Mosquitoes that blood-feed on avian hosts are important vectors of many arthropod-borne viruses (arboviruses). In Europe, these include West Nile virus (WNV), Usutu virus (USUTV) and Sindbis virus.1–3 These are all maintained in enzootic bird-mosquito-bird cycles and are important veterinary and medical threats to the UK.4 5 Principally, veterinary concerns lie with the risks to domestic animals, such as the incidental spillover infection of horses with WNV which may lead to serious neurological sequelae.6 7 Wildlife may also be affected, with certain wild birds being highly susceptible to infection and death with USUTV,8 although poultry are less susceptible.9 10 To date, UK surveillance for these viruses has not yielded evidence of active virus transmission11–14 although serological evidence has been reported.15 16
Farms provide larval habitat for the development of a wide diversity of mosquitoes17–19 close to domestic animals and wildlife. Previously, we reported empirical data of mosquitoes on farms in the UK feeding on both resident and migratory birds.20 Additionally, some of these species feed on humans at farm sites,21 demonstrating the potential for spillover of viruses into these populations.
Studies using animal-baited traps provide data on the biting rates on key hosts.22 Several investigations using bird-baited traps (BBT) have been undertaken in Europe (eg, Czech Republic,23 France,24 25 Portugal26 and Sweden27) but UK data are limited to a single study.28 This investigation aimed to identify the ornithophilic activity of UK farm-associated mosquitoes using BBTs run alongside standard artificial surveillance traps.
The study was conducted between June and October 2013 on four mixed livestock farms in Oxfordshire, Kent, Hampshire and Surrey (see table 1 for habitat classifications according to Laird29). This region is considered to be at high risk of potential outbreaks as it is the warmest part of the country during the summer and early autumn when the biting activity of mosquitoes is likely to be highest. Trapping was conducted overnight (~12 hours) for nine nights on each farm using two BBTs, one set at 1 m and the second set at 4 m from the ground. A Mosquito Magnet Pro trap (MMP) (Midgetech, Stirling, UK) baited with 1-octen-3-ol was placed approximately 100 m away. A one-hour human landing catch (HLC) was additionally performed by one collector starting 30 minutes before sunset.
Table 1.
Details of each farm together with habitat classifications present on each according to Laird,29 as follows: (1) flowing streams; (2) ponded streams; (3) lake edges; (4) swamps and marshes; (5) shallow permanent ponds; (6) shallow temporary pools; (7) intermittent ephemeral puddles; (8) natural containers; (9) artificial containers; (10) natural subterranean waters; (11) artificial subterranean waters
| Farm location | Livestock present | General description | Habitat categories |
| Oxfordshire (51.714399, −1.389034) |
Sheep, cattle, horses | Inland lowland farm surrounded by small villages and other agricultural holdings. Liable to spring and winter flooding due to proximity to the Thames. | 1, 2, 5, 6, 7, 9 |
| Kent (51.377201, 0.783809) |
Sheep, cattle | Coastal grazing marsh in the Thames estuary. Large numbers of UK-resident and local migratory birds present. | 1, 2, 4, 5, 6, 7, 9 |
| Hampshire (50.822415, −0.952401) |
Sheep, cattle | Coastal grazing marsh and mixed arable farm on Hayling Island. | 1, 2, 4, 5, 6, 7, 9 |
| Surrey (51.32052, −0.637904) |
Cattle | Smallholding bordered by woodland and close to Her Majesty’s Prison Coldingley. | 2, 6, 7, 9 |
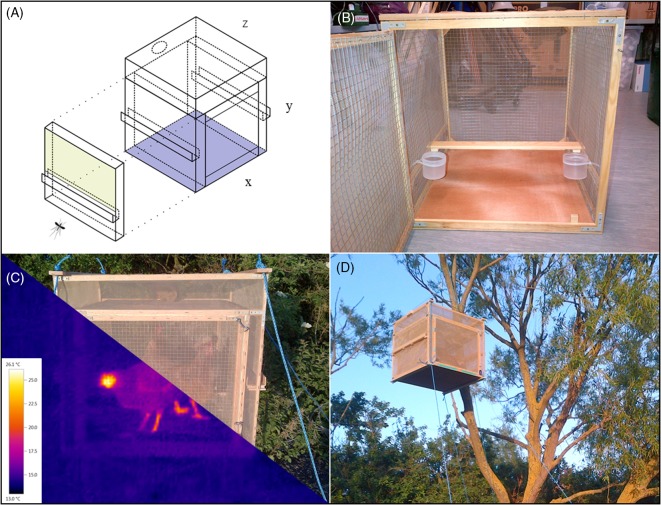
BBTs used chickens as bait and were constructed from pine stripwood, galvanised wire mesh and insect-proof netting (BioQuip, California, USA) (figure 1). Mosquitoes entered the trap via two gutter-like ‘baffles’ and were trapped in the top and side sections from where they were aspirated. The traps were modified from their original design24 following discussion with the Home Office where prevention of biting was recommended. Contact between chickens and mosquitoes was, therefore, prevented via an internal netting layer. Floor space was also increased, and a perch bar added to ensure the chickens were not stressed. Six chickens (ISA/Warren crossbreed) were maintained on a standard diet of layer pellets and two randomly allocated to each trap per evening; food (layer pellets and mixed corn) and water were provided throughout. Preliminary field testing, conducted inside an insect-proof tent (Insectopia, Austrey, UK) and using Culex pipiens sensu lato (sl) from The Pirbright Institute colony placed into the collection section of the trap overnight, showed that the BBTs retained 24–65 per cent of mosquitoes compared with 0–12 per cent when unbaited.
