Lange R, Planky M, Glass EE, Greenfeld DG. Definition of a gastric emptying abnormality in patients with anorexia nervosa. Dig Dis Sci. 1985;30(8):713–722. [DOI] [PubMed] [Google Scholar]
- 91. Domstad PA, Shih WJ, Humphries L, DeLand FH, Digenis GA. Radionuclide gastric emptying studies in patients with anorexia nervosa. J Nucl Med. 1987;28(5):816–819. [PubMed] [Google Scholar]
- 92. Stacher G, Kiss A, Wiesnagrotzki S, Bergmann H, Höbart J, Schneider C. Oesophageal and gastric motility disorders in patients categorised as having primary anorexia nervosa. Gut. 1986;27(10):1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Holsen LM, Lawson EA, Christensen K, Klibanski A, Goldstein JM. Abnormal relationships between the neural response to high- and low-calorie foods and endogenous acylated ghrelin in women with active and weight-recovered anorexia nervosa. Psychiatry Res. 2014;223(2):94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hotta M, Ohwada R, Katakami H, Shibasaki T, Hizuka N, Takano K. Plasma levels of intact and degraded ghrelin and their responses to glucose infusion in anorexia nervosa. J Clin Endocrinol Metab. 2004;89(11):5707–5712. [DOI] [PubMed] [Google Scholar]
- 95.Fazeli PK, Lawson EA, Faje AT, Eddy KT, Lee H, Fiedorek FT, Breggia A, Gaal IM, DeSanti R, Klibanski A. Treatment with a ghrelin agonist in outpatient women with anorexia nervosa: a randomized clinical trial. J Clin Psychiatry. 2018;79(1):17m11585. [DOI] [PMC free article] [PubMed]
- 96. Fukushima N, Hanada R, Teranishi H, Fukue Y, Tachibana T, Ishikawa H, Takeda S, Takeuchi Y, Fukumoto S, Kangawa K, Nagata K, Kojima M. Ghrelin directly regulates bone formation. J Bone Miner Res. 2005;20(5):790–798. [DOI] [PubMed] [Google Scholar]
- 97. Misra M, Miller KK, Stewart V, Hunter E, Kuo K, Herzog DB, Klibanski A. Ghrelin and bone metabolism in adolescent girls with anorexia nervosa and healthy adolescents. J Clin Endocrinol Metab. 2005;90(9):5082–5087. [DOI] [PubMed] [Google Scholar]
- 98. Misra M, Prabhakaran R, Miller KK, Goldstein MA, Mickley D, Clauss L, Lockhart P, Cord J, Herzog DB, Katzman DK, Klibanski A. Prognostic indicators of changes in bone density measures in adolescent girls with anorexia nervosa-II. J Clin Endocrinol Metab. 2008;93(4):1292–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Grinspoon SK, Baum HB, Peterson S, Klibanski A. Effects of rhIGF-I administration on bone turnover during short-term fasting. J Clin Invest. 1995;96(2):900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fazeli PK, Lawson EA, Prabhakaran R, Miller KK, Donoho DA, Clemmons DR, Herzog DB, Misra M, Klibanski A. Effects of recombinant human growth hormone in anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2010;95(11):4889–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nakasaki M, Yoshioka K, Miyamoto Y, Sasaki T, Yoshikawa H, Itoh K. IGF-I secreted by osteoblasts acts as a potent chemotactic factor for osteoblasts. Bone. 2008;43(5):869–879. [DOI] [PubMed] [Google Scholar]
- 102. Canalis E. Effect of insulinlike growth factor I on DNA and protein synthesis in cultured rat calvaria. J Clin Invest. 1980;66(4):709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Misra M, McGrane J, Miller KK, Goldstein MA, Ebrahimi S, Weigel T, Klibanski A. Effects of rhIGF-1 administration on surrogate markers of bone turnover in adolescents with anorexia nervosa. Bone. 2009;45(3):493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5(6):426–437. [DOI] [PubMed] [Google Scholar]
- 105. Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000;1492(1):203–206. [DOI] [PubMed] [Google Scholar]
- 106. Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5(6):415–425. [DOI] [PubMed] [Google Scholar]
