Abstract
There are many determinants of vancomycin clearance, but these have not been analyzed separately in populations with different levels of renal function, which could be why some important factors have been missed. The aim of our study was to compare the pharmacokinetic parameters and factors that may affect vancomycin pharmacokinetics in groups of patients with normal renal function and in those with chronic kidney failure. The study used a population pharmacokinetic modeling approach, based on plasma vancomycin concentrations and other data from 78 patients with chronic kidney failure and 32 patients with normal renal function. The model was developed using NONMEM software and validated by bootstrapping. The final model for patients with impaired kidney function was described by the following equation: CL (L/h) = 0.284 + 0.000596 x DD + 0.00194 x AST, and that for the patients with normal kidney function by: CL (L/h) = 0.0727 + 0.205 x FIB. If our results are confirmed by new studies on two similar populations, these factors could be considered when dosing vancomycin in patients with chronically damaged kidneys, as well as in patients with normal kidneys who frequently require high doses of vancomycin.
Keywords: Vancomycin, Population pharmacokinetics, Renal insufficiency, Non-linear mixed effects model (NONMEM)
1. Introduction
Vancomycin is a hydrophilic antibiotic of the glycopeptide class that cannot pass cell membranes by simple diffusion [1]; therefore, it has to be administered intravenously to achieve systemic action. After intravenous administration it is distributed in extracellular space with an apparent volume of distribution of 0.4–1 L/ kg. Approximately 10% to 50% of the drug in plasma is bound to albumin [1,2]. Inflammation increases penetration of vancomycin to the central nervous system, resulting in increased interstitial fluid concentrations (e.g., from 0–3.45 mg/ L in brain tissue of healthy adults to 6.4–11.1 mg/ L in the brain of patients with meningitis) [3]. About 80%–90% of a vancomycin dose is excreted in urine as the unchanged drug; its clearance is about 2.64 l/h [4].
A number of studies describe the pharmacokinetics (PK) of vancomycin and factors that influence its pharmacokinetics. The study conducted in Spain suggested that clearance of creatinine and mechanical ventilation are related to vancomycin clearance [5]. Another study by Chinese authors discovered that serum creatinine and albumin infusion were significant covariates of vancomycin clearance [6]. Interestingly, patients with hemorrhagic stroke had higher values of vancomycin clearance [7]. Renal function was identified as an important determinant of vancomycin clearance in several other studies, but only in some studies it was observed that declining creatinine clearance with old age contributed to decreased vancomycin clearance [8]. However, determinants of vancomycin clearance were not analyzed separately in populations with different levels of renal function, which could be why some important factors could have been missed. The aim of our study was to compare the pharmacokinetic parameters and factors that may affect vancomycin pharmacokinetics in groups of patients with normal and those with mild/ moderately impaired renal function.
2. Methods
2.1. Patients and data
The study took place at Clinical Center Kragujevac, Serbia, a tertiary care health facility with 1,200 beds, and was conducted from March 1st, 2016 until October 31st, 2017. The two groups of study patients comprised those with normal renal function (n = 32) and those with mild to moderate chronic renal failure (n = 78). Demographic and other characteristics in the study groups are shown in Table 1. The inclusion criteria were: age over 18 years, normal kidney function (Clcr≥90ml/min) or mild to moderate kidney failure (Clcr from 30 to 89 ml/min); and intravenous administration of vancomycin for at least 3 days without changes in the daily dose. Patients excluded were those with severe kidney failure (Clcr < 30 ml/ min, those on dialysis; those younger than 18 years; those who received vancomycin for fewer than 3 days, and those who received vancomycin orally. All patients were prescribed intravenous infusion of vancomycin by their physicians independently from the study investigators, including determination of dose. Vancomycin was measured in serum samples taken from the patients at various time points during the dosing interval, but always after five dose intervals, i.e., after a steady-state was established. Serum concentrations of vancomycin were measured by immunoassay on Cobas® e601 analyzers (Roche Diagnostics, Mannheim, Germany), according to the manufacturer’s instructions. In the group of patients with normal kidney function, 27 serum concentrations were recorded and 5 blood samples were taken 1h to 3h after administration. In the group of patients with kidney failure, 57 blood samples were taken before the drug administration and 21 blood samples were taken 1h to 3 h after administration. The study protocol was approved by the Ethics Committee of the Clinical Center Kragujevac (N0 01-1267); informed written consent was obtained from all participants before the study procedures were undertaken. Principles of the Helsinki Declaration concerning protection of human subjects in clinical trials were strictly followed during the study.
