Abstract
Marine mussels (Mytilus spp.) attach to a wide variety of surfaces underwater using a network of byssal threads, each tipped with a protein-based adhesive plaque that uses the surrounding seawater environment as a curing agent. Plaques undergo environmental post-processing, requiring a basic seawater pH be maintained for up to 8 days for the adhesive to strengthen completely. Given the sensitivity of plaques to local pH conditions long after deposition, we investigated the effect of other aspects of the seawater environment that are known to vary in nearshore habitats on plaque curing. The effect of seawater temperature, salinity and dissolved oxygen concentration were investigated using tensile testing, atomic force microscopy and amino acid compositional analysis. High temperature (30°C) and hyposalinity (1 PSU) had no effect on adhesion strength, while incubation in hypoxia (0.9 mg l−1) caused plaques to have a mottled coloration and prematurely peel from substrates, leading to a 51% decrease in adhesion strength. AFM imaging of the plaque cuticle found that plaques cured in hypoxia had regions of lower stiffness throughout, indicative of reductions in DOPA cross-linking between adhesive proteins. A better understanding of the dynamics of plaque curing could aid in the design of better synthetic adhesives, particularly in medicine where adhesion must take place within wet body cavities.
Keywords: underwater adhesion; mussel foot protein; protein cross-linking; 3,4-dihydroxyphenyl-l-alanine; amino acid composition
1. Introduction
Marine mussels (Mytilus spp.) have mastered the art of underwater adhesion, producing a holdfast comprised of proteinaceous fibres known as byssal threads. Attaching to rocks in the intertidal zone, byssal threads are capable of adhering to a wide variety of substrates with different surface chemistries, hydrophobicities and physical properties [1–3], all while contending with the presence of water, salts and organic films [3]. For these reasons, the mechanisms underlying byssal thread adhesion continue to inspire researchers designing anti-fouling coatings for use in the maritime industry, novel polymers with applications in wet environments and bio-compatible medical adhesives for the repair of sensitive tissues within the human body [4–8].
Each byssal thread is part of a network, extending outwards radially from within the shell, and making contact with a surface at an adhesive plaque [9]. The plaque is made up of adhesive proteins (Mfps) that contain 3,4-dihydroxyphenyl-l-alanine (DOPA) residues, relatively rare post-translationally modified amino acids with adhesive properties [10,11]. Plaques are produced when a mussel extends its foot from the shell, pressing a small depression at the distal end (distal depression) against a surface [12,13]. Once contact is made, the chemical environment within the distal depression is adjusted, producing an acidic microenvironment (pH 1–3) [14] that is also heavily reduced, with a low ionic strength (0.15 M) when compared with seawater (0.7 M) [15–17]. After these conditions are established, Mfps are secreted into the cavity as complex coacervates, oppositely charged polyelectrolyte liquid phases that result from liquid–liquid phase separation [18,19]. Under these conditions, DOPA residues preferentially form interfacial interactions with surfaces (hydrogen bonding, coordination, etc.), rather than interacting with one another [20,21].
Byssal thread formation takes approximately 5 minutes, after which the foot is removed, and the newly formed plaque is exposed to seawater. The chemistry of typical open-ocean seawater, with a pH of 8.1, oxygen saturation of ca 100% and ionic concentration of 0.7 M, is drastically different from the microenvironment that a mussel creates during Mfp secretion. Contact with seawater destabilizes the equilibrium between polyelectrolyte liquid phases by altering the pH, leading to the formation of a desolvated gel/solid, while also paving the way for the oxidation of DOPA residues to dopaquinone through a process called quinone tanning [22]. This switch in the local chemical environment is an essential part of the adhesive formation process, as dopaquinone preferentially forms covalent cross-links between like proteins at a basic pH [2,23] while also establishing increasingly stable (DOPA)Fe3+ complexes within the cuticle of the plaque [24,25].
While the properties of seawater act as a molecular trigger to initiate a phase change during plaque solidification, the local seawater environment continues to be important long after the plaque is deposited. In seawater, plaque adhesion strength doubles 8–12 days after being deposited on a surface, changing from a milky white to dark tan colour [26]. However, this maturation process is arrested when plaques are held at a pH below 5.0 during this time frame. Together, these findings show that environmental post-processing is essential for the adhesive to form correctly and that plaque strengthening requires access to favourable conditions for significantly longer than would be predicted from the cross-linking rates of individual Mfps [27].
With such a long cure window, it is possible that plaques are sensitive to other aspects of the seawater environment that typically vary in nearshore environments. In addition to fluctuations in pH, mussels regularly experience a broad range of seawater temperatures, salinities and dissolved oxygen concentrations, with daily or seasonal variability exceeding even the most extreme values seen in the open ocean [28–31]. Extremes in these parameters have the potential to impact plaque curing positively or negatively. For example, high temperatures could accelerate the kinetics of dopaquinone cross-linking, speeding up the curing process [32] and/or break hydrogen bonds and cause protein unfolding and plaque weakening. Similarly, a hyposaline environment after Mfps have bonded to a surface could reduce the stability of the cross-linking network due to a reduction in charge-balancing counter ions, causing a decrease in the cohesive strength of the adhesive [33,34]. Dopaquinone formation is dependent on oxygen absorption from the surrounding seawater environment [35]; oxygen limitation during the curing process could either slow down DOPA conversion or lead to a reduction in cross-linking densities all together, reducing the cohesive strength of the plaque [36]. Therefore, the aim of this study is to determine the sensitivity of adhesive plaque curing to extremes in seawater temperature, salinity or dissolved oxygen concentration.
To investigate this question, freshly made byssal threads were incubated in seawater treatments (high temperature, hyposaline or hypoxic) for 12 days and then pulled from the substrate with a tensile testing machine. In addition to adhesion strength, the failure mode of each plaque was also recorded to investigate how molecular changes in the material affected the mechanics of the structural failure under tension. For the subset of these seawater parameters that were found to significantly affect adhesion, changes in plaque structure and composition were also characterized using atomic force microscopy and amino acid (AA) analysis. To provide a real-world context for these laboratory treatments, seawater conditions (temperature, salinity and dissolved oxygen concentration) were measured continuously at a mussel farm in Washington State for over 2 years, quantifying the frequency of seasonal variation across depth. By identifying which aspects of the multivariate seawater environment have post-processing effects on adhesive plaques, this study suggests a mechanism by which environmental variability influences mussel attachment in nature, while also informing the design of better medical adhesives that employ DOPA-mediated adhesion to persist in wet and ionically complex environments in the human body [37].