- 107. Fazeli PK, Lun M, Kim SM, Bredella MA, Wright S, Zhang Y, Lee H, Catana C, Klibanski A, Patwari P, Steinhauser ML. FGF21 and the late adaptive response to starvation in humans. J Clin Invest. 2015;125(12):4601–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115(6):1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008;8(1):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, Goetz R, Mohammadi M, Gerard RD, Dechow PC, Mangelsdorf DJ, Kliewer SA, Wan Y. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc Natl Acad Sci USA. 2012;109(8):3143–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fichter MM, Pirke KM. Effect of experimental and pathological weight loss upon the hypothalamo-pituitary-adrenal axis. Psychoneuroendocrinology. 1986;11(3):295–305. [DOI] [PubMed] [Google Scholar]
- 112. Kling MA, Demitrack MA, Whitfield HJ Jr, Kalogeras KT, Listwak SJ, DeBellis MD, Chrousos GP, Gold PW, Brandt HA. Effects of the glucocorticoid antagonist RU 486 on pituitary-adrenal function in patients with anorexia nervosa and healthy volunteers: enhancement of plasma ACTH and cortisol secretion in underweight patients. Neuroendocrinology. 1993;57(6):1082–1091. [DOI] [PubMed] [Google Scholar]
- 113. Morris HA, Need AG, O’Loughlin PD, Horowitz M, Bridges A, Nordin BE. Malabsorption of calcium in corticosteroid-induced osteoporosis. Calcif Tissue Int. 1990;46(5):305–308. [DOI] [PubMed] [Google Scholar]
- 114. Hansen JW, Gordan GS, Prussin SG. Direct measurement of osteolysis in man. J Clin Invest. 1973;52(2):304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Canalis E. Clinical review 83: Mechanisms of glucocorticoid action in bone: implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab. 1996;81(10):3441–3447. [DOI] [PubMed] [Google Scholar]
- 116. Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, Kirilov M, Mandic V, Takacz A, Schmidt-Ullrich R, Ostermay S, Schinke T, Spanbroek R, Zaiss MM, Angel PE, Lerner UH, David JP, Reichardt HM, Amling M, Schütz G, Tuckermann JP. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010;11(6):517–531. [DOI] [PubMed] [Google Scholar]
- 117. McCarthy TL, Centrella M, Canalis E. Cortisol inhibits the synthesis of insulin-like growth factor-I in skeletal cells. Endocrinology. 1990;126(3):1569–1575. [DOI] [PubMed] [Google Scholar]
- 118. Padmanabhan V, Keech C, Convey EM. Cortisol inhibits and adrenocorticotropin has no effect on luteinizing hormone-releasing hormone-induced release of luteinizing hormone from bovine pituitary cells in vitro. Endocrinology. 1983;112(5):1782–1787. [DOI] [PubMed] [Google Scholar]
- 119. Ureña P, Iida-Klein A, Kong XF, Jüppner H, Kronenberg HM, Abou-Samra AB, Segre GV. Regulation of parathyroid hormone (PTH)/PTH-related peptide receptor messenger ribonucleic acid by glucocorticoids and PTH in ROS 17/2.8 and OK cells. Endocrinology. 1994;134(1):451–456. [DOI] [PubMed] [Google Scholar]
- 120. Abrams SA, Silber TJ, Esteban NV, Vieira NE, Stuff JE, Meyers R, Majd M, Yergey AL. Mineral balance and bone turnover in adolescents with anorexia nervosa. J Pediatr. 1993;123(2):326–331. [DOI] [PubMed] [Google Scholar]
- 121. Gordon CM, Grace E, Emans SJ, Feldman HA, Goodman E, Becker KA, Rosen CJ, Gundberg CM, LeBoff MS. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87(11):4935–4941. [DOI] [PubMed] [Google Scholar]
- 122. Bloch M, Ish-Shalom S, Greenman Y, Klein E, Latzer Y. Dehydroepiandrosterone treatment effects on weight, bone density, bone metabolism and mood in women suffering from anorexia nervosa-a pilot study. Psychiatry Res. 2012;200(2-3):544–549. [DOI] [PubMed] [Google Scholar]
