Abstract
Background
Stress urinary incontinence is a common condition in women and can be associated with peripheral nerve injury. Exosomes. derived from Schwann cells, can enhance the regeneration of axons of the peripheral nervous system. This study aimed to investigate the effects of RSC96 Schwann cell-derived exosomes in a novel in vitro model of dorsal root ganglion (DRG) cell injury induced by cyclic mechanical strain (CMS).
Material/Methods
RSC96 Schwann cells and DRG cells were cultured in vitro. CMS in DRG cells involved mechanical stretch trauma with 5333 μ strain. ExoQuick-TC polymer was used to precipitate exosomes from RSC96 Schwann cell culture medium and identified by nanoparticle tracking analysis, electron microscopy, and Western blot for detection of CD9 and tumor susceptibility gene 101 (Tsg101) protein. Cultured DRG cells were treated with RSC96 Schwann cell-derived exosomes, followed by measurement of cell viability, proliferation, senescence, and apoptosis using the cell counting kit-8 (CCK-8) assay, senescence-associated beta-galactosidase (SA-β-gal) staining, and Hoechst 33258 (blue) fluorescence nucleic acid staining using flow cytometry.
Results
Mechanical stretch with 5333 μ strain for 8 hours at 1 Hz decreased the activity of cultured DRG cells. RSC96 Schwann cell-derived exosomes promoted cell proliferation and significantly inhibited apoptosis and senescence of DRG cells following injury induced by CMS.
Conclusions
An in vitro model of DRG cell injury induced by CMS, showed that RSC96 Schwann cell-derived exosomes had a protective effect. The effects of Schwann cell-derived exosomes on peripheral nerve injury, including in stress urinary incontinence, require future in vivo studies.
MeSH Keywords: Exosomes, Peripheral Nerve Injuries, Schwann Cells
Background
Stress urinary incontinence is a common condition in women that can affect the quality of life and results in an economic health burden [1,2]. There have been several theories on the pathophysiology of stress urinary incontinence, including nerve injury associated with childbirth [3]. In 1993, DeLancey proposed that pudendal nerve damage could cause the denervation of the supporting pelvic muscles, leading to stress urinary incontinence [4]. There is evidence from electrophysiological and pathophysiological studies of patients with stress urinary incontinence that nerve injury, and deficiency in neuropeptides, results in denervation of the supporting pelvic muscles [5,6]. Also, a clinical anatomical study has shown that nerve fibers of the anterior vaginal wall in patients with stress urinary incontinence were more slender and density of neuropeptides in the nerve fibers was significantly lower compared with women with normal pelvic floor anatomy [7]. An animal model postpartum stress urinary incontinence in female rats showed that the extent of the genital nerve injury determined the degree of pelvic floor functional impairment and the time required to recover [8]. Therefore, peripheral nerve injury has a possible role in the pathogenesis of stress urinary incontinence.
Axonal regeneration is an important process for functional recovery following nerve damage [9]. In the peripheral nervous system (PNS), signaling between Schwann cells and neurons is crucial for the growth and maintenance of axons. Studies have shown that Schwann cells secrete neurotrophic factors to promote axonal growth, including glial cell line-derived neurotrophic factor (GDNF) and neurotrophin-3 (NT-3) [10,11]. Schwann cells have also been shown to remove myelin debris from the demyelinated areas of injury and regulate macrophage recruitment by secreting cytokines that affect the rate of the process of nerve degeneration [12–14]. Schwann cells, during peripheral nerve damage, can develop a banded appearance, known as Bünger bands, and dedifferentiated Schwann cells provide growth channels for the axonal growth of regenerating neurons [15]. The successful regeneration of injured peripheral nerves results from the intrinsic regenerative capability of the neurons and the activity of supporting Schwann cells.
Exosomes are the smallest identified extracellular vesicles, with documented sizes ranging from between 40–150 nm, and they carry proteins, enzymes, and growth factors, are released by several cell types in vitro, and can be detected in biological fluids in vivo [16]. Exosomes mediate intercellular communication and have recently gained attention for their potential applications in clinical treatment [17,18]. Cortical neurons, microglia, oligodendrocytes, and astrocytes in the central nervous system (CNS) are capable of secreting exosomes [19], indicating that exosomes might be involved in the regulation of nerve activity. In additionthe transfer of vesicles from Schwann cells have been shown to have a protective effect on axonal regeneration [20].
Therefore, this study aimed to investigate the effects of RSC96 Schwann cell-derived exosomes in a novel in vitro model of dorsal root ganglion (DRG) cell injury induced by cyclic mechanical strain (CMS). The potential for functional recovery of damaged peripheral nerves might provide a novel therapeutic approach for the treatment of conditions such as stress urinary incontinence.
