Abstract
Background
Blood culture is the standard diagnostic method for typhoid and paratyphoid (enteric) fever in surveillance studies and clinical trials, but sensitivity is widely acknowledged to be suboptimal. We conducted a systematic review and meta-analysis to examine sources of heterogeneity across studies and quantified the effect of blood volume.
Methods
We searched the literature to identify all studies that performed blood culture alongside bone marrow culture (a gold standard) to detect cases of enteric fever. We performed a meta-regression analysis to quantify the relationship between blood sample volume and diagnostic sensitivity. Furthermore, we evaluated the impact of patient age, antimicrobial use, and symptom duration on sensitivity.
Results
We estimated blood culture diagnostic sensitivity was 0.59 (95% confidence interval [CI], 0.54–0.64) with significant between-study heterogeneity (I2, 76% [95% CI, 68%–82%]; P < .01). Sensitivity ranged from 0.51 (95% CI, 0.44–0.57) for a 2-mL blood specimen to 0.65 (95% CI, 0.58–0.70) for a 10-mL blood specimen, indicative of a relationship between specimen volume and sensitivity. Subgroup analysis showed significant heterogeneity by patient age and a weak trend towards higher sensitivity among more recent studies. Sensitivity was 34% lower (95% CI, 4%–54%) among patients with prior antimicrobial use and 31% lower after the first week of symptoms (95% CI, 19%–41%). There was no evidence of confounding by patient age, antimicrobial use, symptom duration, or study date on the relationship between specimen volume and sensitivity.
Conclusions
The relationship between the blood sample volume and culture sensitivity should be accounted for in incidence and next-generation diagnostic studies.
Keywords: blood culture, bone marrow culture, diagnostic accuracy, paratyphoid fever, typhoid fever
Salmonella enterica serovar Typhi is the causative agent of typhoid fever, for which there are an estimated 17.8 million cases per year; S enterica serovars Paratyphi A, B, and C cause a clinically indistinguishable syndrome and contribute an additional 4.6 million cases per year [1, 2]. Most of this burden occurs in low- and middle-income countries (LMICs), where the clinical presentation of typhoid fever overlaps with the symptoms of many other diseases, such as malaria and dengue [3].
For a definitive diagnosis of typhoid or paratyphoid (enteric) fever, the World Health Organization recommends bacterial isolation from blood or bone marrow [4]. Bone marrow culture (the gold standard) is acquired via aspirate of the iliac crest or sternum and has a suggested sensitivity of ~90% after 4 days of culture [5, 6]. However, due to the invasive nature of bone marrow biopsies, the diagnosis of enteric fever in LMICs typically depends on blood culture or the Widal test, an antibody titer test with markedly poor specificity (~77%) [4, 7, 8]. Blood culture has been the de facto diagnostic test for population-based incidence studies, vaccine trials, as well as the reference standard for novel diagnostics [6, 9, 10].
Blood culture diagnostic sensitivity is characterized by heterogeneity, with estimates ranging between 40% and 87% [6]. Some of this variation could be explained by blood sample volume—a relationship that is commonly acknowledged but has never been quantified [6, 11–14]. Specimen volume varies markedly between studies depending on local regulations and/or the patient’s age [9, 15–17]. Moreover, factors such as patient age (independent of specimen volume), symptom duration, previous antimicrobial use, and laboratory culture conditions (broth and agar used, automated culture systems) are hypothesized to influence blood culture sensitivity [6, 13, 18–22].
We conducted a systematic review and meta-analysis to explore how patient attributes and specimen volume contribute to heterogeneity in estimates of blood culture sensitivity. We investigated how the relationship between blood volume and culture sensitivity could be impacted by the age of the patient, the duration of disease before specimen collection, and prior antimicrobial use.
MATERIALS AND METHODS
We conducted our systematic review according to an a priori-specified protocol (see, Supplement S1 & S2) and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting [23]. One author (M.A.) performed the search and screening. The data extraction and risk of bias assessment were conducted in duplicate (M.A. and N.J.S.), and discrepancies were resolved through consensus or by a third reviewer (V.E.P.).
Inclusion Criteria and Search Strategy
We searched Web of Science, MEDLINE, Embase, Global Health, and PubMed Central for studies published before June 19, 2016, without additional date or language restriction. We identified relevant publications using controlled vocabulary and free text related to enteric fever and blood or bone marrow culture (see Supplement S1). We defined eligible publications as epidemiological studies of any design that assessed the sensitivity of blood culture to detect typhoid or paratyphoid among patients who also had at least 1 sample of blood and bone marrow cultured for either infection. We did not include editorials, commentaries, or reviews (see Supplement S2). We screened the title and abstract of the studies identified, confirmed eligibility by full-text review, and identified additional articles by cross-checking the references of original articles and reviews.
Data Extraction
We extracted data on the number of positive and negative blood and bone marrow cultures, as well as the volume of each sample, if reported (see Supplement S3). We also obtained data on the number of additional patients identified by positive cultures of other sites (rose spots, rectal swab, stool, urine). Because some studies did not report the concordance between blood and bone marrow culture results, we calculated the proportion of blood cultures that grew colonies of S Typhi or S Paratyphi (A, B, or C) among individuals with at least 1 positive culture from any site, thereby leveraging as many studies as possible.
Risk of Bias Assessment
We modified the QUADAS-II tool [24] to fit our research question and assessed the risk of bias in patient recruitment, index test (blood culture), reference test (bone marrow culture), or the flow and timing of the 2 cultures (see Supplement S3).
Statistical Analysis
We used an inverse-variance weighted binomial-normal model to estimate the overall diagnostic sensitivity of blood cultures of S Typhi or S Paratyphi and used a random effects model to account for unmeasured heterogeneity between studies, which was quantified using the I2 statistic [25].
To investigate factors that contribute to heterogeneity of blood culture sensitivity estimates across studies, we performed a meta-regression analysis to assess the relationship between blood specimen volume and sensitivity. We specified the relationship between blood culture sensitivity and specimen volume in 3 ways. First, we tested a “null” model in which diagnostic sensitivity is independent of specimen volume. A second and third model assumed that sensitivity had a log-linear relationship with volume using a slope-only and a slope-and-intercept functional form, respectively (see Supplement S4, section 4.1). We compared the models based on Widely Applicable Information Criterion (WAIC) [26]. We also modeled sensitivity using a linear meta-regression model to assess the impact of the functional form on the relationship between specimen volume and sensitivity (see Supplement S4, section 4.1). We verified our results were robust to the definition of “true positives” (positive culture from any site) by re-estimating blood culture sensitivity and its relationship with specimen volume among patients who were specifically bone marrow culture-positive; studies that did not report the number of patients who were blood culture-positive strictly among those who were also positive by bone marrow culture were excluded from this analysis (n = 3).
Next, we performed a subgroup analysis to test whether heterogeneity across studies was attributable to the differences in the age of patients in the study. To evaluate whether age had a confounding or modifying effect on the relationship between specimen volume and blood culture sensitivity, we included age as an independent predictor and interaction term in the primary meta-regression model (see Supplement S4, section 4.2).
