Abstract
Background
Natural compounds have been utilized in inhibiting metastasis alone or in combination with other anti-tumor agents. Dehydrocostus lactone (DHC), a natural sesquiterpene lactone, was used to investigate its effect on proliferation of lung cancer cells and on the anti-angiogenic efficacy of doxorubicin.
Material/Methods
Cell proliferation was assessed by MTT assay and clonogenic assay. Apoptosis and migration were assessed by flow cytometry and wound-healing assay, respectively. Western blotting and qPCR were performed for gene and protein expression analysis. Matrigel plug assay was performed for angiogenesis assessment.
Results
Results of the study show that DHC inhibited the survival and proliferation of lung cancer cells (A549 and H460) and enhanced the growth-inhibitory properties of DOX. Cotreatment of DHC enhanced the apoptosis-inducing effects of DOX by activating caspase-9 and caspase-3 followed by cleavage of PARP. Treatment of A549 and H460 cells with DHC caused suppression of HIF-1α, Akt and pAkt, GSK-3β and pGSK-3β, as well as ERK, pERK, mTOR, and p-mTOR. DHC enhanced the effect of DOX by inhibiting migration of A549 cells as observed by wound-healing assay. DHC caused synergistic inhibition of MMP-2 and MMP-9 genes when treated in combination with DOX. DHC further enhanced the anti-angiogenic properties of DOX in mice implanted with Matrigel plugs. DHC suppressed the proliferation of lung cancer cells and enhanced the anti-angiogenic properties of DOX.
Conclusions
The putative mechanism behind the metastasis-limiting effects of DHC may involve the suppression of Akt/GSK-3β and inhibition of MMP-2 and MMP-9 in lung cancer cells.
MeSH Keywords: Angiogenesis Inhibitors, Apoptosis, Cell Migration Inhibition, Herbal Medicine, Saussurea, Sesquiterpenes
Background
Lung cancer was diagnosed in about 2 million people and resulted in about 1.6 million deaths worldwide in 2016 [1]. Lung cancer is a malignant tumor characterized by uncontrolled proliferation of cells in lung tissues and is characterized as small-cell lung carcinoma and non-small-cell lung carcinoma (NSCLC). NSCLC has a relatively poor prognosis and accounts for about 80% of all lung cancer cases [1]. Smoking remains the major cause (about 85%) of lung carcinogenesis, mainly due to carcinogens (more than 70 reported), mainly benzo[α]pyrene, 1,3-butadiene, and NNK [2]. Treatments for lung cancer, such as surgical resection, radiation therapy, adjuvant chemotherapy, and targeted therapy, have been conventionally applied and have been advanced with research approaches in diagnosis and therapy for improving health and survival of patients [3]. Among these, chemotherapy has been a critical intervention for lung cancers, but limiting metastasis is still a challenge. Angiogenesis is a normal process of formation of new blood vessels, which is regulated mainly by vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). Tumor cells persistently release these factors and tightly regulate the switch between angiogenesis inhibitors and activators [4]. Thus, inhibition of these angiogenic steps by blocking angiogenesis-related proteins by chemotherapeutic agents could be a novel preventive strategy to arrest tumor growth. Chemotherapeutic agents possessing the ability to induce apoptosis and inhibit migration or invasion may effectively limit metastatic lung carcinoma [5]. Thus, drugs with abilities to suppress malignant tumorigenesis, invasion, and metastasis may intervene through the regulation of cellular apoptosis. Chemotherapy regimens can suppress cancer symptoms and improve quality of life for lung cancer patients [6]. Drugs activating apoptosis and limiting metastasis can selectively inhibit proliferation of cancer cells and are in increasingly high demand. Growing evidence suggests that Traditional Chinese Medicine and phytochemicals may exert promising alternative therapeutic effects on human malignancies, including limiting metastasis [7,8].
Dehydrocostus lactone (DHC) is a sesquiterpene lactone isolated from Chinese herbs like Saussurea lappa and Laurus nobilis. DHC has been used to treat various diseases, including prevention of various types of cancers, such as liver cancer [9,10], breast cancer [11,12], leukemias [13], prostatic cancer [14], ovarian cancer [15], colorectal cancer [16], and bladder cancer [17]. DHC mediates anti-cancer effects by interacting mechanisms like inhibition of telomerase activity, induction of cellular apoptosis pathways (mitochondrial intrinsic and death-receptor dependent extrinsic), induced endoplasmic reticulum (ER) stress [18], and limiting migration-invasion and metastasis [8,16,19]. DHC prevents growth of various types of cancer by specific mechanisms like tumor necrosis factor (TNF)-α-induced degradation and phosphorylation of IkBa [20], inhibition of signal transducers, and activators of transcription 3 (STAT3) [9]. JAK/STAT modulation by DHC has been found to be associated with suppression of human chronic myeloid leukemia cells proliferation [21]. DHC suppresses proliferation of glioma cells through inhibition of the NF-κB/COX-2 signaling pathway by targeting IKKβ [22]. DHC suppressed the angiogenesis of human umbilical vein endothelial cells (HUVEC) in vitro and in vivo through inhibition of Akt/glycogen synthase kinase (GSK-3β) and mechanistic target of rapamycin (mTOR) signaling pathways [23]. DHC was also shown to prevent invasiveness of cervical cancer cells through the PI3K/Akt signaling pathway [24] and inhibited invasion and migration in neuroblastoma cells [25]. These properties indicate that DHC might be a promising anti-tumor agent alone or in combination with other chemotherapeutic agents, and it may modulate tumor metastasis, which also needs validation.
This study investigated the anti-proliferative effects induced by DHC in lung cancer cells in vitro and in vivo. The effects of DHC in combination with doxorubicin (DOX) was also analyzed and the molecular mechanism was elucidated.
Material and Methods
Cell culture
Human lung carcinoma cells A549 and human NSCLC cells NCI-H460 were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Thermo Fisher Scientific Inc., OK, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 μg/mL), all from Sigma (MO, USA). Cells were kept at 37°C in a humidified atmosphere of 5% CO2 and 95% air in a CO2 incubator.
Cell viability assay
A549 and H460 cells were seeded at a density of 4000 cells per well in 96-well plates for 24 h and then treated with or without DHC, DOX, and DHC+DOX for 48 h at various concentrations. DHC and DOX were dissolved in dimethyl sulfoxide (DMSO) and serially diluted for treating cell cultures to a final concentration of 1% DMSO as vehicle control. The MTT cell viability kit (Sigma) was used as per the manufacturer’s instructions. The percentage of viable cells in test sets was calculated against vehicle control. Experiments were performed in triplicate.
