Abstract
Many animals and plants have evolved elaborate water-repellent microstructures on their surface, which often play important roles in their ecological adaptation. Here, we report a unique type of water-repellent structure on a plant surface, which develops as an insect-induced plant morphology in a social context. Some social aphids form galls on their host plant, in which they produce large amounts of hydrophobic wax. Excreted honeydew is coated by the powdery wax to form ‘honeydew balls’, which are actively disposed by soldier nymphs through an opening on their gall. These activities are enabled by a highly water-repellent inner gall surface, and we discovered that this surface is covered with dense trichomes that are not found on normal plant surfaces. The trichomes are coated by fine particles of the insect-produced wax, thereby realizing a high water repellency with a cooperative interaction between aphids and plants. The plant leaves on which the gall is formed often exhibit patchy areas with dense trichomes, representing an ectopic expression of the insect-induced plant morphology. In the pouch-shaped closed galls of a related social aphid species, by contrast, the inner surface was not covered with trichomes. Our findings provide a convincing example of how the extended phenotype of an animal, expressed in a plant, plays a pivotal role in maintaining sociality.
Keywords: gall, aphid, animal–plant interaction, water repellency, hierarchical structure
1. Introduction
The diversity of surface structures in plants and animals often reflects their adaptation to the environment. Water repellency is one of the well-understood adaptive features of biological surfaces. The water-repellent surfaces tend to exhibit microscopic and hierarchical roughness [1,2]. Such hierarchical structures are exemplified by self-cleaning lotus leaves covered by papillose epidermal cells with submicrometre-sized epicuticular waxes [3], floating legs of water striders covered by numerous needle-shaped setae with nanoscale groove structure [4] and others.
Liquid waste management is of critical importance for plant-sucking insects. The water problem is particularly serious for gall-inhabiting species, because they potentially suffer contamination or even drowning with their own liquid waste, which can destroy the colony if experimentally forced to accumulate inside their gall [5,6]. Probably for that reason, most gall-forming aphids produce a large amount of powdery hydrophobic wax from specialized epidermal glands, thereby forming wax-coated ‘honeydew balls’ to protect colony members from wetting [6–8]. In some social aphids, soldier nymphs actively dispose of the wax-coated honeydew balls and other wastes through openings on their gall to keep their habitat clean [6,9,10]. In several social aphids that form completely closed galls, the gall inner wall is specialized for absorption and removal of honeydew, which is regarded as a physiological manipulation of the plant tissue by the gall-forming aphids [5].
Here, we report a previously unrecognized type of hierarchical microstructure that confers hydrophobicity to a specific plant surface, the gall inner wall, induced by gall-forming aphids.
2. Material and methods
(a). Field observation and sampling
The woolly aphid Colophina clematis forms pouch-shaped galls with an opening on the tree Zelkova serrata, in which young nymphs exhibit defensive behaviours against intruders [9]. Galls of C. clematis were observed and collected at Okutama, Tokyo, and Shomaru, Saitama, Japan. In the field, aphids around the gall opening were observed through magnifying glass. Some twigs with a gall-harbouring leaves were brought to the laboratory and put into water, and aphids around the gall opening were video-recorded. Honeydew balls were collected from four galls on two trees using a fine brush and photographed, and from the photographs, 100 balls were randomly chosen for size measurement. Nine gall-harbouring leaves collected from five trees were fixed in FAA (formaldehyde 3.7% and acetic acid 5% in 50% ethanol), dehydrated through an ethanol series and dried. Most of the aphid-derived wax on the gall inner surface was removed during this procedure. The dried samples were examined using a scanning electron microscope and photographed. The density and length of trichomes in a 0.5 × 0.5 mm square area of the sample surface were measured based on the photographs using ImageJ (https://imagej.nih.gov/ij/). Several unfixed galls were examined for distribution of wax particles on the trichomes. Other gall-forming aphids, Colophina arma, Hemipodaphis persimilis and Paracolopha morrisoni, are listed in electronic supplementary material, table S1.