We also performed a subgroup analysis on the sensitivity reported in studies according to the date of publication (before 1980, 1980–1990, and after 1990, which partitions the studies into 3 approximately equal groups). Moreover, we performed meta-analyses on the diagnostic risk ratio of the duration of symptoms and prior antibiotic use, and we tested for a modifying effect of specimen volume on these risk ratios (see Supplement S4, section 4.3).
Analyses were performed in R version 3.3.2 and JAGS version 3.4.0 (details are found in Supplement S4, section 4.4).
RESULTS
Inclusion and Characteristics of Studies
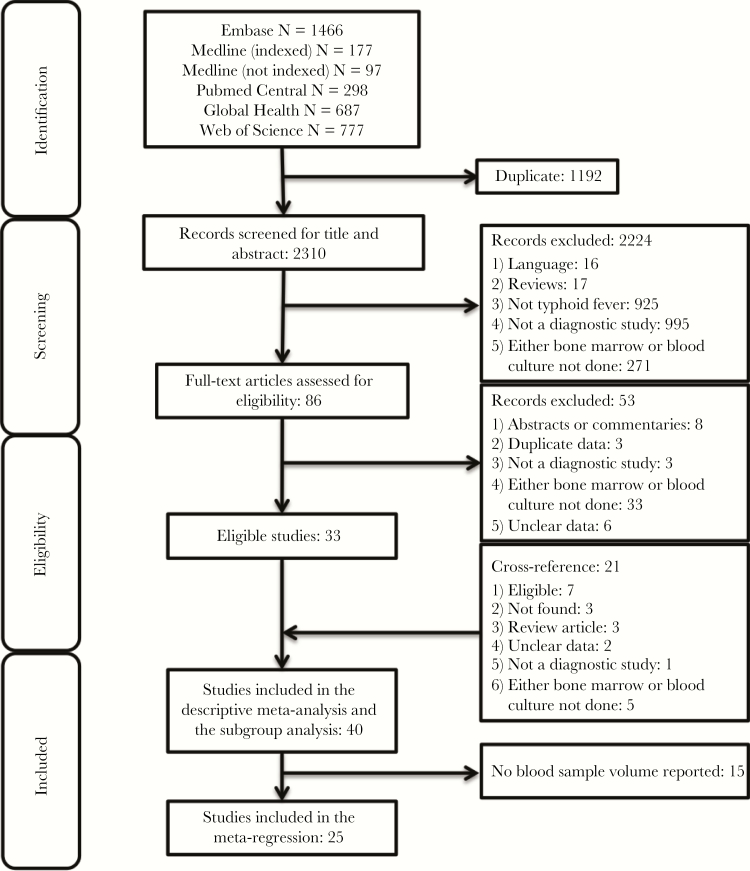
After screening the titles and abstracts of 2310 unique articles identified in our search, 86 articles were identified for full-text screening, yielding a preliminary set of 33 studies for data extraction. After cross-checking the reference lists of the preliminary set and of systematic reviews, we identified an additional 21 articles for full-text review, of which 7 additional studies were eligible (Figure 1). In total, 40 studies were included in the systematic review and meta-analysis, yielding a total of 2051 patients; 25 studies reported blood specimen volume, yielding 1370 patients for the meta-regression analysis. Reasons for exclusion are reported in Figure 1.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram for systematic review. A systematic search in 6 databases yielded 3502 articles, or 2310 unique articles, 86 of which were eligible for full-text review. A total of 40 studies were included in the descriptive synthesis, and 25 studies had blood volume information and were included in our meta-regression analysis of the relationship between blood sample volume and blood culture sensitivity.
Characteristics of the Studies Included
Table 1 presents the characteristics of the included studies; additional details are provided in Supplementary Table S1. All but 4 studies (10%) were conducted in LMICs, and 38 studies (95%) were conducted in urban areas. In most studies, eligibility criteria consisted of “clinical suspicion” of enteric fever (which was rarely described), but in 2 studies (Supplemental Material, Bassily 1980 and Bhutta 1991), the patient populations were individuals suspected of chronic carriage of typhoid or individuals who had prolonged symptoms, respectively. Blood cultures were performed using a variety of techniques (Supplementary Table 1); selective media were generally used, and a few more recent studies have used techniques to improve blood culture sensitivity, such as cell lysis and centrifugation; only 1 study reported using a Bactec (automated) system (see Supplementary Table S1, Escamilla 1982). Three studies (7.5%) were conducted among patients of all ages, 21 studies (52.5%) were performed in a subset of age groups, and 16 studies (40%) did not specify patient age. Seven studies (17.5%) reported sensitivity stratified by antimicrobial use, and 15 studies (37.5%) reported sensitivity according to the time from symptom onset to sample collection.
Table 1.
Study Characteristicsa
| Study (Language, if Other Than English) | Location | Typhi, Paratyphi Included | Eligibility Criteria | Inpatients or Outpatients | Age Distribution of the Sample (in Years)c | How Long the Blood Was Incubated | Broth Used to Culture the Blood | Agar Used to Subculture the Blood | Other Cultures |
|---|---|---|---|---|---|---|---|---|---|
| Akoh 1991 [13] | Zaria, Nigeria | Typhi | Clinical suspicion | Inpatient | Not reported | Not reported | Thioglycolate | Not reported | Urine and stool |
| Avendano 1986 [14] | Santiago, Chile | Typhi, Paratyphi | Clinical suspicion | Inpatient | Children (ages 3–14) | Not reported | Brain heart infusion with sodium polyanetholsulfonate | Salmonella-Shigella, bismuth sulfite, Kliglers triple sugar iron | Bile |
| Baqi Durrani 1996 [15] | Karachi, Pakistan | Typhi, Paratyphi | Clinical suspicion or 4× rise in titers | Both | Older children (ages 5–20) | 7 days | Thioglycolate | Sheep’s blood, MacConkey’s, Salmonella-Shigella agar plates | |
| Barbagallo 1938 (Italian) [16] | Catania, Italy | Typhi, Paratyphi | Clinical suspicion | Inpatient | Not reported | Not reported | Oxoid (trypticase-soy broth) | MacConkey’s, triple sugar iron | |
| Bassily 1980a[17] | Cairo, Egypt | Typhi, Paratyphi | Patients suspected to have chronic salmonellosis | Inpatient | Older children (ages 10–18) | Not reported | Oxgall (oxbile) | Not reported | Urine culture, data not shown |
| Benavente 1981 [18] | Lima, Peru | Unclear or not reported | Not reported | Both | Not reported | Not reported | Oxgall (oxbile) | Not reported | Stool and bile cultures |
| Benavente 1984 [19] | Lima, Peru | Typhi | Clinical suspicion | Unclear or not reported | Not reported | 2+ days | Oxgall (oxbile) | Salmonella-Shigella | Stool cultures, duodenal cultures |
| Bhutta 1991a [20] | Karachi, Pakistan | Typhi | Patients with prior antibiotic treatment or a long duration of illness | Unclear or not reported | Children (age range not reported) | Not reported | Not reported | Not reported | |
| Chaicumpa 1992 [21] | Jakarta, Indonesia | Typhi | Clinical diagnosis | Inpatient | Not reported | 7 days | Oxgall (oxbile) | MacConkey’s, Salmonella-Shigella, desoxycholate-citrate-sucrose-lactose | Urine and stool |
| Chang 1982 (Spanish)a [22] | Lima, Peru | Typhi | Agglutination >1/160 or “significant” rise in titers, or a positive culture | Unclear or not reported | Children (age range not reported) | Not reported | Not reported | Not reported | |
| Chiragh 2005a [23] | Peshawar, Pakistan | Typhi | Clinical suspicion and a fever of more than 4–5 days; no antibiotics taken in previous 3–4 days | Unclear or not reported | Adults (ages 15+) | Not reported | Not reported | Not reported | Urine, data not shown |
| Dance 1991 [24] | Kathmandu, Nepal | Typhi, Paratyphi | Clinical diagnosis | Unclear or not reported | Not reported | Not reported | Brain heart infusion containing liquid | Not reported | |
| Debre 1935 (French)a [25] | Paris, France | Typhi | Not reported | Unclear or not reported | Not reported | Not reported | Meat liver or bile agar | Not reported | |