Material and Methods
Cell culture
Human RSC96 Schwann cells and normal dorsal root ganglion (DRG) cells were purchased from the China Center Type for Culture Collection (CCTCC, Wuhan, China), and were maintained in high glucose Dulbecco’s Modified Eagle’s Medium (DMEM) (Genom Biotech Co. Ltd., Hangzhou, China) supplemented with 10% fetal bovine serum (FBS) (Gemini Bio-Products, Fisher Scientific, Waltham, MA, USA) and 1% penicillin and streptomycin (Beyotime Biotech Co. Ltd., Suzhou, China). The cells were cultured in a humidified incubator (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C with 5% CO2.
Cyclic mechanical strain (CMS)
The DRG cells underwent loaded cyclic mechanical strain (CMS) as previously described, by using a four-point bending device (Miracle Technology Co. Ltd., Chengdu, China) [21]. CMS is an experimental system to study the changes in cells under different mechanical loads [21]. Briefly, DRG cells were trypsinized to prepare a cell suspension and cultured in cell culture plate with a size of 79×40×1.38 mm in high glucose DMEM containing 10% FBS and 1% penicillin and streptomycin until the cells were firmly adherent. The normal DRG cells were stretched by the four-point bending system for 0, 1333, 2666, and 5333 μ strain, using 0 μ strain as the control group, with a frequency set to 1 Hz. Then, the cells were cultured for another 4 h in the incubator and then harvested for the detection of indices of mechanical injury to select the optimal parameters of strain and time to establish the cell model of DRG cell injury for subsequent experiments.
Exosome isolation and characterization
Exosomes from the RSC96 Schwann cell culture supernatant were isolated using ExoQuick-TC™ exosome precipitation solution (EXOTC10A-1) (System Biosciences, Palo Alto, CA, USA). Briefly, RSC96 cells were supplemented with high glucose DMEM containing 10% exosome-free FBS for 24 h. The supernatant was centrifuged at 3,000×g for 15 min to remove cells and cell debris. Then, 5 ml of supernatant was incubated with 1 ml of ExoQuick-TC™ solution and refrigerated overnight (for at least 12 h) at 4°C, and then the mixture was centrifuged at 1,500×g for 30 min. Exosomes were re-suspended in 100 μL of sterile phosphate buffered saline (PBS) and were characterized using nanoparticle tracking analysis (NTA) with a ZetaView® Nanoparticle Tracking Analyzer (Particle Metrix, Meerbusch, Germany), electron microscopy (EM) using an HT7700 transmission electron microscope (Hitachi, Japan), and using Western blot analysis of two well-characterized exosomal protein markers, CD9 and tumor susceptibility gene 101 (Tsg101) protein.
Western blot of cell lysates
The expression levels of RSC96 Schwann cell-derived exosomes and the cell proteins extracted from RSC96 cells using RIPA buffer containing phenylmethylsulfonyl fluoride (PMSF) were detected with Western blot analysis. The protein concentrations were determined using a BCA Protein Assay Kit (Beyotime Biotech Co. Ltd., Shanghai, China). Samples with equal amounts of proteins were fractionated on 15% sodium dodecyl sulfate (SDS) polyacrylamide gels, transferred to polyvinylidene difluoride (PVDF) membranes, and blocked with 5% dried skimmed milk powder for 2 h at room temperature. The membranes were incubated at 4°C overnight with 1: 1000 dilutions (v/v) of primary antibodies, including CD9, Tsg101, and β-actin which was used as a control (Abcam, Cambridge, UK). After washing with Tris-buffered saline (TBS) and Tween 20 (TBST), the membranes were incubated in 1: 4000 dilutions (v/v) of secondary antibodies for 1 h at room temperature. Protein expression was detected using the Li-Cor Odyssey infrared imaging system (Li-Cor Biosciences, Lincoln, NE, USA)
Cell counting kit-8 (CCK-8) assay
The Cell Counting Kit-8 (CCK-8) was purchased from MultiSciences Biotech Co. Ltd. (Hangzhou, China) and was used to assess the cultured DRG cell viability and cell proliferation. Cells were seeded in 96-well cell culture plates at a density of 2×104 cells/ml. The DRG cells were cultured with 10 μl CCK-8 in each well at 37°C for 1 h. Cell viability was measured using a VICTOR 31420 Multilabel plate reader (PerkinElmer, Waltham, MA, USA).
