Abstract
Background
Patients treated with 5-FU can develop rare but potentially severe cardiac effects, including cardiomyopathy, angina pectoris, ventricular tachycardia, heart failure, acute myocardial infarction, and cardiogenic shock. The specific pathologies and mechanisms are not fully understood. Research found that mitochondrial dynamics are widely detected in many angiocardiopathies. Therefore, in the present study we studied the mitochondrial damage and explored the role of mitochondrial fusion/fission proteins on myocardium of rats treated with 5-fluorouracil (5-FU).
Material/Methods
Thirty male SD rats were randomly divided into 3 groups with 10 rats in each group: (1) control group, (2) low 5-FU group (25 mg/kg), (3) high 5-FU group (50 mg/kg). The animals received intraperitoneal injection for 5 consecutive days. We assessed alterations in mitochondrial morphology, ATP content, mitochondrial membrane potential, and mitochondria fusion/fission proteins expression in hearts of rats receiving intraperitoneal injection with different doses of 5-FU.
Results
5-FU intraperitoneal injection induced ultra-structural damage in hearts, such as mitochondrial swelling, cristae disorder, and vacuolization. These changes were accompanied by decreases of mitochondrial membrane potential. The low dose of 5-FU led to a slight increase in ATP content. However, the high 5-FU dose caused a more significant reduction compared with the control group. Furthermore, 5-FU intraperitoneal injection significantly increased specific mitochondrial fission proteins (Drp1 and Fis1) and decreased mitochondrial fusion proteins (Opa1, Mfn1, and Mfn2) in rat hearts. However, no changes in cardiac structure and function were detected by echocardiogram. The high dose caused more damage to mitochondrial function than the low dose.
Conclusions
Mitochondrial damage is a potentially important mechanism and early indicator for 5-FU-induced cardiovascular disease.
MeSH Keywords: Fluorouracil; Mitochondrial Dynamics; Membrane Potential, Mitochondrial
Background
5-fluorouracil (5-FU) is a commonly used synthetic anti-tumor drug, which is widely used in the chemotherapy of gastrointestinal, breast, pancreatic, and some other solid tumors [1–3]. Patients treated with 5-FU can develop rare but potentially severe cardiac effects, including cardiomyopathy, coronary artery disease, tachyarrhythmias, and shock [3–5]. The cardiac toxicity of these events reported in the literature may depend on dose, time, and schedule of chemotherapy [1,6–8]. These can be serious and can affect quality of life of cancer survivors.
Mitochondria are composed of an outer membrane, an inner membrane that forms cristae, and an intermembrane space. Mitochondria occupy about one-third of myocardial cells, providing 30 kg of ATP for myocardial contraction per day [9]. Structural and functional integrity of mitochondria are indispensable for physiological function of the cardiovascular system [10]. Mitochondria not only play an important role in cell energy production, but also play a critical role in cell growth and cell death [10–12]. In the past decade, many studies have focused on mitochondrial dynamics, in which changes in mitochondrial shape, distribution, and movement have been demonstrated to influence cellular physiology and pathology [9,13]. Increased mitochondrial fission can lead to fragmentation of mitochondria and decreased mitochondrial function, which is closely related to cell apoptosis. Mitochondrial fusion is the process of combining adjacent depolarized mitochondria with intact mitochondria, mixing them with their metabolites and mitochondrial DNA, which is conducive to maintaining membrane potential and repairing mitochondrial DNA [14]. Excessive mitochondrial fission is involved in ischemia/reperfusion injury, diabetic cardiomyopathy, pulmonary hypertension, and heart failure [15–17]. Mitochondrial fusion was found to be involved in insufficiency in myocarditis, heart failure, and cardiac hypertrophy [16,18,19]. These processes are essential for the maintenance of healthy mitochondrial networks, which are under the regulation of mitochondrial fusion and fission proteins, respectively [9]. Drp1 and Fis1 play major roles in mitochondrial fission, while Opa1, Mfn1, and Mfn2 are important regulators of fusion. Mfn1 and Mfn2 regulate fusion of the OMM, whereas Opa1 governs the fusion of the IMM [9,16,18,19]. The mechanism of mitochondrial damage of 5-FU-induced heart injury is still unknown. In the present study, we investigated the damage caused by 5-FU in rat cardiac mitochondria and explored the related mechanisms of 5-FU-induced alterations in the expression of mitochondrial fusion/fission proteins.