Table 1.
Baseline demographic, laboratory and clinical data from the study groups.
| Characteristics | Index set (mean values ± standard deviation) - patients with impaired kidney function | Range for the index set | Index set (mean values ± standard deviation) - patients with normal kidney function | Range for the index set |
|---|---|---|---|---|
| Number of patients | 78 | 32 | ||
| Number of observations | 78 | 32 | ||
| Gender (male/female) | 46/32 | 21/11 | ||
| Total body weight (kg) | 78.52±16.64 | 60-180 | 81.37±10.11 | 60-103 |
| Age (years) | 67.00±10.74 | 33-86 | 59.15±14.46 | 27-86 |
| Vancomycin dose (g/day) | 1.65±0.54 | 0.5-3 | 1.93±0.43 | 1-3 |
| Length of vancomycin administration (day) | 6.23±3.27 | 3-23 | 5.78±2.76 | 3-15 |
| Creatinine in serum (mmol/l) | 128.24±47.21 | 57-250 | 61.59±17.12 | 32-99 |
| Creatinine clearance-CKD epi ( ml/min) | 50.00±19.35 | 21.9-89.5 | 99.84±12.58 | 90-120 |
| Creatinine clearance -MDRD (ml/min) | 53.07±20.59 | 23.9-121.2 | 108.53±15.62 | 72-120.0 |
| Creatinine min) clearance-Cockroft-Gault (ml/ | 54.38±17.70 | 30-87 | 112.90±10.94 | 90-120 |
| Serum albumin (g/l) | 32.16.±7.68 | 13-45 | 34.70.±7.68 | 19-46 |
| Total bilirubin (μg/l) | 12.09±8.49 | 4.4-69.0 | 33.35±94.59 | 4.5-493.5 |
| AST concentration (IU/l) | 95.91±543.49 | 9-4810 | 26.68±17.64 | 13-99 |
| ALT concentration (IU/l) | 68.55±317.00 | 5-2790 | 24.96±16.47 | 4-89 |
| C-reactive protein (mg/l) | 94.36±81.22 | 1.04-423.5 | 104.91±85.88 | 5-292 |
| Fibrinogen (g/l) | 3.68±1.55 | 1.59-9.4 | 3.21±0.89 | 1.81-6.77 |
| proBNP (pg/ml) | 1593.44±5575.45 | 300-35000 | 307.90±63.40 | 209-644 |
| Presence of sepsis (yes/no) | 9/69 | 2/30 | ||
| Presence of polytrauma | - | 2/30 | ||
| Vancomycin + comedication with: | ||||
| 5 (6%) | 5 (15%) | |||
| Colistin | ||||
| 29 (37%) | 7 (21%) | |||
| Furosemide | 7 (8.9%) | 1 (3%) | ||
| Piperacillin/tazobactam | ||||
| 15 (19%) | 12 (37%) | |||
| NSAIDs | ||||
| 4 (5.1%) | 1 (3%) | |||
| Aminoglycosides | ||||
| 49 (62%) | 16 (50%) | |||
| Heparin | ||||
| 16 (20%) | 7 (21%) | |||
| ACE inhibitors |
The patients’ data were collected from their histories; these data included records related to patient demographic characteristics (body weight, age, and sex); values of laboratory tests (creatinine clearance, serum albumin, total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), C-reactive protein (CRP), fibrinogen (FB), pro-brain natriuretic peptide (proBNP)); and clinical data (length of vancomycin administration, presence of sepsis, and concomitant medication).