| Del Negro 1960 (Portuguese)a [26] | Sao Paulo, Brazil | Typhi, Paratyphi | Culture or serological confirmation of disease | Inpatient | Children (ages 0–15) | Not reported | Not reported | Not reported | Bile, urine, and stool |
| Farooqui 1991 [27] | Karachi, Pakistan | Typhi | Patients at Aga Khan hospital who had fever of unknown origin | Unclear or not reported | Not reported | 7 days | Brain heart infusion and thioglycolate | MacConkey’s, blood | |
| Gasem 1995 [28] | Semarang, Indonesia | Typhi | Fever for 6+ days and symptoms of typhoid fever | Inpatient | Adults (ages 14–60; mean 23.1) | 7 days | Oxgall (oxbile) | Salmonella Shigella, triple sugar iron | |
| Gasem 2003 [29] | Semarang, Indonesia | Typhi | Participants of an RCT of antibiotic treatment of typhoid. Pregnant women were excluded | Inpatient | Adults (ages 14+; mean 24.6; SD 7.7 years) | Not reported | Not reported | Bactec 9120 | |
| Gilman 1975 [30] | Mexico City, Mexico | Typhi | Patients with a clinical suspicion of typhoid who consented to a clinical trial of antibiotics | Unclear or not reported | Not reported | Not reported | Peptone | Not reported | Urine, stool and rose spot cultures |
| Guerra-Caceres 1979 [31] | Lima, Peru | Typhi | Clinical suspicion | Inpatient | All ages (mean 16.8; median 14; range 2–54) | 10 days | Oxoid (trypticase-soy broth) for half the specimens, and Ruiz-Castaneda for the other half | Not reported | Urine and stool |
| Hirsowitz 1951b [32] | Evaton, Transvaal, South Africa | Unclear or not reported | Culture or serological evidence of typhoid fever, or post-mortem examination consistent with typhoid fever | Inpatient | Older children and adults (ages 10+) | Not reported | Oxgall (oxbile) and nutrient | Not reported | Urine and stool |
| Hoffman 1984 [10] | Jakarta, Indonesia | Typhi, Paratyphi | Clinical suspicion | Inpatient | Older children and adults (ages 4–60; mean 22; SD 10.3) | 7 days | Oxgall (oxbile) | MacConkey’s, Salmonella-Shigella, desoxycholate-citrate-sucrose-lactose | Bile culture, rose-spot culture |
| Hoffman 1986 [33] | Jakarta, Indonesia | Typhi, Paratyphi | Clinical suspicion | Inpatient | All ages (mean 21.6; SD 9.4) | 21 days | Oxgall (oxbile) | MacConkey’s, Salmonella-Shigella, desoxycholate-citrate-sucrose-lactose | Streptokinase clot culture and rectal swab |
| James 1997b [34] | Pondicherry, India | Typhi, Paratyphi | Clinical suspicion and no evidence of chloramphenicol or bone marrow depressant use at the time of specimen collection | Unclear or not reported | Older children and adults (ages 13–42; mean 22.5) | Not reported | Not reported | Not reported | Urine and stool |
| Ling 1940 [35] | Shanghai, China | Typhi, Paratyphi | Clinical suspicion | Inpatient | Not reported | Not reported | Not reported | Not reported | Urine and stool |
| Ling 1948 [36] | Shanghai, China | Typhi, Paratyphi | Clinical suspicion | Inpatient | Not reported | 7 days | Sodium citrate solution | Endo’s agar | Bile, urine, and stool |
| Mehta 1984 [37] | Jamnagar, India | Unclear or not reported | Clinical suspicion | Inpatient | Older children and adults (ages 12–40) | 2 days | Oxgall (oxbile) | MacConkey’s | |
| Ott 1938 (German)b [38] | Berlin, Germany | Typhi, Paratyphi | Clinical suspicion | Unclear or not reported | Not reported | Not reported | Oxgall (oxbile) | Not reported | Urine and stool |
| Rajagopal 1986 [39] | Bangalore, India | Typhi, Paratyphi | Clinical suspicion | Inpatient | Not reported | Not reported | Oxgall (oxbile) | Not reported | Urine and stool |
| Rubin 1989 [40] | Jakarta, Indonesia | Typhi | Clinical suspicion | Inpatient | Older children and adults (ages 6+) | 7 days | Oxgall (oxbile) | MacConkey’s, Salmonella-Shigella, desoxycholate-citrate-sucrose-lactose | Rectal swabs |
| Sacks 1941b [41] | Baltimore, MD | Typhi | Clinical suspicion | Inpatient | Older children (ages 11–14) | Not reported | Not reported | Not reported | Stool and urine culture |
| Schlack 1966 (Spanish) [42] | Santiago, Chile | Typhi, Paratyphi | Clinical suspicion | Inpatient | Children (ages 8 months–13 years) | Unclear | Meat liver broth | Not reported | |
| Seidenstucker 1949 (German) [43] | Oldenburg, Germany | Typhi, Paratyphi | Not reported | Unclear or not reported | Not reported | 2 days | Oxgall (oxbile) | Not reported | Urine and stool |
| Sekarwana 1989 [44] | Bandung, Indonesia | Typhi | Clinical suspicion and fever of more than 7 days | Unclear or not reported | Children (ages 2–13; mean 6; median 5) | 7 days | Oxgall (oxbile) | Urine and stool | |
| Seshadri 1977 [45] | Madras (current-day Chennai), Tamil Nadu, India | Typhi | More than 5 days of fever with toxemia; gastrointestinal symptoms; soft splenomegaly | Unclear or not reported | Older children and adults (ages 13–35; mean 20; median 19) | Unclear | Oxgall (oxbile) | MacConkey’s | Urine and stool |
| Shin 1994b [46] | Seoul, South Korea | Typhi | Not reported | Unclear or not reported | Adults (ages 20–56) | Not reported | Not reported | Not reported | Urine and stool |
| Storti 1937 (French)b [47] | Paris, France | Typhi | Clinical suspicion | Inpatient | Not reported | Not reported | Not reported | Not reported | |
| Terminel 1973 (Spanish)b [48] | Mexico City, Mexico | Typhi | Clinical suspicion among patients who had rose spots | Unclear or not reported | Not reported | Not reported | Not reported | Not reported | Rose spot culture, stool cultures |
| Vallenas 1985 [49] | Lima, Peru | Typhi | Clinical suspicion | Unclear or not reported | Children (ages 2–13) | Not reported | Oxgall (oxbile) | Not reported | Rectal swab and duodenal culture |
| Wain 2008 [50] | Ho Chi Minh City, Vietnam and Dong Thap, Vietnam | Typhi | Clinical diagnosis | Inpatient | All ages (age range not reported) | 10 days | Oxgall (oxbile), brain heart infusion | Columbia with 0.05% sulphpolyanethosulphonate | Stool, data not shown |
| West 1989b [51] | Goroka, Papua New Guinea | Typhi | Febrile illness | Inpatient | Older children and adults (ages 10–60; mean 26.3) | 21 days | Oxoid (trypticase-soy broth) | Not reported |
Abbreviations: RCT, randomized controlled trial; SD, standard deviation.
