Abstract
Comparative phylogeography can inform many macroevolutionary questions, such as whether species diversification is limited by rates of geographical population differentiation. We examined the link between population genetic structure and species diversification in the fully aquatic sea snakes (Hydrophiinae) by comparing mitochondrial phylogeography across northern Australia in 16 species from two closely related clades that show contrasting diversification dynamics. Contrary to expectations from theory and several empirical studies, our results show that, at the geographical scale studied here, rates of population differentiation and speciation are not positively linked in sea snakes. The eight species sampled from the rapidly speciating Hydrophis clade have weak population differentiation that lacks geographical structure. By contrast, all eight sampled Aipysurus–Emydocephalus species show clear geographical patterns and many deep intraspecific splits, but have threefold slower speciation rates. Alternative factors, such as ecological specialization, species duration and geographical range size, may underlie rapid speciation in sea snakes.
Keywords: phylogeography, speciation, sea snake, Australia, marine
1. Background
Speciation biology predicts that if population differentiation and species diversification are limited by similar causal factors, their rates will be linked over macroevolutionary timescales [1,2]. However, the few studies that have examined relationships between rates of intraspecific differentiation and speciation show inconsistent patterns. For example, studies of birds [3] and fish [4] have found positive associations between genetic estimates of population geographical structure and speciation, supporting theory that the generation of differentiated populations contributes to broad-scale species diversity. However, work on orchids has revealed decoupled differentiation and diversification rates [5], indicating that speciation in this group is limited by other factors, such as ecological opportunity or population persistence. Better understanding of the links between population differentiation and species diversification requires phylogeographic comparisons of recently diverged groups that show contrasting diversification dynamics, ideally across a shared landscape. Such examples may be atypical but have the potential to provide important insights into the speciation mechanisms that explain diversity patterns in focal taxa.
Here, we compare phylogeographic patterns in two clades of sea snakes (Hydrophiinae) that share a common ancestor only approximately 6–16 Ma but have undergone very different rates of species diversification. The Hydrophis clade is the most rapidly speciating group of reptiles known, with 47 species that are ecologically diverse and typically have wide geographical distributions in the Indo-West Pacific [6]. By contrast, the Aipysurus–Emydocephalus clade has only nine species, most of which are less ecologically specialized and have narrower geographical ranges restricted to the Australasian region. Estimates of speciation rates based on Bayesian analyses of speciation and extinction (using molecular timetrees and correction for differential sampling across lineages) are more than three times higher for Hydrophis compared to Aipysurus–Emydocephalus: 0.333 versus 0.090 species per million years, respectively [7]. Many species in the two clades have overlapping distributions in various shallow-water habitats across northern Australia. These habitats experienced recurrent cycles of contraction and expansion in response to sea-level fluctuations from the Late Miocene to the Late Pleistocene [8]. Phases of habitat contraction during glacial maxima are thought to explain geographically concordant patterns of population differentiation in many marine taxa, including Australian sea snakes [9], and have been linked to speciation in some groups (e.g. [10]).
In this paper, we generated mitochondrial cytochrome b sequences to analyse phylogeographical histories of 16 sea snake species in the Hydrophis and Aipysurus–Emydocephalus clades. If rates of population geographical differentiation and species diversification are positively linked at the geographical scale studied here, we would expect to find stronger intraspecific differentiation in the Hydrophis taxa because these have threefold higher speciation rates compared with Aipysurus–Emydocephalus.
2. Methods
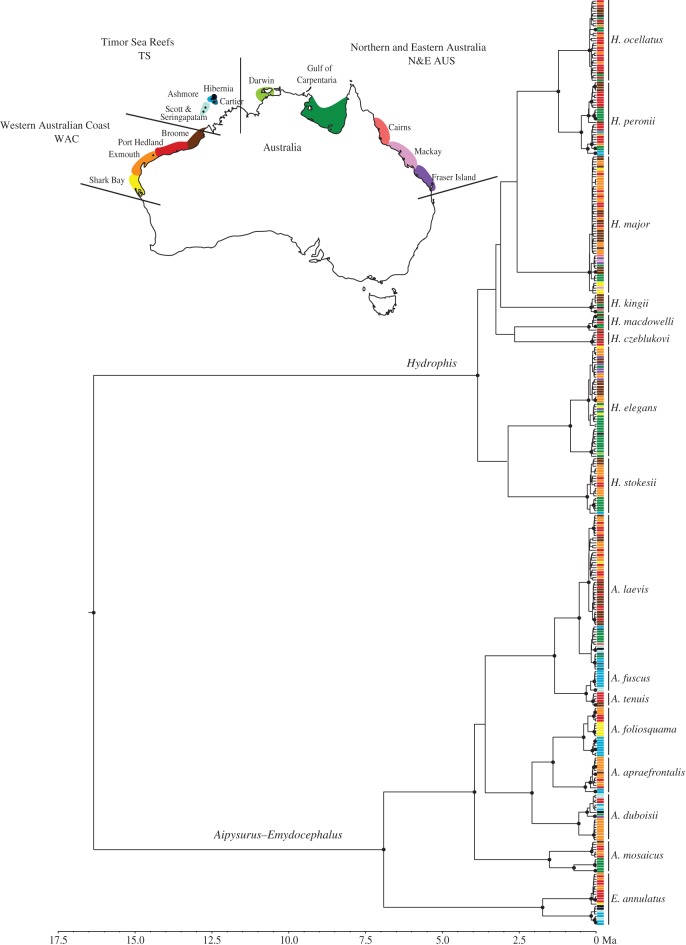
We analysed 373 individual samples from 16 species collected from across their ranges in northern Australia (electronic supplementary material, table S1). These species were initially recognized and described using morphology, but represent monophyletic groupings based on mitochondrial and nuclear genetic markers [11,12]. We sampled eight species from each of the Aipysurus–Emydocephalus and Hydrophis clades. Thirteen species (including one complex of two nominal species) were densely sampled, with 15–63 (mean 29) individuals sampled per species or species complex (table 1). Three Hydrophis species that were less densely sampled (six to eight individuals per species) were included only in the phylogenetic analysis (see below). Sampling localities were grouped into three major regions (figure 1): the Western Australian coast (WAC), Timor Sea Reefs (TS), and northern and eastern Australia (N&E Aus) (figures 1 and 2).
Table 1.
Nei's pairwise population genetic distances between major regions; values in bold are significant (p < 0.05) and are italicized to show monophyletic clades in the BEAST tree. Superscripts denote numbers of haplotypes shared between regions. Shading delimits the Aipysurus–Emydocephalus versus Hydrophis clades.
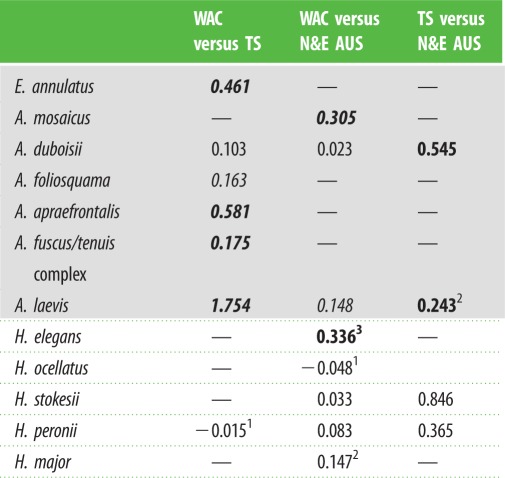 |
Figure 1.
Mitochondrial maximum clade credibility tree for all 16 sampled species. Sampling localities are shown as colours and correspond to the map. Timescale is in millions of years ago (Ma). Posterior probability support values greater than 0.95 are shown as black dots. (Online version in colour.)
Figure 2.
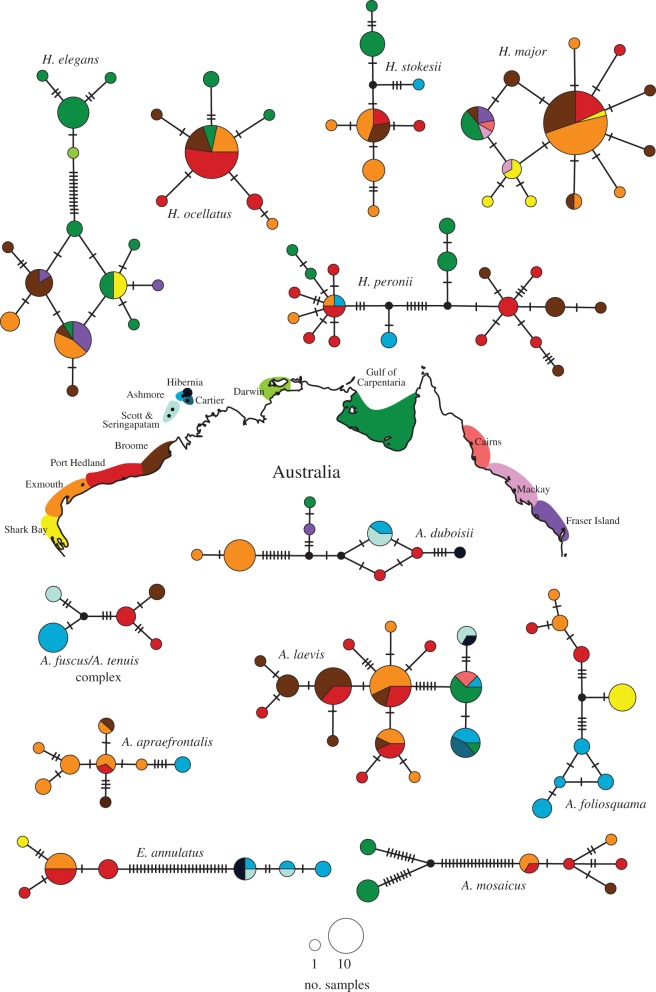
Mitochondrial haplotype networks for 12 densely sampled species or species complexes. Circles represent haplotypes, with sizes of nodes and pie segments proportional to haplotype frequency. Sampling localities are shown as colours based on the corresponding map. (Online version in colour.)
DNA was extracted and the mitochondrial cytochrome b gene was amplified and sequenced using standard protocols. A time-calibrated phylogeny was reconstructed using BEAST v.2.4.7 [13], haplotype networks were created using TCS network methods in PopART [14], and genetic diversity statistics and estimates of pairwise population genetic differentiation were calculated in Arelequin v.3.5.2.2 [15] and DnaSP v.5 [16] (see the electronic supplementary material). An important caveat of our analyses of population differentiation is that they are based on a single mitochondrial gene. However, several previous studies have shown congruent patterns of population structure based on mitochondrial and fast evolving nuclear markers in sea snakes [9,17]. This supports the utility of mitochondrial data in providing meaningful estimates of phylogeographic histories in these species.
3. Results
The final alignment comprised 373 cytochrome b sequences of 1099 base pairs. Divergence time estimates are broadly consistent with previous studies [9,11] and most intraspecific splits are dated within the last approximately 2 Myr (figure 1). Species sampled from the two clades show contrasting phylogeographic patterns. All Aipysurus–Emydocephalus species have strong population structure that is broadly congruent with geographical regions. The BEAST tree (figure 1) recovered well-supported clades corresponding to the WAC versus TS and N&E Aus in A. laevis; WAC versus TS in A. foliosquama, A. fuscus–A. tenuis, A. apraefrontalis, and E. annulatus; and WAC versus N&E Aus in A. mosaicus. A. foliosquama also contained monophyletic groupings within the WAC (Shark Bay versus more northern WAC localities). Haplotype networks for Aipysurus–Emydocephalus species show clear geographical segregation with no haplotypes shared among regions (figure 2), and pairwise comparisons of Nei's genetic distance were significant for 7 of the 11 comparisons among geographical regions (table 1). The only significant Tajima's D-value was for the A. laevis WAC population (−1.66226; p-value: 0.034).
None of the eight Hydrophis species showed clear phylogeographical structure. Two (H. major, H. ocellatus) were recovered in the BEAST tree as shallow clades with no discernable geographical structure (figure 1), and yielded star-shaped haplotype networks with haplotypes shared across distant localities (figure 2). Tajima's D-values were significantly negative for WAC populations of these species, at −2.00107 (p-value: 0.006) and −1.54236 (p-value: 0.02), respectively. The three other densely sampled Hydrophis species (H. peronii, H. elegans, H. stokesii) contained weakly supported clades in the BEAST tree but these did not correspond to geographical regions, and haplotypes were shared among regions in H. peronii and H. elegans. Of the eight pairwise comparisons of Nei's genetic distance in Hydrophis, only one was significant (table 1). Nucleotide and haplotype diversities were high within regions for most species (electronic supplementary material, table S2).
4. Discussion
Contrary to expectations from theory and several empirical studies, our results show that rates of fine-scale population differentiation are not positively linked to speciation in sea snakes. The species sampled from the rapidly speciating Hydrophis clade have weak population differentiation that lacks geographical structure. By contrast, all sampled Aipysurus–Emydocephalus species show clear geographical patterns and many deep intraspecific splits, but have threefold slower speciation rates (figures 1 and 2) [7]. Species in the two groups have diversified across very similar habitats and regions over the past approximately 2 Myr (figure 1). Hence, these lineages' contrasting phylogeographic patterns indicate heritable differences in their responses to historical landscape conditions.
All shallow marine species in northern Australia must have been impacted by the recurrent contractions of their habitats during the Miocene and Pleistocene [8]. However, the persistence of geographical population structure (and therefore the extent that it contributes to species diversity) will depend on the propensity of previously allopatric populations to introgress during expansion phases. Various demographic factors must influence the rate of gene flow in expanding populations that are incompletely reproductively isolated, particularly dispersal-related traits such as population size, intraspecific competition, habitat preference and dispersal ability. Unfortunately, most of these traits are poorly known for sea snakes. However, Hydrophis species typically have large geographical ranges in the Indo-West Pacific, whereas all but two Aipysurus–Emydocephalus species are restricted to Australasian waters. Species’ range sizes are often indicative of their dispersal capacity [18]. If Hydrophis species underwent rapid post-glacial colonization, exporting haplotype diversity over large geographical distances, this may have eroded phylogeographic signal in genetically structured species H. peronii and H. elegans, and could explain the star-shaped haplotype networks and significantly negative Tajima's D-values (indicating recent population expansion) in H. major and H. ocellatus. It is also possible that range expansion of Hydrophis species is less constrained by interspecific competition, given that they are more ecologically specialized than most Aipysurus and often co-occur in diverse assemblages [19]. Future studies are needed to examine dispersal dynamics in sea snakes, and identify whether any clade-specific differences are owing to life-history traits and/or interspecific interactions. It will also be important to identify the locations of refugia (such as the remote Timor Sea reefs) used by the two clades during peak habitat contractions.
Regardless of their causative factors, the phylogeographic patterns reported in this paper have several important implications. It is clear that the anomalously high rates of speciation in Hydrophis are not limited by rates of population genetic differentiation at the geographical scale studied here. Instead, speciation rates may be promoted by greater range sizes in Hydrophis that enhance species' persistence and provide opportunities for divergence across major biogeographic and ecological boundaries. Our previous studies of Hydrophis have shown strong vicariance at inter-regional scales [12], and rapid morphological evolution driven by ecological specialization [20]. However, work is needed to identify links among geographical, ecological and life-history traits in sea snake species formation and diversity limits. Our findings also provide a valuable evolutionary context for sea snake conservation planning. In particular, the contrasting phylogeographic histories of Hydrophis and Aipysurus–Emydocephalus species suggest that they may respond differently to shared threats and require different spatial strategies to preserve genetic diversity and population processes.
Supplementary Material
Acknowledgements
We are grateful to the crews of the RV Naturaliste and RV Investigator for collecting sea snake tissues in the Pilbara region, and the MG Kailis group, especially Jackson Crawford and Paul Moreton, for sample collection in the Exmouth Gulf. PTTEP Australasia funded survey trips to the Timor Sea reefs in 2012 and 2013. We thank the Queensland Museum, South Australian Museum and Western Australian Museum for use of tissue samples in their collections.
Ethics
Work was carried out under the Department of Conservation, Biodivesity and Attactions, Western Australia, Regulation 17 licence no. SF010920, and The University of Adelaide Animal Ethics Committee permit no. S-2014-033.
Data accessibility
Data for all analyses reported in this paper are publicly accessible in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.h1t4h1m [21].
Authors' contributions
K.L.S. conceived the study; all authors contributed to sample collection; C.R.N. carried out laboratory work; C.R.N. and K.L.S. analysed the data and wrote the paper with contributions from M.H. and V.U. All authors approved the final version of the manuscript and agree to be held accountable for its content.
Competing interests
We declare we have no competing interests.
Funding
This work is supported by an Australian Research Council and Australian Biological Resources Study grants to Kate Sanders.
References
- 1.Templeton A. 1986. The relation between speciation mechanisms and macroevolutionary patterns. In Evolutionary processes and theory (eds Karlin S, Nevo E), pp. 497–512. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 2.Turelli M, Barton NH, Coyne JA. 2001. Theory and speciation. Trends Ecol. Evol. 16, 330–343. ( 10.1016/S0169-5347(01)02177-2) [DOI] [PubMed] [Google Scholar]
- 3.Harvey MG, Seeholzer GF, Smith BT, Rabosky DL, Cuervo AM, Brumfield RT. 2017. Positive association between population genetic differentiation and speciation rates in New World birds. Proc. Natl Acad. Sci. USA 114, 6328–6333. ( 10.1073/pnas.1617397114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riginos C, Buckley YM, Blomberg SP, Treml EA. 2014. Dispersal capacity predicts both population genetic structure and species richness in reef fishes. Am. Nat. 184, 52–64. ( 10.1086/676505) [DOI] [PubMed] [Google Scholar]
- 5.Kisel Y, Moreno-Letelier AC, Bogarín D, Powell MP, Chase MW, Barraclough TG. 2012. Testing the link between population genetic differentiation and clade diversification in Costa Rican orchids. Evol. Int. J. Organic Evol. 66, 3035–3052. ( 10.1111/j.1558-5646.2012.01663.x) [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen AR, Murphy JC, Ompi M, Gibbons JW, Uetz P. 2011. Marine reptiles. PLoS ONE 6, e27373 ( 10.1371/journal.pone.0027373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee MS, Sanders KL, King B, Palci A. 2016. Diversification rates and phenotypic evolution in venomous snakes (Elapidae). R. Soc. open sci. 3, 150277 ( 10.1098/rsos.150277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowen BW, Gaither MR, DiBattista JD, Iacchei M, Andrews KR, Grant WS, Toonen RJ, Briggs JC. 2016. Comparative phylogeography of the ocean planet. Proc. Natl Acad. Sci. USA 113, 7962–7969. ( 10.1073/pnas.1602404113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lukoschek V. 2018. Congruent phylogeographic patterns in a young radiation of live-bearing marine snakes: Pleistocene vicariance and the conservation implications of cryptic genetic diversity. Divers. Distrib. 24, 325–340. ( 10.1111/ddi.12687) [DOI] [Google Scholar]
- 10.Shen K-N, Jamandre BW, Hsu C-C, Tzeng W-N, Durand J-D. 2011. Plio-Pleistocene sea level and temperature fluctuations in the northwestern Pacific promoted speciation in the globally-distributed flathead mullet Mugil cephalus. BMC Evol. Biol. 11, 83 ( 10.1186/1471-2148-11-83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders KL, Lee MS, Bertozzi T, Rasmussen AR. 2013. Multilocus phylogeny and recent rapid radiation of the viviparous sea snakes (Elapidae: Hydrophiinae). Mol. Phylogenet. Evol. 66, 575–591. ( 10.1016/j.ympev.2012.09.021) [DOI] [PubMed] [Google Scholar]
- 12.Ukuwela KD, Lee MS, Rasmussen AR, De Silva A, Fry BG, Ghezellou P, Rezaie-Atagholipour M, Sanders KL. 2016. Evaluating the drivers of Indo-Pacific biodiversity: speciation and dispersal of sea snakes (Elapidae: Hydrophiinae). J. Biogeogr. 43, 243–255. ( 10.1111/jbi.12636) [DOI] [Google Scholar]
- 13.Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu C-H, Xie D, Suchard MA, Rambaut A, Drummond AJ. 2014. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 10, e1003537 ( 10.1371/journal.pcbi.1003537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leigh JW, Bryant D. 2015. popart: full-feature software for haplotype network construction. Methods Ecol. Evol. 6, 1110–1116. ( 10.1111/2041-210X.12410) [DOI] [Google Scholar]
- 15.Excoffier L, Lischer HE. 2010. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 10, 564–567. ( 10.1111/j.1755-0998.2010.02847.x) [DOI] [PubMed] [Google Scholar]
- 16.Librado P, Rozas J. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452. ( 10.1093/bioinformatics/btp187) [DOI] [PubMed] [Google Scholar]
- 17.Ukuwela KD, de Silva A, Fry BG, Sanders KL. 2014. Multilocus phylogeography of the sea snake Hydrophis curtus reveals historical vicariance and cryptic lineage diversity. Zool. Scr. 43, 472–484. ( 10.1111/zsc.12070) [DOI] [Google Scholar]
- 18.Jablonski D. 2008. Species selection: theory and data. Annu. Rev. Ecol. Evol. Syst. 39, 501–524. ( 10.1146/annurev.ecolsys.39.110707.173510) [DOI] [Google Scholar]
- 19.Heatwole H, Cogger HG. 1994. Sea snakes of Australia. In Sea snake toxinology (ed. Gopalakrishnakone P.), pp. 167–205. Singapore University Press. [Google Scholar]
- 20.Sherratt E, Rasmussen AR, Sanders KL. 2018. Trophic specialization drives morphological evolution in sea snakes. R. Soc. open sci. 5, 172141 ( 10.1098/rsos.172141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitschke C, Hourston M, Udyawer V, Sanders K. 2018. Data from: Rates of population differentiation and speciation are decoupled in sea snakes Dryad Digital Repository. ( 10.5061/dryad.h1t4h1m) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Nitschke C, Hourston M, Udyawer V, Sanders K. 2018. Data from: Rates of population differentiation and speciation are decoupled in sea snakes Dryad Digital Repository. ( 10.5061/dryad.h1t4h1m) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data for all analyses reported in this paper are publicly accessible in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.h1t4h1m [21].