2.2. Population pharmacokinetics analysis
The data were analyzed separately for the two study groups, using NONMEM software version 7.3.0 (Icon Development Solution, MD,) with the FOCE (first-order conditional estimation) approach with interaction between parameters integrated in our population pharmacokinetics (PPK) modeling [9]. We evaluated two structural models (one-compartment and two-compartment) in accordance with the literature data related to vancomycin pharmacokinetics. The base model was selected based on the range of the minimum objective function (defined as -2 multiplied by the log-likelihood - MOF) and by visual inspection of diagnostic plots. Subroutines ADVAN3 and TRANS4 were used in a two-compartment model to describe pharmacokinetics of vancomycin and its clearance fromthe central compartment. Our model assumed normal distribution of the individual pharmacokinetic parameters. At this phase of the study, we also investigated different models of error to report for both inter-individual and residual variability. The inter-individual variability was tested using additive and exponential error models, whereas residual variability was tested using an additive, exponential, constant coefficient of variation (CCV) and combined (additive and CCV) error models.
The following demographic, clinical, and laboratory test data were collected for evaluation as potential covariates: total body weight (TBW), age and sex of patients, length of vancomycin administration, presence of sepsis, polytrauma, total daily dose of vancomycin, creatinine clearance, serum albumin, total bilirubin, AST, ALT, CRP, fibrinogen, proBNP and co-medication with colistin (COL), furosemide (FUR), piperacillin /tazobactam (PT), nonsteroidal anti-inflammatory drugs (NSAIDs), heparin (HEP) and angiotensin-converting enzyme (ACE) inhibitors. All continuous variables examined in the study were not parameterized. The covariate model was built in stepwise manner where each covariate was added one at a time in a linear or nonlinear manner. To estimate whether a covariate had significant influence on vancomycin clearance, we used change in the MOF values and visual inspection of plotsin comparison to the base model. The decrease in the MOF produced by inclusion of a covariate for at least 3.84 (p<0.05, d.f.=1) and also improvements of the plots were main criteria for inclusion of a covariate in the full model. The full model was created by placing all significant covariates at the same time. This model was further tested by the backward deletion process for each covariate, one at a time, to obtain the final model. An increase in the MOF of at least 6.64 (p<0.01, d.f.=1) was used as the main criterion for retaining a significant covariate in the final model.
To validate the derived population pharmacokinetics (PPK) model and estimate its predictive performance, we applied a bootstrapping analysis. This non-parametric method is a re-sampling technique that includes large number of data replications (several hundreds or thousands) with replacement from the index set using individual patients as the sampling unit. Each of the bootstrap data sets was fitted to the final model to obtain the bootstrap estimated values of pharmacokinetic parameters, and their variability was tested using NONMEM software. The mean values of estimated PK parameters and 2.5th–97.5th percentile of the bootstrap data set were compared to the final pharmacokinetic parameter estimates.
2.3. Statistics
Primary data were described by measures of central tendency (mean) and dispersion (standard deviation and range). Estimates of the model coefficients were calculated and presented as means with 95% confidence intervals (± 1.96 x standard error of the estimate). For estimates obtained by bootstrap analysis, 2.5% and 97.5% percentiles were also calculated and presented. All calculations were performed by SPSS for Windows, version 18. Differences between groups were measured by nonparametric tests.
