Abstract
Chronic subdural hematoma is a frequent type of hemorrhage, which terminates with mortality if not diagnosed and treated early. The aim of this clinical study is to evaluate the patients with unilateral and bilateral recurrent chronic subdural hematoma.
The study group consisted of 13 cases with unilateral and bilateral recurrent chronic subdural hematomas who underwent aggressive wide craniotomy, duraectomy, inner and outer membranectomy, dural border coagulation, incision through cortical vein trace and hang up of dural edge, between 2009 - 2016. All of our patients were diagnosed by preoperative Magnetic Resonance Imaging. We evaluated the age, gender, complaints and neurologic signs, localization and thickness of the hematoma.
We can estimate that wide craniotomy, duraectomy and membranectomy is a good option in preventing recurrent chronic subdural hematoma and complications.
Keywords: Recurrent, Chronic subdural hematoma, Craniotomy, Duraectomy, Membranectomy, Surgical treatment
1. Introduction
Chronic subdural hematoma (CSDH) is a frequent type of hemorrhage that terminates with mortality (10-15%) if not diagnosed and treated early. The incidence is known to be present in 74/1000000 of the population, and increases in elders, babies, alcoholics, and cases with brain atrophy. It predominantly occurs in males and makes a peak at the 7th decade. History of minor head injury is present in 50% of the cases and 1/3 of the patients are unaware of trauma. Besides trauma it can be detected due to coagulopathy, epilepsy, hydrocephalus, arachnoid cyst, hematologic diseases, aneurysm, angioma, vascular malformation, tumor, lumbar puncture and post craniotomy. Neurologic symptoms do not exist in 67.9% of the cases, headache is the symptom in 60.7%. Gait disturbance, hemiparesis, impaired memory, recurrent fall, confusion, mental disorder, weakness, dizziness are the other symptoms respectively. Magnetic Resonance Imaging (MRI) and Computed Tomography (CT) are the diagnostic methods [1,2,3,4,5].
Recurrence develops in 20% of the cases with CSDH. Residual inner and outer capsule, blood at the subdural space, fibrin and degradation products, re vascularization of the capsule and residual subdural space are important factors for recurrence of CSDH [6,7,8].
We evaluated the results of wide craniotomy, duraectomy membranectomy and coagulation of dural border in cases with unilateral or bilateral recurrent chronic subdural hematoma.
2. Materials and methods
We present 13 cases with recurrent CSDH who under-went wide craniotomy, duraectomy and membranectomy between 2009-2016 in two centers. The study group consisted of 8 males, 5 females with a mean age of 61.2 (48-90) for males, 35 (4-80) for females. The most prominent complaint of our cases was headache and was present in all of the cases. We performed wide craniotomy and duraectomy, coagulated the junction between outer and inner capsule and duramater (dural border) following the evacuation of the hematoma.
We evaluated the age, gender, duration of hospitalisation, complaints and neurologic deficits, localization and thickness of the hematoma at admission and discharge from the hospital and at the 3rd month postoperatively. We separated the dura from the outer capsule of the hematoma and removed the dura by cutting circumferentially 0.5 cm away from the bone flap. Outer capsule of hematoma was followed up through the dural border and removed.
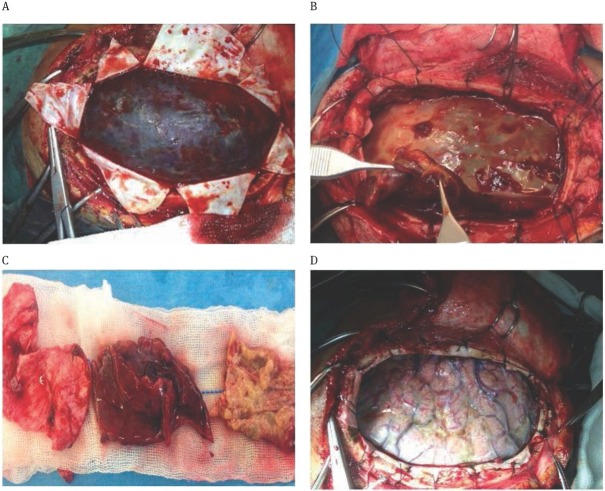
We washed and aspirated the blood and blood products inside the capsule of the hematoma. We then separated the inner capsule from the arachnoid except the parts firmly adherent to veins, and paid attention not to damage the arachnoid. We cut and suspended the dura along the trace of veins, supported areas of venous pressure with absorbable hemostate, applied bipolar coagulation to the dural border circumferentially, washed the cortex and applied drainage (Figure 1).