Material and Methods
Animals and treatments
Thirty adult male Sprague Dawley (SD) rats (6–8 weeks old) were purchased from the Experimental Animal Center of Hebei Medical University (Shijiazhuang, China). The animals were kept under standard laboratory conditions (12 h light: 12 h dark and 24±3°C). Feed and water were provided ad libitum. The rats were randomly divided into 3 groups: (1) the control group, (2) the low 5-FU group, and (3) the high 5-FU group. The animals were treated by intraperitoneal injection for 5 consecutive day. Dosage of 5-FU was based on a previous study [20]. 5-FU was purchased from MCE. The experiment was approved by the Laboratory Animal Ethics Committee of the Fourth Hospital of Hebei Medical University (No. 201711).
Echocardiographic measurements
Rats were anesthetized (sodium pentobarbital, 36 mg/kg, ip) 24 h after the last treatment. Transthoracic echocardiographic measurement was performed using echocardiography (Visual Sonics Vevo 2100 with 21 MHz transducer, Canada). Left ventricular end-systolic and end-diastolic dimensions, as well as systolic and diastolic wall thickness, were measured from M-mode tracings at the midpapillary level. For each M-mode operation and measurement by a trained electrocardiographer of small animals, at least 3 consecutive cardiac cycles were sampled. Studies and analysis were performed by investigators in a blinded fashion.
The ultrastructure of mitochondria
Rats were killed by sodium pentobarbital injection (36 mg/kg, ip) at 24 h after the last treatment, then myocardium from the heart apex were cut into 1-mm3 pieces and fixed in 4% glutaraldehyde followed by osmic acid. Subsequently, specimens were dehydrated by acetone and then embedded. Thin and semi-thin specimen slices were made, and transmission electron microscopy was carried out to observe the cellular mitochondrial structure. We examined samples at a magnification of 5000× and 15 000× with a transmission electron microscope (HITACHI-S7500, Tokyo, Japan).
Mitochondrial membrane potentials and ATP content assay
One section of the heart tissue was used to isolated mitochondria using the myocardial mitochondria isolation kit (c3606; Beyotime Institute of Biotechnology, Shanghai, China). The isolated mitochondria were used to detect the mitochondrial membrane potential (c2006; Beyotime Institute of Biotechnology, Shanghai, China). Mitochondrial ATP content was analyzed using the ATP content kit (s0026; Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer’s instructions.
Western blotting analysis
The frozen heart tissue samples were thawed. Protein concentrations were determined using a BCA protein assay kit (Multisciences, Shanghai, China). Samples were mixed with 1× loading buffer and denatured at 95°C for 10 min. We resolved 30 ug of protein by sodium dodecyl sulfate polyacrylamide gel electrophoresis on a 10% or 15% SDS-PAGE and electrophoretically transferred it to a polyvinylidene difluoride (PVDF) membrane. After blocking with 5% milk in TBST for 4 h at room temperature, the rat monoclonal antibodies specific for rat Opa1 (A9833, ABclonal, USA), Mfn1 (A9880, ABclonal, USA), Mfn2 (A5750, ABclonal, USA), Drp1 (A2586, ABclonal, USA) and Fis1 (GTX111010, GeneTex, USA) and alpha Tubulin (GTX628802, GeneTex, USA) were incubated at a concentration of 1: 500 (for Opa1, Mfn1, Drp1), or 1: 1000 (for Mfn2, Fis1, and Tubulin) in 1×TBST overnight at 4°C. The infrared-labeled anti-rat secondary antibodies (26055, Rockland, USA) at a concentration of 1: 10 000 were added to PVDF membranes and incubated for 2 h at 37°C. The membranes were scanned and the band densities were quantified using the Odyssey Infrared Imaging System.