3. Results
The baseline demographic, laboratory, and clinical data of the study groups are shown in Table 1. Mean values of total body weight were similar (78.52kg and 81.37kg) for patients in both groups (Мann-Whitney U Test, p>0.05), whereas the mean value for age was higher in the group with impaired renal function (67.00 versus 59.15years) (Мann-Whitney U Test, p<0,05). Vancomycin was administered intravenouslyto all patients, but the length of drug administration and its mean daily dose were different between the groups. Patients with impaired renal function were receiving lower daily doses of vancomycin (1.65±0.54g/ day) for longer time periods (up to 23 days) compared to patients with normal renal function(Мann-Whitney U Test, p<0,05). A two-compartment model best described serum vancomycin concentration-time data in the base data set. Analysis of various types of error showed that an exponential model best described the inter-individual variability, whereas an additive error type was more appropriate for residual error in both base models. The estimated typical population clearance of vancomycin was lower in the patients with normal renal function (0.655 L/h) compared to the patients with impaired renal function (1.31 L/ h). In the base models, the central volume of distribution was 3-times larger in the group with renal impairment (22.7L vs. 7.12L). Inter-individual variability and residual variability were expressed as the coefficient of variation (%). The group with normal renal function had values of 37.41% and 22.64% for inter-individual and residual variability of vancomycin clearance, respectively. Inter-individual and residual variability of the drug clearance were 57.65% and 22.64% in the patients with impaired renal function, respectively.
The effects of in total twenty-four covariates on PK parameters were explored in the base models of both groups, with one covariate more (presence of polytrauma) in patients with normal renal function (Table 1). The full PPK model of vancomycin clearance had four significant covariates (fibrinogen, presence of polytrauma, creatinine clearance estimated by CKDepi, MDRD) in the group of patients with normal renal function, and three significant covariates in patients with mild-to-moderate renal failure: a daily dose of vancomycin, aspartate aminotransferase, and co-medication with aminoglycoside antibiotics. However, after backward deletion, only three covariates remained as significant determinants of vancomycin clearance in both groups:
CL (L/h) = 0.0727 + 0.205 x FIB (normal renal function)
CL (L/h) = 0.284 + 0.000596 x DD + 0.00194 x AST (impaired renal function)
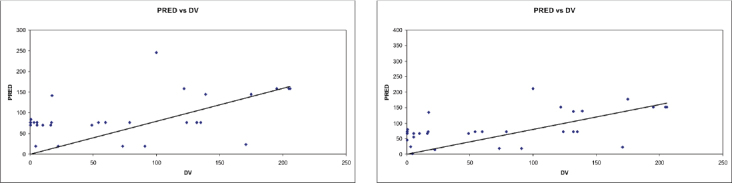
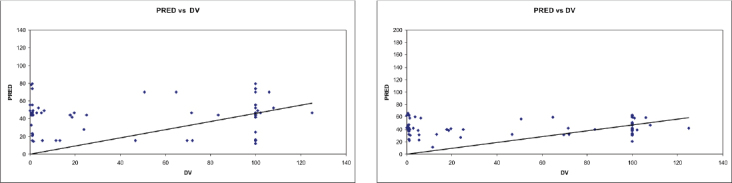
The goodness-of-fit plots indicated fair fit of the data from the final regression model. Population-predicted (PRED) values of vancomycin concentrations versus its observed concentrations (DV) in the base model and the final model for patients with normal and impaired renal function are shown in the Figures 1 and 2, respectively.
Figure 1.
Predicted vancomycin concentrations versus measured concentrations for population with normal kidneys in the base model (A) and the final model (B), respectively.
Figure 2.
Predicted vancomycin concentrations versus measured concentrations for population with impaired kidney function in the base model (A) and the final model (B), respectively
Estimates of parameters in the final models are shown in the Tables 2 and 3. The final models led to reduction of objective function values for 9.517 and 43.247 units in comparison to the base models of vancomycin clearance in groups with normal and impaired renal functions, respectively. Moreover, decrease of variability was recorded in the final models. Inter-individual and residual variability were 24.65% and 22.64%, respectively, in the group of patients with normal renal function. Conversely, inter-individual variability was 38.02% and residual variability 21.45% in patients with impaired renal function. Two hundred bootstrap runs were included in the bootstrap analysis for validation purposes. Tables 2 and 3 show summaries of parameter estimates and their 95% confidence intervals for the final PPK models. Mean values of parameter estimates using the bootstrap method were comparable with the values obtained from the original NONMEM analysis, indicating accuracy and stability of the models.