(b). Hydrophobicity measurement
We compared the following three areas: (i) thin-sliced gall inner surface areas (n = 17, from 11 leaves on four trees); (ii) hairy leaf underside areas (n = 17, from 10 leaves on four trees); and (iii) normal leaf underside areas (n = 13, from eight leaves on three trees). Each sample was affixed to an experimental table by double-sided adhesive tape to ensure an even surface. For contact angle measurement, 1.6–1.8 µl of distilled water were placed on the sample, which was then photographed using a digital camera attached to a horizontally mounted dissection microscope. The photographs were converted into greyscale and subjected to contact angle measurement using the low-bond axisymmetric drop shape analysis plugin [11] implemented for ImageJ.
(c). Gall surface manipulation
A total of 18 mature galls of C. clematis were collected from four trees and cut in half with a knife. From one half, aphid-derived wax was collected into a plastic tube using a fine brush. The other half was further cut into an approximately 5 × 5 mm square. To remove aphid wax, the gall slice was soaked in 1 ml hexane for 1 min, taken out and left until residual hexane completely evaporated. Then, 1.6–1.8 µl of distilled water were placed on the sample and photographed. After removing the distilled water, the aphid wax collected in the plastic tube was spread onto the sample surface. Again, the same amount of distilled water was placed on the same location of the sample and photographed. The photographs were subjected to contact angle measurement as described above.
(d). Water absorption by galls of Paracolopha morrisoni
In the field, on each of six galls of P. morrisoni formed on leaves of Z. serrata, a 1×1 mm square hole was bored using a fine edge of chisel. Then, 3 µl of food dye water (0.2% Food Red no. 102, Kyoritsu Foods) were injected into each gall using a micropipette. The hole was immediately filled with an adhesive [5]. After 15 h, the galls were brought to the laboratory and inspected for the injected solution.
3. Results and discussion
(a). Housekeeping behaviour of young nymphs in Colophina clematis galls
In five of eight C. clematis galls (63%) examined in the field (figure 1a), honeydew balls came out through a slit-like opening during 30 min observation (figure 1b), where first and second instar nymphs actively pushed honeydew balls out of the galls (figure 1c; electronic supplementary material, movie S1). These observations indicate that young nymphs of C. clematis perform not only defence against enemies but also housekeeping by disposing of colony wastes, as previously reported in other social aphids [6,9].
Figure 1.
Gall of C. clematis. (a) A mature gall on a leaf of Z. serrata. (b) A slit-like gall opening (arrow). (c) Young nymphs and honeydew balls in a mature gall. The gall inner cavity is full of aphid-derived powdery wax. (d) A scanning electron micrograph of trichomes on the gall inner surface. Note that aphid-derived wax is removed during fixation. (e) A fresh cross-section image of the gall inner surface. Note that trichomes are coated with aphid-derived white wax. (f) A scanning electron micrograph of the wax-coated trichomes. (Online version in colour.)
(b). Inner surface structure of Colophina clematis galls
Microscopic observations revealed that the inner surface of the galls of C. clematis was covered with minute trichomes (figure 1d; electronic supplementary material, figure S1). The trichome density was 221.7 ± 61.3 mm−2 (n = 36), which was 30 times higher than the trichome density on the opposite underside of the same leaf (7.2 ± 5.9 mm−2) (n = 36, table 1). The average pairwise distance between two neighbouring trichomes was 42.1 ± 14.3 µm (n = 149, table 1), which was far smaller than the diameter of honeydew balls (405.3 ± 176.8 µm, n = 100). Hence, a honeydew ball is expected to sit on several tens or hundreds of trichomes in the gall of C. clematis. In mature galls, the trichomes were coated with fine wax particles, which were obviously aphid-derived, thereby forming a unique hierarchical microstructure (figure 1e,f). Notably, we found that 14 of 31 galled leaves (45%) exhibited a patchy hairy area outside the gall, where the trichome density was as high as 203.1 ± 35.9 mm−2 (n = 36, table 1; electronic supplementary material, figure S2a), whereas none of the ungalled leaves we observed contained such hairy area. The hairy region may represent a remote effect of the galling activity by C. clematis, as observed in some insects whose galls are induced at a plant part distant from their infesting site [13].