2. Material and methods
2.1. Byssal thread experiments
Adult mussels (Mytilus trossulus, Gould 1850; ca 4–6 cm shell length) were collected from aquaculture lines at Penn Cove Shellfish's mussel aquaculture operation located in Quilcene Bay, Quilcene, Washington, USA (47°47′42.0″ N, 122°51″10.8″ W) during the winter of 2016 (December–February). Mussels were kept in 50 l aquaria for up to two weeks, filled with 0.2 µm filtered seawater and fed Shellfish Diet 1800 (Reed Mariculture, Campbell, CA, USA) up to 5% of wet tissue mass day−1 at an algal concentration of 2000 cells ml−1. Upon collection, the shell length (±0.1 cm) of each mussel was determined using a vernier calliper, while the reproductive condition (gonad index (GI)) and physiological condition (condition index (CI)) were determined for a subset of the collected population. GI was calculated as the ratio of dried gonadal to total tissue mass [38], while the CI was calculated as the total dry tissue mass divided by shell length cubed [39]. Gonadal tissue was dissected from somatic tissue and subsequently dried at 60°C to a constant dry weight (ca 3 days). The remaining mussels were haphazardly assigned to treatment groups and at the end of the experiment, the GI and CI for each mussel were determined.
Mussels were secured to mica plates with rubber bands and allowed to produce byssal threads for up to 4 h in typical open-ocean seawater conditions (pH = 8.1, T = 10°C, Sal = 31 PSU, O2 = 8 mg l−1), after which threads were cut away from the animal in the proximal region of the thread (at the shell margin). Only mussels that produced three or more attachments were included in a treatment group. A subset of threads was tested immediately, serving as a 4 h, ‘freshly made’ control. The remainder of mica plates with attached threads were placed in one of four treatments (Control, N2, 30°C and DI water) and allowed to mature for 12 days, removing a subset of plates at 3, 5, 8 and 12 days.
Seawater treatments were designed to mimic open-ocean conditions in all ways but one, pushing temperature, dissolved oxygen, or salinity to the most extreme values seen in estuarine systems that are metabolically driven by the local biota [40]. A hypoxia treatment (O2 < 2 mg l−1) was achieved through the injection of N2 gas into a 3 l container, using an aerator. The dissolved oxygen concentration of seawater treatments was monitored in real-time with a DirectLine DL5000 equilibrium probe (accuracy ± 1%) attached to a UDA2182 analyser (Honeywell, Fort Washington, PA, USA), which controlled the injection of N2 by dynamically opening a solenoid valve in-line with a nitrogen gas cylinder. A high temperature treatment (30°C) was achieved using a 500-Watt titanium aquarium heater and accompanying PID controller (Aquatop Aquatic Supplies, Brea, CA, USA). A low salinity treatment (less than 1 PSU) was achieved by placing plaques in deionized water. DI water was chosen for two reasons: (i) deionized water approximates the salinity and pH of riverine inputs that lead to the stratification of the water column in nearshore habitats [41] and, (ii) byssal threads are commonly stored in DI water before mechanical testing is performed (JH Waite 2018, personal communication). Only one sample was collected for the low salinity treatment after 12 days of incubation. Seawater pH and temperature were monitored in each treatment with a Honeywell Durafet III pH electrode ([42]; accuracy ± 0.01), while salinity was monitored with a DL4000 conductivity cell (accuracy ± 1 PSU). Treatment means (± s.d.) for seawater pH (NBS scale), temperature (°C), salinity (PSU) and dissolved oxygen (mg l−1) are reported in table 1.
Table 1.
Mean seawater conditions (±s.d.) in each treatment during 12-day exposures. pH, temperature (T), salinity (Sal) and dissolved oxygen (O2) where recorded at 10-min intervals.
| treatment | pH (NBS) | T (°C) | Sal (PSU) | O2 (mg l−1) |
|---|---|---|---|---|
| control | 8.01 ± 0.03 | 9.7 ± 1.4 | 29 ± 1 | 8.5 ± 0.5 |
| N2 | 8.00 ± 0.03 | 9.8 ± 1.0 | 28 ± 2 | 0.9 ± 0.6 |
| 30°C | 8.01 ± 0.04 | 29.7 ± 0.5 | 29 ± 1 | 8.7 ± 0.4 |
| DI | 7.22 ± 0.12 | 10.1 ± 0.6 | 1 ± 2 | 8.6 ± 0.3 |
2.2. Mechanical testing and atomic force microscopy
The adhesion strength of individual plaques was determined by gripping each byssal thread in the distal region, 1 mm away from the adhesive plaque, and pulling perpendicular to the substrate until failure using a tensometer [26]. Adhesion strength (kPa) was calculated as the maximum of the force extension curve (N), normalized by the planform area of the attachment plaque measured in mm2 [43]. The adhesion strength for 3–5 plaques was averaged and reported as a single value for each mussel. During mechanical testing, the failure mode of each plaque was also visually scored as an adhesive, peeling or tearing failure, as outlined by Young and Crisp [44]. Adhesive failure occurred when a plaque disengaged from a surface uniformly at the adhesive–substrate interface, while a peeling failure characteristically began at a single point along the outer edge of the plaque, propagating to the rest of the structure. A tearing failure was evident when a portion of the adhesive remained attached to the surface after the test had completed.