Senescence-associated beta-galactosidase (SA-β-gal) cytochemical assay
The DRG cells were cultured on sterile glass coverslips, incubated in six-well plates, and washed twice with PBS. Cells were then fixed with 4% neutral buffered formalin for 15 min at room temperature. The cells were then washed twice for 3 min with PBS, and senescence-associated beta-galactosidase (SA-β-gal) staining solution was added (1 ml per 35 mm plate). Cells were incubated with staining solution at 37°C in an incubator without CO2 for at least 8 h. The cells were washed twice with PBS and the SA-β-gal-positive cells (stained blue-green) were counted under a BX51 light microscope (Olympus, Japan).
Hoechst 33258 fluorescence nucleic acid staining using flow cytometry
A Hoechst 33258 Staining Kit (Beyotime Biotech Co. Ltd., Shanghai, China) was used to evaluate the DRG cell nuclear apoptotic changes. Cells were seeded on sterile glass coverslips, incubated in the six-well plates, and then fixed, washed and stained according to the manufacturer’s instructions. The DRG cells were observed under an inverted fluorescence microscope (Olympus, Japan). Cells were analyzed based on changes in nuclear morphology; for example, cells with bright blue nuclear fluorescence and condensed cell nuclei were considered to be apoptotic. The apoptosis rate was defined as the ratio of the number of apoptotic cells number to the total cell number.
Flow cytometry analysis for cell apoptosis with Annexin-V phycoerythrin (PE)/7-Amino Actinomycin D (7-AAD)
Apoptotic cells were quantified using an Annexin-V phycoerythrin (PE)/7-Amino Actinomycin D (7-AAD) kit (Becton Dickinson, Franklin Lakes, NJ, USA) using a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). Adherent cells were harvested, washed, re-suspended in 0.5 ml PBS buffer and co-stained with 5 μl Annexin-V PE and 5 μl 7-AAD for analysis by flow cytometry. The density plots using this method illustrate four cell populations according to the fluorescence characteristics: live; early apoptotic; late apoptotic; and necrotic (or dead). In this assay, live cells would be expected to be both PE-negative and 7-AAD-negative; early apoptotic cells would be PE-positive and 7-AAD-negative; late apoptotic cells would be PE-negative and 7-AAD-positive; and necrotic (dead) cells would be both PE-positive and 7-AAD-positive.
Statistical analysis
The results were analyzed using GraphPad Prism 5 InStat Software (GraphPad, San Diego, CA, USA). Comparisons between groups were performed with one-way analysis of variance (ANOVA). All data were presented as the mean ±SEM. P-values <0.05 were considered to be statistically significant.
Results
Dorsal root ganglion (DRG) cell injury induced by cyclic mechanical strain (CMS)
To investigate the effects of cyclic mechanical strain (CMS) on dorsal root ganglion (DRG) cells, the cultured DRG cells underwent 0, 1333, 2666, 5333 μ strain for 0, 4, 8, 12 h at 1 Hz. DRG cells were then cultured in an incubator for another 4 h, and the cell viability was measured by the cell counting kit-8 (CCK-8) assay. As shown in Figure 1A, mechanical stress significantly decreased the cell viability of DRG cells in the 5333 μ strain group in a time-dependent manner. However, no significant differences were observed in the 1333 μ strain group. Cell viability was also reduced in DRG cells stretched for 12 h in the 2666 μ strain group. Compared with the 5333 μ strain group, the stretching time was too lengthy, and the damage effect was less than for the 5333 μ strain. Therefore, the parameters of the injury model used in this study were set as 5333 μ strain at 1 Hz.
Figure 1.
Effects of cyclic mechanical strain (CMS) on dorsal root ganglion (DRG) cell activity. (A) The effect of mechanical strain (1 Hz) on dorsal root ganglion (DRG) cell viability. (B) The effect of mechanical strain (1 Hz, 5,333 μ strain) on DRG cell senescence. Bar=50 μm. (C) The effect of mechanical strain (1 Hz, 5333 μ strain) on DRG cell apoptosis. (D) The effect of mechanical strain (1 Hz, 5333 μ strain) for 8 hours on cell proliferation in DRG cells. 0 h, 4 h, 8 h, 12 h represent DRG cells stretched for 0 h, 4 h, 8 h, 12 h, respectively. The values presented are the mean ± SEM (n=4). * p<0.05, **p<0.01.
To further investigate the effects of mechanical stress on DRG cell senescence and apoptosis, senescence-associated beta-galactosidase (SA-β-gal)-positive cells increased significantly in the 8-hour group (8 h), and a large number of DRG cells changed morphology in the 12-hour group (12 h) (Figure 1B). The apoptosis results also showed that mechanical stress in the 12-hour group induced an increased proportion of apoptotic cells (Figure 1C). Therefore, cell injury in DRG cells occurred at 5333 μ strain for a duration of 8 hours at 1 Hz in this study.