- 123. Divasta AD, Feldman HA, Giancaterino C, Rosen CJ, Leboff MS, Gordon CM. The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa. Metabolism. 2012;61(7):1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. DiVasta AD, Feldman HA, Beck TJ, LeBoff MS, Gordon CM. Does hormone replacement normalize bone geometry in adolescents with anorexia nervosa? J Bone Miner Res. 2014;29(1):151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Moshang T Jr, Parks JS, Baker L, Vaidya V, Utiger RD, Bongiovanni AM, Snyder PJ. Low serum triiodothyronine in patients with anorexia nervosa. J Clin Endocrinol Metab. 1975;40(3):470–473. [DOI] [PubMed] [Google Scholar]
- 126. Miyai K, Yamamoto T, Azukizawa M, Ishibashi K, Kumahara Y. Serum thyroid hormones and thyrotropin in anorexia nervosa. J Clin Endocrinol Metab. 1975;40(2):334–338. [DOI] [PubMed] [Google Scholar]
- 127. Croxson MS, Ibbertson HK. Low serum triiodothyronine (T3) and hypothyroidism in anorexia nervosa. J Clin Endocrinol Metab. 1977;44(1):167–174. [DOI] [PubMed] [Google Scholar]
- 128. Leslie RD, Isaacs AJ, Gomez J, Raggatt PR, Bayliss R. Hypothalamo-pituitary-thyroid function in anorexia nervosa: influence of weight gain. BMJ. 1978;2(6136):526–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Casper RC, Frohman LA. Delayed TSH release in anorexia nervosa following injection of thyrotropin-releasing hormone (TRH). Psychoneuroendocrinology. 1982;7(1):59–68. [DOI] [PubMed] [Google Scholar]
- 130. Moore R, Mills IH. Serum T3 and T4 levels in patients with anorexia nervosa showing transient hyperthyroidism during weight gain. Clin Endocrinol (Oxf). 1979;10(5):443–449. [DOI] [PubMed] [Google Scholar]
- 131. Onur S, Haas V, Bosy-Westphal A, Hauer M, Paul T, Nutzinger D, Klein H, Müller MJ. L-tri-iodothyronine is a major determinant of resting energy expenditure in underweight patients with anorexia nervosa and during weight gain. Eur J Endocrinol. 2005;152(2):179–184. [DOI] [PubMed] [Google Scholar]
- 132. Fraser SA, Anderson JB, Smith DA, Wilson GM. Osteoporosis and fractures following thyrotoxicosis. Lancet. 1971;1(7707):981–983. [DOI] [PubMed] [Google Scholar]
- 133. Bassett JH, Williams GR. Critical role of the hypothalamic-pituitary-thyroid axis in bone. Bone. 2008;43(3):418–426. [DOI] [PubMed] [Google Scholar]
- 134. Abu EO, Bord S, Horner A, Chatterjee VK, Compston JE. The expression of thyroid hormone receptors in human bone. Bone. 1997;21(2):137–142. [DOI] [PubMed] [Google Scholar]
- 135. Kindblom JM, Gevers EF, Skrtic SM, Lindberg MK, Göthe S, Törnell J, Vennström B, Ohlsson C. Increased adipogenesis in bone marrow but decreased bone mineral density in mice devoid of thyroid hormone receptors. Bone. 2005;36(4):607–616. [DOI] [PubMed] [Google Scholar]
- 136. Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(6):2129–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ecklund K, Vajapeyam S, Feldman HA, Buzney CD, Mulkern RV, Kleinman PK, Rosen CJ, Gordon CM. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res. 2010;25(2):298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Miell JP, Taylor AM, Zini M, Maheshwari HG, Ross RJ, Valcavi R. Effects of hypothyroidism and hyperthyroidism on insulin-like growth factors (IGFs) and growth hormone- and IGF-binding proteins. J Clin Endocrinol Metab. 1993;76(4):950–955. [DOI] [PubMed] [Google Scholar]
- 139. Misra M, Miller KK, Tsai P, Gallagher K, Lin A, Lee N, Herzog DB, Klibanski A. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91(3):1027–1033. [DOI] [PubMed] [Google Scholar]
- 140. Utz AL, Lawson EA, Misra M, Mickley D, Gleysteen S, Herzog DB, Klibanski A, Miller KK. Peptide YY (PYY) levels and bone mineral density (BMD) in women with anorexia nervosa. Bone. 2008;43(1):135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Misra M, Miller KK, Cord J, Prabhakaran R, Herzog DB, Goldstein M, Katzman DK, Klibanski A. Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J Clin Endocrinol Metab. 2007;92(6):2046–2052. [DOI] [PubMed] [Google Scholar]