Clonogenic assay
A549 and H460 cells were seeded at a density of 4000 cells per well in 96-well plates for 24 h and then treated with or without DHC, DOX, and DHC+DOX at indicated concentrations for 24 h. Culture media was removed from wells and 100 μl methanol added. Plates were incubated at 37°C for 30 min, then cells were stained with crystal violet for 1 h. Cells were then washed with PBS and counted under a light microscope (40×). Experiments were performed in triplicates.
Flow Cytometric assessment of apoptosis
A549 and H460 cells were seeded at a density of 2×106 cells for 24 h and then treated with or without DHC, DOX, and DHC+DOX at indicated concentrations for 24 h. The pattern of apoptotic cell death in cells was assayed by flow cytometry using the Annexin V-FITC Apoptosis Detection Kit (BD Bioscience, USA) as per the manufacturer’s instructions. Cells treated for 24 h were subjected to staining with FITC-conjugated annexin V and analysis by flow cytometry using FACSCalibur (BD Bioscience, USA).
Wound-healing assay
A549 cells were seeded in 6-well plates at a density of 1×106 cells per well for 24 h. Cells could grow in a monolayer confluence and wounds were created with a sterile micropipette tip of 200 μl volume. Cells in different wells were treated with or without DHC, DOX, and DHC+DOX at indicated concentrations for 24 h. Wells were photographed under phase-contrast microscopy at 0 h (within 30 min of creating the wound) and at the end of incubation (24 h). The images of wound scratches were comparatively analyzed in different treatment groups.
Protein isolation and western blotting
A549 and H460 cells were seeded in 6-well plates for 24 h and then treated with or without DHC, DOX, and DHC+DOX at indicated concentrations for 24 h. Protein was isolated from cells and quantified by using the BCA protein assay kit (Sigma, USA). An equal amount of protein was electrophoresed by SDS-PAGE followed by blot transfer onto the PVDF membrane. Membranes were incubation with specific primary antibodies overnight at 4°C followed by treatment with HRP-conjugated secondary antibodies. Protein bands were visualized by using an enhanced chemiluminescence (ECL) detection system (Thermo Fisher, USA). Primary antibodies with the following technical details were used for Western blotting: caspase-9 (Cat#9508, Cell Signaling Technology, USA), caspase-3 (Cat#9662, Cell Signaling Technology, USA), PARP (Cat#9542, Cell Signaling Technology, USA), NF-κB (Cat# NB100-2176, Novus Biologicals, USA), BCL-2 (Cat#2876, Cell Signaling Technology, USA), GAPDH (Cat#sc-166574, Santacruz Biotechnology, USA), HIF-1α (Cat# NB100-105, Novus Biologicals, USA), AKT (Cat#4691, Cell Signaling Technology, USA), pAKT (Cat#9271, Cell Signaling Technology, USA), ERK1/2 (Cat#9102, Cell Signaling Technology, USA), pERK1/2 (Cat#9106, Cell Signaling Technology, USA), GSK-3β (Cat#9366, Cell Signaling Technology, USA), pGSK-3β (Cat#9323, Cell Signaling Technology, USA), mTOR (Cat#2983, Cell Signaling Technology, USA), p-mTOR (Cat#2971, Cell Signaling Technology, USA).
RNA isolation and quantitative real-time PCR (qPCR)
A549 cells were seeded in 6-well plates for 24 h and then treated with or without DHC, DOX, and DHC+DOX at indicated concentrations for 24 h. Total cellular RNA was extracted using TRIzol reagent (Thermo Fisher, USA) and cDNA was synthesized using the Superscript III reverse transcription kit (Thermo Fisher, USA). The qPCR amplification for target genes was performed on an Applied Biosystems 7500 Real-Time PCR system. A total of 2 μl cDNA per sample was used for target amplification in a 20-μl reaction using 10-pmol primers (forward and reverse each) and 10 μL of SYBR Green dye (Thermo Fischer, USA). SYBR Green binding to double-stranded DNA resulted in the fluorescence, which was measured and quantified by threshold cycle (Ct) value method. Gene-specific primers used for PCR analysis were: MMP-1, 5′-AGCTAGCTCAGGATGACATTGATG-3′ (forward) and 5′-GCCGATGGG CTGGACAG-3′ (reverse); MMP-2, 5′-TGAGCTCCCG GAAAAGATTG-3′ (forward) and 5′-TCAGCAGCC TAGCCAGTCG-3′ (reverse); MMP-9, 5′-CAACATCAC CTATTGGATCC-3′ (forward) and 5′-CGGGTGTAG AGTCTCTCGCT-3′ (reverse); GAPDH, 5′-ACCACAGTCC ATGCCATCAC-3′ (forward) and 5′-TCCACCACC CTGTTGCTGTA-3′ (reverse). The relative quantification of genes was performed by normalizing their levels to that of 18S in the same cDNA. Data are represented as relative fold change in expression of target genes in treatment group versus control. Each experiment was performed in triplicate.
Animals
The animal experimental procedures were performed in accordance with the guidelines of the Animal Ethics Committee of the institute. Animal ethics clearance was obtained for each experiment performed in this study. Female C57BL/6 mice (5 weeks old and 22 g average weight) were included in the study for Matrigel assay. Mice were housed in a climate-controlled room maintained at 22–24°C and 50–60% humidity with a 12-h light/dark cycle.
In vivo anti-angiogenesis (Matrigel plug) assay
The anti-angiogenic effect of DHC alone or in combination with DOX was investigated by the in vivo angiogenesis assay in an exogenous Matrigel plug injected into C57BL/6 mice (n=5, each group). Matrigel (BD Bioscience, San Jose, CA) was injected in mice after mixing with heparin (10 units/ml), VEGF (40 ng/ml), IGF-1 (40 ng/ml), EGF (40 ng/ml), and bFGF (40 ng/ml), all from Sigma. The mixture was mixed with: (i) vehicle control, (ii) DHC (5 mg/kg), and (iii) DHC (5 mg/kg) + DOX (2 mg/kg) and the resulting mixture was injected subcutaneously into the abdomens under cold conditions. One week later, mice in the 3 groups were sacrificed and the Matrigel plugs were carefully dissected and photographed. Angiogenesis was assayed by determining blood vessel growth in the Matrigel plugs. The quantification of the formation of blood vessels and hemoglobin content was analyzed using Drabkin’s reagent kit (Sigma, USA). To visualize endothelial infiltration and to assess the microvascular density (MVD) in treatment groups, Masson’s Trichrome (M-T) staining was performed. Matrigel plugs were sectioned to 4-μm thickness followed by staining with M-T solution. The blood vessels distribution was visualized under a light microscope.