The stiffness of the plaque cuticle was determined by following the protocol outlined by George and Carrington (2018) [26]. Briefly, stiffness (DMT modulus) was measured using a Dimension ICON atomic force microscope (AFM), fitted with a ScanAsyst-Air probe with a silicon-nitride tip (Bruker, Billerica, MA, USA). Prior to testing, plaques were rinsed with DI water and allowed to dry for 5 min. Efforts were taken to probe smooth patches away from the thread–plaque junction, avoiding the innervating roots of the thread. The DMT modulus (GPa) was calculated as the slope of the force curve during tip-sample separation [45]. To obtain a representative stiffness of the cuticle, the DMT modulus was averaged over a 10 nm2 scan area, with a sampling rate of 512 per line. Multiple locations (3–5) were scanned for each plaque and then averaged. Images of the plaque surface were taken with a resolution of 10 µm2. The DMT modulus was calibrated against a fused silica standard (Veeco, Plainview, NY, USA).
2.3. Biochemical characterization of adhesive plaques
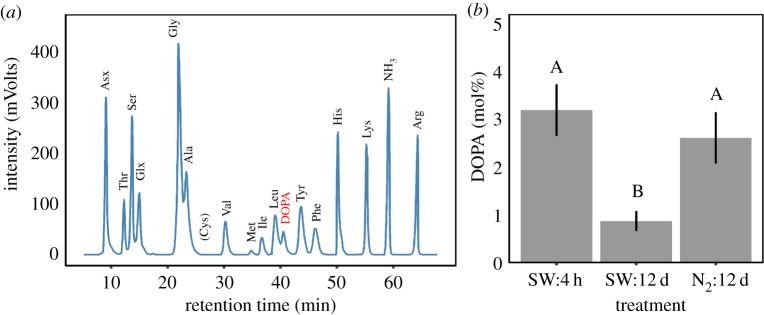
In preparation for AA analysis, adhesive plaques were collected from three different seawater treatments (4 h and 12 days in open-ocean conditions; 12 days in nitrogen infused seawater) and stored in nitrogen flushed microfuge tubes at −80°C for up to four weeks. Acid hydrolysis was then performed in vacuo at 110°C for 48 h in 6 M HCl, with 5% phenol added to preserve DOPA residues. The hydrolysate of each plaque was flash-evaporated against DI water and methanol, dissolving the precipitate in 0.02 M HCl. One hundred microlitres of the mixture was then analysed using an AA analyser system based on ninhydrin-based chemistry (Hitachi L-8900; Tokyo, Japan). A typical spectrum obtained from the analyser with identified peaks is presented in figure 4a. The integral of each AA peak was divided by the integral of all peaks to determine the relative molar concentration of each AA, normalizing against a background of 0.02 M HCl and subtracting the ammonia peak.
Figure 4.
AA composition analysis of adhesive plaques aged in control and nitrogen infused seawater treatments. (a) A characteristic AA spectrum denoting the typical intensity (mVolts) and retention time (min) of each AA reported in table 2. (b) The molar concentration of DOPA (mol %) within plaques aged under control conditions for 4 h (SW:4 h), 12 days (SW:12 d), or in hypoxic conditions until maturity (N2:12 d). Capital letters represent the result of Tukey's HSD comparisons between treatments. (Online version in colour.)
2.4. Seawater monitoring
Environmental monitoring took place from March 2015 to September 2017 at Penn Cove Shellfish's mussel aquaculture operation located in Quilcene Bay, Quilcene, Washington, USA (47°47′42.0″ N, 122°51″10.8″ W). Two YSI EXO2 water quality sondes (YSI no. 599502-00; Yellow Springs, OH, USA) were suspended from ropes in the centre of a mussel raft, deployed at −1 and −7 m below the surface. The mussel raft was approximately 15 × 18 m, supported ca 1500 lines with 20 kg of mussels per line. Sensors were deployed in the centre of the raft, surrounded by mussel lines. Each sonde was equipped with an EXO pH smart sensor (accuracy ± 0.1 pH units; YSI no. 599701), an EXO optical dissolved oxygen smart sensor (accuracy ± 1%; YSI no. 599100-01) and an EXO conductivity and temperature smart sensor (accuracy ± 0.5%; YSI no. 599870). Water temperature (°C), salinity (PSU) and dissolved oxygen concentration (mg l−1) were reported hourly, transmitting data to a logger onshore using radio telemetry. Electrodes were cleaned and calibrated monthly against NBS pH standards (YSI no. 3822), a 50 000 µS cm−1 conductivity standard (YSI no. 3169) and air-saturated DI water.
2.5. Statistical analyses
2.5.1. Laboratory experiments
All statistical analyses were performed in R (v. 3.4.1; http://www.r-project.org/) with the RStudio IDE (v. 1.0.153; http://www.rstudio.com/). When appropriate, datasets were transformed using the Johnson Transformations package (v. 1.4) to achieve normality. To control for any impact of mussel physiology on plaque strength within treatments, multiple linear regression models were used to investigate the effect of adhesive age (days), shell length (cm), GI, CI (×10−3 g cm−3) and plaque planform area (mm2) on adhesion strength (kPa). Across treatments, plaques sampled at 12 days were compared using ANOVA, listing seawater treatment, shell length, GI, CI and plaque planform area as factors. For significant effects, a Tukey's HSD test was performed to compare treatment groups. Adhesion strength is reported as the average of 3–5 plaque pulls per individual, while the failure mode of each plaque was pooled as part of a treatment. The effect of treatment on plaque failure mode was also evaluated using a chi-squared test, using the open-ocean control treatment as the expected distribution at each time point.
2.5.2. Seawater conditions under a mussel raft
Field measurements of seawater temperature (°C), salinity (PSU) and dissolved oxygen (mg l−1) were pooled across years into seasons (spring, summer, autumn and winter), using the spring equinox, summer solstice, autumn equinox and winter solstice of each year as the onset of each respective season. The time series for each parameter measured was transformed using the normal quantile transformation to achieve normality [46], and subsequently analysed using a two-way ANOVA with depth and season as factors.