aCitation numbers correspond to the reference list in Supplement S8. Additional details are provided in Supplementary Table S1.
bIndicates that the study will only be included in the summary of the systematic review but not in the analysis examining blood sample volume and sensitivity.
cWe categorized the age of study populations according to the following criteria: children, 0–4 years; older children, 5–15 years; adults, 15+ years.
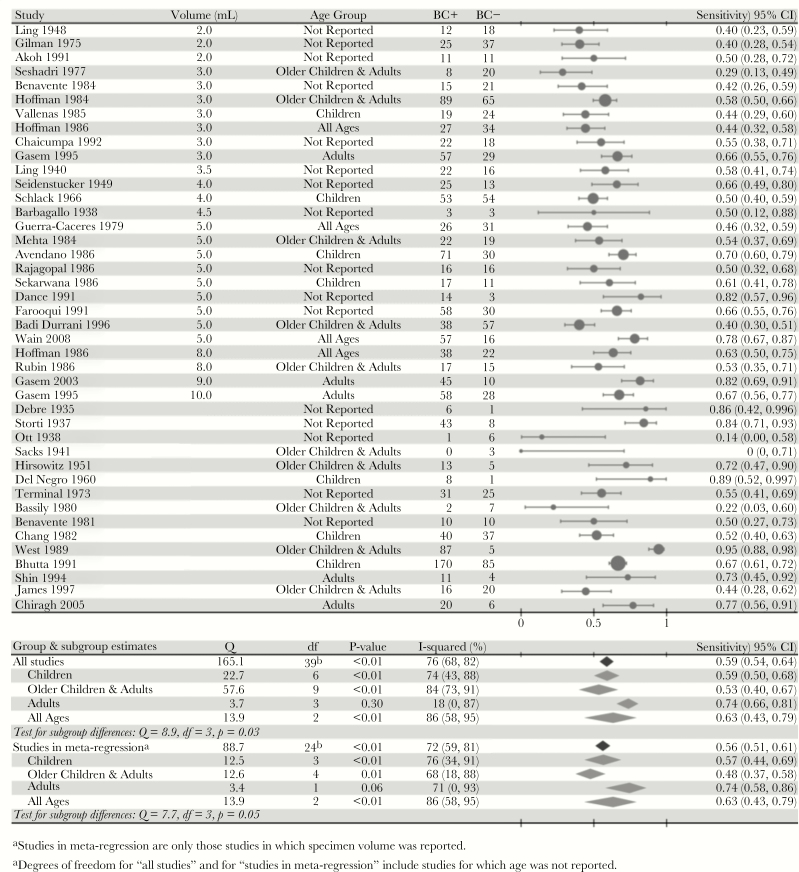
Meta-Analysis
The overall estimate of blood culture sensitivity was 0.59 (95% CI, 0.54–0.64). Figure 2 shows blood culture sensitivity for each study and subgroup estimates of sensitivity according to the age group of patients. Sensitivity was not markedly different among the subset of studies included in our meta-regression analysis (0.56; 95% CI, 0.51–0.61) or among the 34 studies that reported sensitivity among bone-marrow culture-positive patients only (0.60; 95% CI, 0.53–0.65) (Supplementary Figure S1). However, between-study heterogeneity was statistically significant (P < .01) for all of these estimates (Figure 2, Supplementary Figure S1).
Figure 2.
Sensitivity of blood culture to detect typhoid fever. The sensitivity of blood culture is expressed as the proportion of patients who tested positive by blood culture among patients who had at least 1 positive culture (bone marrow, blood, rose spots, stools, or urine) for Salmonella Typhi or Salmonella Paratyphi. The size of the markers is proportional to the number of patients in the study. We reported the midpoint volume of the blood sample for studies that reported specimen volume as a range. We tested for heterogeneity and age-related subgroup differences via the Q-statistic, which is assumed to have a χ2 distribution with degrees of freedom equal to the number of studies minus 1 with noncentrality parameter equal to 0. Hoffman (1986) and Gasem (1995) (Supplemental Material) reported sensitivity on the same patient population using specimens of 2 different volumes per patient, so we have taken only the results from the larger specimen in each study for the subgroup analysis by age to avoid double-counting. Abbreviations: BC+, blood culture-positive; BC−, blood culture-negative; CI, confidence interval.