Figure 1.
Intraoperative pictures;
A: Hematoma following the opening of duramater; B: Thickness of the capsule is approximately 1cm; C: The amount of removed inner and outer capsule; D: Intact arachnoid following the removal of the capsule.
Informed consent was obtained from all patients included in this study. The study was approved by the University Bioethical Committee and followed the rules and principles of the Helsinki Declaration.
3. Results
The study group consisted of 13 cases. The most prominent complaint was headache and it was present in all of the cases. However one case had unconsciousness, 2 cases had extremity weakness, and one case had epilepsy. Six cases had a history of trauma and two of these cases had hemiparesis. Data of the study population are summarized in Table 1.
Table 1.
Demographic data, clinical and imaging findings of the patients.
| Patient | Age | Gender | Clinical Presentation | GCS, Neurological Signs | History of head trauma | Previous disease and number of previous operations | MRI T1 Weight | MRI T2 Weight |
|---|---|---|---|---|---|---|---|---|
| 1 | 14 | F | H | 15 | No | Operated Hydroceplahus, 3 | Bilateral FP Inhomogenous RCSDH | Bilateral FP Inhomogenous RCSDH |
| 2 | 58 | M | H, Extremity weakness | 13, Right hemiparesis | No | Operated Hydroceplahus, 2 | Left FP Hyperintense RCSDH | Left FP Hyperintense RCSDH |
| 3 | 4 | F | H | 14 | No | Operated Left Arachnoid Cyst, 4 | Right FP and Left F Inhomogenous RCSDH | Right FP and Left F Inhomogenous RCSDH |
| 4 | 48 | M | H, Epilepsy | 12, Epilepsy | Yes | Left Temporal Arachnoid Cyst, 2 | Right FP İsointense RCSDH | Right FP İsointense RCSDH |
| 5 | 22 | F | H | 14 | No | Caesarean Section Under Spinal Anesthesia, 1 | Left FP İsointense RCSDH | Left FP İsointense RCSDH |
| 6 | 54 | M | H, Extremity weakness, unconciousness. | 7, Right hemiparesis and unconciousness | Yes | No, 4 | Left FP Inhomogenous RCSDH | Left FP Inhomogenous RCSDH |
| 7 | 51 | M | H | 15 | Yes | AAT, 3 | Bilateral FP Inhomogenous RCSDH | Bilateral FP Inhomogenous RCSDH |
| 8 | 58 | M | H | 15 | No | AAT, 1 | Right FP Inhomogenous RCSDH | Right FP Inhomogenous RCSDH |
| 9 | 69 | M | H | 15 | Yes | AAT, 2 | Bilateral FP Inhomogenous RCSDH | Bilateral FP Inhomogenous RCSDH |
| 10 | 80 | F | H | 14 | No | AAT, 1 | Bilateral FP Inhomogenous RCSDH | Bilateral FP Inhomogenous RCSDH |
| 11 | 55 | F | H | 15 | Yes | AAT, 2 | Bilateral FP Inhomogenous RCSDH | Bilateral FP Inhomogenous RCSDH |
| 12 | 90 | M | H | 14 | Yes | AAT, 2 | Bilateral FP Hyperintense RCSDH | Bilateral FP Hyperintense RCSDH |
| 13 | 62 | M | H | 14 | No | AAT, 1 | Bilateral FP Inhomogenous RCSDH | Bilateral FP Inhomogenous RCSDH |
AAT:Anti agregan treatment, H:Headache, CSDH: Chronic subdural hematomas, GCS: Glasgow comascale, F: Frontal, FP: Frontoparietal, RCSDH: Recurrent chronic subdural hematoma.
We performed wide craniotomy and duraectomy. Following the evacuation of the hematoma we coagulated the dural border.
A fourteen year old girl (Case 1) with hydrocephalus underwent ventriculoperitoneal shunt operation when born, the shunt was changed 5 years ago, and the patient was operated on for bilateral CSDH 5 months ago. She was admitted to our hospital with a complaint of headache. Her MRI revealed bilateral recurrent CSDH. She did not have a history of trauma and signs of overdrainage. We recommended operation (Figure 2).