Table 2.
The final model parameter estimates in population with normal renal function
| Parameter | NONMEM Estimate | 95% CI* | Bootstrap analysis Estimate | 95% CI** |
|---|---|---|---|---|
| Clearance of vancomycin – CL (L/h) | 0.0727 | 0.0586–0.0868 | 0.0754 | 0.0599-0.0909 |
| Central volume of distribution – V1 (L) | 7.47 | 5.90–9.04 | 7.55 | 5.87–9.23 |
| Fibrinogen (g/L) | 0.205 | 0.156–0.254 | 0.201 | 0.143–0.259 |
| Interindividual variance of clearance - ω2CL | 0.059 | 0.042–0.076 | 0.056 | 0.032–0.080 |
| Residual variance - σ2 | 0.05 | 0.026–0.074 | 0.055 | 0.024–0.086 |
(Estimate)±1.96*(standard error of the estimate)
2.5th and 97.5th percentile of the ranked bootstrap parameter estimates
Table 3.
The final model parameter estimates in population with impaired renal function
| Parameter | NONMEM Estimate | 95% CI* | Bootstrap analysis Estimate | 95% CI** |
|---|---|---|---|---|
| Clearance of vancomycin – CL (L/h) | 0.284 | 0.216–0.352 | 0.281 | 0.216-0.343 |
| Central volume of distribution – V1 (L) | 29.9 | 23.86–35.94 | 30.7 | 22.69–38.71 |
| Daily dose (mg/day) | 0.000596 | 0.00045–0.00074 | 0.000602 | 0.000444–0.00076 |
| AST (IU/L) | 0.00194 | 0.00122-0.00266 | 0.00191 | 0.00121-0.00261 |
| Interindividual variance of clearance - ω2CL | 0.135 | 0.092–0.178 | 0.137 | 0.082-0.192 |
| Residual variance - σ2 | 0.045 | 0.021–0.069 | 0.041 | 0.019–0.062 |
(Estimate)±1.96*(standard error of the estimate)
2.5th and 97.5th percentile of the ranked bootstrap parameter estimates
4. Discussion
Our study showed difference in factors affecting clearance of vancomycin among patients both with normal and reduced renal function. Main determinants of vancomycin clearance in patients with normal renal function were levels of fibrinogen in plasma, whereas elimination of the same drug in patients with mild or moderate chronic kidney failure was influenced by daily dose and serum levels of AST.
Although serum values of AST and ALT in our patients with chronic renal failure were within the normal limits in most cases (mean AST and ALT values were above the upper limit of normal values in only 6.7% of patient), the actual level of AST was linked with extent of vancomycin clearance. It has recently been shown that reduced serum aminotransferase levels (within the normal limits) were proportional to the decrease of the glomerular filtration rate in chronic kidney disease patients [10], which might explain why the opposite was observed in our study: AST levels were associated with elevated vancomycin clearance in the final model.
Higher doses of vancomycin were associated with larger clearance of vancomycin and lower trough-serum concentration of vancomycin in our study. This result is not the first one reported, as complex relationship between vancomycin dose and mode of administration on one side, and its plasma concentrations and clearance on the other side has been observed in many studies [11]. Campassi et al. demonstrated that patients with augmented renal clearance had lower serum concentrations of vancomycin during the first days of therapy despite higher doses, and none of the patients reached therapeutic levels on the first day of therapy [12]. Some authors have proposed that increased loading doses and higher dose frequencies or continuous infusions are necessary to achieve higher success rates [13]. On the other hand, vancomycin serum concentrations during the first days of therapy will also depend on creatinine clearance, and low creatinine clearance levels can result in supratherapeutic vancomycin concentrations [14]. One of the possible explanations of the relationship between higher doses of vancomycin and its larger clearance could be reduced reabsorption of vancomycin from ultra-filtrate resulting from the tubular toxicity of this drug. Indeed, necrosis of tubular cells has been confirmed in histological studies of kidney biopsies taken from the patients who experienced vancomycin-in-duced renal toxicity, and vancomycin is both secreted and reabsorbed by renal tubular cells [15,16]. In addition, a correlation between daily doses of vancomycin and renal toxicity was demonstrated when some authors used daily doses up to 4 grams [11].