Figure 1.
The bird-baited trap design: (A) isometric projection (produced using Google SketchUp) with one side additionally isolated to show the route of mosquito entry via the gutter baffle (x=700 mm, y=1200 mm, z=500 mm); (B) the interior portion of the trap showing double protective mesh, perch bar and food/water containers; (C) front view of trap with chickens inside, shown during the day and at night via thermal image taken with a Testo 875-1 Thermal Imaging Camera; (D) the trap secured using guide ropes in the high position (~4 m).
Collected mosquitoes were identified morphologically using standard keys.30 31 Specimens identified as Cx pipiens sl/Cx torrentium and Anopheles maculipennis sl were then delineated using previously described molecular methods.20 32
A total of 610 unfed female mosquitoes, of 16 species or species complexes, were collected (table 2). All farms, except the Oxfordshire site, yielded mosquitoes. The BBTs collected three species/species complexes: Cx pipiens sl/Cx torrentium (of all specimens collected in the study, 37/40 were Cx pipiens form (f) pipiens; three specimens were not fully identifiable), Cx modestus and Coquillettidia richiardii. The latter two species were also collected by HLC and in the MMP. Collectively, this supports their role as potential enzootic20 and bridge21 vectors for arboviruses in the UK,5 33 and validates the utility of the MMP as a tool for collecting them.14 The ornithophilic species Cx pipiens f pipiens was the most numerous species collected in the BBTs; it was, however, also collected by HLC at the Kent site, providing further evidence for the occurrence of human-biting by this ecoform in Kent.21
Table 2.
The total number of mosquitoes collected during the study
| Species | Kent farm | Hampshire farm | Surrey farm | Total | |||||||||
| BBH | BBL | HLC | MMP | BBH | BBL | HLC | MMP | BBH | BBL | HLC | MMP | ||
| Aedes geniculatus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| Ae cantans/annulipes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 8 | 13 |
| Ae caspius/dorsalis | 0 | 0 | 1 | 7 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 10 |
| Ae detritus | 0 | 0 | 6 | 11 | 0 | 0 | 32 | 30 | 0 | 0 | 0 | 0 | 79 |
| Ae flavescens | 0 | 0 | 22 | 80 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 102 |
| Ae punctor | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 35 | 9 | 44 |
| Ae rusticus | 0 | 0 | 5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 10 |
| Aedes species (damaged) | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Anopheles atroparvus | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| An claviger | 0 | 0 | 7 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| An plumbeus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| Coquillettidia richiardii | 0 | 2 | 23 | 141 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 166 |
| Culiseta annulata | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 31 |
| Cu morsitans | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Cu subochrea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 |
| Culex modestus | 0 | 1 | 42 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 93 |
| Cx pipiens form (f) pipiens | 8 | 6 | 3 | 14 | 2 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 37 |
| Cx pipiens sensu lato (sl)* | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Cx pipiens sl/Cx torrentium† | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Totals per farm | 8 | 10 | 114 | 316 | 2 | 1 | 33 | 33 | 0 | 1 | 43 | 49 | 610 |
No mosquitoes were collected from the Oxfordshire farm, therefore this site is omitted from the table.
*Specimens separated from Cx torrentium but could not be separated to ecoform.
†Specimens which could not be separated.
BBH, bird-baited trap ‘high’ position; BBL, bird-baited trap ‘low’ position; HLC, human landing catch; MMP, Mosquito Magnet Pro trap, baited with 1-octen-3-ol.