Figure 2.
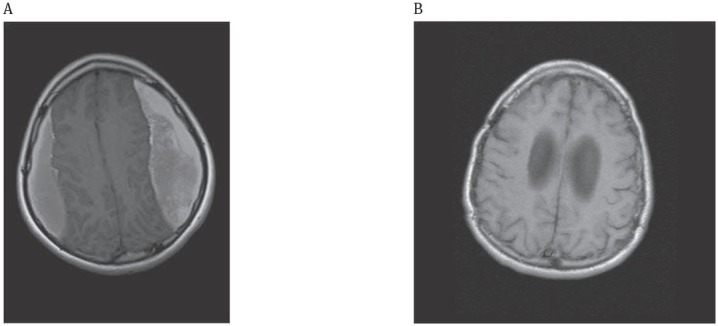
Bilateral residual chronic subdural hematoma (14 yearoldgirl);
A: Preoperative, Axial T1-weighted MRI; Bilateral hyperintense parietal subdural hematoma
B: Postoperative, Axial T1-weighted MRI; Disappearance of the hematoma following operation
A 58 year old man (Case 2) was diagnosed with adult hydrocephalus and had undergone ventriculo-peritoneal shunt operation. He was operated on for CSDH 3 months ago. He was admitted to our hospital with a complaint of right hemiparesis and his cranial MRI revealed recurrent CSDH.
A 4 year old girl (Case 3) had undergone cysto peritoneal shunt operation 2 years ago following the diagnosis of left large frontotemporoparietal arachnoid cyst shifting to the right. She had undergone three operations in 5 months for right CSDH and was admitted to our hospital with the continuation of her complaints, headache and continuous knockdown. Her cranial MRI revealed right CSDH and we performed the 5th operation (Figure 3).
Figure 3.
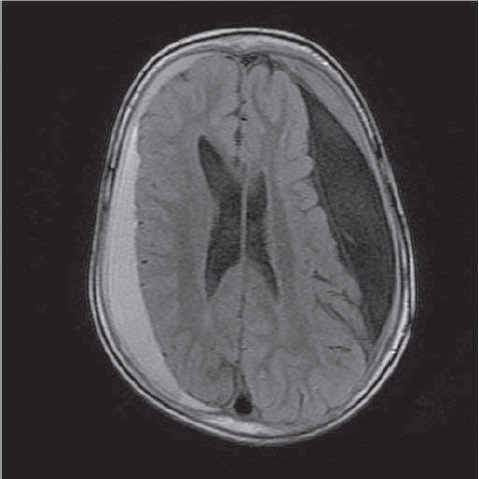
Axial T1-weighted MRI; Hyperintense right frontoparietal and left frontal residual chronic subdural hematoma, left arachnoid cyst.
A 48 year old male (Case 4) had left temporal arachnoid cyst following trauma. He was operated on for CSDH 2 months ago. His complaints continued and was operated again 1 month ago. He was admitted to our clinic with epilepsy and his MRI revealed recurrent CSDH at the same area.
A 22 year old female (Case5) who gave birth with caesarean section under spinal anesthesia 4 months ago, had signs of intracranial hypotension following anesthesia. She underwent MRI examination with a complaint of headache and was diagnosed as CSDH. She was operated 2 months ago. Her complaints did not recover and she was admitted to our hospital, she was diagnosed with recurrent CSDH and was operated on.
The case aged 54 (Case 6) had trauma 8 months ago and had been operated on for 4 times previously. He was admitted to our hospital following the 4th operation with right hemiparesis and unconsciousness. His MRI revealed left recurrent CSDH and we performed the 5th operation.
Other 7 patients (Case 7,8,9,10,11,12,13) were undergoing anti-aggregant therapy and all were over 50 years of age. Before admitting to our hospital one of them had three operations for SDH (Case 7), three of them had 2 (Patients 9,11,12), and 3 of them had 1(Patients 8,10,13). Antiaggregant therapy was ceased 2 days before the operation. Hematoma was bilateral in five of the cases and its mean thickness was 2.5 cm.
On MRI, while hematoma was isointense in two and hyperintense in two cases, it was inhomogenous in nine cases. (Figure 4). At time of hospitalisation GCS of 12 cases was 12 to 15, only in case 6, the GCS was 7 during operation.
Figure 4.