Statistical analysis
All data were collected in triplicate and are presented as mean±SD (standard deviation). Data were analyzed using SPSS v15.0 statistical software (SPSS, Chicago, IL, USA) and statistical comparisons were performed between the groups by the one-way analysis of variance (ANOVA) or t test, as per experimental requirements. P values <0.05 were considered statistically significant.
Results
DHC suppresses proliferation of lung cancer cells
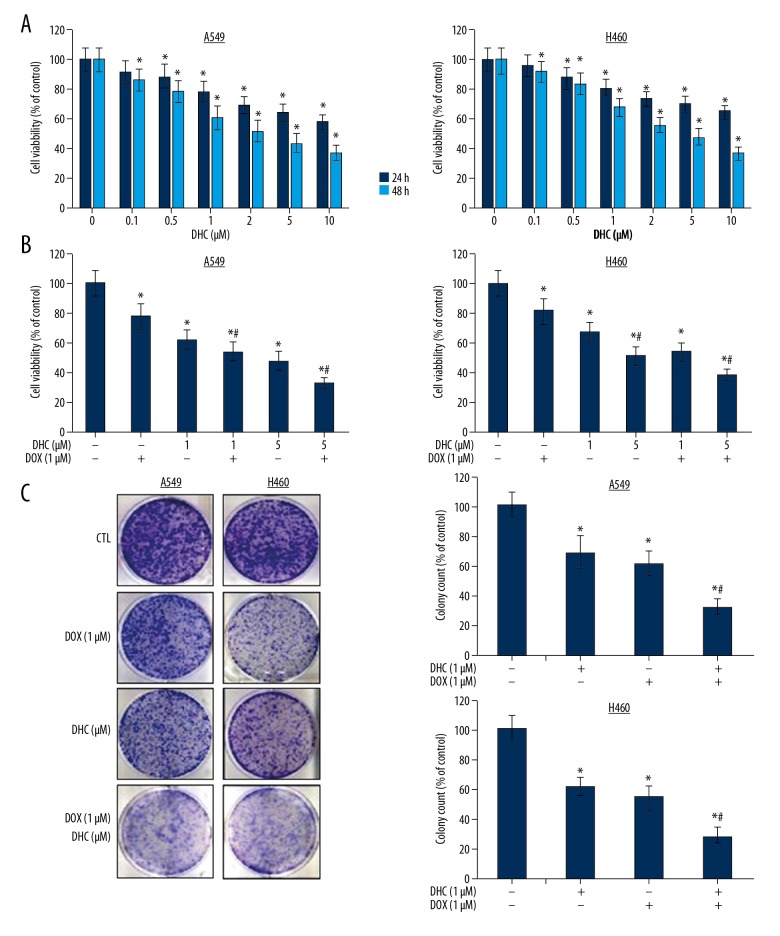
The effect of DHC on survival and proliferation of lung cancer cells was investigated by treating A549 and H460 cells with DHC alone or in combination with DOX. The cell growth analysis demonstrates that DHC suppressed the growth of both cells in time- and dose-dependent manners (Figure 1A). The growth-inhibitory concentration (IC50) determined for A549 and H460 in both cell lines was about 2 μM at 24 h and about 1 μM at 48 h. DHC has time-dependent pharmacological effects on lung cancer cells. DHC was effective on both cell lines at 24 h, which was further enhanced at 48 h of treatment (Figure 1A). Next, we assessed the effect of the combination of DHC (1 and 5 μM) with DOX (1 μM) by analyzing cell viability (Figure 1B). The treatment of A549 with DOX caused 15.8% growth inhibition (in 3 quadrants), which was significantly enhanced to 25.4% growth inhibition (in 3 quadrants) at 1 μM of DHC. The growth inhibition was synergistically high in the combination of 1 μM DOX and 5 μM DHC with 42.8% cells in early apoptosis, 16.2% in late apoptosis, and 6.8% in necrosis stage (Figure 2B). The treatment of H460 cells with DOX (1 μM) caused growth inhibition in a similar manner with 13.2% growth inhibition with only DOX and 20.6% growth inhibition with only DHC. The combination of DOX and DHC lead to a high growth inhibition with 22.4% of cells in early apoptosis and 34.2% in late apoptosis stage (Figure 2B). The effect of DOX and its combination with DHC on H460 cells was similar in pattern to A549 cells, yet slightly lower; however, this assessment is not comparable. Further, the clonogenic assay was performed to analyze the effect of DHC on proliferation of A549 lung cancer cells proliferation. Colony formation assay results demonstrated that DHC (1 μM) treatment (24 h) suppressed the proliferation of A549 by 38%, DOX (1 μM) suppressed colony formation by 26%, and the combination of both at 1 μM each synergistically suppressed A549 cells proliferation to 48% (Figure 1C). These results show that DHC enhances the chemotherapeutic potentials of DOX in lung cancer cells.
Figure 1.
Effect of DHC on cell survival and proliferation. (A) A549 and H460 cells were treated with DHC at indicated concentrations for 24 and 48 h and cell viability was assayed. The MTT cell viability assessment is represented as percent of control. * P<0.05 vs. control for 24 or 48 h. (B) Cells were treated with DHC and DOX for 48 h and cell viability was assayed. * P<0.05 vs. control; # P<0.05 vs. DOX and DHC. (C) A549 cells were treated with DHC and DOX for 24 h and clonogenic assay was performed. Percentage of cells per well is represented as compared to control. DHC – dehydrocostus lactone; DOX – doxorubicin. * P<0.05 vs. control; # P<0.05 vs. DOX or DHC.