Cell proliferation assays showed that mechanical strain under the above conditions significantly affected the proliferation of DRG cells (Figure 1D). These results identified varied effects of CMS on DRG cells, including reducing cell proliferation and promoting cell senescence and apoptosis.
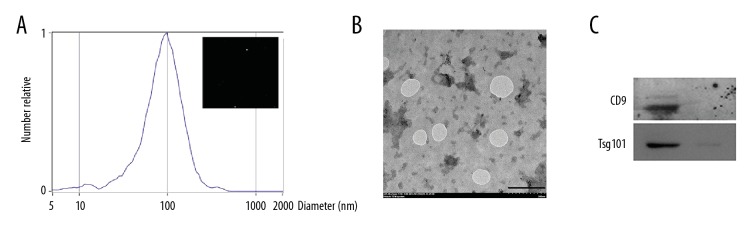
Isolation and characterization of RSC96 Schwann cell exosomes
The characteristics of exosomes that were isolated from RSC96 Schwann cells, as previously described [22] are shown in Figure 2. Nanoparticle tracking analysis (NTA) showed vesicles with a maximum diameter of 95.6 nm (Figure 2A). Transmission electron microscopy (TEM) identified particles with an average particle diameter size of between 40–150 nm, which is characteristic of exosomes (Figure 2B). Western blot showed that exosomes were positive for the expression of exosomal protein markers CD9 and Tsg101 (Figure 2C).
Figure 2.
Characterization of exosomes derived from RSC96 Schwann cells. (A) Nanoparticle tracking analysis measurements of the concentration and size distribution of isolated RSC96 Schwann cell exosomes. (B) Representative transmission electron microscopy TEM images of negative staining of exosomes obtained from RSC96 Schwann cells. Bar=200 nm. (C) Western blot analysis showing anti-CD9 and anti-tumor susceptibility gene 101 (Tsg101) of 40 μg of exosome lysates derived from RSC96 Schwann cells.
RSC96 Schwann cell-derived exosomes increased cell proliferation of injured DRG cells
DRG cells underwent mechanical 5333 μ strain (1 Hz for 8 h), were then cultured in an incubator for another 4 h, followed by supplementation with RSC96 Schwann cell-derived exosomes (20 ug/ml) or vehicle solution (PBS) for 12 h, 24 h, and 36 h, followed by measurement of cell proliferation using the CCK-8 assay. The results showed that the proliferation in DRG cells was significantly decreased after being stretched for 8 h compared with the normal group, but DRG cell proliferation was increased by incubation with exosomes (24 h) (p<0.05). Also, injured DRG cells returned to almost normal levels of proliferation following treatment with RSC96 Schwann cell-derived exosomes for 36 h (Figure 3). These results showed that Schwann cell-derived exosomes enhanced cell proliferation of injured DRG cells.
Figure 3.
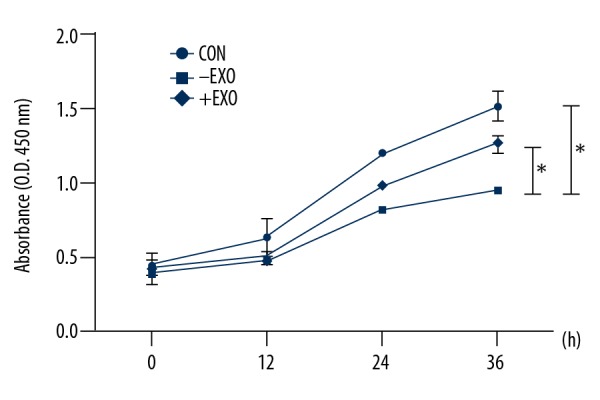
The effect of RSC96 Schwann cell-derived exosomes on cell proliferation of injured dorsal root ganglion (DRG) cells. Dorsal root ganglion (DRG) cells were stretched for 8 h (1 Hz, 5333 μ strain) and then cultured with RSC96 Schwann cell-derived exosomes (20 μg/ml) for 12 h, 24 h, and 36h, respectively. Cell proliferation was determined with a cell counting kit-8 (CCK-8) assay. CON – control group; +EXO – DRG injury group cultured with RSC96 Schwann cell-derived exosomes; −EXO – DRG injury group cultured with vehicle solution. The values presented are the mean ±SEM (n=3). * p<0.05, ** p<0.01.