To determine how frequently mussels were exposed to periods of high temperature, hyposalinity and hypoxia under a mussel raft, a threshold analysis was performed across all the time points available, grouped by depth. High temperature was defined as greater than 20°C, matching the induction temperature required for the production of heat-shock transcription factor 1 (HSF-1) for Mytilus trossulus living in subtidal conditions [47], as well as the temperature after which mussels have been shown to produce defective byssal threads [48]. Salinities capable of causing hypo-osmotic stress in Mytilus galloprovincialis (less than 10 PSU) were considered hyposaline [49], while hypoxia was defined by a dissolved oxygen concentration of less than 2 mg l−1, representing conditions that are usually lethal for pelagic invertebrates and fishes [50], but have been shown to be tolerated by Mytilus edulis for up to 1000 h [51,52]. The proportion of days that experienced at least one instance of heat stress, hyposalinity, or hypoxia was determined using the Quantmod package [53]. The mean, mode and maximum excursion duration were also determined using the length of time each parameter remained below the threshold before returning.
3. Results
3.1. Plaques cured in open-ocean conditions
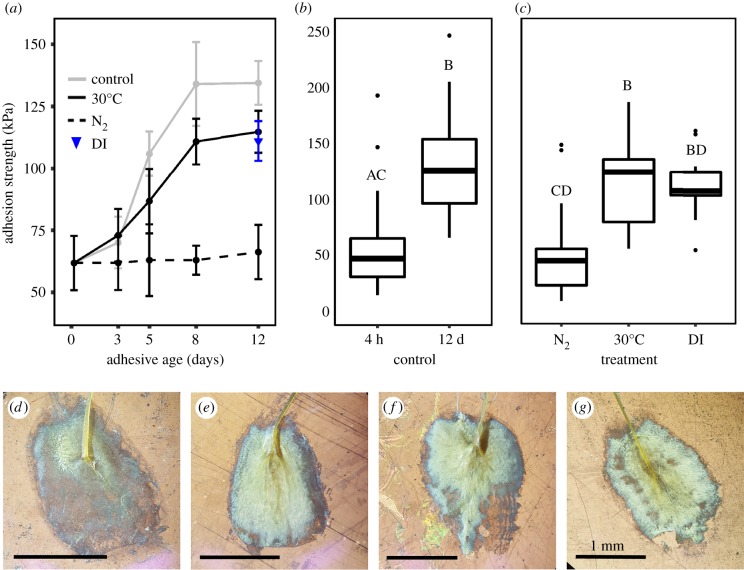
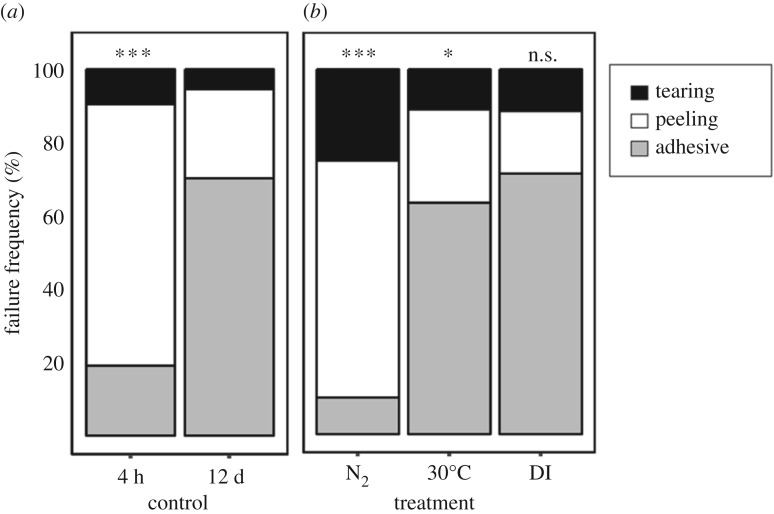
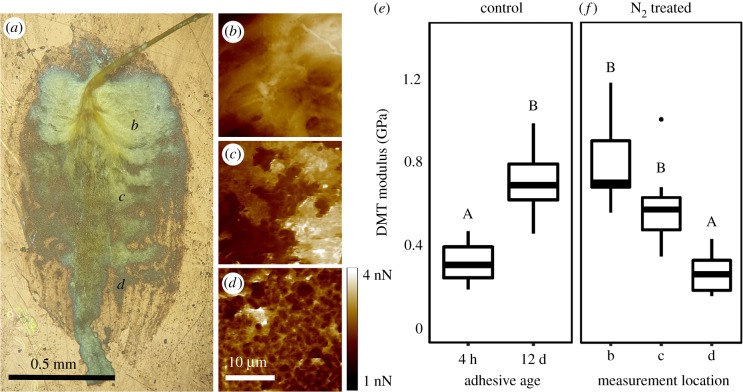
Plaque adhesion strength was influenced by the length of time the adhesive spent in seawater after being deposited on a surface (figure 1a). Freshly deposited plaques were milky white, with an average adhesion strength of 62 ± 11 kPa. However, after immersion in ‘typical open-ocean conditions' for 12 days, adhesion strength increased by 117% (p < 0.001; electronic supplementary material, table S2; figure 1a,b), with an average of 134 ± 9 kPa. Strengthening was paired with a change in physical appearance, with fresh plaques changing from translucent white to yellow over time (figure 1d,e), along with a shift in the mode of plaque failure under tension. Peeling failure was initially the most common failure mode observed (4 h: 71%), but adhesive failure steadily became more prevalent as the material cured (12 days: 70%; p < 0.001; figure 2). Strengthening was also observed at the nanometre scale, with AFM measurements of stiffness (DMT modulus) increasing 145% as the material aged from 4 h (0.31 ± 0.03 GPa) to 12 days (0.77 ± 0.08 GPa; p < 0.001; figure 3e). This increase in strength and stiffness over time was accompanied by a decrease in the molecular concentration of DOPA (mol%) within the plaque, from 3.2% in freshly made plaques to only 0.8% in the plaques aged in seawater for 12 days (p < 0.001; table 2 and figure 4). Plaques aged in the control treatment for 12 days were enriched in glycine (18.1–19.6%) and histidine (6.2–9.1%) relative to those that were freshly made and contained less alanine (10.1–8.4%; table 2). None of the physiological metrics measured affected plaque strength in the control treatment (electronic supplementary material, table S2).
Figure 1.