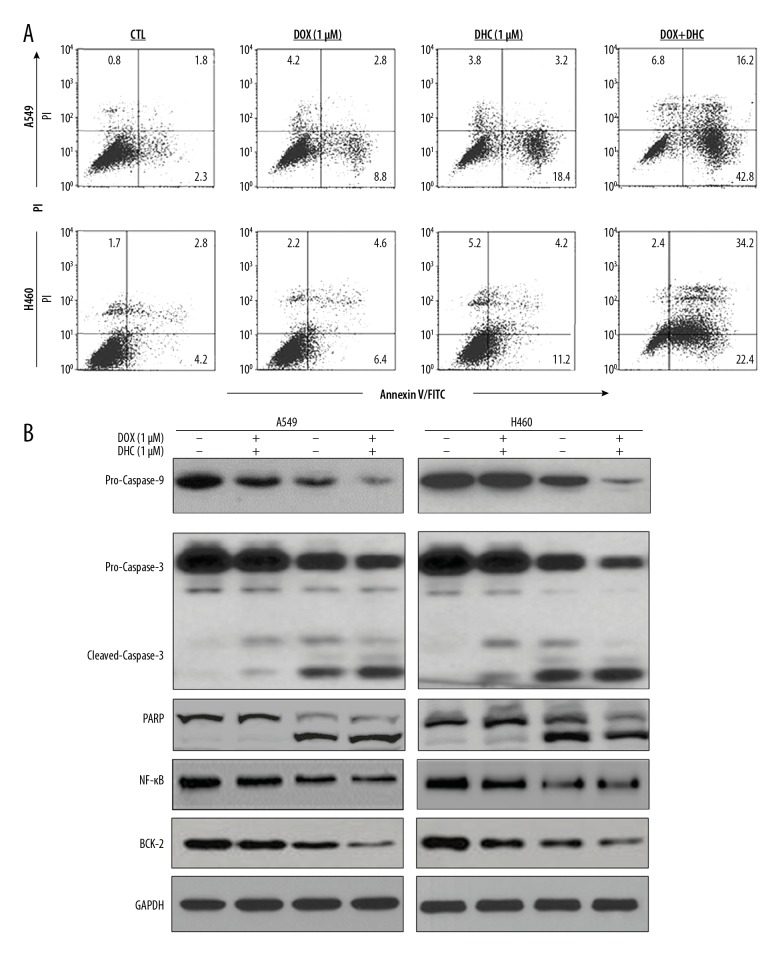
Figure 2.
Effect of DHC on apoptosis of lung cancer cells. (A) Flow cytometry analysis of A549 and H460 cells after annexin V/PI-FITC staining. (B) Western blot analysis of apoptosis-related proteins from A549 and H460 cells treated with or without DOX and DHC. DHC – dehydrocostus lactone; DOX – doxorubicin.
DHC enhances pro-apoptotic and anti-angiogenic properties of DOX
To understand the mechanism behind the growth-inhibitory effects of DHC and DOX, A549 and H460 cells were stained with Annexin V and PI and subjected to FACS analysis (Figure 2A). DHC (1 μM)-treated cells showed induction of apoptosis by 16.8% and 16.4% for early and late apoptosis, respectively. DOX (1 μM)-treated A549 cells also showed induction of apoptosis by 6.8% (early apoptosis) and 11.6% (late apoptosis). A combination of both showed a drastic increase in early apoptosis (18.4%) and late apoptosis (23.5%). Similar observations and trends in apoptosis were recorded for H460 cells. These results show that DHC enhanced the apoptosis-inducing effects of DOX in A549 and H460 cells, which was further confirmed by Western blotting of protein proliferation signaling (Figure 2B). DHC (1 μM) induced apoptosis by causing activation of pro-caspase-9 followed by cleavage of pro-caspase-3 to cleaved-caspase-3. DHC (1 μM) further caused cleavage of PARP, a nuclear substrate of caspase-3, leading to cell death. DOX (1 μM) also induced apoptosis via the caspase-9-dependent pathway. The combination of DHC significantly enhanced the apoptosis-inducing effects DOX. The activation of caspases (9 and 3) and cleavage of PARP was drastic with the combination of DHC with DOX (Figure 2B). These results collectively demonstrate that DHC enhanced the apoptosis-inducing properties of DOX in lung cancer cells, which caused growth inhibition.
DHC modulates growth-regulatory and angiogenesis signaling pathways
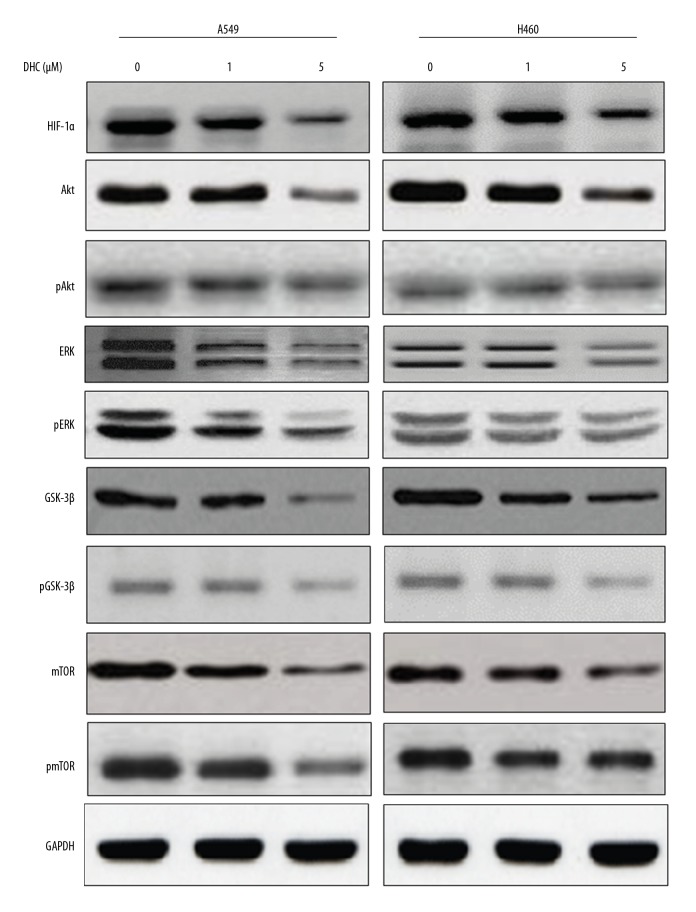
Rigorous tumor growth causes hypoxia, which upregulates hypoxia-inducible factor-1 alpha (HIF-1α) levels, leading to cell survival and angiogenesis via activation of PI3K/Akt and ERK as well as GSK-3β signaling cascades [26,27]. We assessed the effect of DHC on expression of proteins in cell growth and proliferation signaling, as well as proteins involved in maintaining invasiveness and migration of cells. Western blotting results (Figure 3) show that treatment of A549 and H460 cells with DHC suppressed the levels of HIF-1α in a dose-dependent manner. DHC suppressed the levels of Akt in both cell lines, but more clearly at the 5-μM concentration. The levels of pAkt were also similarly reduced in both cell lines by DHC treatment. Likewise, ERK and pERK were reduced by the treatment of DHC, in a dose-dependent manner. GSK-3β was highly expressed in both cell lines and was subsequently suppressed by treatment with DHC in a dose-dependent manner. The phosphorylated form of GSK-3β was also suppressed by DHC treatment in both cell lines. In addition, mTOR and p-mTOR levels were also reduced by DHC treatment in both cell lines. A549 cells were more responsive to DHC, especially at the 5-μM dose. These results clearly indicate that DHC has inhibitory effects against growth-regulatory and cell proliferation signaling molecules. DHC also suppressed HIF-1α, which supports our hypothesis that DHC interfere in angiogenic processes in lung cancer cells.