Specimen Volume
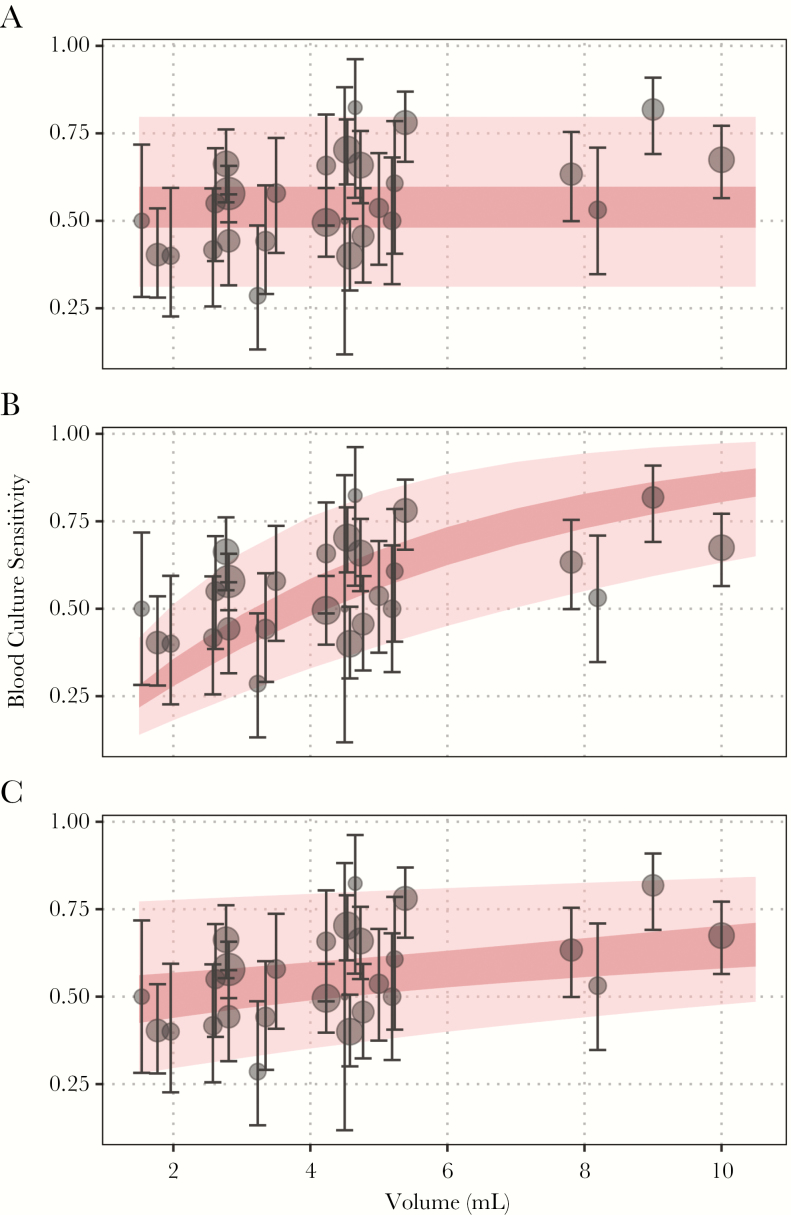
We found support for a positive relationship between sample volume and blood culture sensitivity. The observed and model-predicted relationship between sensitivity and sample volume is plotted in Figure 3; model parameters and goodness-of-fit statistics are displayed in Supplementary Table S2. In the preferred model (the model with the lowest WAIC), blood culture sensitivity shows a marginal gain in sensitivity for each additional milliliter of blood and a “baseline” sensitivity for very small samples. The second-best model was one in which sensitivity did not depend on sample volume, whereas the worst fit was obtained for a model that parameterized the relationship between volume and sensitivity using only a log-linear slope term and a fixed (zero) intercept. The results were similar when we modeled blood culture sensitivity among bone-marrow culture-positive patients only, although the baseline sensitivity was higher and the relationship with blood sample volume was attenuated in this analysis (Supplementary Table S2, Supplementary Figure S2). The results were unchanged when we assumed a linear relationship between specimen volume and blood culture sensitivity (Supplementary Table S6).
Figure 3.
Relationship between sample volume and model estimates of blood culture sensitivity. The observed blood culture sensitivity among all culture-positive cases is plotted in black (with corresponding 95% confidence intervals), whereas the mean model-predicted blood culture sensitivity is plotted in dark pink. The lighter pink regions correspond to the model-predicted population response. (A) The model assumes no correlation with blood volume; (B) the model assumes sensitivity increases with increasing sample volume and is constrained to be zero for a hypothetical 0-mL sample; (C) the model assumes sensitivity could vary with sample volume and estimates an intercept for a hypothetical 0-mL sample. All models account for heterogeneity between studies using random effects (see Supplement S4.1).
The mean sensitivity of blood culture was predicted to vary from 0.51 (95% CI, 0.44–0.57) for a 2-mL specimen to 0.65 (95% CI, 0.58–0.70) for a 10-mL specimen (Table 2). Sensitivity increased by 3% (95% CI, 1%–6%) for each additional milliliter of blood cultured (between 1 and 10 mL) according to the linear model (Supplementary Table S6).
Table 2.
Estimates of Sensitivity by Blood Sample Volumea
| Volume | Mean Prediction | Population Response |
|---|---|---|
| 2 mL | 0.51 (0.44–0.57) | 0.51 (0.30–0.78) |
| 5 mL | 0.56 (0.51–0.61) | 0.56 (0.38–0.80) |
| 7 mL | 0.60 (0.54–0.65) | 0.60 (0.42–0.82) |
| 10 mL | 0.65 (0.58–0.70) | 0.65 (0.46–0.84) |
aThe posterior mean prediction and population response of sensitivity, as well as the corresponding 95% credible intervals, from the log-linear meta-regression model are presented for 2, 5, 7, and 10 mL of blood. The mean prediction is the sensitivity from all studies given a specific volume of blood, whereas the population response is the estimated sensitivity that can be expected from a new study that measures sensitivity with samples of a given volume.
Age
Differences between studies could be significantly (P = .03) attributed to the differences in age groups recruited. The diagnostic sensitivity was comparable in studies of children (0.59; 95% CI, 0.50–0.68) and in studies that included both older children and adults (0.53; 95% CI, 0.40–0.67), but studies that contained only adults had higher sensitivity (0.74; 95% CI, 0.66–0.81). Between-study heterogeneity within each age group was significant except among the studies that recruited adults only (Figure 2). Results of the subgroup analysis restricted to studies in the meta-regression were comparable, as were the results among patients who were bone marrow culture-positive only (Figure 2, Supplementary Figure S1). When we added age to the best-fit model of specimen volume and sensitivity, the model containing an indicator for age category did not provide a better fit (Supplementary Table S3). There were insufficient data to test for age-related effect modification between specimen volume and sensitivity.
Publication Date
There was a subtle trend toward higher sensitivity among studies published after 1990 compared with studies published in 1980–1989 and before 1980 (Supplementary Figure S4), which was only significant among the group of studies included in the meta-regression analysis using data among all culture-positive patients (but not among bone marrow culture-positive patients specifically). There was no evidence that this trend was confounded by patient age or use of improved techniques for culture (Supplementary Table S8). Restricting our analysis of sample volume and sensitivity to studies published after 1980 did not change our choice of best-fit model (Supplementary Table S7, Supplementary Figure S5, and Supplementary Figure S6).
Antimicrobial Use and Duration of Illness
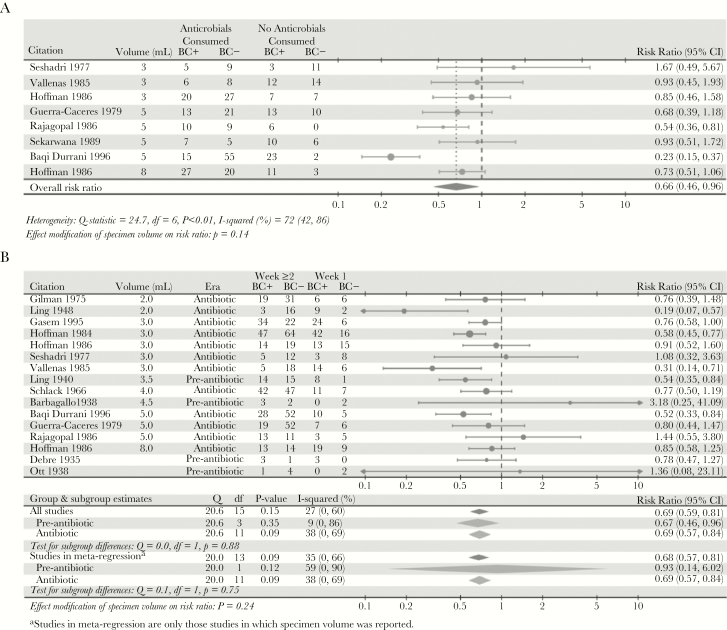
Cultures of specimens taken from patients who had been exposed to antimicrobials were 34% (95% CI, 4%–54%) less sensitive than those from patients with no prior antimicrobial use (Figure 4). Furthermore, cultures performed on specimens collected after the first week of illness were 31% (95% CI, 19%–41%) less sensitive than cultures of specimens collected during the first week (Figure 4). We found no modifying effect of antimicrobial use (P = .14) or duration of symptoms (P = .24) on the relationship between specimen volume and sensitivity. Subgroup analyses showed that the impact of symptom duration on sensitivity was independent of antimicrobial use (P = .88) (Figure 4, Supplementary Table S4).