- 142. Tagami T, Satoh N, Usui T, Yamada K, Shimatsu A, Kuzuya H. Adiponectin in anorexia nervosa and bulimia nervosa. J Clin Endocrinol Metab. 2004;89(4):1833–1837. [DOI] [PubMed] [Google Scholar]
- 143. Housova J, Anderlova K, Krizová J, Haluzikova D, Kremen J, Kumstyrová T, Papezová H, Haluzik M. Serum adiponectin and resistin concentrations in patients with restrictive and binge/purge form of anorexia nervosa and bulimia nervosa. J Clin Endocrinol Metab. 2005;90(3):1366–1370. [DOI] [PubMed] [Google Scholar]
- 144. Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome DT, Soliman SS, DelProposto JL, Lumeng CN, Mitra A, Pandit SV, Gallagher KA, Miller JD, Krishnan V, Hui SK, Bredella MA, Fazeli PK, Klibanski A, Horowitz MC, Rosen CJ, MacDougald OA. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20(2):368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wojcik MH, Meenaghan E, Lawson EA, Misra M, Klibanski A, Miller KK. Reduced amylin levels are associated with low bone mineral density in women with anorexia nervosa. Bone. 2010;46(3):796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lawson EA, Donoho DA, Blum JI, Meenaghan EM, Misra M, Herzog DB, Sluss PM, Miller KK, Klibanski A. Decreased nocturnal oxytocin levels in anorexia nervosa are associated with low bone mineral density and fat mass. J Clin Psychiatry. 2011;72(11):1546–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Schorr M, Marengi DA, Pulumo RL, Yu E, Eddy KT, Klibanski A, Miller KK, Lawson EA. Oxytocin and its relationship to body composition, bone mineral density, and hip geometry across the weight spectrum. J Clin Endocrinol Metab. 2017;102(8):2814–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Maïmoun L, Guillaume S, Lefebvre P, Philibert P, Bertet H, Picot MC, Gaspari L, Paris F, Courtet P, Thomas E, Mariano-Goulart D, Bringer J, Renard E, Sultan C. Role of sclerostin and dickkopf-1 in the dramatic alteration in bone mass acquisition in adolescents and young women with recent anorexia nervosa. J Clin Endocrinol Metab. 2014;99(4):E582–E590. [DOI] [PubMed] [Google Scholar]
- 149. Grinspoon S, Gulick T, Askari H, Landt M, Lee K, Anderson E, Ma Z, Vignati L, Bowsher R, Herzog D, Klibanski A. Serum leptin levels in women with anorexia nervosa. J Clin Endocrinol Metab. 1996;81(11):3861–3863. [DOI] [PubMed] [Google Scholar]
- 150. Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351(10):987–997. [DOI] [PubMed] [Google Scholar]
- 151. Chou SH, Chamberland JP, Liu X, Matarese G, Gao C, Stefanakis R, Brinkoetter MT, Gong H, Arampatzi K, Mantzoros CS. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci USA. 2011;108(16):6585–6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lee NJ, Nguyen AD, Enriquez RF, Doyle KL, Sainsbury A, Baldock PA, Herzog H. Osteoblast specific Y1 receptor deletion enhances bone mass. Bone. 2011;48(3):461–467. [DOI] [PubMed] [Google Scholar]
- 153. Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, Herzog H. Hypothalamic Y2 receptors regulate bone formation. J Clin Invest. 2002;109(7):915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wong IP, Driessler F, Khor EC, Shi YC, Hörmer B, Nguyen AD, Enriquez RF, Eisman JA, Sainsbury A, Herzog H, Baldock PA. Peptide YY regulates bone remodeling in mice: a link between gut and skeletal biology. PLoS One. 2012;7(7):e40038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83. [DOI] [PubMed] [Google Scholar]
- 156. Luo XH, Guo LJ, Xie H, Yuan LQ, Wu XP, Zhou HD, Liao EY. Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res. 2006;21(10):1648–1656. [DOI] [PubMed] [Google Scholar]
- 157. Ealey KN, Kaludjerovic J, Archer MC, Ward WE. Adiponectin is a negative regulator of bone mineral and bone strength in growing mice. Exp Biol Med (Maywood). 2008;233(12):1546–1553. [DOI] [PubMed] [Google Scholar]