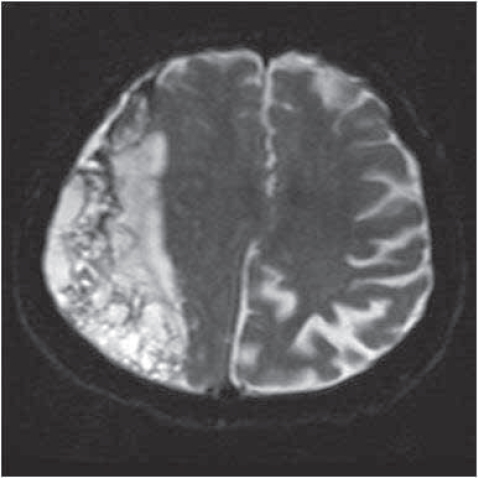
Axial T2-weighted MRI; Right frontoparietal inhomogenous residual chronic subdural hematoma,
The patients laid at semi-fowler position, their heads were wrapped with elastic bandage. Subcutaneous collection developed in three cases. We performed percutanenous tapping and aspiration to one of the cases on the 5th day. In all of the patients except one, the skin flap settled on the 7th day. The flap settled following excessive lumbar puncture every other day for 15 days in 80 years old case (Case 10) with bilateral CSDH (Figure 5).
Figure 5.
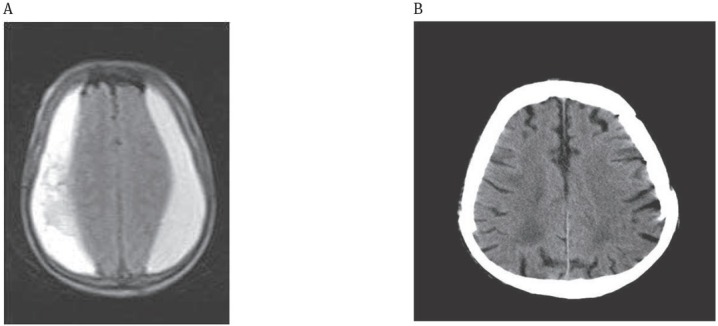
Bilateral residual chronic subdural hematoma (80 year old case);
A: Preoperative, Axial T1-weighted MRI; Bilateral hyperintense parietal subdural hematoma B: Postoperative, Computed Tomography (CT); Disappearance of subdural hematoma
The patient who was unconscious recovered on the 5th day. Paralysis of two patients started to improve by the 3rd day. One patient (Case 6) had 1/5 paresis at the 3rd month. 2 cases (Case 9,11) had residual hematoma of 0.5-1cm by the 1st month, and low dose dexamethasone was recommended. We did not observe any recurrence of CSDH in any patients at the 3rd-month control examination.
4. Discussion
CSDH frequently develops following minor head injury resultingin cortical laceration, thorn bridging veins, enforcement of clot material, sinus tear due to direct sinus trauma, laceration of cortical vessels due to weak support of the arachnoid trabeculae. Neomembrane composed of fibroblasts surrounds the nucleus of the hematoma and lies as outer and inner capsule between the duramater and the arachnoid [2,3,8,9,10].
There is no consensus about the mechanism of enlargement of the hematoma. Blood and fibrin degradation products inside the capsule increase the osmotic pressure. The capsule is vascularised from dural border. Numerous blood components can be seen at the Gap junction, squeezing or spilling leakage. Therefore, liquid flows from the low dense extracapsular area to the intercapsular area which has high density media due to intercapsular fragmentation of products, and CSDH goes on growing. The internal part of the duramater and external part of capsule are (dural layer) highly vascular with excessive fibrinolytic activity and inflammatory structure, which secretes various cytokines, especially interleukin 6 8 (IL6 8)1,11,12,13,14].
Following fragmentation of blood elements at the subdural area thrombin, fibrin, D dimer C reactive protein, TAT, fibropeptit A, IL which are protease activators, increase and cause hypercoagulation.