The MMP collected the greatest number of mosquitoes overall (n=398), while the BBTs collected far fewer specimens (n=22), averaging 1.00 mosquito/trap/night (range 0–6) in Kent, 0.17 (0–2) in Hampshire and 0.06 (0–1) in Surrey. These numbers were too low to permit a comparison between trap heights (high position n=10, low position n=12). Vertical stratification of mosquito populations has been reported across Europe, including the UK,23 25 28 34 35 although results are difficult to compare directly between the different trapping strategies used. Here, the absence of many mosquitoes in the BBTs, despite their collection by other methods, may reflect a low intrinsic ornithophily, the unattractiveness of chickens to these species (although chickens are widely used as arbovirus sentinels16 36), or most likely result from the constraints of trap design. Anopheles atroparvus, for example, was previously found to feed on chickens in Kent20 but here was absent from the BBTs. Anopheles species generally fly upwards upon hitting a vertical surface37 and thus the gutter design may have lessened the chances of entry for mosquitoes of this genus. Furthermore, unlike in the original design, mosquitoes were prevented from feeding on the birds which may have resulted in greater escape from the trap, as shown in other studies38 39 and as indicated by the variability in observed retention rates in the preliminary experiments. The recorded numbers may, therefore, be underestimates of true ornithophilic mosquito activity on these sites. Conversely, the numbers do fall within the range of the previous UK bird-baited trapping study which reported a combined mean of 1.05 mosquitoes/night for Cx pipiens sl and Culiseta morsitans.28
Despite the challenges of using animal-baited mosquito traps, the data generated using BBTs in this study are important to complement and validate data on mosquito host-seeking and feeding behaviour gained from surveillance studies, intensive HLCs21 and blood meal analyses.20 The results also demonstrate that farms with the same apparent habitat types present (Kent and Hampshire) may support a vastly different mosquito species diversity. Collectively, the ornithophilic and anthropophilic behaviour of farm-associated mosquitoes highlights their potential importance in enzootic and bridge arbovirus transmission in the event of a UK outbreak. Given current concerns regarding the invasion of exotic arboviruses,40 it would be prudent to increase awareness among the equine veterinary community in particular of clinical signs of mosquito-borne arboviruses in horses. These workers can play a key role in maintaining expertise in the wider community41 and offer preventive advice in the event of an outbreak. The simplest practical control measure targeted at mosquitoes would be to regularly empty stagnant water sources to disrupt larval habitats,42 which would be particularly important in reducing populations of key vector species Cx pipiens sl.43 44
Acknowledgments
We gratefully acknowledge the support of all the farms, farm workers and research groups involved in the study, particularly those involved with routine chicken care. We thank Thomas Balenghien for providing details of the original bird-baited trap design.
Footnotes
Funding: The project was conducted as part of VAB’s PhD funded by the UK’s Biotechnology and Biological Sciences Research Council (BBSRC, grant number BB/F016492/1), and The Pirbright Institute. ARF and NJ were financially supported by the UK Department for Environment, Food and Rural Affairs, Scottish and Welsh governments (Defra grant SV3045).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Rizzoli A, Jimenez-Clavero MA, Barzon L, et al. . The challenge of West Nile virus in Europe: knowledge gaps and research priorities. Euro Surveill 2015;20:21135 10.2807/1560-7917.ES2015.20.20.21135 [DOI] [PubMed] [Google Scholar]
- 2.Hesson JC, Lundström JO, Tok A, et al. . Temporal variation in sindbis virus antibody prevalence in bird hosts in an endemic area in Sweden. PLoS One 2016;11:e0162005 10.1371/journal.pone.0162005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cadar D, Lühken R, van der Jeugd H, et al. . Widespread activity of multiple lineages of Usutu virus, western Europe, 2016. Euro Surveill 2017;22:30452 10.2807/1560-7917.ES.2017.22.4.30452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medlock JM, Snow KR, Leach S. Potential transmission of West Nile virus in the British Isles: an ecological review of candidate mosquito bridge vectors. Med Vet Entomol 2005;19:2–21. 10.1111/j.0269-283X.2005.00547.x [DOI] [PubMed] [Google Scholar]