Statistical analysis
All results are expressed as means ±SD. The data obtained from various groups were statistically analyzed using one-way ANOVA using SPSS21.0. The least significant difference (LSD) test was performed as post hoc analysis for multiple comparisons between groups. Dunnett’s T3 test was performed when the variance was irregular. P<0.05 was considered to be statistically significant.
Results
Echocardiography
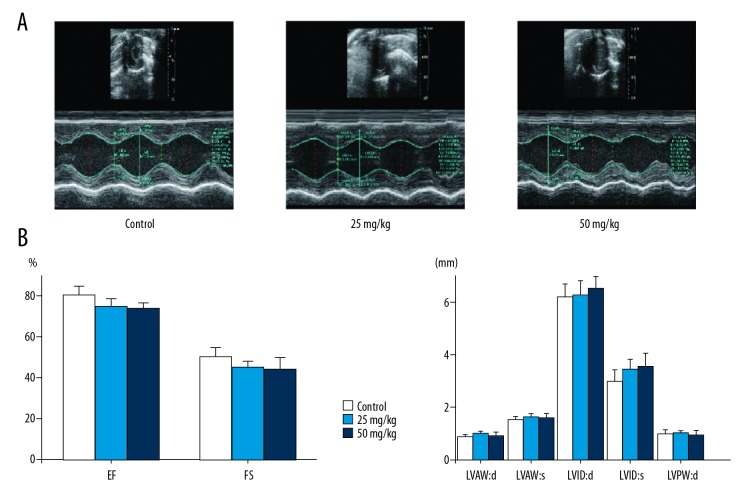
Echocardiography was performed to examine the effect of 5-FU on cardiac structure and function. The data showed that 5-FU rats presented no significant left ventricular systolic dysfunction. Specifically, the most important parameters of cardiac structure and function were not significantly changed in 5-FU rats, and there was no significant change in the low- and high-dose groups (Figure 1).
Figure 1.
Effects of 5-FU on cardiac function of rats. (A) Representative M-mode images and changes of echocardiographic parameters in each group. (B) The left ventricular paramenters were detected by echocardiography; EF ejection fraction; FS – fractional shortening; LVAW – d, left ventricular end-diastolic anterior wall thickness; LVAW:s – left ventricular end-systolic anterior wall thickness; LVID:d – left ventricular end-diastolic diameter; LVID:s – left ventricular end-systolic diameter; LVPW:d – left ventricular end-diastolic posterior wall thickness. Data are expressed as mean ±SD from ten individual samples. Using one-way ANOVA, comparing with control group,significant difference is indicated by * P<0.05 and ** P<0.01.