Thrombocytes secrete platelet-derived growth factor, transforming growth factor, PL selectin, adenosin triphosphate and von willebrant factor [1,2,15,16,17]. These substances participate in coagulation, stimulate aggregation, and support the capsule matrix and stroma. Sympathetic innervation, lymphatic system, basal lamina, laminin, and fibronectin are not present in these new vascular areas. They have vascular integrity and high vascular permeability, and are extremely fragile. Furthermore they secrete plasminogen activators, thrombomodulin and plasminogen activator inhibitor. Therefore the balance between plasminogen and plasmin activity corrupts. Development of thrombus, fibrinolysis and tendency for repeated microhemorrhage result in enlargement of sub-arachnoid hematoma. These capsule and blood elements support reccurrence, in nine of our cases, bleeding and clotting in different areas was present and MRI revealed inhomogenous intensity (Figure 4). Furthermore these sinusoidal vessels are continuously injured and bled easily [1,5,11,12,13,15,18,19,20,21,22,23].
Microglia and macrophages responsible for the resorption of brain hematoma are less in subdural area and resorption of hematoma is highly difficult. Removal of dura and capsule facilitates resorption.
The aim of CSDH therapy is to drain the hematoma and the fluid and to remove procoagulants, fibrin and degradation products from the medium. However, the recurrence rates of CSDH are particularly high [6,7,8]. There is no consensus especially about the management of recurrent CSDH. One or two burr hole, enlarged burr hole, trepanation, twist drill craniostomy, percutaneous evacuation, subdural- peritoneal shunt, craniotomy are the known surgical approaches.
Therefore, we performed a wide craniotomy, duraectomy, membranectomy, and suspended the edges of dura. We also performed an incision to the dura through the trace of cortical veins, and coagulated dural borders by bipolar to facilitate venous drainage.
Mohammed [4] suggested the dura and outer membrane as sources of hematoma and he did not detect residual hematoma following excision of these tissues. He applied subcutaneous trapping and aspiration only to 5% of his patients.
We applied percutaneous tapping to one case (Case 3) and lumber puncture to another case (Case 10), and the flap was settled in all of our cases without complication.
The extent of this rate may be explained with harming the arachnoid membrane during the removal of inner membrane which results in leakage of BOS when we performed puncture. Mohammed [4] did not remove the inner membrane.
From our 13 cases, two had undergone five operations, two had undergone four, five had undergone three, four had undergone two. CT revealed subdural collection (0.5-1cm) in two cases at the postoperative second month and was found to have disappeared at the 3rd month CT control.
Because there is no serious trauma in CSDH brain injury does not develop, neurologic deficit is minimal and most of the patients do not remember the trauma. Only six of our patients had trauma history.
In CSDH neurologic deficit occurs due to increased intracranial pressure, mechanic distortion of a region of the brain such as thalamus and decrease in blood flow. Of the cases 67.9% do not have neurologic symptoms. Most common symptoms are headache 60.7%, gait disturbances 57.1%, seizures 22%. Hemiparesis, aphasia, dizziness are the symptoms respectively. All our patients had headache, but benign headache can be observed in 20% of normal population. Persistence of headache at the third month following operation in seven of our cases can be attributed to this phenomenon. Hemiparesis recovered substantially in one of two cases at the 3rd month, 1/5 paresis of the 6th case still existed . As seen in our cases with arachnoid cyst, ventriculo-arachnoidoperitoneal shunt, cortical atrophy are more sensitive to trauma and CSDH can develop easily [8,9,10,13,18,24,28].
Intracranial hypotension occurred due to cerebrospinal fluid leakage in the case who was delivered with epidural anesthesia and CSDH developed. Apart from these symptoms we could not correlate the development, complications and recovery of recurrent CSDH with age, gender, antiaggregant drug use and thickness of subdural hematoma.
Lee [16] reported that reoperation and recurrence occur following partial membranectomy with burrhole (16%), enlarged craniectomy (23%), and in cases with coagulopaty (41%). Therefore, extended surgical approach with partial membranectomy has no advantage regarding the rate of reoperation and the outcome, burrhole drainage with irrigation of hematoma and closed system drainage is recommended. We observed successful results with enlarged craniotomy, duraectomy, membranectomy, coagulation of dural borders and incision of cortical vein trace.
5. Conclusion
Our aim in performing this aggressive operation was to achieve the chance to find the bleeding vessel, easy control of sinus laceration and bridging veins, maintain blood fluidity, minimise residual subdural space and blood products. We also coagulated the joint of CSDH inner and outer capsule with dural border suggesting that existence of high level inflammatory cells in this area promote angiogenesis and residual subdural hematoma by cytokin secretion.