Table 1.
Trichomes on the different areas of Z. serrata leaves harbouring a C. clematis gall. Statistical significance was analysed using linear mixed model (lmer function in the lme4 package in R) with gall identity treated as a random factor, followed by Tukey's post hoc test using the glht function in the multcomp package in R [12]. Values indicate mean ± s.d. Note: values within a column with different superscript letters are significantly different (p < 0.01). The leaf areas are illustrated in electronic supplementary material, figure S1. Details of the statistical analyses are shown in electronic supplementary material, table S2.
| area | trichome density (no. trichomes mm−2) |
trichome length (μm) |
distance between trichomes (μm) |
|---|---|---|---|
| gall inner surface | 221.7a ± 61.3 (N = 36) |
104.9a ± 35.5 (N = 160) |
42.1a ± 14.3 (N = 149) |
| trichome-dense area on the underside | 203.1a ± 35.9 (N = 36) |
197.4b ± 70.0 (N = 180) |
37.9a ± 11.8 (N = 180) |
| on the underside | 7.2b ± 5.9 (N = 36) |
111.1a ± 66.1 (N = 178) |
244.2b ± 176.9 (N = 175) |
| on the upperside | 8.7b ± 5.3 (N = 36) |
115.1a ± 68.4 (N = 126) |
286.7c ± 119.2 (N = 115) |
(c). Comparison of inner gall structure between galls formed by different aphid species on the same plant
Not only C. clematis but also closely related aphids, including C. arma, H. persimilis and P. morrisoni, form galls on leaves of the same plant Z. serrata (electronic supplementary material, figure S3a,d and g). In the pouch-shaped open galls of C. arma and also in the leaf-roll open galls of H. persimilis, the inner surface was covered with dense trichomes (table 2; electronic supplementary material, figure S3b,e). The trichomes were significantly denser and longer in C. clematis and C. arma than in H. persimilis (table 2). In the pouch-shaped closed galls of P. morrisoni, by contrast, the inner surface was not covered with trichomes (table 2; electronic supplementary material, figure S3h). The different surface structures of the galls on the same plant strongly suggest that these morphological traits of the plant are controlled by the insects and regarded as their extended phenotypes, consistent with the previous phylogenetic study that demonstrates that aphids determine the gall morphology [14].
Table 2.
Differences among the gall inner surfaces of Eriosomatini aphids on Zelkova serrata. Values indicate mean ± s.d. Note: values within a column with different superscript letters are significantly different (p < 0.001). Paracolopha morrisoni was excluded from the statistical analyses. Details of the statistical analyses are shown in electronic supplementary material, table S2.
| species | no. galls | gall morphology | trichome density (trichomes mm−2) |
Trichome length (μm) |
|---|---|---|---|---|
| Colophina clematis | 9 | open pouch | 221.7a±61.3 (N = 36) |
104.9a ± 35.5 (N = 160) |
| Colophina arma | 3 | 254.3a ± 66.2 (N = 12) |
87.6a ± 18.4 (N = 30) |
|
| Hemipodaphis persimilis | 5 | open leaf-roll | 114.1b ± 44.4 (N = 19) |
37.8b ± 15.0 (N = 48) |
| Paracolopha morrisoni | 4 | closed pouch | 0 (N = 16) |
n.a. |
(d). Functional difference between hairy inner wall of open galls and smooth inner wall of closed galls
When food dye solution was introduced into open galls of C. clematis and H. persimilis, the solution was repelled by the waxy and trichome-covered inner surface, thereby forming round droplets (electronic supplementary material, figure S3c,f). By contrast, the dye solution introduced into closed galls of P. morrisoni was not repelled by the inner surface (electronic supplementary material, figure S3i). Notably, when 3 µl of the dye solution were injected into six galls of P. morrisoni, the solution was completely absorbed in five galls within 15 h, whereas the solution was covered with aphid-derived wax and remained as a honeydew ball in one gall after 15 h. These observations suggest that gall openness, surface trichomes and waste managing strategies are ecologically interconnected in these gall-forming aphids: namely, the aphids forming open galls induce water-repelling inner surface covered with dense trichomes and facilitate disposal of honeydew droplets from the opening [8], whereas the aphids forming closed galls induce water-absorbing inner surface with few trichomes and remove honeydew through the plant vascular system [5].