Ultrastructure of mitochondria
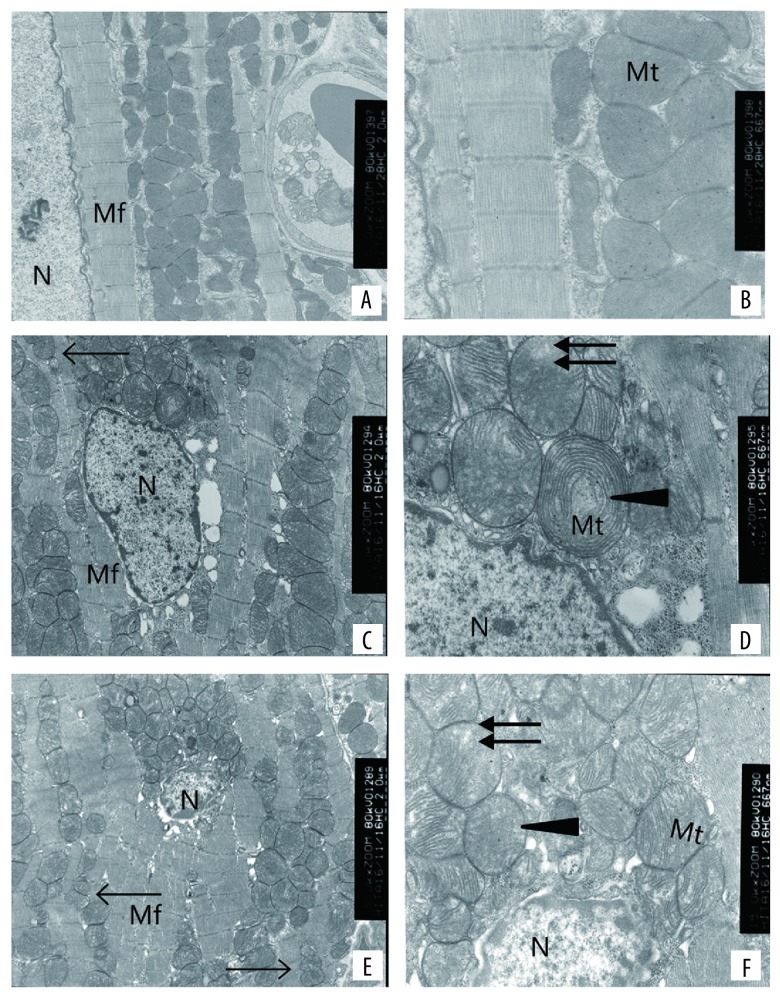
Mitochondria were scanned with transmission electron microscopy. In the control group, the ultra-structural features showed normal architecture of myocardium structures, complete structures of the mitochondria, and obvious cristae of the mitochondria (Figure 2A, 2B). In the low 5-FU group, the ultra-structural features showed myocardial myofibril disorder, mitochondrial swelling, cristae decrease, and mitochondrial vacuolation (Figure 2C, 2D). In the high 5-FU group, the ultra-structural features showed myocardial myofibril disorder, mitochondrial swelling, cristae decrease, and mitochondrial vacuolation (Figure 2E, 2F). Swelling degree and crest fracture degree were more serious in the high-dose group than in the low-dose group. A smaller volume was associated with, the number change much more in the high dose group than low dose group.
Figure 2.
Ultrastructural damage effects of 5-FU on myocardial cells and mitochondria of rats from control 5,000×magnification (A), 15,000×magnification (B), 25 mg/kgb.w. 5,000×magnification (C), 15,000×magnification (D) and 50 mg/kg b.w. 5,000×magnification (E), 15,000×magnification (F). The arrows indicate sites of mitochondrial fission, the triangles indicates site of mitochondrial swelling and crista disorder, and the double-headed arrow indicate vacuolization, respectively. N – nucleus; Mt – mitochondrion; Mf – myocardial fibers.
ATP content and mitochondrial membrane potentials
Compared with the control group, the mitochondrial ATP content of myocardial tissue in the low 5-FU group was slightly increased, while in the high 5-FU group it was decreased. The results were different in the high 5-FU group compared with the control group. Compared with the control group, the mitochondrial membrane potential showed a downward trend, and only the results of the high-dose 5-FU group had significant differences compared with the control group (p<0.05) (Figure 3). Mitochondrial function damage was not noticeable in the low-dose group but was obvious in the high-dose group.
Figure 3.