We estimate that especially in recurrent chronic sub-dural hematomas, this operation is a good option in preventing residual/recurrent chronic subdural hematoma and complications.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interests.
References
- [1].Frati A., Salvati M., Mainiero F., Ippoliti F., Rocchi G., Raco A., Caoli E., Cantore G., Delfini R.. Iflammation markers and risk factors for reccurence in 35 patients with a post traumatic subdural hematoma: a prospective study. Journal of Neurosurgery. 2004;100(1):24. doi: 10.3171/jns.2004.100.1.0024. –. [DOI] [PubMed] [Google Scholar]
- [2].Lee Z.M., Muizelaar P.S. Clinical pathophysiology of traumatic brain injury, Neurological Surgery ed. New York: 2004. pp. 5044–5045. Winn EH. –. [Google Scholar]
- [3].Maggio W.W. Chronic subdural hematoma in adults, Brain Surgery Apuzzo JLM (eds) Churchill Livingstore. New York: 1993. pp. 1299–1313. –. [Google Scholar]
- [4].Mohamed E.H.. Chronic subdural hematoma treated by craniotomy, durectomy, outer membranectomy and subgaleal suction drainage. Personal experience in 39 patients. 2003;17(3):244. doi: 10.1080/0268869031000153134. Research Article. –. [DOI] [PubMed] [Google Scholar]
- [5].Prabhu S.S., Zauner A., Bullock R.R.M. Surgical Management of traumatic brain injury chronic subdural hematoma, Neurological Surgery ed: winn RH. New York: 2004. pp. 5145–5180. –. [Google Scholar]
- [6].Chon K.H., Lee J.M., Koh E.J., Choi H.Y.. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir. 2012;154:1541. doi: 10.1007/s00701-012-1399-9. –. [DOI] [PubMed] [Google Scholar]
- [7].Leroy H.A., Aboukais R., Reyns N., Bourgeois P., Labreuche J., Duhamel A, Lejeune JP. Predictors of functional outcomes and recurrence of chronic subdural hematomas. J. Clin. Neurosci. 2015;22:1895. doi: 10.1016/j.jocn.2015.03.064. –. [DOI] [PubMed] [Google Scholar]
- [8].Bernardi J.R., Smith R.K. Traumatic hematomas. Brain Surgery, Apuzzo JLM. (eds) Churchill Living Store. New York: 1993. pp. 1931–1965. –. [Google Scholar]
- [9].Kontopoulos V., Foroglou N., Patsalas J., Magras J., Foroglou G., Yiannakou-Pephtoulidou M., Sofianos E., Anastassiou H., Tsaoussi G.. Decompressive craniectomy for the management of patients with refractory hypertension: should it be reconsidered. Acta Neurochirurgical. 2002;144:791. doi: 10.1007/s00701-002-0948-z. –. [DOI] [PubMed] [Google Scholar]
- [10].Münch E., Horn P., Schürer L., Piepgras A., Paul T., Schmiedek P.. Manangement of severe traumatic brain injury by decompressive craniectomy. Neurosurgery. 2000;47(2):315. doi: 10.1097/00006123-200008000-00009. –. [DOI] [PubMed] [Google Scholar]
- [11].Fay P.W., Leung K.L.L., Tirnaver S.J. Thrombotic and hemorrhagic disorders due to abnormal fibrinolysis. 2013. pp. 1–18.htt://www.uptodate.com –.