(a) Adhesion strength (mean ± s.e.; kPa) of plaques curing in either a hypoxic (N2) or high temperature (30°C) seawater treatment, sampled over time (days). (b) Adhesion strength of plaques aged under control conditions for 4 h (freshly made) and 12 days (mature). (c) Adhesion strength of plaques aged for 12 days in either hypoxic, high temperature or hyposaline seawater (DI). Light microscopy images of plaques freshly deposited on a surface (d) or aged for 12 days in either ‘open ocean’ (e), hypoxic (f) or high temperature (g) seawater conditions. Letters represent the result of Tukey's HSD comparisons.
Figure 2.
The frequency of plaque detachment (failure frequency, %) during tensile testing of adhesive plaques aged to maturity (12 days) under either ‘open ocean’ (a), hypoxic (b, N2), high temperature (b, 30°C) or hyposaline (b, DI water) seawater conditions. The failure mode frequency of freshly made plaques is also presented in (a) as the 4-h control. Stars represent the results of chi-squared tests comparing treatment failure distributions to the 12-day control (***p < 0.001; **p < 0.01; *p < 0.1).
Figure 3.
(a) Light microscopy image of a plaque aged to maturity (12 days) under hypoxic seawater conditions. (b–d) AFM adhesion images of the plaque surface at three different positions along the plaque pictured in (a). The material stiffness (DMT modulus) of the plaque surface was determined using AFM for both plaques incubated under control (e) and hypoxic (f) seawater conditions. Axis labels in (f) denote the location in (a) where stiffness measurements were taken. Capital letters above bar and whisker plots denote the results of Tukey's HSD pairwise comparisons between treatments.
Table 2.
Mean AA composition (mol % ± s.e.) of adhesive plaques that were freshly deposited (SW:4 h), aged in seawater for 12 days (SW:12 d) and matured in nitrogen infused seawater for 12 days (N2:12 d). The resulting p-values of ANOVA comparing the mol % of each AA across treatments are reported below, with letters representing the output of Tukey's HSD comparisons (α = 0.05). Asx is Asp and/or Asn; Glx is Glu and/or Gln. DOPA is 3,4-dihydroxyphenyl-l-alanine. The asterisk denotes a p-value <0.05.
| AA | SW:4 h | SW:12 d | N2:12 d | p-value |
|---|---|---|---|---|
| Asx | 9.1 ± 1.7a | 9.8 ± 1.8a | 10.7 ± 2.0b | <0.001* |
| Thr | 3.3 ± 0.6 | 3.2 ± 0.6 | 3.6 ± 0.7 | 0.249 |
| Ser | 8.5 ± 1.6ab | 9.2 ± 1.7b | 8.0 ± 1.5a | 0.027* |
| Glx | 5.3 ± 1.0 | 5.2 ± 1.0 | 5.1 ± 0.9 | 0.715 |
| Gly | 18.1 ± 1.7b | 19.6 ± 3.6a | 18.0 ± 3.3b | 0.003* |
| Ala | 10.1 ± 1.8a | 8.4 ± 1.6b | 8.4 ± 1.5b | <0.001* |
| Cys | 0 | 0 | 0 | |
| Val | 3.3 ± 0.6 | 3.6 ± 0.7 | 3.5 ± 0.6 | 0.587 |
| Met | 0.8 ± 0.2a | 0.2 ± 0.1b | 0.2 ± 0.1b | 0.002* |
| Ile | 1.6 ± 0.3a | 1.5 ± 0.3b | 2.1 ± 0.4b | 0.001* |
| Leu | 4.8 ± 0.9 | 4.6 ± 0.8 | 4.9 ± 0.9 | 0.294 |
| DOPA | 3.2 ± 0.6a | 0.8 ± 0.2b | 2.6 ± 0.6a | <0.001* |
| Tyr | 5.3 ± 1.0a | 6.3 ± 1.2b | 6.1 ± 1.1b | 0.016* |
| Phe | 3.6 ± 0.7a | 4.3 ± 0.8b | 3.7 ± 0.7a | 0.045* |
| His | 6.2 ± 1.1a | 9.1 ± 1.7b | 6.0 ± 1.4a | <0.001* |
| Lys | 4.7 ± 0.9a | 5.3 ± 1.0b | 5.4 ± 1.0b | 0.004* |
| Arg | 6.3 ± 1.1 | 6.5 ± 1.2 | 6.7 ± 1.2 | 0.239 |
| Pro | 4.1 ± 0.8ab | 3.7 ± 0.7a | 4.5 ± 0.8b | 0.031* |
| n = 5 | n = 5 | n = 5 |
3.2. Plaques cured under hypoxia
Plaques aged in hypoxic seawater conditions (0.9 ± 0.6 mg l−1; table 1) for 12 days failed to increase in strength over time (p = 0.86; electronic supplementary material, table S2; figure 1a), were 63% weaker than the 12-day control treatment (N2 treated = 66 ± 11 kPa; p < 0.001; figure 1b) and failed significantly more frequently by peeling (65%; p < 0.001; figure 2b). Physical differences were also evident, with plaques displaying a mottled coloration of yellow and white spots (figures 1f and 3a). Closer examination of the plaque cuticle using atomic force microscopy demonstrated that the yellow regions were smooth in texture (figure 3b), with a stiffness (0.80 ± 0.07 GPa) like those aged in open-ocean conditions for 12 days (p = 0.99; figure 3f). By contrast, the white regions were significantly softer than the yellow (0.27 ± 0.03 GPa; p < 0.001; figure 3f) and had a porous architecture at the micron scale. In transition zones between yellow and white material (figure 3c), material stiffness was not statistically different from regions that were yellow (0.59 ± 0.07 GPa; p = 0.053; figure 3f). As with the DMT modulus, the DOPA concentration of plaques aged for 12 days under hypoxia was significantly higher (2.6%) than those matured in the control treatment (0.8%; p < 0.001; figure 4b). By contrast, the molar concentration of glycine (18.0%) and histidine (6.0%) was not significantly different from the 4-h-old control (18.1% and 6.2%), while alanine (8.4%) was not significantly different from the 12-day control (8.4%; table 2). None of the physiological metrics measured affected plaque strength in the hypoxia treatment (electronic supplementary material, table S2).