- 5.Medlock JM, Snow KR, Leach S. Possible ecology and epidemiology of medically important mosquito-borne arboviruses in Great Britain. Epidemiol Infect 2007;135:466–82. 10.1017/S0950268806007047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunning ML, Bowen RA, Cropp CB, et al. . Experimental infection of horses with West Nile virus. Emerg Infect Dis 2002;8:380–6. 10.3201/eid0804.010239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman GE, Baylis M, Archer D, et al. . The challenges posed by equine arboviruses. Equine Vet J 2018;50:436–45. 10.1111/evj.12829 [DOI] [PubMed] [Google Scholar]
- 8.Becker N, Jöst H, Ziegler U, et al. . Epizootic emergence of Usutu virus in wild and captive birds in Germany. PLoS One 2012;7:e32604 10.1371/journal.pone.0032604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swayne DE, Beck JR, Zaki S. Pathogenicity of West Nile virus for turkeys. Avian Dis 2000;44:932 10.2307/1593067 [DOI] [PubMed] [Google Scholar]
- 10.Chvala S, Bakonyi T, Hackl R, et al. . Limited pathogenicity of Usutu virus for the domestic chicken (Gallus domesticus). Avian Pathol 2005;34:392–5. 10.1080/03079450500268500 [DOI] [PubMed] [Google Scholar]
- 11.Phipps LP, Duff JP, Holmes JP, et al. . Surveillance for West Nile virus in British birds (2001 to 2006). Vet Rec 2008;162:413–5. 10.1136/vr.162.13.413 [DOI] [PubMed] [Google Scholar]
- 12.Brugman VA, Horton DL, Phipps LP, et al. . Epidemiological perspectives on West Nile virus surveillance in wild birds in Great Britain. Epidemiol Infect 2013;141:1134–42. 10.1017/S095026881200177X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horton DL, Lawson B, Egbetade A, et al. . Targeted surveillance for Usutu virus in British birds (2005–2011): TABLE 1:. Vet Rec 2013;172:17.2–17. 10.1136/vr.101275 [DOI] [PubMed] [Google Scholar]
- 14.Vaux AG, Gibson G, Hernandez-Triana LM, et al. . Enhanced West Nile virus surveillance in the North Kent marshes, UK. Parasit Vectors 2015;8:91 10.1186/s13071-015-0705-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckley A, Dawson A, Moss SR, et al. . Serological evidence of West Nile virus, Usutu virus and Sindbis virus infection of birds in the UK. J Gen Virol 2003;84:2807–17. 10.1099/vir.0.19341-0 [DOI] [PubMed] [Google Scholar]
- 16.Buckley A, Dawson A, Gould EA. Detection of seroconversion to West Nile virus, Usutu virus and Sindbis virus in UK sentinel chickens. Virol J 2006;3:71 10.1186/1743-422X-3-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brugman VA. Host selection and feeding preferences of farm-associated mosquitoes (Diptera: Culicidae) in the United Kingdom, 2016. [Google Scholar]
- 18.Boukraa S, de La Grandiere MA, Bawin T, et al. . Diversity and ecology survey of mosquitoes potential vectors in Belgian equestrian farms: A threat prevention of mosquito-borne equine arboviruses. Prev Vet Med 2016;124:58–68. 10.1016/j.prevetmed.2015.12.013 [DOI] [PubMed] [Google Scholar]
- 19.Chapman GE, Archer D, Torr S, et al. . Potential vectors of equine arboviruses in the UK. Vet Rec 2017;180:19 10.1136/vr.103825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brugman VA, Hernández-Triana LM, England ME, et al. . Blood-feeding patterns of native mosquitoes and insights into their potential role as pathogen vectors in the Thames estuary region of the United Kingdom. Parasit Vectors 2017;10:163 10.1186/s13071-017-2098-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brugman VA, England ME, Stoner J, et al. . How often do mosquitoes bite humans in southern England? A standardised summer trial at four sites reveals spatial, temporal and site-related variation in biting rates. Parasit Vectors 2017;10:420 10.1186/s13071-017-2360-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silver JB. Mosquito ecology: field sampling methods. 3rd edn Springer: Netherlands, 2008. [Google Scholar]
- 23.Cerný O, Votýpka J, Svobodová M. Spatial feeding preferences of ornithophilic mosquitoes, blackflies and biting midges. Med Vet Entomol 2011;25:104–8. 10.1111/j.1365-2915.2010.00875.x [DOI] [PubMed] [Google Scholar]