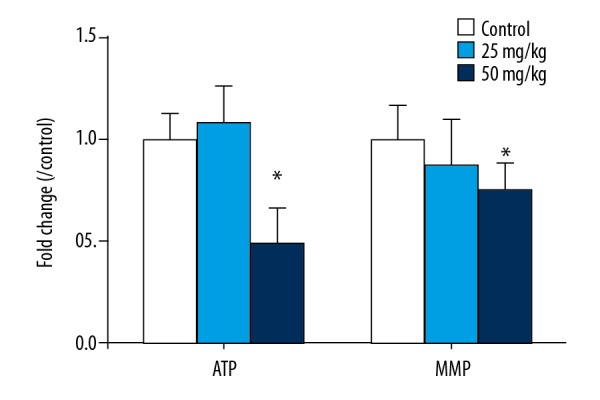
The MMP in cardiac mitochondria among each group. Data are expressed as mean ±SD from six individual samples. Using one-way ANOVA, comparing with control group, significant difference is indicated by * P<0.05.
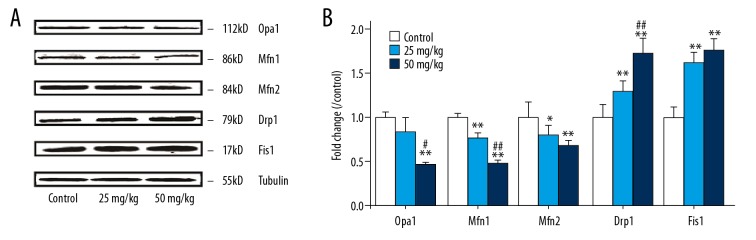
Mitochondria fission/fusion proteins expression
The fission protern Drp1 level in the hearts of 5-FU rats in the low 5-FU group and high 5-FU group were significantly increased compared with the control group (p<0.01 or p<0.01). Meanwhile, the fission protern Fis1 was significant changed in the low 5-FU group and high 5-FU group (p<0.01 or p<0.01), whereas, the fusion-related proterns Opa1, Mfn1, and Mfn2 were significantly decreased in the high 5-FU group (p<0.01, p<0.01, or p<0.01). Mfn1 was obviously decreased in the low 5-FU group (p<0.01) and Mfn2 was decreased in the low 5-FU group (p<0.05), but no significant change in Opa1 were observed in the low 5-FU group (Figure 4). The increase of fission protein was greater than the degree of fusion protein decrease.
Figure 4.
(A, B) Expression of protein of OPA1, Mfn1, Mfn2, Drp1 and Fis1 in heart mitochondria of rats treated with different 5-FU concentrations. The control group was intraperitonealled injection with same amount of physiological saline. Mean expression in each treated group is shown as increase/decrease compared to mean expression in control group which has been ascribed an arbitrary value of 1. The values are mean ±SD from five individual samples. Using one-way ANOVA, comparing with control group, significant difference is indicated by * P<0.05 and ** P<0.01, comparing with 25 mg/kg group, significant difference is indicated by # P<0.05 and ## P<0.01.
Discussion
The pathogenesis of 5-FU-induced cardiotoxicity is multi-factorial and is not fully understood. Oxidative stress is a possible mechanistic hypothesis explaining the cardiotoxicity effects of 5-FU [21]. Mitochondria are one of the major cellular sources of ROS and are also the target organ of ROS [12]. Previous studies reported that the increase in anaerobic metabolism and the increase in oxygen uptake could be due to reduced aerobic efficiency resulting from mitochondrial uncoupling. Uncoupling of the mitochondrial respiratory chain results in increased basal oxygen consumption and decreased ATP-production [22]. This mitochondrial damage wants further study. There is no data on the ultrastructure of mitochondria. Mitochondrial morphologic change has recently been recognized as an integral factor in the energetic efficiency of mitochondria in maintaining cellular homeostasis [12]. The maintenance of mitochondrial health partly depends on mitochondrial dynamics, a process involving continual fission and fusion of mitochondria. The process involves changes in mitochondrial morphology, promoting the transfer of chemical and electronic signals, some pathological damages can lead to a fusion/fission imbalance, resulting in mitochondrial morphology changes and decreased mitochondrial function [9,12,23]. Excessive mitochondrial fission is widely detected in ischemia/reperfusion injury, myocardial infarction, diabetic cardiomyopathy, and heart failure [24,25]. Thus, we studied the mitochondrial damage and explored the role of mitochondrial fusion/fission proteins in myocardium in rats treated with 5-fluorouracil (5-FU).