RSC96 Schwann cell-derived exosomes prevented or delayed senescence of DRG cells after mechanical injury
SA-β-gal is the most widely used cytochemical biomarker for senescent cells [23]. In this study, cellular SA-β-gal in varied numbers DRG cells under different treatment conditions. As shown in Figure 4, significantly increased the rates of SA-β-gal cytochemical staining were present in injured DRG cells compared with the non-injured control group (p<0.05). However, following treatment of RSC96 Schwann cell-derived exosomes (20 ug/ml) for 24 h, the rate of positive staining for SA-β-gal was reduced to approximately 10%, which was closer to that of the control group. Therefore, Schwann cell-derived exosomes prevented or delayed senescence of DRG cells after mechanical injury.
Figure 4.
The effect of RSC96 Schwann cell-derived exosomes on cell senescence in dorsal root ganglion (DRG) cells after the induction of mechanical trauma. Cell senescence was determined with a senescence-associated beta-galactosidase (SA-β-gal) staining assay. Bar=50 μm. (A) Control group; (B) Dorsal root ganglion (DRG) cell injury group cultured with vehicle solution; (C) DRG injury group cultured with RSC96 Schwann cell-derived exosomes.
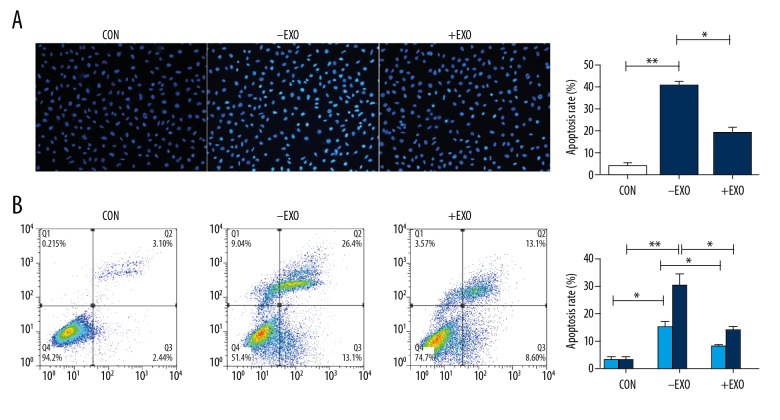
RSC96 Schwann cell-derived exosomes inhibited apoptosis in DRG cells after mechanical injury
The anti-apoptotic effects of RSC96 Schwann cell-derived exosomes on DRG cell after mechanical strain were assessed following incubated with or without supplementation with RSC96 Schwann cell-derived exosomes. Incubation with exosomes was undertaken for 24 h, and apoptosis was assessed with Hoechst 33258, which showed that the injured DRG cells had more bright blue fluorescent and condensed nuclei than the control group, and the proportion of these cells were reduced by treatment with RSC96 Schwann cell-derived exosomes (Figure 5A).
Figure 5.
The effect of RSC96 Schwann cell-derived exosomes on apoptosis of dorsal root ganglion (DRG) cells after the induction of mechanical injury. (A) Apoptotic cells were stained with Hoechst 33258 (blue) fluorescent nucleic acid stain using flow cytometry. Cells with nuclear morphological changes of bright-blue fluorescent and condensed nuclei were identified as apoptotic cells (×100). The apoptosis rate was defined as the ratio of the apoptotic cell number to the total cell number. (B) Cell apoptosis was analyzed by flow cytometry with Annexin-V phycoerythrin (PE)/7-aminoactinomycin D (7-AAD) double staining. CON – control group; +EXO – DRG injury group cultured with RSC96 Schwann cell-derived exosomes; −EXO – DRG injury group cultured with vehicle solution. The values presented are the mean ±SEM (n=3). * p<0.05, ** p<0.01.
Also, the rate of apoptosis in exosome-supplemented DRG cells after mechanical trauma was determined by flow cytometry, which showed similar changes to those shown by Hoechst 33258 staining (Figure 5B). Mechanical injury significantly increased the apoptosis rate of DRG cells (46%) compared with the control group (p<0.05). The rate of apoptosis was decreased (to 18.0%) following treatment with RSC96 Schwann cell-derived exosomes. These results showed that the supplementation of Schwann cell-derived exosomes was capable of inhibiting apoptosis in injured DRG cells in vitro.
Discussion
Worldwide, up to 40% of women suffer from stress urinary incontinence, which tends to become more common with advancing age and which can affect the quality of life for women, in terms of social, psychological, physical, domestic, and sexual function [24]. The pathogenesis of stress urinary incontinence remains unclear. However, previous studies have shown that peripheral nerve damage might induce the development of stress urinary incontinence [4–9]. New and effective treatments for conditions caused by peripheral nerve injury have become a challenge for medical research. The aims of this study were to further develop and use in vitro model of dorsal root ganglion (DRG) cell injury induced by cyclic mechanical strain (CMS) to investigate the effects of RSC96 Schwann cell-derived exosomes. The findings showed that RSC96 Schwann cell-derived exosomes had a protective effect on DRG cells after mechanical injury, improving cell proliferation, delaying senescence, and inhibiting apoptosis.