There are many reports about increased clearance of hydrophilic antibiotics like vancomycin in patients with sepsis, provided that renal function remains unaffected by complications of the infection itself [17]. Increased heart output and hyperkinetic circulation increase perfusion of kidneys, elevating the glomerular filtration rate and bringing a greater number of drug molecules to the tubule lumen; if a molecule of an antibiotic is hydrophilic, it could not be reabsorbed and will be excreted in the urine. Therefore, it is not surprising that plasma levels of fibrinogen, which is elevated in infection [18], are associated with clearance of vancomycin (which is a hydrophilic drug)—i.e., increased fibrinogen levels and increased vancomycin clearance go together. Indeed, the mean serum level of C-reactive protein, another marker of sepsis, was above 100 mg/l in patients with normal renal function (104.91±85.88 [SE] mg/l). Although sepsis was present in our patients with renal failure as well (mean CRP 95.4 ± 9.2 [SE] mg/l), their vancomycin clearance remained unaffected by its extent (fibrinogen did not enter final model), as increased renal perfusion could not result with large enough increasein the glomerular filtration rate.
Authors in Thailand have found a relationship between creatinine and vancomycin clearance [19]. Similar results were also described by authors in other countries [20, 21, 22]. We did not observe this relationship in our patients; furthermore, other studies supported our observation [23, 24]. The existence of a nonrenal mechanism for vancomycin elimination may explain the relatively high values of vancomycin clearances observed in patients with compromised renal function. Hepatic conjugation of vancomycin would seem the most possible nonrenal route of excretion. The vancomycin particle has a molecular weight of 1,450 and has structural chemical groups for essential conjugation with other compounds [25]. Some authors have reported measurable vancomycin concentrations in the bile after intravenous administration of vancomycin, which also supports the possibility of an extrarenal path for vancomycin elimination [26].
In some earlier pharmacokinetic studies, it was suggested that patients with malignancy had increased clearance of vancomycin [25]. Conversely, other authors reported that patients with acute myeloid leukemia had lower clearance of vancomycin [26]. It was also noted that body weight may affect clearance of vancomycin, as an increase in weight was related to higher values of both clearance and volume of distribution [27, 28]. Finally, some authors showed that furosemide may influence vancomycin clearance, whereas others concluded that concomitant drugs had no influence on clearance [29, 21].
The main limitations of our study are the relatively small number of patients, and only one measurement of vancomycin concentration per patient. This could be a reason why so many covariates with significant influence after univariate analyses were eliminated in the backward deletion phase, indicating a wider array of influences on vancomycin clearance than we were unable to demonstrate.
5. Conclusion
In conclusion, our study generates the hypothesis that elimination of vancomycin is dependent on different covariates in patients with normal renal function and mild-to-moderate chronic kidney failure Clearance of vancomycin in patients with chronically impaired kidney function was positively correlated with the administered daily dose of that drug and significantly increased by serum level of AST. Clearance of vancomycin in patients with normal kidney function was increased in patients with higher levels of fibrinogen. If our hypothesis is confirmed by future studies using two similar but larger populations, when dosing vancomycin, clinicians should account for the differences between the populations in factors that have influence on clearance of this drug.
Acknowledgements
This study was partially financially supported by the Grant No 175007 given by the Ministry of Education, Science and Technological Development, Republic of Serbia.