- 158. Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides. 1989;10(1):89–93. [DOI] [PubMed] [Google Scholar]
- 159. Björkstrand E, Uvnäs-Moberg K. Central oxytocin increases food intake and daily weight gain in rats. Physiol Behav. 1996;59(4-5):947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Lawson EA, Marengi DA, DeSanti RL, Holmes TM, Schoenfeld DA, Tolley CJ. Oxytocin reduces caloric intake in men. Obesity (Silver Spring). 2015;23(5):950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Colaianni G, Tamma R, Di Benedetto A, Yuen T, Sun L, Zaidi M, Zallone A. The oxytocin-bone axis. J Neuroendocrinol. 2014;26(2):53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Tamma R, Colaianni G, Zhu LL, DiBenedetto A, Greco G, Montemurro G, Patano N, Strippoli M, Vergari R, Mancini L, Colucci S, Grano M, Faccio R, Liu X, Li J, Usmani S, Bachar M, Bab I, Nishimori K, Young LJ, Buettner C, Iqbal J, Sun L, Zaidi M, Zallone A. Oxytocin is an anabolic bone hormone. Proc Natl Acad Sci USA. 2009;106(17):7149–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Elabd C, Basillais A, Beaupied H, Breuil V, Wagner N, Scheideler M, Zaragosi LE, Massiéra F, Lemichez E, Trajanoski Z, Carle G, Euller-Ziegler L, Ailhaud G, Benhamou CL, Dani C, Amri EZ. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells. 2008;26(9):2399–2407. [DOI] [PubMed] [Google Scholar]
- 164. Dacquin R, Davey RA, Laplace C, Levasseur R, Morris HA, Goldring SR, Gebre-Medhin S, Galson DL, Zajac JD, Karsenty G. Amylin inhibits bone resorption while the calcitonin receptor controls bone formation in vivo. J Cell Biol. 2004;164(4):509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Misra M, Tsai P, Anderson EJ, Hubbard JL, Gallagher K, Soyka LA, Miller KK, Herzog DB, Klibanski A. Nutrient intake in community-dwelling adolescent girls with anorexia nervosa and in healthy adolescents. Am J Clin Nutr. 2006;84(4):698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Haagensen AL, Feldman HA, Ringelheim J, Gordon CM. Low prevalence of vitamin D deficiency among adolescents with anorexia nervosa. Osteoporos Int. 2008;19(3):289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Isley WL, Underwood LE, Clemmons DR. Dietary components that regulate serum somatomedin-C concentrations in humans. J Clin Invest. 1983;71(2):175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Garn SM, Rohmann CG, Behar M, Viteri F, Guzman MA. Compact bone deficiency in protein-calorie malnutrition. Science. 1964;145(3639):1444–1445. [DOI] [PubMed] [Google Scholar]
- 169. Fontana L, Shew JL, Holloszy JO, Villareal DT. Low bone mass in subjects on a long-term raw vegetarian diet. Arch Intern Med. 2005;165(6):684–689. [DOI] [PubMed] [Google Scholar]
- 170. Fernstrom MH, Weltzin TE, Neuberger S, Srinivasagam N, Kaye WH. Twenty-four-hour food intake in patients with anorexia nervosa and in healthy control subjects. Biol Psychiatry. 1994;36(10):696–702. [DOI] [PubMed] [Google Scholar]
- 171. Mehler PS, Blalock DV, Walden K, Kaur S, McBride J, Walsh K, Watts J. Medical findings in 1,026 consecutive adult inpatient-residential eating disordered patients. Int J Eat Disord. 2018;51(4):305–313. [DOI] [PubMed] [Google Scholar]