It has been documented that vaginal delivery can cause denervation of the pelvic floor, particularly involving the pudendal nerve, as well as injury of muscles and connective tissues, and can lead to the development of stress urinary incontinence [25,26]. However, the specific mechanism of pudendal nerve injury remains unclear and may be associated with partial injury, traction, or hypoxia of the peripheral nerves during the vaginal delivery process. Several in vitro and in vivo models of peripheral nerve injury have been studied in the past few decades. In 1995, Eils et al. developed a model of stretch-induced neuronal injury by stretching cells growing on a membrane that could undergo controlled deformation and cell injury to exert a rapid positive pressure [27]. Morrison et al. designed a device for mechanical trauma on cells, based on existing systems producing deformation of a clamped membrane on which cells were cultured [28].
In the present study, to develop a model of injured nerves at the cellular level, DRG cells underwent cyclic mechanical strain induced by a four-point bending system, based on a previously described method of cell injury induced by CMS [29]. This study showed that an established degree and duration of mechanical strain could reduce DRG cell proliferation, cell senescence, and significantly increase the rate of apoptosis in DRG cells compared with the non-strain control group. These findings were consistent with previous research [30]. However, the mechanism of mechanical damage to nerve cells remains unclear. Some previous studies have shown that excessive mechanical stress could result in the accumulation of reactive oxygen species (ROS) and cause oxidative damage, which is involved in trauma-induced pelvic floor dysfunction [31,32]. However, there is no direct evidence for the mechanism of mechanical damage in DRG cells and in vivo mechanical nerve damage is likely to be far more complex than can be studied by single cell populations in vitro.
Although many clinical approaches, such as pelvic floor muscle training [33], electrical stimulation [34], and surgical treatment [35], have been effectively used in the treatment of stress urinary incontinence, treatment approaches remain to be improved. Therefore, it is important to consider new approaches to peripheral nerve repair and regeneration. Exosomes are nanovesicles that are involved in biological processes by delivering their contents to target cells, including contents such as DNA, RNA, and proteins [36]. Recent studies have shown that some exosomal molecules may be potential therapeutic agents in human disease [37,38]. Xin et al. demonstrated that exosome-mediated transfer of miR-133b contributed to neurite outgrowth [39], which suggested that exosomes might also be involved in nerve growth in the central nervous system (CNS). Also, Lopez-Verrilli et al. showed that Schwann cell-derived exosomes enhanced axonal growth both in vitro and in vivo and found that Schwann cells associated with damaged axons released large numbers of exosomes [40]. Sequencing analysis has shown that Schwann cell-derived exosomes released in regions of axonal injury were rich in regeneration-related miRNAs [40]. Therefore, there is some support for the view that Schwann cell-derived exosomes might be involved in the regulation of axonal regeneration and growth, promoting repair after peripheral nerve injury. Mechanical damage has previously been reported to be associated with Nrf2/antioxidant response element (ARE) signaling suppression mediating transforming growth factor (TGF)-β1/Smad3 signaling, which might be the pathogenic mechanism for trauma-induced stress urinary incontinence [21]. However, there is a lack of direct evidence of the mechanistic damage mechanisms on nerve cells. Therefore, the underlying mechanism of injury to the pudendal nerve in patients with stress urinary incontinence remains unknown and requires further study.
The findings of the present study provide support for the conclusions from previous studies and have shown that, in vitro, RSC96 Schwann cell-derived exosomes could significantly improve the condition of DRG cells subjected to mechanical strain, by promoting DRG cell proliferation, and significantly inhibiting apoptosis and cell senescence. However, this study had several limitations and should be regarded as preliminary in nature, due to the limitations of time and support for a study from a single center. Although the findings showed that Schwann cell-derived exosomes had a potential repair effect on DRG cell injury, the specific regulatory mechanisms involved have not been studied. Relevant in vivo data remain to be obtained to support the findings of this study.
Conclusions
The effects of RSC96 Schwann cell-derived exosomes, in a novel in vitro model of dorsal root ganglion (DRG) cell injury induced by cyclic mechanical strain (CMS), showed multiple beneficial effects enhancing cell proliferation, delaying senescence, and inhibiting apoptosis. These exosome-mediated effects add a new dimension to our understanding of peripheral nerve function and support the potential role of exosomes in the treatment of diseases due to peripheral nerve damage, including stress urinary incontinence.
Acknowledgments
The authors would like to thank all the staff in the Department of Gynecology and Obstetrics and Central Laboratory, Renmin Hospital of Wuhan University, for their technical assistance.
Footnotes
Conflicts of interests
None.