Figure 3.
Effect of DHC on cell survival and growth-regulatory proteins. Western blotting of apoptosis-related proteins from A549 and H460 cells treated with or without DOX and DHC. DHC – dehydrocostus lactone; DOX – doxorubicin.
DHC enhances anti-angiogenic effects of DOX
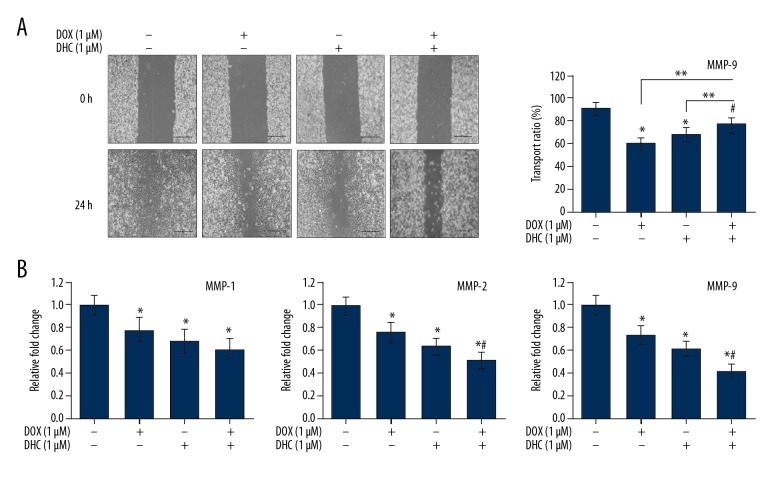
Results in Figure 3 indicate the probable anti-angiogenic role of DHC in lung cancer cells, so we assessed the effect of DHC in combination with DOX on migration and invasion of lung cancer cells. The wound-healing assay was performed to evaluate the effects of DHC in combination with DOX on migration of A549 cells. Results in Figure 4A demonstrate that wound-healing was very high in control cells and cells almost reached confluence at 24 h after wound creation. A549 cells treated with DOX at the 1-μM concentration showed dramatic wound healing at 24 h. Likewise, treatment of A549 cells with DHC at the 1-μM concentration showed high wound healing at 24 h, while the combination of DHC and DOX (1 μM each) drastically increased the wound-healing pattern in A549 cells at 24 h after wound creation. The quantitative estimation of cells transport ratio shows that control cells reached 92±6% confluence at 24 h after wound creation. Treatment of cells with DOX and DHC (1 μM each) caused transport of 61±5% and 69±6% cells (* P<0.003) as compared to control, while the combination of DOX and DHC (1 μM each) caused 78±6% (** P<0.02) cells transport as compared to control (Figure 4A). The high migration of cells in combination of DOX with DHC was close to that of the control (78%) with a marginal statistical difference (# P=0.041 vs. control) (Figure 4A).
Figure 4.
Effects of DHC and DOX migration and invasion of lung cancer cells. (A) Representative photomicrographs of cell migration analyzed by wound-healing assay (40×). Quantification of the number of migrated cells at 24 h of treatment is represented as the percentage of the vehicle control. DHC – dehydrocostus lactone; DOX – doxorubicin. * P<0.05 vs. control; ** P<0.05 vs. DOX or DHC. # P=0.041 vs. control. (B) A549 cells were treated with DOX and/or DHC and MMPs expression levels were analyzed by qPCR.
Cancer cell metastasis is characterized by the degradation of ECM, which is closely associated with overexpression of proteolytic enzymes like matrix metalloprotease (MMPs) [28]. Activation of MMPs 1, 2, and 9 has been found elevated in the metastasis of several carcinomas. Thus, we examined the effect of DHC in combination with DOX on A549 cells by analyzing gene expression of MMP-1, MMP-2, and MMP-9 by qPCR (Figure 4B). Results show that treatment of A549 cells with DOX (1 μM) and DHC (1 μM) alone caused reduction in the levels of MMP-1, MMP-2, and MMP-9 to significant levels as compared to that in control. The combination of DOX (1 μM) with DHC (1 μM) caused synergistic reduction in the levels of MMP-1, MMP-2, and MMP-9 to significant levels (Figure 4B). These observations suggest that DHC enhances the anti-angiogenic effects of DOX in lung cancer.