Figure 4.
The relative probability of a positive blood culture according to patient history. (A) Relative probability of a positive blood culture for enteric fever patients who took antimicrobials versus patients who did not take antimicrobials before specimen collection. (B) Relative probability of a positive blood culture for enteric fever patients who had blood samples taken for culture in the second week of illness or later versus patients who had blood samples taken for culture in the first week of illness. To assess the possible impact of antibiotics on the relationship between duration of symptoms and sensitivity, we tested for a difference in risk ratio of the duration of illness stratified by studies carried out in the pre-antibiotic era and in the antibiotic era. All studies published before 1945 were considered to report the results of sensitivity in the absence of antimicrobial use, and studies published after 1945 were considered to report results that could show an interaction with antimicrobial use.
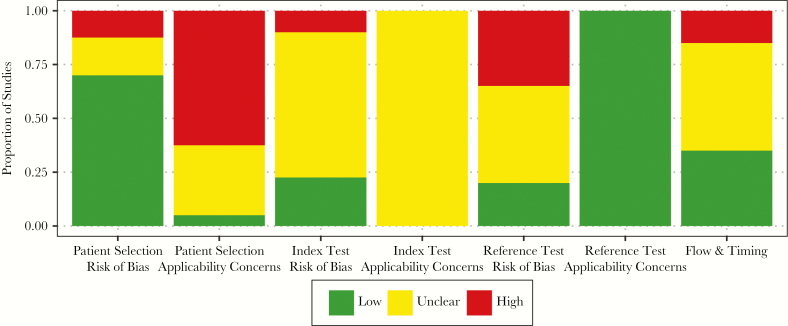
Risk of Bias Assessment
The greatest risk of bias involved concerns over the applicability of patient selection (Figure 5, Supplementary Table S5). Twenty-five studies (62.5%) were performed in inpatient (hospitalized) populations and/or in high-income countries. There was also a high risk of bias regarding the reference test (bone marrow culture) in 14 studies (35%) that cultured less than 1 mL of bone marrow (Supplementary Table S1), potentially missing cases with lower bacteremia. For a detailed discussion of the risk of bias assessment, see Supplement S5.
Figure 5.
Summary findings of the risk of bias assessment using a modified QUADAS-II tool. We evaluated the risk of bias for the 7 domains of the QUADAS-II tool. The modified QUADAS-II tool was integrated into our data extraction form, found in Supplement S3. Detailed findings on the risk of bias assessment are found in Supplementary Table S6 and discussed in Supplement S5.
DISCUSSION
Our systematic review and meta-analysis synthesized the findings of 40 studies and examined factors affecting blood culture diagnostic sensitivity. We identified a significant, although modest, relationship between specimen volume and blood culture sensitivity, resonating with observations that have been made for other infections [27–29], and confirming a relationship that has been postulated but never quantified [6, 11–14]. We estimated that sensitivity of blood culture as a typhoid diagnostic method increases from 51% for 2 mL of blood to 65% for 10 mL, or by 3% for each additional milliliter. Patient age, publication date, antimicrobial use, and duration of symptoms were also associated with changes in blood culture sensitivity, but we found no evidence that these factors confounded or modified the relationship between specimen volume and sensitivity.
Our estimate of the overall sensitivity of blood culture (irrespective of sample volume) is consistent with another recent analysis [14], although the authors of that analysis only identified 10 papers and did not examine the relationship between sensitivity and blood sample volume, antibiotic use, symptom duration, or publication date. Three studies (Supplementary Material; Hoffman 1986, Gasem 1995, Wain 2008) previously examined the relationship between sample volume and blood culture sensitivity directly, but one of these studies did not find conclusive evidence to support the hypothesized relationship (Supplementary Material, Gasem 1995) and another (Supplementary Material, Hoffman 1986) was borderline significant (P = .046 by Pearson’s χ2 test). We believe these studies were underpowered to detect such an effect. In these 2 studies, between 60 and 86 people were recruited, and the difference in volume between the 2 blood samples collected from each patient was 5–7 mL; according to our analysis, over 400 bone-marrow typhoid-positive patients would need to be recruited to detect a significant difference in sensitivity. In all studies that collected 2 blood samples from the same patient, there was no patient for whom a larger sample of blood failed to confirm diagnosis after isolation of bacteria from the smaller sample; this would be highly unlikely if sensitivity were independent of blood volume (P = 3.12 × 10−36 assuming 56% sensitivity, as predicted by the meta-analysis) (Figure 2).
To our knowledge, only 1 study has quantified the relationship between blood sample volume and culture sensitivity for other pathogens; it found that each additional milliliter yielded a 3% increase in sensitivity—consistent with our findings—but the relationship could not be established for any one specific pathogen [28]. However, such an effect has been suggested for Neisseria meningitidis and Streptococcus pneumoniae [30–32]. Only 1 study (which was excluded from this analysis because bone marrow culture was not conducted concurrently) has quantified the effect of age on blood culture sensitivity to detect typhoid [33]. That study found that the density of bacteria is inversely related to age; thus, patient age may confound the relationship between culture volume and sensitivity if smaller samples from children achieve similar sensitivity to larger samples from adults [33, 34]. However, we found the opposite relationship: there was comparable sensitivity in studies consisting of children only and of older children and adults, but higher sensitivity in studies consisting of adults only (Figure 2). Furthermore, there was no evidence of confounding by age when it was included as a predictor in the meta-regression model (Supplementary Table S3).
Sensitivity was also 31% lower among patients whose samples were collected more than 1 week after symptom onset and 34% lower among patients who had been previously exposed to antimicrobials, relationships that had not been assessed in the previous meta-analysis [14]. Similar effects of antimicrobial use on blood culture sensitivity have been observed for N meningitidis [28]. The estimated impact of prior antimicrobial use was consistent with the findings from one study in our review (Supplementary Material, Gasem 2003) found that sensitivity decreased by 60% (95% CI, 39%–80%) after 3 days of antimicrobial use and by 73% (95% CI, 50%–89%) after 5 days of antimicrobial use in patients who were blood and bone marrow culture-positive before treatment. Antimicrobial use in that study was defined as a full course of treatment in a setting with limited antimicrobial resistance and completely observed compliance, whereas other studies defined antimicrobial use differently or in vague terms, which may explain the greater effect. Emerging antimicrobial resistance may diminish the negative impact of prior antimicrobial use on blood culture sensitivity.