3.3. Plaques cured in high temperature and hyposalinity
The adhesion strength of plaques matured in high temperature (30°C; 115 ± 9 kPa) and DI water (ca 1 PSU; 111 ± 8 kPa) was not significantly different from the 12-day control treatment (p = 0.06 and p = 0.50; electronic supplementary material, table S3; figure 1c). The failure mode of plaques aged in DI water was also like the 12-day control (p = 0.21), while high temperature marginally increased the prevalence of peeling failure (p = 0.07; figure 2b). Incubating plaques at 30°C marginally slowed adhesive strengthening, with no significant difference observed at any time point when compared with the control treatment (figure 1a). Plaques aged in either treatment did not appear different in colour compared to the 12-day control, although high temperature caused uneven tanning in rare cases (figure 1g). There was no treatment level effect of mussel size, plaque area or reproductive and physiological condition on adhesion strength. None of the physiological metrics measured affected plaque strength in either treatment (electronic supplementary material. table S2).
3.4. Seawater conditions under a mussel aquaculture raft
Field measurements of seawater temperature varied seasonally and with depth (electronic supplementary material, figure S1; table S4; p < 0.001), with a summer maximum of 24.1°C at the surface (1 m) and a low of 5.3°C in autumn at depth (7 m; electronic supplementary material, figure S2). Higher mean temperatures were observed at the surface during the spring and summer, while in the autumn and winter months the trend reversed, with cooler surface temperatures at the surface than at depth (electronic supplementary material, figures S1 and S2). High temperature excursions were only observed at the surface in the summer and spring, with 12% of days displaying at least one temperature spike above 20°C (table 3). The average length of each high temperature excursion was 7.3 h, with the longest excursion occurring over 18 h. Most commonly, excursions lasted for 2 h. Although season and depth were both significant factors driving temperature in this system, the interaction between the two was also significant, indicating that the effect of one depends on the context of the other (p < 0.001; electronic supplementary material, table S4).
Table 3.
The frequency of extreme excursions in seawater temperature (°C), salinity (PSU) and dissolved oxygen (mg l−1) at two depths (1 and 7 m) beneath a mussel aquaculture raft located in Quilcene Bay, Quilcene, Washington. Water conditions were monitored hourly from March 2015 through September 2017. Sample size (n) reflects the exclusion of data compromised by sensor fouling or communication errors. The proportion of days that experienced at least one instance of heat stress (greater than 20°C), hyposalinity (less than 10 PSU) or hypoxia (less than 2 mg l−1) is reported as n%. The mean, mode and maximum excursion durations (hours) are also reported for each condition, at each depth.
| excursion duration (hours) |
||||||
|---|---|---|---|---|---|---|
| condition | depth (m) | n | n% | mean | mode | max |
| T > 20°C | 1 | 656 | 12.2 | 7.3 | 2 | 18 |
| 7 | 701 | — | — | — | ||
| Sal < 10 PSU | 1 | 506 | 4 | 2.9 | 2 | 6 |
| 7 | 528 | — | — | — | ||
| O2 < 2 mg l−1 | 1 | 646 | 3.7 | 3.3 | 1 | 10 |
| 7 | 641 | 14 | 3.3 | 1 | 12 | |
Salinity also varied seasonally (p < 0.001) and with depth (p < 0.001), with excursions as low as 2.1 and 2.6 PSU occurring in autumn and winter at the surface (electronic supplementary material, table S4; figures S1 and S2). Low salinity excursions were limited to the surface and were relatively rare, with only 4% of days experiencing a salinity less than 10 PSU. The average length of a salinity excursion was 2.9 h, with the longest bout of hyposalinity occurring in autumn and lasting 6.3 h (table 2). Salinity at depth (7 m) remained above 10 PSU year-round (electronic supplementary material, figure S2; table 3). As with temperature, the differences in salinity between depths depended on season (depth × season interaction, electronic supplementary material, table S4) and were most prominent in autumn and winter.
Variability in dissolved oxygen was observed in the spring and summer, with lower concentrations observed at depth (electronic supplementary material, figures S1 and S2). By contrast, less variation was seen in the autumn and winter, although the depth trend was maintained (electronic supplementary material, figure S2). As with temperature and salinity, dissolved oxygen varied significantly with depth (p < 0.001) and season (p < 0.001), with a significant interaction between the two factors (p < 0.001; electronic supplementary material, table S4). Hypoxic excursions were routinely observed at both depths, with 3.7% of the days included in this study experiencing a hypoxic event at the surface, compared to 14% at depth (table 3). The average length of an excursion was 3.3 h for both depths, with the most common being 1 h. The longest excursions observed lasted for 10 h at the surface and 12 h at depth (table 3). The dissolved oxygen minima for each depth was 0.4 and 0.1 mg l−1 for the surface and at depth, respectively, with both conditions occurring during the summer (electronic supplementary material, figures S1 and S2).
4. Discussion
Of the three seawater parameters tested, dissolved oxygen was the only one that was required for environmental post-processing. Plaques deprived of oxygen were 51% weaker than those cured in open-ocean conditions for 12 days and developed a mottled yellow coloration with translucent regions commonly found along the perimeter. While dopaquinone formation did occur under hypoxia, atomic force microscopy imaging of translucent patches produced adhesion maps that looked different from white patches and had lower material stiffness. These results suggest that, in addition to a basic pH, environmental oxygen must be available during the curing process for the complex protein structure of the plaque to form correctly, even long after the structure has transitioned from a fluid to a bulk solid.
Mussel plaques represent a special case where both the adhesive and cohesive properties of an adhesive are linked to a single functional group. DOPA residues comprise 2–30% of the molecular structure of adhesive proteins (Mfps) within the plaque [54], with the highest molecular concentrations of DOPA found in Mfps localized at the adhesive–substratum interface [21,55] and in the cuticle [24,25]. While DOPA residues are initially responsible for substrate-level adhesion, the conversion of DOPA to dopaquinone after adsorption can be just as important for overall adhesion strength [56]. In this way, adhesion strength is optimized when a balance is maintained between the adhesive and cohesive interactions within the plaque and the material distributes load evenly, preferentially breaking bonds at the adhesive–substrate interface (adhesive failure) [57,58].