In the present study, we observed no significant changes in cardiac structure and function. It may be that the administration time was too short and the dosage was too low to cause cardiac dysfunction. In attempting to prolong the duration of administration or increase in the administered dosage of the drug, there were significant gastrointestinal events causing increased mortality. Therefore, we use previous methods of administration and doses [20] to investigate the heart injury.
Our results showed some abnormal morphological changes in mitochondria, confirming the abnormality of mitochondrial ultrastructure in 5-FU-treated rats. Previous studies have shown that mitochondrial damage is associated with apoptosis, necrosis, and autophagy in cardiomyocytes [8,26,27]. In this study, we found only mitochondrial damage and no damage in myocardial cells in the rat hearts. This suggests that mitochondrial damage in 5-FU-treated rats may be associated with mitochondrial dysfunction, and that there is a possible role of mitochondrial dynamics in its pathological progression. The main function of mitochondria is to provide energy for metabolism. MMP reflects the integrity of mitochondrial function and is a sensitive index for evaluating the functional integrity of mitochondria. Normal MMP ensure mitochondrial oxidative phosphorylation, which is a necessary condition for ATP generation, and membrane potential dissipation leads to cells that cannot synthesize enough ATP to complete normal life activities [12]. Low concentrations of 5-FU can damage mitochondria, but due to mitochondrial compensatory function, ATP content increased [28], and high concentrations cause damage beyond the mitochondrial compensatory function, leading to decreased ATP [22].
In our study, we found that the fission proterns expression of Drp1 and Fis1 were significantly increased in the low 5-FU group and high 5-FU group. Abnormal expression of Drp1 protein may cause the abnormal division of mitochondria. When the expression level of Drp1 is low, it causes decreased mitochondrial division, and the mitochondria cannot be transported to where the energy is needed. When the expression level of Drp1 is high, the abnormal fission increased, the membrane potential decreased, and the biological function decreased. After the fusion of mitochondria is inhibited, the damaged mtDNA cannot be repaired by fusion with normal mitochondria [29]. Previous research has shown that cardiac myocyte-specific knockout of Mfn1 and Mfn2 in adults causes cardiomyopathy [23]. Lack of Mfn1 and Mfn2 leads to insufficient mitochondrial membrane fusion, and myocardial cells will be more sensitive to a variety of injuries. It has been demonstrated that Opa1-mediated mitochondrial fusion requires the involvement of Mfn1, while Mfn2 has no direct effect on Opa1 [30]. Another study showed mitochondrial fusion depends on the presence of mitochondrial membrane potential [31]. In our experiments, mitochondrial fusion was consistent with the change in membrane potential. Knockdown of Drp1 inhibits the fission of mitochondria, which become elongated. Upregulation of Drp1 results in fragmentation of mitochondria, which affects mitochondrial electron transport, and the 3 carboxylic acid cycle [32]. The level of Fis1 may determine the division of mitochondria [33]. With excessive fission of mitochondria and the lack of fusion, the synthesis of mitochondria is blocked and the mitochondrial function is destroyed. It was recently reported that inhibiting mitochondrial fission with Mdivi-1 or siRNA can attenuate mitochondrial damage and reduce myocardial injury [34,35]. We plan to further study whether inhibiting mitochondrial fission can improve 5-FU-induced mitochondrial damage.
Conclusions
Our results revealed that mitochondrial damage is a potentially important mechanism and is an early indicator for 5-FU-induced cardiovascular disease. It may be caused by increase in fission and decrease in fusion proteins. This novel finding will not only help us understand the pathological of 5-FU, but also help develop new therapeutic strategies.