- 24.Balenghien T, Fouque F, Sabatier P, et al. . Horse-, bird-, and human-seeking behavior and seasonal abundance of mosquitoes in a West Nile virus focus of southern France. J Med Entomol 2006;43:936–46. 10.1093/jmedent/43.5.936 [DOI] [PubMed] [Google Scholar]
- 25.L’Ambert G, Ferré JB, Schaffner F, et al. . Comparison of different trapping methods for surveillance of mosquito vectors of West Nile virus in Rhône Delta, France. J Vector Ecol 2012;37:269–75. 10.1111/j.1948-7134.2012.00227.x [DOI] [PubMed] [Google Scholar]
- 26.Ventim R, Ramos JA, Osório H, et al. . Avian malaria infections in western European mosquitoes. Parasitol Res 2012;111:637–45. 10.1007/s00436-012-2880-3 [DOI] [PubMed] [Google Scholar]
- 27.Jaenson TGT, Niklasson B. Feeding patterns of mosquitoes (Diptera: Culicidae) in relation to the transmission of Ockelbo disease in sweden. Bull Entomol Res 1986;76:375 10.1017/S0007485300014863 [DOI] [Google Scholar]
- 28.Service MW. The use of traps in sampling mosquito populations. Entomol Exp Appl 1969;12:403–12. 10.1111/j.1570-7458.1969.tb02536.x [DOI] [Google Scholar]
- 29.Laird M. The natural history of larval mosquito habitats: Academic Press Ltd, 1988. [Google Scholar]
- 30.Cranston PS, Ramsdale CD, Snow KR, et al. . Adults, larvae and pupae of British mosquitoes (Culicidae) – A Key: Freshwater Biological Association, 1987. [Google Scholar]
- 31.Snow KR. Mosquitoes. (Naturalists’ Handbook 14): Richmond Publishing Co. Ltd, 1990. [Google Scholar]
- 32.Brugman VA, Hernández-Triana LM, Prosser SW, et al. . Molecular species identification, host preference and detection of myxoma virus in the Anopheles maculipennis complex (Diptera: Culicidae) in southern England, UK. Parasit Vectors 2015;8:421 10.1186/s13071-015-1034-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medlock JM, Vaux AG. Distribution of West Nile virus vector, Culex modestus, in England. Vet Rec 2012;171:278.4–278. 10.1136/vr.e6123 [DOI] [PubMed] [Google Scholar]
- 34.Lundström JO, Chirico J, Folke A, et al. . Vertical distribution of adult mosquitoes (Diptera: Culicidae) in southern and central Sweden. J Vector Ecol 1996;21:159–66. [Google Scholar]
- 35.Bellini R, Veronesi R, Draghetti S, et al. . Study on the flying height of Aedes caspius and Culex pipiens females in the Po Delta area, Italy. J Am Mosq Control Assoc 1997;13:356–60. [PubMed] [Google Scholar]
- 36.Morris CD, Baker WG, Stark L, et al. . Comparison of chickens and pheasants as sentinels for eastern equine encephalitis and St. Louis encephalitis viruses in Florida. J Am Mosq Control Assoc 1994;10:545–8. [PubMed] [Google Scholar]
- 37.Snow RW, Jawara M, Curtis CF. Observations on Anopheles gambiae Giles s.l. (Diptera: Culicidae) during a trial of permethrin-treated bed nets in The Gambia. Bull Entomol Res 1987;77:279 10.1017/S0007485300011755 [DOI] [Google Scholar]
- 38.Service MW. A critical review of procedures for sampling populations of adult mosquitoes. Bull Entomol Res 1977;67:343 10.1017/S0007485300011184 [DOI] [Google Scholar]
- 39.Darbro JM, Harrington LC. Bird-baited traps for surveillance of West Nile mosquito vectors: effect of bird species, trap height, and mosquito escape rates. J Med Entomol 2006;43:83–92. 10.1093/jmedent/43.1.83 [DOI] [PubMed] [Google Scholar]
- 40.Medlock JM, Leach SA. Effect of climate change on vector-borne disease risk in the UK. Lancet Infect Dis 2015;15:721–30. 10.1016/S1473-3099(15)70091-5 [DOI] [PubMed] [Google Scholar]
- 41.Chapman GE, Baylis M, Archer DC. Survey of UK horse owners' knowledge of equine arboviruses and disease vectors. Vet Rec 2018:1–9. 10.1136/vr.104521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Floore TG. Mosquito larval control practices: past and present. J Am Mosq Control Assoc 2006;22:527–33. 10.2987/8756-971X(2006)22%5B527%5BMLCPPA%5D2.0.CO%3B2 [DOI] [PubMed] [Google Scholar]
- 43.Department of health. West Nile virus: a contingency plan to protect the public’s health, 2004. [Google Scholar]
- 44.Brugman V, Hernández-Triana L, Medlock J, et al. . The role of culex pipiens l. (diptera: culicidae) in virus transmission in Europe. Int J Environ Res Public Health 2018;15:389 10.3390/ijerph15020389 [DOI] [PMC free article] [PubMed] [Google Scholar]