Typically, temperature plays a pivotal role in modulating the balance between adhesive and cohesive interactions within adhesives by altering the kinetics of molecular interactions [32]. However, plaques cured in seawater heated to 30°C, exceeding even the most extreme temperatures seen in surface waters in this study, failed to alter either the curing rate or adhesion strength of plaques after 12 days. This result is consistent with the cross-linking activity of DOPA-functionalized synthetic polypeptide mimics of mussel adhesive, which can be achieved up to 60°C [57]. Similar results were seen with hyposalinity, which also failed to affect plaque curing. It should be noted that this result is testing a different aspect of plaque adhesion than other studies, which have investigated the adhesion of Mfp films to mica in solutions of varied ionic strength [21]. Our results show the resilience of whole plaque curing to changes in environmental salinity after deposition. The robust nature of the curing process with regard to salinity could be due, in part, to the fact that ionic interactions in the plaque are limited mainly to Fe3+ in the cuticle [59,60], and Zn2+ and Cu2+ in the plaque–thread junction [61,62], which are supplied by the mussel during protein secretion, in the ionically sparse distal depression [63].
By contrast, when separated from the mussel after deposition and aged to maturity under hypoxia, plaques displayed several indicators that cohesive interactions within the structure either did not form or formed improperly, during the curing process. First, plaques incubated in hypoxia for 12 days had a different physical appearance than the 12-day control, with regions along the perimeter that resembled the 4-h control. Second, hypoxia reared plaques lacked characteristic shifts in AA composition that are typical of biomaterials that undergo schlerotization. Under hypoxia, the molar concentration of DOPA residues within the plaque remained elevated (2.6%) after 12 days, resembling the composition of freshly made plaques (3.2%), rather than the 12-day control (0.8%). Unfortunately, studies reporting the DOPA composition within the distal region of the thread are more prevalent than the plaque, although a concentration of ca 1.4% for the plaque disc of Mytilus edulis has been reported [64]. While the per cent DOPA composition undoubtedly varies between species [65], individuals [66] and even with the physiological health of the animal [67], the fact that DOPA composition decreased over 12 days in the control testifies that our method was precise enough to measure DOPA residue oxidation.
The histidine composition of the plaque under hypoxia (6.0%) displayed a close similarity to freshly made plaques (6.2%) and was significantly lower than the 12-day control (9.1%). Histidine is often involved in metal ion coordination in proteins and is a likely partner for either Zn2+ or Cu2+ in the distal region of the thread [61,68], while also serving as a DOPA cross-linking partner [69]. In the plaque, histidine has been proposed as an important component of the gap-junction protein mfp-4 [70], which acts at the interface between the bulk of the plaque (mfp-2) and the preCOL components of the distal region. In this context, the histidine content of a plaque would be expected to decrease over time as cross-links are formed; mcfp-4 extracted from the foot (19.2%) contains more histidine than when extracted directly from the plaque after deposition (18.3%) [70], and preCol-P contains significantly more histidine than Col-P (0.98%) [71]. However, the results of this study are not consistent with these findings. One possible explanation is that the relative contribution of the distal region root structure to each plaque varies [72]. Differences in the relative contribution of preCOLs to the molecular mass of hydrolysed plaque samples could be significant without the careful removal of the distal stem. Either way, considerable uncertainty remains regarding the wide array of possible interactions between proteins and AA residues within quinone-tanned adhesives [73,74], making this an exciting area for further research.
A reduction in quinone tanning under hypoxia could be the result of direct oxygen limitation at the site of covalent cross-linking or due to the disruption of the activity of oxidizing enzymes in the plaque after secretion. DOPA cross-linking in mussel byssus adhesive is aided by the enzyme catechol oxidase [75], as well as undoubtedly others that have yet to be discovered (JH Waite 2018, personal communication). Catechol oxidase facilitates cross-linking through the oxidative dehydrogenation of o-diphenols, acting as a catalyst across the entirety of the mussel byssus [76]. While enzyme activity is known to be pH sensitive, with a kinetic optimum at seawater pH [75], the oxidation rate and aggregation of Mfp-1 in solution have been shown to rely much more heavily on the nature of the oxidator that is present [27]. For this reason, further work is needed to properly investigate the role that catechol oxidase plays in DOPA cross-linking under oxygen limitation, perhaps through the supplementation of oxidase inhibitors such as phenylthiourea or salicylhydroxamic acid.
Compositional similarities between plaques aged in hypoxia and those that were freshly made in a seawater control could help to explain why their failure dynamics were also so similar. Peeling was the most common failure mode for both the 4-h control and hypoxia incubated plaques. One reason for this similarity could be the prevention of dopaquinone cross-linking formation under oxygen limitation, leading to a reduction in the integrity of the plaque structure. AFM scans of the surface of hypoxia matured plaques looked different from the 12-day control treatment, indicating a possible malformation of the plaque cuticle. However, a more definitive assessment of the integrity of the plaque cuticle would require the use of higher resolution imaging, such as TEM, with greater depth penetration. Nevertheless, the stiffness measurements of smooth, yellow regions near the plaque-thread junction were like those reported for the cuticle of Mytilus galloprovincialis (ca 1.5 GPa), while the white, porous regions had moduli consistent with the interior of the plaque (ca 400 MPa) [77], supporting the hypothesis that the cuticle is missing in some regions. Alternatively, this result could be due to the reduction in cross-linking density that comes as a result of oxygen depletion, as the cuticle of the plaque is made up of Mfp-1 (15% DOPA), while the core comprises Mfp-2 (5% DOPA) [78,79]. A reduction in dopaquinone formation and the cohesive strength that comes with proper cuticle formation could explain why plaques frequently peeled from substrates under hypoxia, as areas of low stiffness can act as regions of concentrated stress while under tension.