Footnotes
Source of support: This work was supported by Hebei province in 2016 through the Government Aid Clinical Medical Talents Cultivation and Basic Research Projects [2015] (No. 188)
References
- 1.Polk A, Vistisen K, Vaage-Nilsen M, Nielsen DL. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacology and Toxicology. 2014;15(1):47. doi: 10.1186/2050-6511-15-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorrentino MF, Kim J, Foderaro AE, Truesdell AG. 5-fluorouracil induced cardiotoxicity: Review of the literature. Cardiol J. 2012;19(5):453–57. doi: 10.5603/cj.2012.0084. [DOI] [PubMed] [Google Scholar]
- 3.Polk A, Vaage-Nilsen M, Vistisen K, Nielsen DL, et al. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: A systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev. 2013;39(8):974–84. doi: 10.1016/j.ctrv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Ray JC, Cho P, Dragon M, Graham CG. A Case of 5-fluorouracil-induced cardiac arrest. J Emerg Med. 2016;50(1):e1–6. doi: 10.1016/j.jemermed.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Kim SM, Kwak CH, Lee B, et al. A case of severe coronary spasm associated with 5-fluorouracil chemotherapy. Korean J Intern Med. 2012;27(3):342–45. doi: 10.3904/kjim.2012.27.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saif MW, Shah MW, Shah AR. Fluoropyrimidine-associated cardiotoxicity: Revisited. Expert Opin Drug Saf. 2009;8(2):191–202. doi: 10.1517/14740330902733961. [DOI] [PubMed] [Google Scholar]
- 7.Tsibiribi P, Bui-Xuan C, Bui-Xuan B, et al. Cardiac lesions induced by 5-fluorouracil in the rabbit. Hum Exp Toxicol. 2006;25(6):305–9. doi: 10.1191/0960327106ht628oa. [DOI] [PubMed] [Google Scholar]
- 8.Lamberti M, Porto S, Marra M, et al. 5-Fluorouracil induces apoptosis in rat cardiocytes through intracellular oxidative stress. J Exp Clin Cancer Res. 2012;31(1):60. doi: 10.1186/1756-9966-31-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong SB, Kalkhoran SB, Cabrera-Fuentes HA, Hausenloy DJ. Mitochondrial fusion and fission proteins as novel therapeutic targets for treating cardiovascular disease. Eur J Pharmacol. 2015;763:104–14. doi: 10.1016/j.ejphar.2015.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominic EA, Ramezani A, Anker SD, et al. Mitochondrial cytopathies and cardiovascular disease. Heart. 2014;100(8):611–18. doi: 10.1136/heartjnl-2013-304657. [DOI] [PubMed] [Google Scholar]
- 11.Pei H, Yang Y, Zhao H, et al. The role of mitochondrial functional proteins in ROS production in ischemic heart diseases. Oxid Med Cell Longev. 2016;2016(1) doi: 10.1155/2016/5470457. 54707457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy E, Ardehali H, Balaban RS, et al. Mitochondrial function, biology, and role in disease. Circ Res. 2016;118(12):1960–91. doi: 10.1161/RES.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorn IG. Mitochondrial fission/fusion and cardiomyopathy. Curr Opin Genet Dev. 2016;38:38–44. doi: 10.1016/j.gde.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy PH1, Reddy TP, Manczak M, et al. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev. 2011;67(1–2):103–18. doi: 10.1016/j.brainresrev.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galloway CA, Yoon Y. Mitochondrial dynamics in diabetic cardiomyopathy. Antioxidant Redox Signal. 2015;22(17):1545–62. doi: 10.1089/ars.2015.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Givvimani S, Pushpakumar S, Veeranki S, Tyagi SC. Dysregulation of Mfn2 and Drp-1 proteins in heart failure1. Can J Physiol Pharmacol. 2014;92(7):583–91. doi: 10.1139/cjpp-2014-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsboom G, Toth PT, Ryan JJ, et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res. 2012;110(11):1484–97. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall AR, Burke N, Dongworth RK, et al. Hearts deficient in both Mfn1 and Mfn2 are protected against acute myocardial infarction. Cell Death and Disease. 2016;7(5):e2238. doi: 10.1038/cddis.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wai T, García-Prieto J, Baker MJ, et al. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science. 2015;350(6265) doi: 10.1126/science.aad0116. aad0116. [DOI] [PubMed] [Google Scholar]