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- [1].Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;1(42):S35. doi: 10.1086/491712. –. [DOI] [PubMed] [Google Scholar]
- [2].Jeffres MN. The Whole Price of Vancomycin: Toxicities, Troughs, and Time. Drugs. 2017;77:1143. doi: 10.1007/s40265-017-0764-7. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Albanèse J, Léone M, Bruguerolle B, Ayem ML, Lacarelle B, Martin C. Cerebrospinal fluid penetration and pharmacokinetics of vancomycin administered by continuous infusion to mechanically ventilated patients in an intensive care unit. Antimicrob Agents Chemother. 2000;44:1356. doi: 10.1128/aac.44.5.1356-1358.2000. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Šíma M, Hartinger J, Cikánková T, Slanař O. Importance of vancomycin loading doses in intermittent infusion regimens. J Infect Chemother. 2018;24:247. doi: 10.1016/j.jiac.2017.11.002. –. [DOI] [PubMed] [Google Scholar]
- [5].Medellín-Garibay SE, Romano-Moreno S, Tejedor-Prado P, Rubio-Álvaro N, Rueda-Naharro A, Blasco-Navalpotro MA. Influence of Mechanical Ventilation on the Pharmacokinetics of Vancomycin Administered by Continuous Infusion in Critically Ill Patients. Antimicrob Agents Chemother. 2017;22(61):e01249. doi: 10.1128/AAC.01249-17. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yang W, He B, Deng CH. Population pharmacokinetics of vancomycin from severe in patients with lower respiratory tract infection. Zhonghua Jie He He Hu Xi Za Zhi. 2017;12(40):205. doi: 10.3760/cma.j.issn.1001-0939.2017.03.012. –. [DOI] [PubMed] [Google Scholar]
- [7].Morbitzer KA, Jordan JD, Sullivan KA, Durr EA, Olm-Shipman CM, Rhoney DH. Vancomycin Pharmacokinetic Parameters in Patients with Hemorrhagic Stroke. Neurocrit Care. 2016;25:250. doi: 10.1007/s12028-016-0264-8. –. [DOI] [PubMed] [Google Scholar]
- [8].Ji XW, Ji SM, He XR, Zhu X, Chen R, Lu W. Influences of renal function descriptors on population pharmacokinetic modeling of vancomycin in Chinese adult patients. Acta Pharmacol Sin. 2018;39:286. doi: 10.1038/aps.2017.57. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Beal SL, Boeckmann AJ, Sheiner LB. NONMEM users guide. Parts I–VIII ICON Development Solutions.
- [10].Sette LHBC, Lopes EP de A. The reduction of serum aminotransferase levels is proportional to the decline of the glomerular filtration rate in patients with chronic kidney disease. Clinics (Sao Paulo) 2015;70:346. doi: 10.6061/clinics/2015(05)07. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrobial Agents Chemother. 2008;52:1330. doi: 10.1128/AAC.01602-07. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Campassi ML, Gonzalez MC, Masevicius FD, Vazquez AR, Moseinco M, Navarro NC. Augmented renal clearance in critically ill patients: incidence, associated factors and effects on vancomycin treatment. Rev Bras Ter Intensiva. 2014;26:13. doi: 10.5935/0103-507X.20140003. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49:325. doi: 10.1086/600877. et al. –. [DOI] [PubMed] [Google Scholar]
- [14].Saugel B, Gramm C, Wagner JY, Messer M, Lahmer T, Meidert AS. Evaluation of a dosing regimen for continuous vancomycin infusion in critically ill patients: an observational study in intensive care unit patients. J Crit Care. 2014;29:351. doi: 10.1016/j.jcrc.2013.12.007. et al. –. [DOI] [PubMed] [Google Scholar]