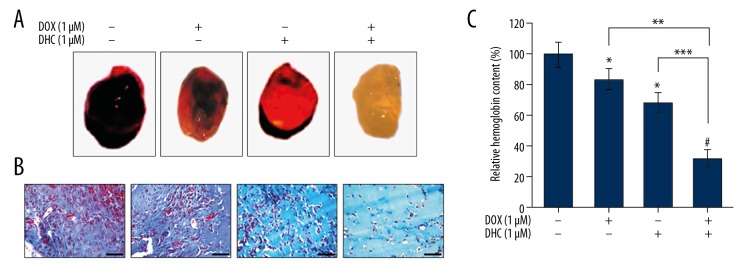
DHC enhances anti-angiogenic properties of DOX in mice
The anti-angiogenic effects of DHC were evaluated in a Matrigel plug in the mouse model (Figure 5). Matrigel plugs in control mice (containing VEGF alone) were very dark red, suggesting vessel formation by induction of the angiogenic process (Figure 5A). The Matrigel plugs in DOX-treated mice were lighter than in control mice, followed by lighter Matrigel plugs in DHC-treated mice. Conversely, the Matrigel plugs in mice treated with the combination of DOX and DHC were avascular and pale white (Figure 5A). These observations suggest that Matrigel plugs in control mice have abundant functional vasculatures formed due to VEGF supplementation, which was subsequently suppressed by DOX and DHC or the combination of both. These results were further supported by M-T staining of the sections of Matrigel plugs and by analyzing the number of functional vessels within the Matrigel plugs (Figure 5B). Control mice showed very high number of blood vessels formed in the Matrigel plugs, while the vasculature was comparatively lower in DOX- and DHC-treated mice. Furthermore, the section of Matrigel plugs in mice treated with the combination of DOX and DHC showed drastically reduced numbers of vessels (Figure 5B). The analysis of hemoglobin content in Matrigel plugs was further confirmation of angiogenesis (Figure 5C). The relative hemoglobin (Hb) content in control mice averaged 100±8%. The relative Hb content was comparatively lower in DOX- and DHC-treated mice, at 84±7% and 68±7%, respectively, while the combination of DOX with DHC showed drastically lower levels of Hb content (32±6%) (Figure 5C). The statistical analysis of these data shows that Hb content was significantly lower in mice individually treated with DOX and DHC (* P<0.05) as compared to control. The combination of DOX with DHC produced significantly lower Hb levels (# P<0.001) compared to control. The combination of DHC with DOX caused highly significant reduction in Hb levels as compared to DOX (** P<0.038) and DHC (*** P<0.022) alone. Overall, these results demonstrate that DHC suppresses angiogenesis in a mouse model and it enhances the anti-angiogenic properties of DOX when treated in combination.
Figure 5.
DHC inhibits angiogenesis and enhances effects of DOX. (A) Matrigel plugs were implanted in C57BL/6 mice (n=5). (B) Masson-Trichrome staining from sections of the Matrigel plug (40×). Scale bar 100 μM. (C) Hemoglobin content was analyzed data represented as mean ±SD. DHC – dehydrocostus lactone; DOX – doxorubicin. * P<0.05 vs. control; # P<0.001 vs. control; ** P<0.038 vs. DOX; *** P<0.022 vs. DHC.
Discussion
Lung cancer therapy requires prevention of invasion and metastasis of carcinoma. Thus, there is a need for therapeutic modalities limiting lung cancer metastasis, along with a need to reduce the associated adverse effects. Natural chemical agents have been a great source of cancer therapy drugs and several phytochemical treatments have showed metastasis-limiting potential. In this study, we demonstrated that DHC induced apoptotic cell death in lung cancer cell lines A549 and H460. Whether DHC-induced apoptotic machinery affects proliferation and invasive potential of lung cancer was further explored. Based on the available knowledge of tumor biology, plant molecules with novel and specific mechanisms of action may serve as alternative therapies, even in drug-resistant tumors.
Network-based pharmacological approaches have been successful in determining the active components of plants and have led to identification of bioactive molecules, such as the identification of over 70 compounds from Curculigo orchioides as potential targets for the treatment of osteoporosis [29]. Diterpenoids are interesting molecules for cancer therapy and prevention. Recently, 2 diterpenoids (Jolkinolide A and B) isolated from Euphorbia fischeriana were shown to have anti-tumor and anti-metastatic activity against lung the cancer cell line A549. They prevented the invasion of tumor cells to blood vessels and thus metastasis [30]. Dehydrocostus lactone, a sesquiterpene lactone, has shown potent therapeutic effects, including anti-cancer effects [9–15,17]. DHC was reported to trigger oxidative endoplasmic reticular stress, leading to apoptotic cell death in lung carcinoma cells [18]. However, studies relating the effects of DHC on angiogenesis and metastasis need validation. The present study found that DHC inhibited the proliferation of lung cancer cell lines (A549 and H460) and it enhanced the growth-inhibitor properties of DOX against both cell lines. DHC was found to induce mitochondrial intrinsic apoptosis with enhanced pro-apoptotic effects of DOX in both cell lines. DHC was shown to induce apoptosis in liver [9,10], breast [11,12], leukemia [13], prostate [14], ovary [15], colon [16], and bladder cancer [17]. DHC caused reduction in HIF-1α levels in A549 and H460 cells followed by suppression of the levels of Akt and pAkt, GSK-3β and pGSK-3β, and mTOR and p-mTOR. It is noteworthy that Akt levels were remarkably reduced by DHC at 5-μM concentration in cell lines, while 1-μM DHC was only fairly effective. However, pAkt levels were effectively reduced at 1-μM DHC in both cell lines. DHC was also earlier reported to modulate Akt signaling in cervical cancer cells [24]. These observations indicate that DHC may act as anti-angiogenic agent in treatment conditions and it may also enhance the effects of DOX. HIF-1α plays an essential role in the development of NSCLC by regulating important signaling events in response to hypoxia for cell survival and angiogenesis [26,27]. HIF-1α is a heterodimer consisting of 2 subunits (α and β). It is stabilized during hypoxia but metabolized by the ubiquitin-proteasomal pathway under normoxia. Thus, it acts as a marker signaling molecule activated under hypoxia, and is, in turn, a marker of tumor environments where oxygen deficiency is due to high cell proliferation. The HIF-1α stabilization and activation process is affected by specific signal cascades, such as PI3K/Akt and ERK [31,32]. A natural diterpene, andrographolide, also suppressed metastasis of lung cancer cells by suppressing HIF-1α through suppression of PI3K/Akt signaling [33]. PI3K/Akt and ERK signaling cascades were also reported to be involved in HIF-1α-mediated metastasis in human malignancies like ovarian carcinoma [34], breast tumor [35], hepatoma [36], and prostate cancer [37]. Tumor growth is critically regulated by Akt activation, which simultaneously causes activation of phosphorylation in a number of substrates, including GSK-3β [38], which regulates cell cycle progression by phosphorylating cyclin D1 [39]. DHC was reported to suppress proliferation and invasiveness of cervical cancer cells through downregulation of the I3K/Akt signaling pathway [24]. Our results are comparable to these observations that DHC inhibited the HIF-1α followed by inhibition of Akt/GSK-3β signaling pathways, leading to limiting the rate of metastasis in lung cancer cells.