- 20.Millart H, Brabant L, Lorenzato M, et al. The effects of 5-fluorouracil on contractility and oxygen uptake of the isolated perfused rat heart. Anticancer Res. 1992;12(2):571–76. [PubMed] [Google Scholar]
- 21.Eskandari MR, Moghaddam F, Shahraki J, Pourahmad J, et al. A comparison of cardiomyocyte cytotoxic mechanisms for 5-fluorouracil and its pro-drug capecitabine. Xenobiotica. 2015;45(1):79–87. doi: 10.3109/00498254.2014.942809. [DOI] [PubMed] [Google Scholar]
- 22.Millart H, Brabant L, Lorenzato M, et al. The effects of 5-fluorouracil on contractility and oxygen uptake of the isolated perfused rat heart. Anticancer Res. 1992;12(2):571–76. [PubMed] [Google Scholar]
- 23.Chen Y, Liu Y, Dorn GN. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. 2011;109(12):1327–31. doi: 10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesnefsky EJ, Moghaddas S, Tandler B, et al. Mitochondrial dysfunction in cardiac disease: Ischemia – reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33(6):1065–89. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 25.Wüst RC, Myers DS, Stones R, et al. Regional skeletal muscle remodeling and mitochondrial dysfunction in right ventricular heart failure. Am J Physiol Heart Circ Physiol. 2012;302(2):H402–11. doi: 10.1152/ajpheart.00653.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Focaccetti C, Bruno A, Magnani E, et al. Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes. PLoS One. 2015;10(2):e0115686. doi: 10.1371/journal.pone.0115686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Filgueiras MC, Morrot A, Soares PM, et al. Effects of 5-fluorouracil in nuclear and cellular morphology, proliferation, cell cycle, apoptosis, cytoskeletal and caveolar distribution in primary cultures of smooth muscle cells. PLoS One. 2013;8(4):e63177. doi: 10.1371/journal.pone.0063177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picard M, Gentil BJ, McManus MJ, et al. Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. J Appl Physiol (1985) 2013;115(10):1562–71. doi: 10.1152/japplphysiol.00819.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy PH, Reddy TP, Manczak M, et al. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev. 2011;67(1–2):103–18. doi: 10.1016/j.brainresrev.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA. 2004;101(45):15927–32. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meeusen S, McCaffery JM, Nunnari J. Mitochondrial fusion intermediates revealed in vitro. Science. 2004;305(5691):1747–52. doi: 10.1126/science.1100612. [DOI] [PubMed] [Google Scholar]
- 32.Ishihara N, Nomura M, Jofuku A, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11(8):958–66. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 33.James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278(38):36373–79. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 34.Lin L, Zhang M, Yan R, et al. Inhibition of Drp1 attenuates mitochondrial damage and myocardial injury in Coxsackievirus B3 induced myocarditis. Biochem Biophys Res Commun. 2017;484(3):550–56. doi: 10.1016/j.bbrc.2017.01.116. [DOI] [PubMed] [Google Scholar]
- 35.Dong Y, Undyala VVR, Przyklenk K. Inhibition of mitochondrial fission as a molecular target for cardioprotection: Critical importance of the timing of treatment. Basic Res Cardiol. 2016;111(5):59. doi: 10.1007/s00395-016-0578-x. [DOI] [PubMed] [Google Scholar]