The relationship between blood specimen volume and diagnostic sensitivity is weak, and considerable uncertainty remains in blood culture sensitivity even after adjusting for specimen volume. Blood cultures may have a low sensitivity for enteric fever because of low bacterial density in the blood, which is often observed in diseases that lack a specific focus of infection [12, 27, 35–37]. In our analysis, only 1 study used an automated culture system (Supplementary Material, Gasem 2003); there was no secular trend in the use of techniques to improve sensitivity (Supplementary Tables S1 and S8), although there was a weak trend towards higher sensitivity in more modern studies (Supplementary Figure S5). Although the use of automated systems may help to improve culture sensitivity for typhoid, this may not be feasible in many resource-constrained settings where typhoid is endemic; thus, it is also necessary to understand and estimate sensitivity according to the culture methods that are typically used in such settings. Future studies assessing the sensitivity of blood cultures should ideally collect 2 or more blood samples of randomly selected volumes from patients, a sample of bone marrow, and data on age, symptom duration, and antimicrobial use. Our meta-regression framework could then be extended to test the joint effects of patient characteristics on the relationship between specimen volume and sensitivity.
The studies featured in this analysis likely represent a typical typhoid patient population. However, most studies (38 of 40) were exclusively conducted among hospitalized patients, potentially representing more severe cases, whereas only 1%–20% of cases were hospitalized in population-based surveillance studies [16, 38–40]. Moreover, no studies reported the range of symptoms experienced by patients, and, consequently, it remains unclear whether more severely affected patients (potentially with higher bacteremia) were overrepresented in study samples. Furthermore, the sensitivity of bone marrow culture (the gold-standard diagnostic) is itself only ~90%, and <1 mL of bone marrow was collected in 14 studies (Figure 5, Supplementary Table S5). Although we attempted to correct for this by considering patients who were positive according to any specimen as true positives in our primary analysis, some cases may still have been missed, and thus blood culture sensitivity may have been overestimated (Supplementary Figure S8).
The evaluation of next-generation serological diagnostics depends on the appropriate selection of a “gold” (reference) standard to estimate diagnostic accuracy [9, 41–43]. Recent studies of rapid-test diagnostics that used blood cultures as the reference standard collected specimens ranging from 1 to 10 mL [9, 44–46], obfuscating comparisons between studies. Studies that use small blood volumes for culture diagnosis may be selecting for subjects with higher bacteremia, potentially overestimating the sensitivity of the novel diagnostic. Furthermore, such studies may underestimate the specificity of the novel diagnostic test by misclassifying true cases as “false positive” results, especially in high-incidence settings, as shown in a previous modeling study [9, 42]. The data underscores the importance of recruiting typhoid-negative patients from healthy populations or those with a different confirmed infection. Bayesian latent class models have recently been described to account for the imperfect nature of blood culture when evaluating novel diagnostics [44, 47, 48]; our findings can inform prior distributions on the sensitivity of blood culture according to sample volume.
Our findings have important implications for estimates of the burden of typhoid fever and clinical recommendations for the diagnosis of enteric fever. Because the degree of underreporting in surveillance studies depends on the volume of blood collected, meta-regression studies that estimate the global burden of typhoid fever should adjust for differences in culture sensitivity across surveillance studies and between age groups [16, 17, 40]. Finally, wherever ethical and regulatory guidelines permit, more than 7 mL of blood should be drawn for the diagnosis of enteric fever, a recommendation that has been echoed in other hospital-based studies [28].
CONCLUSIONS
In conclusion, our analysis demonstrates that the sensitivity of blood culture for detecting infection with S Typhi and S Paratyphi is dependent on sample volume, as well as patient age, duration of symptoms, and prior antimicrobial use. Further prospective studies are needed to better characterize these relationships. The evaluation of novel diagnostics and efforts to integrate data across incidence studies of typhoid fever should take into account the potential influence of these factors.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Kate Nyhan at the Yale Medical Library for advice on constructing the systematic review search strategy. We also thank Andrea Torneri for screening and extracting data from Italian publications as well as Katharine Owers and Kathryn Hacker for aiding in screening and extracting data from Portuguese publications.
Disclaimer. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Financial support. This work was funded by the Bill and Melinda Gates Foundation (Grants OPP1116967 and OPP1151153; to V. E. P. and M. A.), the Wellcome Trust (Strategic Award 106158/Z/14/Z; to N. J. S., S. B., A. J. P., and V. E. P.), and the Belgian American Educational Foundation Postdoctoral Fellowship program (to N. J. S. and M. A.).
Supplement sponsorship. This article is part of the supplement “Surveillance for Enteric Fever in Asia Project,„ sponsored by the Sabin Vaccine Institute.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Antillón M, Warren JL, Crawford FW, et al. . The burden of typhoid fever in low- and middle-income countries: a meta-regression approach. PLoS Negl Trop Dis 2017; 11:e0005376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388:1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crump JA. Typhoid fever and the challenge of nonmalaria febrile illness in sub-Saharan Africa. Clin Infect Dis 2012; 54:1107–9. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. Background document: the diagnosis, treatment and prevention of typhoid fever. 2003. http://www.who.int/iris/handle/10665/68122. Accessed January 2018. [Google Scholar]
- 5. Gilman RH, Terminel M, Levine MM, Hernandez-Mendoza P, Hornick RB. Relative efficacy of blood, urine, rectal swab, bone-marrow, and rose-spot cultures for recovery of Salmonella typhi in typhoid fever. Lancet 1975; 1:1211–3. [DOI] [PubMed] [Google Scholar]
- 6. Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev 2015; 28:901–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crump JA, Mintz ED. Global trends in typhoid and paratyphoid fever. Clin Infect Dis 2010; 50:241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levine MM, Grados O, Gilman RH, Woodward WE, Solis-Plaza R, Waldman W. Diagnostic value of the Widal test in areas endemic for typhoid fever. Am J Trop Med Hyg 1978; 27:795–800. [DOI] [PubMed] [Google Scholar]
- 9. Maheshwari V, Kaore NM, Ramnani VK, Sarda S. A comparative evaluation of different diagnostic modalities in the diagnosis of typhoid fever using a composite reference standard: a tertiary hospital based study in Central India. J Clin Diagnostic Res 2016; 10:DC01–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naheed A, Ram PK, Brooks WA, et al. . Clinical value of Tubex and Typhidot rapid diagnostic tests for typhoid fever in an urban community clinic in Bangladesh. Diagn Microbiol Infect Dis 2008; 61:381–6. [DOI] [PubMed] [Google Scholar]
- 11. Stoesser N, Moore CE, Pocock JM, et al. . Pediatric bloodstream infections in Cambodia, 2007 to 2011. Pediatr Infect Dis J 2013; 32:e272–6. [DOI] [PubMed] [Google Scholar]