DHC was further found to exert anti-angiogenic effects against A549 cells and it enhanced the effects of DOX. DHC treatment of A549 cells caused transport of cells for wound-healing. DOX-treated cells, when co-treated with DHC, showed dramatically high transport of cells in synergistic manner. Cancer cell metastasis is characterized by the overexpression of proteolytic enzymes like MMP-2 and MMP-9, controlling the rate of cell invasion and metastasis [28,40,41]. Higher levels of MMPs are critically associated with metastasis in many tumors, including lung cancer [41], and MMP-9 overexpression is especially associated with the progression of several tumor types [42,43]. MMP-2 is constitutively expressed in tissues and its level is abrogated during neoplastic transformation, especially at the occurrence of metastasis leading to migration and invasion [42]. Therefore, pharmacological inhibition of MMP-2 and MMP-9 is a critical target in therapeutic strategies against malignant tumors, including lung cancer. DHC inhibited the levels of MMP-2 and MMP-9 and it also caused synergistic inhibition of both MMPs when treated in combination with DOX.
DHC was further found to enhance the anti-angiogenic properties of DOX in mice implanted with Matrigel plugs. DHC-treated Matrigel plugs showed highly reduced vasculature as compared to control and similar observation reported from the DOX-treated Matrigel plugs. The combination of DHC enhanced the vessel-growth inhibitor properties of DOX in plugs. The M-T staining of Matrigel plugs sections and the Hb content analysis confirms our observation. DHC was reported to suppress proliferation and invasiveness of cervical cancer cells [24] and suppressed proliferation, migration, and invasion of colorectal carcinoma [16]. Overall results demonstrate that DHC suppresses angiogenesis in a mouse model and enhances the anti-angiogenic properties of DOX when treated in combination. The possible mechanism of action is suppression of Akt/GSK-3β and inhibition of MMPs (2 and 9) in lung cancer cells.
Conclusions
Results of the study demonstrate that DHC could be a potent anti-tumor agent with pro-apoptotic properties, which suppresses proliferation of lung cancer cells. DHC enhances the apoptosis-inducing and growth-inhibitory properties of DOX. DHC suppressed the expression of HIF1α, GSK-3β, Akt, ERK, MMP-2, and MMP-9 and limited the metastasis of lung tumors. DHC enhanced the anti-angiogenic properties of DOX through a probable mechanism of the inhibition of Akt/GSK-3β and MMPs (2 and 9) signaling in lung cancer cells. Our findings emphasize the therapeutic potential of DHC in targeting Akt/GSK-3β signaling and for limiting metastasis of lung carcinoma. These observations have high clinical relevance in developing therapeutic regimens containing DHC for individual treatment or to enhance the efficacy of known anti-cancer agents such as DOX.
Footnotes
Conflict of interests
None.
Source of support: Departmental sources
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131:2724–32. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaric B, Stojsic V, Tepavac A, et al. Adjuvant chemotherapy and radiotherapy in the treatment of non-small cell lung cancer (NSCLC) J Thorac Dis. 2013;5(Suppl 4):S371–77. doi: 10.3978/j.issn.2072-1439.2013.05.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 5.Weber GF. Why does cancer therapy lack effective anti-metastasis drugs? Cancer Lett. 2013;328:207–11. doi: 10.1016/j.canlet.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Saba NF, Khuri FR. Chemoprevention strategies for patients with lung cancer in the context of screening. Clin Lung Cancer. 2005;7:92–99. doi: 10.3816/CLC.2005.n.023. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Yang G, Li X, et al. Traditional Chinese medicine in cancer care: A review of controlled clinical studies published in chinese. PLoS One. 2013;8:e60338. doi: 10.1371/journal.pone.0060338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye L, Jia Y, Ji KE, et al. Traditional Chinese medicine in the prevention and treatment of cancer and cancer metastasis. Oncol Lett. 2015;10:1240–50. doi: 10.3892/ol.2015.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu YL, Wu LY, Kuo PL. Dehydrocostuslactone, a medicinal plant-derived sesquiterpene lactone, induces apoptosis coupled to endoplasmic reticulum stress in liver cancer cells. J Pharmacol Exp Ther. 2009;329:808–19. doi: 10.1124/jpet.108.148395. [DOI] [PubMed] [Google Scholar]
- 10.Liu CY, Chang HS, Chen IS, et al. Costunolide causes mitotic arrest and enhances radiosensitivity in human hepatocellular carcinoma cells. Radiat Oncol. 2011;6:56. doi: 10.1186/1748-717X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng Z, Wang Y, Fan J, et al. Costunolide and dehydrocostuslactone combination treatment inhibit breast cancer by inducing cell cycle arrest and apoptosis through c-Myc/p53 and AKT/14-3-3 pathway. Sci Rep. 2017;7:41254. doi: 10.1038/srep41254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitchai D, Roy A, Banu S. In vitro and in silico evaluation of NF-kappaB targeted costunolide action on estrogen receptor-negative breast cancer cells – a comparison with normal breast cells. Phytother Res. 2014;28:1499–505. doi: 10.1002/ptr.5155. [DOI] [PubMed] [Google Scholar]
- 13.Butturini E, Cavalieri E, de Prati AC, et al. Two naturally occurring terpenes, dehydrocostuslactone and costunolide, decrease intracellular GSH content and inhibit STAT3 activation. PLoS One. 2011;6:e20174. doi: 10.1371/journal.pone.0020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim EJ, Lim SS, Park SY, et al. Apoptosis of DU145 human prostate cancer cells induced by dehydrocostus lactone isolated from the root of Saussurea lappa. Food Chem Toxicol. 2008;46:3651–58. doi: 10.1016/j.fct.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 15.Choi EJ, Ahn WS. Antiproliferative effects of dehydrocostuslactone through cell cycle arrest and apoptosis in human ovarian cancer SK-OV-3 cells. Int J Mol Med. 2009;23:211–16. [PubMed] [Google Scholar]