- 172. Verbalis JG, Barsony J, Sugimura Y, Tian Y, Adams DJ, Carter EA, Resnick HE. Hyponatremia-induced osteoporosis. J Bone Miner Res. 2010;25(3):554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Barsony J, Sugimura Y, Verbalis JG. Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss. J Biol Chem. 2011;286(12):10864–10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Kinsella S, Moran S, Sullivan MO, Molloy MG, Eustace JA. Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol. 2010;5(2):275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Jamal SA, Arampatzis S, Harrison SL, Bucur RC, Ensrud K, Orwoll ES, Bauer DC. Hyponatremia and fractures: findings from the MrOS study. J Bone Miner Res. 2015;30(6):970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Lawson EA, Fazeli PK, Calder G, Putnam H, Misra M, Meenaghan E, Miller KK, Klibanski A. Plasma sodium level is associated with bone loss severity in women with anorexia nervosa: a cross-sectional study. J Clin Psychiatry. 2012;73(11):e1379–e1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Etherington J, Harris PA, Nandra D, Hart DJ, Wolman RL, Doyle DV, Spector TD. The effect of weight-bearing exercise on bone mineral density: a study of female ex-elite athletes and the general population. J Bone Miner Res. 1996;11(9):1333–1338. [DOI] [PubMed] [Google Scholar]
- 178. Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med. 2006;166(22):2502–2510. [DOI] [PubMed] [Google Scholar]
- 179. Rigotti NA, Nussbaum SR, Herzog DB, Neer RM. Osteoporosis in women with anorexia nervosa. N Engl J Med. 1984;311(25):1601–1606. [DOI] [PubMed] [Google Scholar]
- 180. DiVasta AD, Feldman HA, O’Donnell JM, Long J, Leonard MB, Gordon CM. Effect of exercise and antidepressants on skeletal outcomes in adolescent girls with anorexia nervosa. J Adolesc Health. 2017;60(2):229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Nagata JM, Carlson JL, Golden NH, Murray SB, Long J, Leonard MB, Peebles R. Associations between exercise, bone mineral density, and body composition in adolescents with anorexia nervosa [published online ahead of print 8 June 2018]. Eat Weight Disord. Accessed 17 September 2018. [DOI] [PMC free article] [PubMed]
- 182. Bachrach LK, Guido D, Katzman D, Litt IF, Marcus R. Decreased bone density in adolescent girls with anorexia nervosa. Pediatrics. 1990;86(3):440–447. [PubMed] [Google Scholar]
- 183. Joyce JM, Warren DL, Humphries LL, Smith AJ, Coon JS. Osteoporosis in women with eating disorders: comparison of physical parameters, exercise, and menstrual status with SPA and DPA evaluation. J Nucl Med. 1990;31(3):325–331. [PubMed] [Google Scholar]
- 184. Frisch RE, Gotz-Welbergen AV, McArthur JW, Albright T, Witschi J, Bullen B, Birnholz J, Reed RB, Hermann H. Delayed menarche and amenorrhea of college athletes in relation to age of onset of training. JAMA. 1981;246(14):1559–1563. [PubMed] [Google Scholar]
- 185. Konstantynowicz J, Kadziela-Olech H, Kaczmarski M, Zebaze RM, Iuliano-Burns S, Piotrowska-Jastrzebska J, Seeman E. Depression in anorexia nervosa: a risk factor for osteoporosis. J Clin Endocrinol Metab. 2005;90(9):5382–5385. [DOI] [PubMed] [Google Scholar]
- 186. Fazeli PK, Calder GL, Miller KK, Misra M, Lawson EA, Meenaghan E, Lee H, Herzog D, Klibanski A. Psychotropic medication use in anorexia nervosa between 1997 and 2009. Int J Eat Disord. 2012;45(8):970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Warden SJ, Robling AG, Sanders MS, Bliziotes MM, Turner CH. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology. 2005;146(2):685–693. [DOI] [PubMed] [Google Scholar]
- 188. Feuer AJ, Demmer RT, Thai A, Vogiatzi MG. Use of selective serotonin reuptake inhibitors and bone mass in adolescents: An NHANES study. Bone. 2015;78:28–33. [DOI] [PubMed] [Google Scholar]
- 189. Diem SJ, Blackwell TL, Stone KL, Yaffe K, Haney EM, Bliziotes MM, Ensrud KE. Use of antidepressants and rates of hip bone loss in older women: the study of osteoporotic fractures. Arch Intern Med. 2007;167(12):1240–1245. [DOI] [PubMed] [Google Scholar]
- 190. Wu Q, Bencaz AF, Hentz JG, Crowell MD. Selective serotonin reuptake inhibitor treatment and risk of fractures: a meta-analysis of cohort and case-control studies. Osteoporos Int. 2012;23(1):365–375. [DOI] [PubMed] [Google Scholar]