While the cross-linking behaviour of specific Mfps is well understood [27,36,80,81], the kinetics of cross-linking in a protein network as complex as the one in the plaque remains unclear. In this study, plaques were incubated in containers with constant aeration in the laboratory, without the addition of circulating pumps. It is, therefore, likely that the curing window presented here approximates still or gently flowing water conditions found underneath mussel rafts [82–84], rather than the turbulent, high flow conditions mussels may experience in the intertidal zone [85,86]. The increased flux of oxygen to the plaque in turbulent habitats may either speed up cross-linking or flush the local microenvironment, effectively rescuing adhesion stalled by localized regions of oxygen depletion [35]. Oxygen availability could explain why the tenacity of solitary mussels [87], and those located on the margins of mussel beds [88], are typically stronger than those in aggregations, although additional work in needed in order to separate this effect from any physiological response of mussels to flow or food availability [89,90].
The observed decrease in plaque adhesion strength with hypoxia is particularly relevant for mussels hanging from ropes in raft aquaculture, where robust adhesion is necessary for survival. Seasonal comparisons of byssal thread mechanics have found that threads decay in as little as two weeks in the summer [91], necessitating that mussels perpetually produce threads in order to remain attached. Even if mussels refrain from byssus production in response to disadvantageous conditions, closing their shells to reduce physiological stress [92,93], freshly made threads must contend with local seawater conditions during an 8–12 days curing period for quinone tanning to fully occur [26]. Given the sensitivity of the curing process to oxygen availability, further work is needed to tease apart whether the curing process is delayed or irrevocably altered. If delayed, tidal fluctuations in oxygen saturation could serve to rescue adhesion. Alternatively, if the ultimate cohesive strength of the plaque is determined by the rate of dopaquinone formation, then the curing process could have a window of time wherein cross-linking needs to occur to serve its function as a load bearing structure.
Mussels have adapted an adhesion strategy that circumvents the challenges of adhesion underwater, using the chemistry of their surroundings to their advantage. However, seawater conditions in nearshore environments often vary dramatically from the basic, oxygen saturated conditions of the open-ocean that are conducive for adhesion [28,29,41,94]. For example, field observations outlining the prevalence of hypoxia excursions where mussels were collected in this study show that 14% of the days sampled experienced at least one hypoxic event (less than 2 mg l−1), with the longest event lasting for 12 h. Given the susceptibility of plaque curing to oxygen availability, experiments where the curing process is inundated by environmentally relevant excursions in dissolved oxygen are needed to determine whether hypoxia irrevocably damages the protein network or if adhesion strength can recover after favourable conditions return.
The molecular mechanisms underlying DOPA-mediated adhesion have been a source of inspiration for researchers developing medical adhesives that work under wet conditions in the human body. Currently, many of the advancements have come from functionalizing polymers [4,23,95], hydrogels [96–98] and thin films [3] with DOPA, in an effort to produce reliable adhesion and cross-linking. However, an exciting new direction has been made possible by the development of new methods for the production of coacervate films, which can be easily stored and administered in a clinical setting [18,19,99]. A better understanding of the role that environmental post-processing plays in plaque curing can aid in the design of medically relevant coacervate adhesives.
During coacervate formulation, the mixture of catechol varieties used to functionalize the poly-cation and poly-anion components of the coacervate need to be optimized, along with their density, so that the length of the curing window is relevant for the application [100]. Optimization can be accomplished by controlling the ratio of covalent and supramolecular interactions by including catechol analogues that are more or less prone to oxidation [101–103]. In contrast to single molecule studies where cross-linking is achieved over minutes to hours [36], the relatively long curing window reported in this study for the byssus plaque could be a result of the diversity of Mfps present, as well as how they are patterned in the cuticle and core of the plaque. Therefore, a formulation that mimics the structure, composition and mechanics of the entire plaque may be useful for sutures applied to delicate tissues where rapid dopaquinone formation would cause tearing or scarring.
Supplementary Material
Acknowledgements
We thank MacKenzie Edelsward, Chandana Kulkarni, Chloe Peterschmidt, Benjamin Makhlouf and Jonathan Huie for the assistance with mechanical testing, Micah Glaz for support with atomic force microscopy analysis, Dr Daniel DeMartini for assistance with amino acid analysis and Dr J. Herbert Waite for sharing his equipment, time and expertise. We also thank Cinde Donaghue, Ian Jefferds, Dominic Pangelinan, Brad Woolf, and all of the mussel growers at Penn Cove Shellfish, without whom this project would not have been possible.
Data accessibility
Data are archived under project no. 2250 at www.bco-dmo.org.
Authors' contributions
M.N.G. wrote the manuscript and conducted the research, including the monthly calibration of water sensors, tensometer testing, atomic force microscopy and AA analysis. B.P. contributed to experimental design and performed mechanical testing for the temperature assays. E.C. helped to conceive of the study and edited drafts of the manuscript. All authors gave final approval for publication.
Competing interests
The authors of the manuscript have no competing interests.
Funding
This work was supported by an NSF GRFP fellowship [no. DGE-1256082] to M.N.G., the Washington Research Foundation Benjamin Hall Fellowship to M.N.G., an Alan and Marian Kohn FHL Fellowship to M.N.G., a Mary Gates Fellowship to B.P. and an NSF grant no. [OCE-1041213] to E.C. Part of this work was conducted at the Molecular Analysis Facility, a National Nanotechnology Coordinated Infrastructure site at the University of Washington which is supported in part by the National Science Foundation [no. ECC-1542101], the University of Washington, the Molecular Engineering and Sciences Institute, the Clean Energy Institute and the National Institutes of Health. The efforts of E.C. were supported while serving at the National Science Foundation. Any opinion, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. Additional support was provided by the Washington Sea Grant Program (NA14OAR4170078, to E.C. and C. S. Friedman) and the Washington State Department of Natural Resources.
References
- 1.Lu Q, Danner E, Waite JH, Israelachvili JN, Zeng H, Hwang DS. 2013. Adhesion of mussel foot proteins to different substrate surfaces. J. R. Soc. Interface 10, 20120759 ( 10.1098/rsif.2012.0759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu J, Wei W, Menyo MS, Masic A, Waite JH, Israelachvili JN. 2013. