Abstract
Species that are able to solve novel problems through social learning from either a conspecific or a heterospecific may gain a significant advantage in new environments. We tested the ability of a highly successful invasive species, the Italian wall lizard Podarcis sicula, to solve a novel foraging task when social information was available from both a conspecific and an unfamiliar heterospecific (Podarcis bocagei). We found that Italian wall lizards that had access to social information made fewer errors, regardless of whether the demonstrator was a conspecific or a heterospecific, compared to Italian wall lizards that individually learnt the same task. We suggest that social learning could be a previously underappreciated, advantageous mechanism facilitating invasions.
Keywords: Podarcis sicula, biological invasions, social learning, heterospecific learning, cognition
1. Introduction
Invasive species are a global problem with severe economic and environmental impacts. Despite all the attention, the mechanisms underlying successful invasions are often unclear, although behaviour and cognition are thought to play a key role [1,2]. The ability to learn from conspecifics (social learning) may give individuals an advantage in novel environments [3]. For example, black rats (Rattus rattus) were able to invade a new patch of pine forest after innovating a feeding method that spread to the rest of the population through social learning [3]. Social learning thus has the potential to influence a species' invasive success, although this is rarely considered. Animals can use cues provided by conspecifics to minimize risk and make a wide range of decisions that may impact fitness [4]. We propose that the same can be true when learning from a different species. It is likely that heterospecific learning is more common than previously believed [5] because so many species make use of heterospecific cues to make decisions about escaping predators [6], where to find suitable habitat [7], or food sources [8]. During an introduction event, where conspecifics may be present in low numbers and unfamiliar with their new environment [4], the ability to learn from a different species could represent a powerful shortcut to individual learning.
To the best of our knowledge, the use of heterospecific learning by an invasive species has never previously been tested. To investigate this, we used the invasive Italian wall lizard Podarcis sicula, which has established populations in several countries outside its native range and that commonly interacts with native lizards, through competition or hybridization [9]. Many introductions of the Italian wall lizard occur because individuals have been accidentally stowed away in human cargo and transported long distances from their native range [9]. A robust test of heterospecific learning in the context of an invasion is to select a species that they have never encountered before. There are 23 species of wall lizards spread across the Mediterranean Basin, which means it is highly likely that in any invasion an Italian wall lizard will encounter another Podarcis. We tested the hypothesis that Italian wall lizards use social information to learn a novel foraging task. We predicted that they will solve a foraging task more rapidly when social information is available from either a conspecific or heterospecific, compared to a control in which social information is absent.
2. Material and methods
(a). Treatments and social learning task
We collected 43 female Podarcis sicula from Lisbon and 10 Podarcis bocagei from Vairão, Portugal (details in electronic supplementary material). We randomly allocated lizards to two different treatments: social and individual (control) learners. In each learning treatment, the focal species P. sicula was paired with either a conspecific or heterospecific (P. bocagei; table 1). Each pair shared an opaque enclosure (320 mm W × 480 mm L × 300 mm H) that was divided by both a fixed transparent barrier (Plexiglas) and a removable opaque (wood) barrier. Each lizard occupied one side of the enclosure in a protocol similar to [10,11].
Table 1.
The number of animals in each treatment and with each demonstrator species (N), the latency to the correct choice (s), the number that reached learning criterion (Nlearners), and the number that were successful in the first trial (Nfirst trial). Standard error (s.e.) follows all means.
| observer | treatment | demonstrator | N | proportion of correct choices | latency | Nlearners | no. trials to reach learning criterion | Nfirst trial | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. sicula | social | P. sicula | 10 | 21 | 0.48 ± 0.04 | 0.46 ± 0.03 | 348 ± 62 | 384 ± 44 | 4 | 8 | 26.00 ± 5.37 | 24.13 ± 2.98 | 4 | 6 (28.6%) |
| P. sicula | social | P. bocagei | 11 | 0.44 ± 0.03 | 416 ± 63 | 4 | 22.25 ± 3.22 | 2 | ||||||
| P. sicula | individual | P. sicula | 8 | 16 | 0.35 ± 0.04 | 0.34 ± 0.02 | 583 ± 96 | 400 ± 67 | 1 | 2 | 25.00 ± n.a. | 30.00 ± 5.00 | 1 | 4 (25.0%) |
| P. sicula | individual | P. bocagei | 8 | 0.33 ± 0.02 | 218 ± 27 | 1 | 35.00 ± n.a. | 3 | ||||||
Lizards had to solve a discrimination task in which they had a choice of three dishes with different coloured (blue, yellow and red) removable lids. Only one dish (blue) contained an accessible food reward (a live mealworm). The dishes were placed on an elevated platform (4 cm tall) with a ramp that provided easy access (figure 1a). The location of the colours was randomized between pairs and trials, but the observers in the social treatment always had the same arrangement of dishes as the respective demonstrator. All demonstrators were trained to remove the blue lid to receive a reward before experiments began. Demonstrators were only able to remove the blue lid (yellow and red lids were fixed) to ensure that the observer only received reliable information during trials. At the same time, all experimental lizards were trained to eat from a dish.
Figure 1.
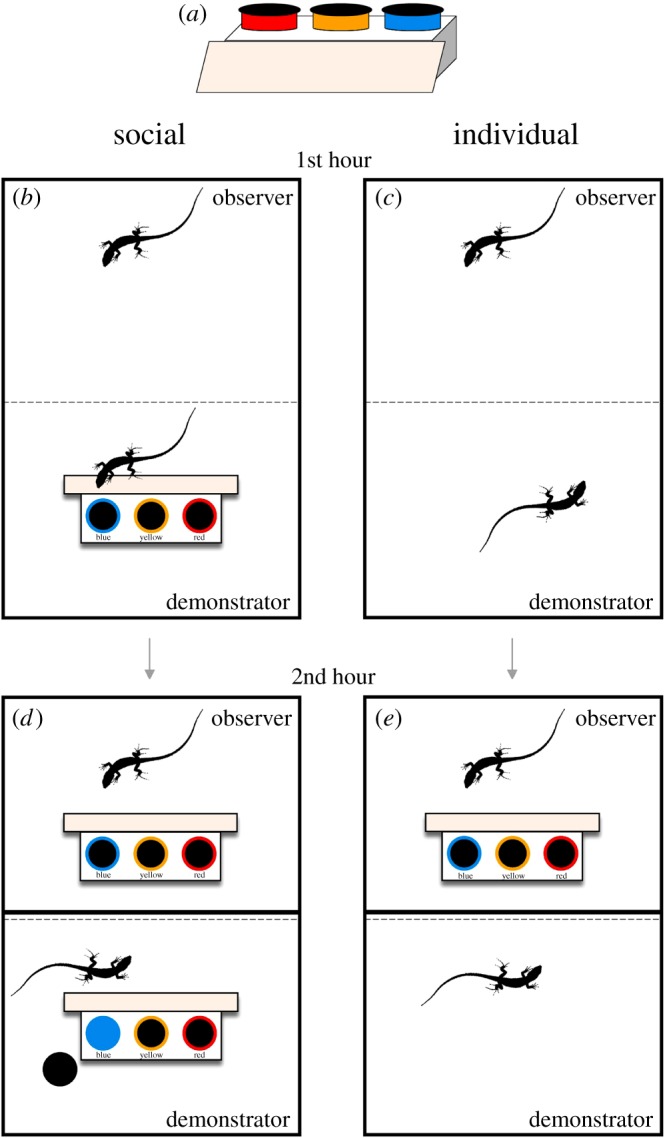
The experimental apparatus (a) and protocol. The social treatment (b) observed a demonstrator performing the discrimination task for 1 h, while the individual treatment (c) observed another lizard in the absence of the apparatus. After the opaque barrier was reinserted all observers were presented with the task for another hour (d,e). (Online version in colour.)
(b). Experimental set-up
Each trial began by removing the opaque barrier between a pair, leaving only the transparent barrier. For the social treatment, the apparatus was placed in the demonstrator's area with the ramp facing the observers (figure 1b). For the individual treatment (control), the observer was able to view another lizard in the absence of a task (figure 1c). Details in the electronic supplementary material.
After 1 h, the opaque barrier between the pair was reinserted and the apparatus was placed in the observer's area, mirroring the placement in the demonstrator's area (figure 1d,e). Observers performed the task correctly if they opened the blue lid first and ate the mealworm. If lizards did not make a choice, the trial was not counted. If a demonstrator did not perform the task, we halted the trial and did not give the task to the focal lizard. We gave the lizards 40 trials to reach our learning criterion and they were considered to have learnt the task once they made 7/7 or 7/8 correct choices [11]. Although we did not test for robustness, this learning criterion is significant according to a binomial probability, which is conservative for this experiment because the task consisted of three choices. All trials were remotely video recorded with CCTV cameras and scored by I.D.-M. From each video, we recorded whether the lizard performed the task correctly (1, opened the blue dish) or incorrectly (0, opened the red or yellow dishes), and the latency (s) from the moment the apparatus was available until the correct dish was opened.
(c). Statistical analyses
Statistical analyses were performed in R v. 3.4.2 [12], to examine if there were differences between learning treatments (social or individual) and demonstrator species (P. sicula or P. bocagei) in: (a) the number of lizards that learnt the task (learnt = 1, not learnt = 0) using a generalized linear model (GLM) with a binomial distribution (by using the function glm from the R package stats [13]); (b) the number of trials until learning criterion using a GLM with a Poisson distribution; (c) the proportion of correct choices (the number of correct choices over the number of total trials each lizard performed) using a GLM with a binomial distribution; (d) the probability of making a correct choice within a trial (correct = 1 and incorrect = 0) using a generalized linear mixed effect model (GLMM) with a binomial distribution (using the function glmer from the lme4 R package [14]). Trial number was included as an additional predictor variable in this model. We also included a random intercept and slope for lizard identity across trials to account for dependency among repeated observations of the same individual; (e) latency until the correct choice within a trial using a linear mixed effect model (LMM; Gaussian distribution), with the function lmer from the lme4 package [14]. The LMM contained the same variables, fixed and random, as the GLMM above (d). Details in the electronic supplementary material.
3. Results
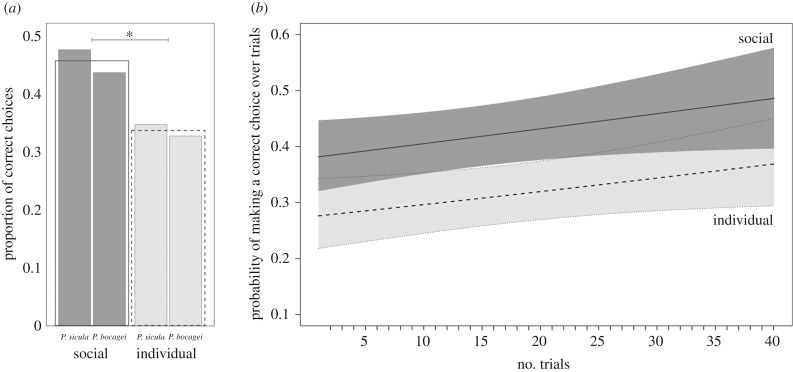
In the social treatment, 38% (8/21) of lizards met the learning criterion, while in the individual treatment 13% (2/16) of lizards reached the learning criterion (table 1). Neither the number of lizards that learnt between treatments, nor demonstrator species, were significantly different (table 2a). Similarly, the treatment or demonstrator species did not affect the number of trials needed to reach the learning criterion (table 2b). The social treatment had a significantly higher proportion of correct choices, but no effect regarding which species was demonstrating the task (table 2c and figure 2a). The probability of making the correct choice within a trial was significantly higher in the social treatment, while this did not differ between demonstrator species (table 2d and figure 2b). Latency to make a correct choice within a trial (s) was affected by an interaction between demonstrator species and treatment (table 2e); P. sicula (social treatment) that were observing P. bocagei took significantly longer to correctly complete the trials (β = −209.440 ± 61.601, z-value = −3.400, p = 0.004). All other treatment and species interaction comparisons were non-significant (electronic supplementary material, table S1).
Table 2.
Outcomes of statistical models. Number of individuals (Nind) and of observations (Nobs) are indicated; significant values are in italics.
| β | s.e. | z-value | P | |
|---|---|---|---|---|
| (a) number of lizards that learnt Nind = 37 | ||||
| intercept (Individual − P. bocagei) | −2.004 | 0.857 | −2.339 | 0.019 |
| treatment (Social) | 1.464 | 0.880 | 1.663 | 0.096 |
| demonstrator (P. sicula) | 0.114 | 0.773 | 0.147 | 0.883 |
| (b) number of trials taken to reach criterion Nind = 10 | ||||
| intercept (Individual − P. bocagei) | 3.379 | 0.224 | 15.083 | <0.001 |
| treatment (Social) | −0.222 | 0.229 | −0.967 | 0.333 |
| demonstrator (P. sicula) | 0.050 | 0.188 | 0.267 | 0.789 |
| (c) proportion of correct choices Nind = 37 | ||||
| intercept (Individual − P. bocagei) | −1.119 | 0.095 | −11.729 | <0.001 |
| treatment (Social) | 0.240 | 0.105 | 2.287 | 0.022 |
| demonstrator (P. sicula) | 0.073 | 0.104 | 0.703 | 0.482 |
| (d) probability of learning within a trial Nind = 37, Nobs = 1333 | ||||
| intercept (Individual − P. bocagei) | −0.974 | 0.164 | –5.950 | <0.001 |
| trial number | 0.011 | 0.006 | 1.900 | 0.057 |
| treatment (Social) | 0.481 | 0.145 | 3.322 | 0.001 |
| demonstrator (P. sicula) | 0.126 | 0.134 | 0.943 | 0.345 |
| (e) latency until correct choice within a trial Nind = 37, Nobs = 1333 | ||||
| intercept (Individual − P. bocagei) | 382.199 | 45.096 | 8.475 | <0.001 |
| trial number | −6.392 | 1.910 | −3.347 | 0.001 |
| treatment (Social) | 141.742 | 57.529 | 2.464 | 0.014 |
| demonstrator (P. sicula) | 209.440 | 61.601 | 3.400 | 0.001 |
| treatment : demonstrator (Social : P. sicula) | −222.267 | 82.356 | −2.699 | 0.007 |
Figure 2.
The proportion of correct choices during the task (a), and the probability of making a correct choice across 40 trials (b). Social treatment is represented in dark grey (solid lines), and the individual treatment in light grey (dashed lines). In (a), the outlined bars show treatment average of raw data, whereas the shaded bars are demonstrator-specific treatment averages. In (b), we plotted data predicted from models; shaded polygons on either side of the fitted lines are 95% CIs.
4. Discussion
We show that the invasive Italian wall lizard is able to use social information to solve a novel foraging task. While the proportion of individuals that reached the learning criterion was relatively low, lizards in the social treatment made fewer errors and had a higher probability of making a correct choice within a trial. Notably, Italian wall lizards used social information from both conspecifics and heterospecifics. However, the number of lizards that learnt, and the number of trials taken to learn the task, were not significantly different between social and individual learning treatments. While there is a relatively rich literature on how animals use heterospecific cues to make decisions on where to forage [8] or when to seek refuge [6], the idea that animals learn from other species has received little attention [5,15]. Overall, our results add to the accumulating evidence that non-avian reptiles can learn from conspecifics (e.g. [10,16,17]) and we report the first instance of heterospecific learning in an invasive species.
Biological invasions can be complex and dynamic. It may be over-simplistic to simply focus on an invasive species' traits and abilities, without considering the community into which an organism is introduced. If for example, their new environment contains closely related native species, they may make use of subtle behaviours to obtain important information about the location of food and resources. This information can form the basis for later social learning.
Our results have important implications for the field of invasion biology because they not only support previous findings that cognitive ability can play an important role in determining the success of an invasion [2], but that social learning may be an additional mechanism facilitating the establishment of invasive species in novel environments. During the course of an invasion, invasive species interact with a host of species that include conspecifics, native species, predators and prey [7,18]. Some of these organisms are in competition for resources. By using social information from both conspecifics and heterospecifics, invasive species may gain a small but significant advantage needed for success.
Supplementary Material
Acknowledgements
We thank Bernardino Silva, Miguel Carretero and Bruno Pleno for their help and support.
Ethics
Research approved by the Macquarie University Animal Ethics Committee (ARA2015/038) and by the Portuguese Institute for Conservation of Nature and Forests (ICNF) (License 695/2016/CAPT and 157/2017/CAPT).
Data accessibility
Data and R code for this study are accessible at Figshare: https://doi.org/10.6084/m9.figshare.7082948.
Authors' contributions
I.D.-M., D.J.H. and M.J.W. conceived the study. I.D.-M., D.O. and J.L.S. collected the data. I.D.-M. and J.L.R. analysed the data. All authors contributed to the manuscript, agree to be held accountable for the content therein, and approved the final version of the manuscript
Competing interests
We declare we have no competing interests.
Funding
This research was funded by Macquarie University (PhD scholarship to I.D.-M.).
References
- 1.Chapple DG, Simmonds SM, Wong BB. 2012. Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol. Evol. 27, 57–64. ( 10.1016/j.tree.2011.09.010) [DOI] [PubMed] [Google Scholar]
- 2.Sol D, Timmermans S, Lefebvre L. 2002. Behavioural flexibility and invasion success in birds. Anim. Behav. 63, 495–502. ( 10.1006/anbe.2001.1953) [DOI] [Google Scholar]
- 3.Terkel J. 1995. Cultural transmission in the black rat: pine cone feeding. Adv. Stud. Behav. 24, 119–154. ( 10.1016/s0065-3454(08)60393-9) [DOI] [Google Scholar]
- 4.Laland KN. 2004. Social learning strategies. Anim. Learn. Behav. 32, 4–14. ( 10.3758/bf03196002) [DOI] [PubMed] [Google Scholar]
- 5.Avarguès-Weber A, Dawson EH, Chittka L. 2013. Mechanisms of social learning across species boundaries. J. Zool. 290, 1–11. ( 10.1111/jzo.12015) [DOI] [Google Scholar]
- 6.Vitousek MN, Adelman JS, Gregory NC, St Clair JJ. 2007. Heterospecific alarm call recognition in a non-vocal reptile. Biol. Lett. 3, 632–634. ( 10.1098/rsbl.2007.0443). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szymkowiak J, Robert LT, Lechosław K. 2017. Interspecific social information use in habitat selection decisions among migrant songbirds. Behav. Ecol. 28, 767–775. ( 10.1093/beheco/arx029) [DOI] [Google Scholar]
- 8.Whiting MJ, Greeff JM. 1999. Use of heterospecific cues by the lizard Platysaurus broadleyi for food location. Behav. Ecol. Sociobiol. 45, 420–423. ( 10.1007/s002650050579) [DOI] [Google Scholar]
- 9.CABI. 2018. Podarcis sicula (Italian wall lizard) [original text by Silva-Rocha I]. In Invasive species compendium. Wallingford, UK: CAB International; www.cabi.org/isc. [Google Scholar]
- 10.Noble DWA, Byrne RW, Whiting MJ. 2014. Age-dependent social learning in a lizard. Biol. Lett. 10, 20140430 ( 10.1098/rsbl.2014.0430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riley JL, Küchler A, Damasio T, Noble DW, Byrne RW, Whiting MJ. 2018. Learning ability is unaffected by isolation rearing in a family-living lizard. Behav. Ecol. Sociobiol. 72, 20 ( 10.1007/s00265-017-2435-9) [DOI] [Google Scholar]
- 12.Core Team R. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://Rproject.org. [Google Scholar]
- 13.Crawley MJ. 2012. The R book. New York, NY: John Wiley & Sons Ltd. [Google Scholar]
- 14.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. (doi:10.18637/jss.v067.i01) [Google Scholar]
- 15.Dawson EH, Chittka L. 2012. Conspecific and heterospecific information use in bumblebees. PLoS ONE 7, e31444 ( 10.1371/journal.pone.0031444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkinson A, Kuenstner K, Mueller J, Huber L. 2010. Social learning in a non-social reptile (Geochelone carbonaria). Biol. Lett. 6, 614–616. ( 10.1098/rsbl.2010.0092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kis A, Huber L, Wilkinson A. 2014. Social learning by imitation in a reptile (Pogona vitticeps). Anim. Cogn. 18, 325–331. ( 10.1007/s10071-014-0803-7) [DOI] [PubMed] [Google Scholar]
- 18.Mennen GJ, Laskowski KL. 2018. Defence is the best offence: invasive prey behaviour is more important than native predator behaviour. Anim. Behav. 138, 157–164. ( 10.1016/j.anbehav.2018.02.017) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and R code for this study are accessible at Figshare: https://doi.org/10.6084/m9.figshare.7082948.