- [15].Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7:136. doi: 10.1177/2042018816638223. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nakamura T, Hashimoto Y, Kokuryo T, Inui KI. Effects of fosfomycin and imipenem/cilastatin on nephrotoxicity and renal excretion of vancomycin in rats. Pharm Res. 1998;15:734. doi: 10.1023/a:1011971019868. –. [DOI] [PubMed] [Google Scholar]
- [17].Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37:840. doi: 10.1097/CCM.0b013e3181961bff. –. [DOI] [PubMed] [Google Scholar]
- [18].Moore JX, Zakai NA, Mahalingam M, Griffin RL, Irvin MR, Safford MM. Hemostasis biomarkers and risk of sepsis: the REGARDS cohort. J ThrombHaemost. 2016;14:2169. doi: 10.1111/jth.13446. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Purwonugroho TA, Chulavatnatol S, Preechagoon Y, Chindavijak B, Malathum K, Bunuparadah P. Population Pharmacokinetics of Vancomycin in Thai Patients. Scientific World Journal. 2012;2012:762649. doi: 10.1100/2012/762649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Adane ED, Herald M, Koura F.. Pharmacokinetics of Vancomycin in Extremely Obese Patients with Suspected or Confirmed Staphylococcus aureus Infections. Pharmacotherapy. 2015;35:127. doi: 10.1002/phar.1531. –. [DOI] [PubMed] [Google Scholar]
- [21].Medellín-Garibay SE, Ortiz-Martín B, Rueda-Naharro A, García B, Romano-Moreno S, Barcia E. Pharmacokinetics of vancomycin and dosing recommendations for trauma patients. J AntimicrobChemother. 2016;71:471. doi: 10.1093/jac/dkv372. –. [DOI] [PubMed] [Google Scholar]
- [22].Sánchez JL, Dominguez AR, Lane JR, Anderson PO, Capparelli EV, Cornejo-Bravo JM. Population pharmacokinetics of vancomycin in adult and geriatric patients: Comparison of eleven approaches. Int J ClinPharmacolTher. 2010;48:525. doi: 10.5414/cpp48525. –. [DOI] [PubMed] [Google Scholar]
- [23].Garaud JJ, Regnier B, Inglebert F, Faurisson F, Bauchet J, Vachon F. Vancomycin pharmacokinetics in critically ill patients. J AntimicrobChemother. 1984;14:53. doi: 10.1093/jac/14.suppl_d.53. –. [DOI] [PubMed] [Google Scholar]
- [24].Rotschafer JC, Crossley K, Zaske DE, Mead K, Sawchuk RJ, Solem LD. Pharmacokinetics of Vancomycin: Observations in 28 Patients and Dosage Recommendations. Antimicrob Agents Chemother. 1982;22:391. doi: 10.1128/aac.22.3.391. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Al-Kofide H, Zaghloul I, Al-Naim L. Pharmacokinetics of vancomycin in adult cancer patients. J Oncol Pharm Pract. 2010;16:245. doi: 10.1177/1078155209355847. –. [DOI] [PubMed] [Google Scholar]
- [26].Jarkowski A, Forrest A, Sweeney RP, Tan W, Segal BH, Almyroudis N. Characterization of vancomycin pharmacokinetics in the adult acute myeloid leukemia population. J Oncol Pharm Pract. 2012;18:91. doi: 10.1177/1078155211402107. et al. –. [DOI] [PubMed] [Google Scholar]
- [27].Moore JN, Healy JR, Thoma BN, Peahota MM, Ahamadi M, Schmidt L. A Population Pharmacokinetic Model for Vancomycin in Adult Patients Receiving Extracorporeal Membrane. CPT Pharmacometrics Syst Pharmacol. 2016;5:495. doi: 10.1002/psp4.12112. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Adane ED, Herald M, Koura F.. Pharmacokinetics of vancomycin in extremely obese patients with suspected or confirmed Staphylococcus aureus infections. Pharmacotherapy. 2015;35:127. doi: 10.1002/phar.1531. –. [DOI] [PubMed] [Google Scholar]
- [29].Lin WW, Wu W, Jiao Z, Lin RF, Jiang CZ, Huang PF. Population pharmacokinetics of vancomycin in adult Chinese patients with post-craniotomy meningitis and its application in individualised dosage regimens. Eur J Clin Pharmacol. 2016;72:29. doi: 10.1007/s00228-015-1952-6. et al. –. [DOI] [PubMed] [Google Scholar]