- [12].Gottlieb A.L., Lanqille B.L., Wong M.K., Kim D.W.. Structure and function of the endothelial cytoskeleton. Lab. Invest. 1991;65(2):123. –. [PubMed] [Google Scholar]
- [13].Schin V.B., Vanhoutte P.M. Endothelium–derived vasoactive factors in thrombosis and hemorrhage, Eds. J. Loscal, I Schafer Black well Scientitic Publications. Oxford: 1994. pp. 349–367. –. [Google Scholar]
- [14].Vaquero J., Zurita M., Cincu R.. Acta Neurochirurgica. 2002;144:Vascular endothelial growth. 343. doi: 10.1007/s007010200047. –. –. [DOI] [PubMed] [Google Scholar]
- [15].Ducruet A.F., Grobelny B.T., Zacharia B.E., Hickman Z.L., DeRosa P.L., Anderson K., Sussman E., Carpenter A., Connolly E.S.. The surgical management of chronic subdural hematoma. Neurosurgical Review. 2012;35:155. doi: 10.1007/s10143-011-0349-y. –. [DOI] [PubMed] [Google Scholar]
- [16].Lee J.Y., Ebel H., Ernestus R.I., Klug N.. Various surgical treatments of chronic subdural hematoma and outcome in 172 patients:is membranectomy necessary? Surgical Neurology. 2004;61(6):523. doi: 10.1016/j.surneu.2003.10.026. –. [DOI] [PubMed] [Google Scholar]
- [17].Decompressive craniectomy in trauma patients with severe brain injury. The American Surgeon. 2002;68(12):1066. Soukiasian H.J. Hui T. Avital|I. Eby J. Thompson R. Kleisli T. Margulies D.R. Cunneen S. –. [PubMed] [Google Scholar]
- [18].Bohem S.K., Mc Conalogue K., Kong W., Bunnett W.N.. Proteinase-activated receptors. Newsphysiol. Sci. 1998;13:331. doi: 10.1152/physiologyonline.1998.13.5.231. Ç. –. [DOI] [PubMed] [Google Scholar]
- [19].Brokinkel B., Evelf C., Holling M., Hesselmann J., Heindel L.W., Stummer W., Fischer K.B. Routine postoperative CT scan after burr hole trepanation for chronic subdural hematoma. Turkish Neurosurgery. 2013. pp. 458–463. –. [DOI] [PubMed]
- [20].Junge C.E., Lee C.J., Hubbard K.B., Zhang Z., Olson J.J., Hepler J.R., Brat D.J., Traynelis S.F.. Protease-activated receptor-1 in human brain: localization and functional expression in astrocytes. Exp. Neureol. 2004;188(1):94. doi: 10.1016/j.expneurol.2004.02.018. –. [DOI] [PubMed] [Google Scholar]
- [21].Liu L., Freedman J., Hornstein A., Fenton J.W., Song Y., Ofosu A.F.. Binding of thrombin to the G-protein–linked receptor and not to glycoprotein lb, precedes thrombin-mediated platelet activation. The Journal of Biological Chemistry. 1997;277:1997. doi: 10.1074/jbc.272.3.1997. –. [DOI] [PubMed] [Google Scholar]
- [22].Mostofi K., Marnet D.. Percutaneous evacuation for treatment of subdural hematoma and outcome in 28 patient. Turkish Neurosurgery. 2011;21(5):522. –. [PubMed] [Google Scholar]
- [23].Murakami H., Hirose Y., Sagoh M., Shimizu K., Kojima M., Gotoh K., Mine Y., Hayashi T., Kawase T.. Why do chronic subdural hematomas continue to grow slowly and not coagulate? Role in thrombomodulin in the mechanism, J. Neurosurgery. 2002;96(5):877. doi: 10.3171/jns.2002.96.5.0877. –. [DOI] [PubMed] [Google Scholar]
- [24].Csόkay A., Nagy L., Novoth B.. Avoidance of vascular compression in decompressive surgery for brain edema caused by trauma and tumor ablation. Neurosurgical Rev. 2001;24:209. doi: 10.1007/s101430100158. –. [DOI] [PubMed] [Google Scholar]
- [25].Fang H., Chen J., Liu S., Wang P., Wang Y., Xiong X., Yang Q.. CD36 mediate hematoma absorption following intracerebral hemorrhage negative regulation by TLR4 signaling. J. Immunol. 2014;15(192):5984. doi: 10.4049/jimmunol.1400054. (2) –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nayıl K., Altal R., Shoaib Y., Wani A., Laharwal M., Zahoor A.. Chronic subdural hematomas. Turkish Neurosurgery. 2014;24(2):246. doi: 10.5137/1019-5149.JTN.8465-13.0. –. [DOI] [PubMed] [Google Scholar]
- [27].Taylor A., Butt W., Rosenfeld J., Shann F., Ditchfield M., Lewis E., Klug G., Wallace D., Henning R., Tibballs J.. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Child’s Nervous-System. 2001;17:154. doi: 10.1007/s003810000410. –. [DOI] [PubMed] [Google Scholar]
- [28].Roth J., Costantini S., Rosenfeld U.S. Management of the brain tumors in the pediatric patient, Ed: Kaye HA, Laws RE. Brain Tumors Elsevier. Edinburg: 2012. pp. 339–346. –. [Google Scholar]