- 191. Misra M, Le Clair M, Mendes N, Miller KK, Lawson E, Meenaghan E, Weigel T, Ebrahimi S, Herzog DB, Klibanski A. Use of SSRIs may impact bone density in adolescent and young women with anorexia nervosa. CNS Spectr. 2010;15(9):579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Wudarsky M, Nicolson R, Hamburger SD, Spechler L, Gochman P, Bedwell J, Lenane MC, Rapoport JL. Elevated prolactin in pediatric patients on typical and atypical antipsychotics. J Child Adolesc Psychopharmacol. 1999;9(4):239–245. [DOI] [PubMed] [Google Scholar]
- 193. Bolton JM, Targownik LE, Leung S, Sareen J, Leslie WD. Risk of low bone mineral density associated with psychotropic medications and mental disorders in postmenopausal women. J Clin Psychopharmacol. 2011;31(1):56–60. [DOI] [PubMed] [Google Scholar]
- 194. Lee SH, Hsu WT, Lai CC, Esmaily-Fard A, Tsai YW, Chiu CC, Wang J, Chang SS, Lee CC. Use of antipsychotics increases the risk of fracture: a systematic review and meta-analysis. Osteoporos Int. 2017;28(4):1167–1178. [DOI] [PubMed] [Google Scholar]
- 195. Schellinger D, Lin CS, Hatipoglu HG, Fertikh D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol. 2001;22(8):1620–1627. [PMC free article] [PubMed] [Google Scholar]
- 196. Abdallah BM, Jensen CH, Gutierrez G, Leslie RG, Jensen TG, Kassem M. Regulation of human skeletal stem cells differentiation by Dlk1/Pref-1. J Bone Miner Res. 2004;19(5):841–852. [DOI] [PubMed] [Google Scholar]
- 197. Fazeli PK, Bredella MA, Freedman L, Thomas BJ, Breggia A, Meenaghan E, Rosen CJ, Klibanski A. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. J Bone Miner Res. 2012;27(9):1864–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Li X, Warmington KS, Niu QT, Asuncion FJ, Barrero M, Grisanti M, Dwyer D, Stouch B, Thway TM, Stolina M, Ominsky MS, Kostenuik PJ, Simonet WS, Paszty C, Ke HZ. Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J Bone Miner Res. 2010;25(12):2647–2656. [DOI] [PubMed] [Google Scholar]
- 199. McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–420. [DOI] [PubMed] [Google Scholar]
- 200. Langdahl BL, Libanati C, Crittenden DB, Bolognese MA, Brown JP, Daizadeh NS, Dokoupilova E, Engelke K, Finkelstein JS, Genant HK, Goemaere S, Hyldstrup L, Jodar-Gimeno E, Keaveny TM, Kendler D, Lakatos P, Maddox J, Malouf J, Massari FE, Molina JF, Ulla MR, Grauer A. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet. 2017;390(10102):1585–1594. [DOI] [PubMed] [Google Scholar]
- 201. Faje AT, Fazeli PK, Katzman DK, Miller KK, Breggia A, Rosen CJ, Mendes N, Klibanski A, Misra M. Sclerostin levels and bone turnover markers in adolescents with anorexia nervosa and healthy adolescent girls. Bone. 2012;51(3):474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Fazeli PK, Wang IS, Miller KK, Herzog DB, Misra M, Lee H, Finkelstein JS, Bouxsein ML, Klibanski A. Teriparatide increases bone formation and bone mineral density in adult women with anorexia nervosa. J Clin Endocrinol Metab. 2014;99(4):1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. DiVasta AD, Feldman HA, Rubin CT, Gallagher JS, Stokes N, Kiel DP, Snyder BD, Gordon CM. The ability of low-magnitude mechanical signals to normalize bone turnover in adolescents hospitalized for anorexia nervosa. Osteoporos Int. 2017;28(4):1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Miller KK, Lee EE, Lawson EA, Misra M, Minihan J, Grinspoon SK, Gleysteen S, Mickley D, Herzog D, Klibanski A. Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab. 2006;91(8):2931–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Schilcher J, Koeppen V, Aspenberg P, Michaëlsson K. Risk of atypical femoral fracture during and after bisphosphonate use. N Engl J Med. 2014;371(10):974–976. [DOI] [PubMed] [Google Scholar]