- 16.Sun X, Kang H, Yao Y, et al. Dehydrocostus lactone suppressed the proliferation, migration, and invasion of colorectal carcinoma through the downregulation of eIF4E expression. Anticancer Drugs. 2015;26:641–48. doi: 10.1097/CAD.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 17.Rasul A, Bao R, Malhi M, et al. Induction of apoptosis by costunolide in bladder cancer cells is mediated through ROS generation and mitochondrial dysfunction. Molecules. 2013;18:1418–33. doi: 10.3390/molecules18021418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung JY, Hsu YL, Ni WC, et al. Oxidative and endoplasmic reticulum stress signaling are involved in dehydrocostuslactone-mediated apoptosis in human non-small cell lung cancer cells. Lung Cancer. 2010;68:355–65. doi: 10.1016/j.lungcan.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Lin X, Peng Z, Su C. Potential anti-cancer activities and mechanisms of costunolide and dehydrocostuslactone. Int J Mol Sci. 2015;16:10888–906. doi: 10.3390/ijms160510888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh GS, Pae HO, Chung HT, et al. Dehydrocostus lactone enhances tumor necrosis factor-alpha-induced apoptosis of human leukemia HL-60 cells. Immunopharmacol Immunotoxicol. 2004;26:163–75. doi: 10.1081/iph-120037712. [DOI] [PubMed] [Google Scholar]
- 21.Cai H, Qin X, Yang C. Dehydrocostus lactone suppresses proliferation of human chronic myeloid leukemia cells through Bcr/Abl-JAK/STAT signaling pathways. J Cell Biochem. 2017;118:3381–90. doi: 10.1002/jcb.25994. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Yu Z, Wang C, et al. Dehydrocostus lactone, a natural sesquiterpene lactone, suppresses the biological characteristics of glioma, through inhibition of the NF-kappaB/COX-2 signaling pathway by targeting IKKbeta. Am J Cancer Res. 2017;7:1270–84. [PMC free article] [PubMed] [Google Scholar]
- 23.Wang CY, Tsai AC, Peng CY, et al. Dehydrocostuslactone suppresses angiogenesis in vitro and in vivo through inhibition of Akt/GSK-3beta and mTOR signaling pathways. PLoS One. 2012;7:e31195. doi: 10.1371/journal.pone.0031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang E, Sun X, Kang H, et al. Dehydrocostus lactone inhibits proliferation, antiapoptosis, and invasion of cervical cancer cells through PI3K/Akt signaling pathway. Int J Gynecol Cancer. 2015;25:1179–86. doi: 10.1097/IGC.0000000000000474. [DOI] [PubMed] [Google Scholar]
- 25.Tabata K, Nishimura Y, Takeda T, et al. Sesquiterpene lactones derived from Saussurea lappa induce apoptosis and inhibit invasion and migration in neuroblastoma cells. J Pharmacol Sci. 2015;127:397–403. doi: 10.1016/j.jphs.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Axelson H, Fredlund E, Ovenberger M, et al. Hypoxia-induced dedifferentiation of tumor cells – a mechanism behind heterogeneity and aggressiveness of solid tumors. Semin Cell Dev Biol. 2005;16:554–63. doi: 10.1016/j.semcdb.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Harris AL. Hypoxia – a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 28.Steeg PS. Tumor metastasis: Mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 29.Wang N, Zhao G, Zhang Y, et al. A network pharmacology approach to determine the active components and potential targets of curculigo orchioides in the treatment of osteoporosis. Med Sci Monit. 2017;23:5113–22. doi: 10.12659/MSM.904264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen L, Zhang SQ, Liu L, et al. Jolkinolide A and Jolkinolide B inhibit proliferation of A549 cells and activity of human umbilical vein endothelial cells. Med Sci Monit. 2017;23:223–37. doi: 10.12659/MSM.902704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minet E, Arnould T, Michel G, et al. ERK activation upon hypoxia: Involvement in HIF-1 activation. FEBS Lett. 2000;468:53–58. doi: 10.1016/s0014-5793(00)01181-9. [DOI] [PubMed] [Google Scholar]
- 32.Zhong H, Chiles K, Feldser D, et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: Implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–45. [PubMed] [Google Scholar]
- 33.Lin HH, Tsai CW, Chou FP, et al. Andrographolide down-regulates hypoxia-inducible factor-1alpha in human non-small cell lung cancer A549 cells. Toxicol Appl Pharmacol. 2011;250:336–45. doi: 10.1016/j.taap.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Yang N, Katsaros D, et al. The oncogene phosphatidylinositol 3′-kinase catalytic subunit alpha promotes angiogenesis via vascular endothelial growth factor in ovarian carcinoma. Cancer Res. 2003;63:4225–31. [PubMed] [Google Scholar]
- 35.Yin F, Giuliano AE, Law RE, Van Herle AJ. Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP kinase activation in breast carcinoma cells. Anticancer Res. 2001;21:413–20. [PubMed] [Google Scholar]
- 36.Arsham AM, Plas DR, Thompson CB, Simon MC. Phosphatidylinositol 3-kinase/Akt signaling is neither required for hypoxic stabilization of HIF-1 alpha nor sufficient for HIF-1-dependent target gene transcription. J Biol Chem. 2002;277:15162–70. doi: 10.1074/jbc.M111162200. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q, Tang X, Zhang ZF, et al. Nicotine induces hypoxia-inducible factor-1alpha expression in human lung cancer cells via nicotinic acetylcholine receptor-mediated signaling pathways. Clin Cancer Res. 2007;13:4686–94. doi: 10.1158/1078-0432.CCR-06-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HS, Skurk C, Thomas SR, et al. Regulation of angiogenesis by glycogen synthase kinase-3beta. J Biol Chem. 2002;277:41888–96. doi: 10.1074/jbc.M206657200. [DOI] [PubMed] [Google Scholar]
- 39.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–70. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 41.Decock J, Thirkettle S, Wagstaff L, Edwards DR. Matrix metalloproteinases: Protective roles in cancer. J Cell Mol Med. 2011;15:1254–65. doi: 10.1111/j.1582-4934.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park SK, Hwang YS, Park KK, et al. Kalopanaxsaponin A inhibits PMA-induced invasion by reducing matrix metalloproteinase-9 via PI3K/Akt- and PKCdelta-mediated signaling in MCF-7 human breast cancer cells. Carcinogenesis. 2009;30:1225–33. doi: 10.1093/carcin/bgp111. [DOI] [PubMed] [Google Scholar]
- 43.Chung TW, Moon SK, Chang YC, et al. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: Complete regression of hepatoma growth and metastasis by dual mechanism. FASEB J. 2004;18:1670–81. doi: 10.1096/fj.04-2126com. [DOI] [PubMed] [Google Scholar]