(e). Trichomes and wax jointly contribute to water-repellent inner surface of Colophina clematis galls
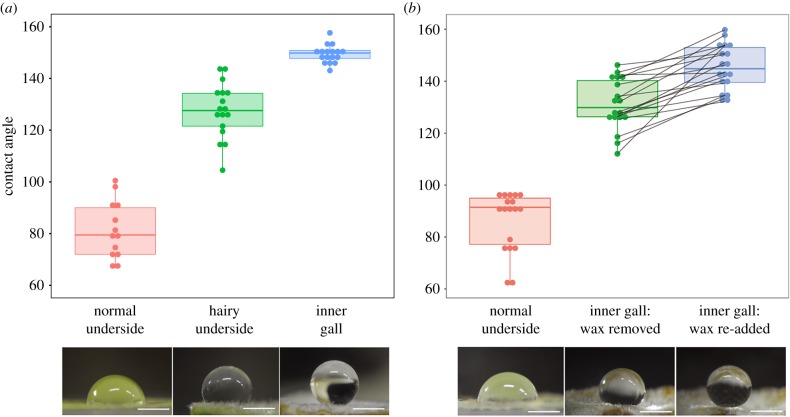
By using contact angle measurements, we quantitatively evaluated the water-repelling properties of the inner surface of the galls of C. clematis in comparison with other plant parts of Z. serrata. The gall inner surface (with both trichomes and wax) was highly water-repellent, with contact angles of 149.5 ± 3.5° (n = 17); the hairy underside area of the leaf (with trichomes but no wax) was also water-repellent, with slightly lower contact angles of 127.6 ± 10.6° (n = 17), and the normal underside area of the leaf (with neither trichomes nor wax) showed remarkably smaller contact angles of 81.5 ± 11.1° (n = 13) (figure 2a). The differences between these three areas were all statistically significant (Tukey's HSD test, p < 0.001), indicating that both factors, mainly trichomes and additionally wax, contribute to the water repellency. The hierarchically rough surface consisting of trichomes and wax reduces contact area of a liquid drop with the surface, thereby attaining higher contact angle and increased water repellency than smoothed surface [2,8]. Wax removal and re-addition experiments reproduced the significant shift of contact angles between 131.1 ± 9.8° and 145.3 ± 8.6° (n = 18, paired t-test, t17 = −6.28, p < 0.001), confirming the cooperative contribution of trichomes and wax to the water repellency of the gall inner surface (figure 2b).
Figure 2.
Hydrophobic effects of trichome wax on the galls and gall-harbouring leaves of C. clematis. (a) Contact angles of water droplets measured on normal underside areas (left, n = 13), hairy underside areas (middle, n = 17) and gall inner surface areas (right, n = 17). (b) Contact angles of water droplets measured on normal underside areas (left, n = 18), gall inner surface areas from which aphid-derived wax was removed by hexane (middle, n = 18) and gall inner surface areas from which the wax was removed and then re-added (right, n = 18). Lines indicate the changes of contact angle values measured on the same gall inner surface. The box plots depict median, quartiles, and minimum and maximum values. Corresponding water droplet images are shown below (bars 1 mm). (Online version in colour.)
4. Conclusion
Colophina clematis and closely related aphids induce dense trichomes on the inner surface of their galls, and by adding the aphid-derived fine wax particles, the trichome–wax complex constitutes a highly water-repellent surface, thereby facilitating waste management in combination with behavioural honeydew disposal by soldier nymphs. Our finding highlights the ecological relevance of gall openness, the inner surface structure and the waste management strategies, in which the intricate manipulation of plant morphology plays a pivotal role in the aphid social system. A larger comparative study across aphids and host plants will clarify the general applicability of this unrecognized animal–plant interaction.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank S. Akimoto for the information on H. persimilis, and three anonymous reviewers for helpful comments. K.U. was supported by a JSPS Postdoctoral Fellowship for Young Scientists.