Source of support: This study was supported by the National Natural Science Foundation of China (No. 81471442)
References
- 1.Coyne KS, Wein A, Nicholson S, et al. Economic burden of urgency urinary incontinence in the United States: A systematic review. J Manag Care Pharm. 2014;20(2):130–40. doi: 10.18553/jmcp.2014.20.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poomalar GK, Priyadharshini M. Prevalence of urinary incontinence in reproductive women and its impact on quality of life. Int J Reprod Contracept Obstet Gynecol. 2015;4(5):1353–58. [Google Scholar]
- 3.Kranz J, Schmidt S. Mid-urethral sling operations for stress urinary incontinence in Women. Urologe A. 2016;55(5):654–57. doi: 10.1007/s00120-016-0098-1. [DOI] [PubMed] [Google Scholar]
- 4.DeLancey JO. Anatomy and biomechanics of genital prolapse. Clin Obstet Gynecol. 1993;36(4):897–909. doi: 10.1097/00003081-199312000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Bakas P, Liapis A, Karandreas A, Creatsas G. Pudendal nerve terminal motor latency in women with genuine stress incontinence and prolapse. Gynecol Obstet Invest. 2001;51(3):187–90. doi: 10.1159/000052922. [DOI] [PubMed] [Google Scholar]
- 6.Zhu L, Lang J, Chen J, Chen J. Study on nerve fiber density in anterior vaginal epithelium for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15(4):272–75. doi: 10.1007/s00192-004-1155-7. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida S, Koyama M, Kimura T, et al. A clinicoanatomical study of the novel nerve fibers linked to stress urinary incontinence: The first morphological description of a nerve descending properly along the anterior vaginal wall. Clin Anat. 2007;20(3):300–6. doi: 10.1002/ca.20415. [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto K, Smith GM, Storer PD, et al. Neuroregeneration and voiding behavior patterns after pudendal nerve crush in female rats. Neurourol Urodyn. 2000;19(3):311–21. doi: 10.1002/(sici)1520-6777(2000)19:3<311::aid-nau11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: Interactions at the axon level. Prog Neurobiol. 2012;98(1):16–37. doi: 10.1016/j.pneurobio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Jessen KR, Mirsky R. Schwann cells and their precursors emerge as major regulators of nerve development. Trends Neurosci. 1999;22(9):402–10. doi: 10.1016/s0166-2236(98)01391-5. [DOI] [PubMed] [Google Scholar]
- 11.Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat. 1999;194(Pt 1):1–4. doi: 10.1046/j.1469-7580.1999.19410001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Zheng Z, Hu D. Inhibition of EphA4 expression promotes Schwann cell migration and peripheral nerve regeneration. Neurosci Lett. 2013;548(35):201–5. doi: 10.1016/j.neulet.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Yi S, Wang QH, Zhao LL, et al. miR-30c promotes Schwann cell remyelination following peripheral nerve injury. Neural Regen Res. 2017;12(10):1708–15. doi: 10.4103/1673-5374.217351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLean NA, Verge VM. Dynamic impact of brief electrical nerve stimulation on the neural immune axis-polarization of macrophages toward a pro-repair phenotype in demyelinated peripheral nerve. Glia. 2016;64(9):1546–61. doi: 10.1002/glia.23021. [DOI] [PubMed] [Google Scholar]
- 15.Zhang YG, Sheng QS, Qi FY, et al. Schwann cell-seeded scaffold with longitudinally oriented micro-channels for reconstruction of sciatic nerve in rats. J Mater Sci Mater Med. 2013;24(7):1767–80. doi: 10.1007/s10856-013-4917-2. [DOI] [PubMed] [Google Scholar]
- 16.Marqués-García F, Isidoro-García M. Protocols for exosome isolation and RNA profiling. Methods Mol Biol. 2016;1434:153–67. doi: 10.1007/978-1-4939-3652-6_11. [DOI] [PubMed] [Google Scholar]
- 17.Ju Z, Ma J, Wang C, et al. Exosomes from iPSCs delivering siRNA attenuate intracellular adhesion Molecule-1 expression and neutrophils adhesion in pulmonary microvascular endothelial cells. Inflammation. 2017;40(2):486–96. doi: 10.1007/s10753-016-0494-0. [DOI] [PubMed] [Google Scholar]