- 12. Wang SK, Chu CJ, Sun PS, et al. . Study on blood cultures and bacteria counts in the blood of paratyphoid fever A patients. Eur J Clin Microbiol Infect Dis 2009; 28:1259–61. [DOI] [PubMed] [Google Scholar]
- 13. Andrews JR, Ryan ET. Diagnostics for invasive Salmonella infections: current challenges and future directions. Vaccine 2015; 33(Suppl 3):C8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mogasale V, Ramani E, Mogasale VV, Park J. What proportion of Salmonella Typhi cases are detected by blood culture? A systematic literature review. Ann Clin Microbiol Antimicrob 2016; 15:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brooks WA, Hossain A, Goswami D, et al. . Bacteremic typhoid fever in children in an urban slum, Bangladesh. Emerg Infect Dis 2005; 11:326–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Breiman RF, Cosmas L, Njuguna H, et al. . Population-based incidence of typhoid fever in an urban informal settlement and a rural area in Kenya: implications for typhoid vaccine use in Africa. PLoS One 2012; 7:e29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ochiai RL, Acosta CJ, Danovaro-Holliday MC, et al. . A study of typhoid fever in five Asian countries: disease burden and implications for controls [published correction appears in Bull World Health Organ 2015;93:440 and Bull World Health Organ 2015;93:284]. Bull World Health Organ 2008; 86:260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffman SL, Punjabi NH, Rockhill RC, Sutomo A, Rivai AR, Pulungsih SP. Duodenal string-capsule culture compared with bone-marrow, blood, and rectal-swab cultures for diagnosing typhoid and paratyphoid fever. J Infect Dis 1984; 149:157–61. [DOI] [PubMed] [Google Scholar]
- 19. Wain J, Diep TS, Bay PV, et al. . Specimens and culture media for the laboratory diagnosis of typhoid fever. J Infect Dev Ctries 2008; 2:469–74. [DOI] [PubMed] [Google Scholar]
- 20. Escamilla J. Isolation of enteric fever agents from the blood. Philipp J Microbiol Infect Dis 1982; 11:14–23. [Google Scholar]
- 21. Watson KC. Laboratory and clinical investigation of recovery of Salmonella typhi from blood. J Clin Microbiol 1978; 7:122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Escamilla J, Santiago LT, Uylangco CV, Cross JH. Evaluation of sodium polyanethanol sulfonate as a blood culture additive for recovery of Salmonella typhi and Salmonella paratyphi A. J Clin Microbiol 1983; 18:380–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG. Reprint–preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther 2009; 89:873–80. [PubMed] [Google Scholar]
- 24. Whiting PF, Rutjes AW, Westwood ME, et al. . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155:529–36. [DOI] [PubMed] [Google Scholar]
- 25. Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med 2010; 29:3046–67. [DOI] [PubMed] [Google Scholar]
- 26. Gelman A, Hwang J, Vehtari A. Understanding predictive information criteria for Bayesian models. Stat Comput 2014;24:997. [Google Scholar]
- 27. Isaacman DJ, Karasic RB, Reynolds EA, Kost SI. Effect of number of blood cultures and volume of blood on detection of bacteremia in children. J Pediatr 1996; 128:190–5. [DOI] [PubMed] [Google Scholar]
- 28. Mermel LA, Maki DG. Detection of bacteremia in adults: consequences of culturing an inadequate volume of blood. Ann Intern Med 1993; 119:270–2. [DOI] [PubMed] [Google Scholar]
- 29. Cockerill FR 3rd, Wilson JW, Vetter EA, et al. . Optimal testing parameters for blood cultures. Clin Infect Dis 2004; 38:1724–30. [DOI] [PubMed] [Google Scholar]
- 30. Iroh Tam PY, Bernstein E, Ma X, Ferrieri P. Blood culture in evaluation of pediatric community-acquired pneumonia: a systematic review and meta-analysis. Hosp Pediatr 2015; 5:324–36. [DOI] [PubMed] [Google Scholar]
- 31. Heckenberg SG, de Gans J, Brouwer MC, et al. . Clinical features, outcome, and meningococcal genotype in 258 adults with meningococcal meningitis. Medicine 2008; 87:185–92. [DOI] [PubMed] [Google Scholar]
- 32. Tacon CL, Flower O. Diagnosis and management of bacterial meningitis in the paediatric population: a review. Emerg Med Int 2012; 2012:320309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saha SK, Baqui AH, Hanif M, et al. . Typhoid fever in Bangladesh: implications for vaccination policy. Pediatr Infect Dis J 2001; 20:521–4. [DOI] [PubMed] [Google Scholar]
- 34. Dutta P, Mitra U, Datta S, et al. . Ciprofloxacin susceptible Salmonella typhi with treatment failure. J Trop Pediatr 2001; 47:252–3. [DOI] [PubMed] [Google Scholar]
- 35. Wain J, Diep TS, Ho VA, et al. . Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol 1998; 36:1683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bell LM, Alpert G, Campos JM, Plotkin SA. Routine quantitative blood cultures in children with Haemophilus influenzae or Streptococcus pneumoniae bacteremia. Pediatrics 1985; 76:901–4. [PubMed] [Google Scholar]
- 37. Hall MM, Ilstrup DM, Washington JA 2nd. Effect of volume of blood cultured on detection of bacteremia. J Clin Microbiol 1976; 3:643–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sinha A, Sazawal S, Kumar R, et al. . Typhoid fever in children aged less than 5 years. Lancet 1999; 354:734–7. [DOI] [PubMed] [Google Scholar]
- 39. Lin FY, Ho VA, Van Bay P, et al. . The epidemiology of typhoid fever in the Dong Thap Province, Mekong Delta region of Vietnam. Am J Trop Med Hyg 2000; 62:644–8. [DOI] [PubMed] [Google Scholar]
- 40. Sur D, von Seidlein L, Manna B, et al. . The malaria and typhoid fever burden in the slums of Kolkata, India: data from a prospective community-based study. Trans R Soc Trop Med Hyg 2006; 100:725–33. [DOI] [PubMed] [Google Scholar]
- 41. Reller ME, Zaidi AK, Sultana S, et al. . Controlled evaluation of Bactec Peds Plus/F and Bactec lytic/10 anaerobic/F media for isolation of Salmonella enterica serovars typhi and paratyphi A from blood. J Clin Microbiol 2009; 47:245–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Storey HL, Huang Y, Crudder C, Golden A, de los Santos T, Hawkins K. A meta-analysis of typhoid diagnostic accuracy studies: a recommendation to adopt a standardized composite reference. PLoS One 2015; 10:e0142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Darton TC, Jones C, Dongol S, et al. . Assessment and translation of the antibody-in-lymphocyte supernatant (ALS) assay to improve the diagnosis of enteric fever in two controlled human infection models and an endemic area of Nepal. Front Microbiol 2017; 8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moore CE, Pan-Ngum W, Wijedoru LP, et al. . Evaluation of the diagnostic accuracy of a typhoid IgM flow assay for the diagnosis of typhoid fever in Cambodian children using a Bayesian latent class model assuming an imperfect gold standard. Am J Trop Med Hyg 2014; 90:114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kawano RL, Leano SA, Agdamag DM. Comparison of serological test kits for diagnosis of typhoid fever in the Philippines. J Clin Microbiol 2007; 45:246–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Siba V, Horwood PF, Vanuga K, et al. . Evaluation of serological diagnostic tests for typhoid fever in Papua New Guinea using a composite reference standard. Clin Vaccine Immunol 2012; 19:1833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tennant SM, Toema D, Qamar F, et al. . Detection of typhoidal and paratyphoidal Salmonella in blood by real-time polymerase chain reaction. Clin Infect Dis 2015; 61(Suppl 4):S241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Van Smeden M, Naaktgeboren CA, Reitsma JB, Moons KG, De Groot JA. Latent class models in diagnostic studies when there is no reference standard - a systematic review. Am J Epidemiol 2014; 179:423–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.