Data accessibility
Additional data, details of statistical analyses and a movie are available as electronic supplementary material and in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.q9p2q59 [15].
Authors' contributions
All authors designed the study. K.U. and M.K. collected data and performed analysis. K.U. and T.F. wrote the manuscript. All authors revised the manuscript, gave their final approval and agree to be held accountable for the content therein.
Competing interests
We have no competing interests.
Funding
This study was supported by the Sasakawa Scientific Research Grant from The Japan Science Society and JSPS KAKENHI grant no. 14J00015.
References
- 1.Barthlott W, Mail M, Neinhuis C. 2016. Superhydrophobic hierarchically structured surfaces in biology: evolution, structural principles and biomimetic applications. Phil. Trans. R. Soc. A 374, 20160191 ( 10.1098/rsta.2016.0191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhushan B. 2016. Biomimetics. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 3.Barthlott W, Neinhuis C. 1997. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202, 1–8. ( 10.1007/s004250050096) [DOI] [Google Scholar]
- 4.Gao X, Jiang L. 2004. Biophysics: water-repellent legs of water striders. Nature 432, 36 ( 10.1038/432036a) [DOI] [PubMed] [Google Scholar]
- 5.Kutsukake M, Meng X-Y, Katayama N, Nikoh N, Shibao H, Fukatsu T. 2012. An insect-induced novel plant phenotype for sustaining social life in a closed system. Nat. Commun. 3, 1187 ( 10.1038/ncomms2187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benton TG, Foster WA. 1992. Altruistic housekeeping in a social aphid. Proc. R. Soc. Lond. B 247, 199–202. ( 10.1098/rspb.1992.0029) [DOI] [Google Scholar]
- 7.Smith RG. 1999. Wax glands, wax production and the functional significance of wax use in three aphid species (Homoptera: Aphididae). J. Nat. Hist. 33, 513–530. ( 10.1080/002229399300227) [DOI] [Google Scholar]
- 8.Pike N, Richard D, Foster W, Mahadevan L. 2002. How aphids lose their marbles. Proc. R. Soc. Lond. B 269, 1211–1215. ( 10.1098/rspb.2002.1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoki S. 1980. Occurrence of a simple labor in a gall aphid Pemphigus dorocola. Kontyû 48, 71–73. [Google Scholar]
- 10.Abbot P, Chapman T. 2017. Sociality in aphids and thrips. In Comparative social evolution (eds Rubenstein DR, Abbot P), pp. 124–153. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 11.Stalder AF, Melchior T, Müller M, Sage D, Blu T, Unser M. 2010. Low-bond axisymmetric drop shape analysis for surface tension and contact angle measurements of sessile drops. Colloids Surf. A Physicochem. Eng. Asp. 364, 72–81. ( 10.1016/j.colsurfa.2010.04.040) [DOI] [Google Scholar]
- 12.R Core Team. 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ .
- 13.Matsukura K, Matsumura M, Tokuda M. 2009. Host manipulation by the orange leafhopper Cicadulina bipunctata: gall induction on distant leaves by dose-dependent stimulation. Naturwissenschaften 96, 1059–1066. ( 10.1007/s00114-009-0566-1) [DOI] [PubMed] [Google Scholar]
- 14.Stern DL. 1995. Phylogenetic evidence that aphids, rather than plants, determine gall morphology. Proc. R. Soc. Lond. B 260, 85–89. ( 10.1098/rspb.1995.0063) [DOI] [Google Scholar]
- 15.Uematsu K, Kutsukake M, Fukatsu T. 2018. Data from: Water-repellent plant surface structure induced by gall-forming insects for waste management Dryad Digital Repository. ( 10.5061/dryad.q9p2q59) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Uematsu K, Kutsukake M, Fukatsu T. 2018. Data from: Water-repellent plant surface structure induced by gall-forming insects for waste management Dryad Digital Repository. ( 10.5061/dryad.q9p2q59) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Additional data, details of statistical analyses and a movie are available as electronic supplementary material and in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.q9p2q59 [15].