- 18.Yang T, Martin P, Fogarty B, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32(6):2003–14. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Verrilli MA, Picou F, Court FA. Schwann cell derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61(11):1795–806. doi: 10.1002/glia.22558. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-verrilli MA, Court FA. Transfer of vesicles from Schwann cells to axons: A novel mechanism of communication in the peripheral nervous system. Front Physiol. 2012;3:205. doi: 10.3389/fphys.2012.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang J, Li B, Liu C, et al. Mechanism of mechanical trauma-induced extracellular matrix remodeling of fibroblasts in association with Nrf2/ARE signaling suppression mediating TGF-β1/Smad3 signaling inhibition. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/8524353. 8524353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun L, Li D, Song K, et al. Exosomes derived from human umbilical cord mesenchymal stem cells protect against cisplatin-induced ovarian granulosa cell stress and apoptosis in vitro. Sci Rep. 2017;7(1):2552. doi: 10.1038/s41598-017-02786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee BY, Han JA, Im JS, et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5(2):187–95. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 24.Song P, Wen Y, Huang C, et al. The efficacy and safety comparison of surgical treatments for stress urinary incontinence: A network meta-analysis. Neurourol Urodyn. 2018;2018 doi: 10.1002/nau.23468. 29331033. [DOI] [PubMed] [Google Scholar]
- 25.Hadi E, Groutz A, Gold R, et al. [Pregnancy, labor and delivery: The pelvic floor injury]. Harefuah. 2004;143(7):525–29. [in Hebrew] [PubMed] [Google Scholar]
- 26.Clark MH, Scott M, Vogt V, Benson JT. Monitoring pudendal nerve function during labor. Obstet Gynecol. 2001;97(4):637–39. doi: 10.1016/s0029-7844(00)01207-2. [DOI] [PubMed] [Google Scholar]
- 27.Ellis EF, Mckinney JS, Willoughby KA, et al. A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. J Neurotrauma. 1995;12(3):325–39. doi: 10.1089/neu.1995.12.325. [DOI] [PubMed] [Google Scholar]
- 28.Morrison B, 3rd, Meaney DF, Mcintosh TK. Mechanical characterization of an in vitro device designed to quantitatively injure living brain tissue. Ann Biomed Eng. 1998;26(3):381–90. doi: 10.1114/1.61. [DOI] [PubMed] [Google Scholar]
- 29.LI BS, Guo WJ, Hong L, et al. Role of mechanical strain-activated PI3K/Akt signaling pathway in pelvic organ prolapse. Mol Med Rep. 2016;14(1):243–53. doi: 10.3892/mmr.2016.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong S, Li H, Wu D, et al. Oxidative damage to human parametrial ligament fibroblasts induced by mechanical stress. Mol Med Rep. 2015;12(4):5342–48. doi: 10.3892/mmr.2015.4115. [DOI] [PubMed] [Google Scholar]
- 31.Ghantous CM, Kobeissy FH, Soudani N, et al. Mechanical stretch-induced vascular hypertrophy occurs through modulation of leptin synthesis-mediated ROS formation and GATA-4 nuclear translocation. Front Pharmacol. 2015;6:240. doi: 10.3389/fphar.2015.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim EJ, Chung N, Park SH, et al. Involvement of oxidative stress and mitochondrial apoptosis in the pathogenesis of pelvic organ prolapse. J Urol. 2013;189(2):588–94. doi: 10.1016/j.juro.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 33.Luginbuehl H, Lehmann C, Baeyens JP, et al. Involuntary reflexive pelvic floor muscle training in addition to standard training versus standard training alone for women with stress urinary incontinence: study protocol for a randomized controlled trial. Trials. 2015;16:524. doi: 10.1186/s13063-015-1051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson D, Cardozowan L. Urinary incontinence in the young woman: Treatment plans and options available. Womens Health (Lond) 2014;10(2):201–17. doi: 10.2217/whe.14.1. [DOI] [PubMed] [Google Scholar]
- 35.Jovan HD, Uroš B, Aleksandar A, et al. Etiopathogenesis, diagnostics and history of surgical treatment of stress urinary incontinence. Acta Chir Iugosl. 2014;61(1):85–90. [PubMed] [Google Scholar]
- 36.Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82–88. doi: 10.1016/j.semcdb.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Yang T, Martin P, Fogarty B, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in danio rerio. Pharm Res. 2015;32(6):2003–14. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ju Z, Ma J, Wang C, et al. Exosomes from iPSCs delivering siRNA attenuate intracellular adhesion molecule-1 expression and neutrophils adhesion in pulmonary microvascular endothelial cells. Inflammation. 2017;40(2):486–96. doi: 10.1007/s10753-016-0494-0. [DOI] [PubMed] [Google Scholar]
- 39.Xin H, Li Y, Buller B, et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30(7):1556–64. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Leal R, Court FA. Schwann cell exosomes mediate neuron-glia communication and enhance axonal regeneration. Cell Mol Neurobiol. 2016;36(3):429–36. doi: 10.1007/s10571-015-0314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]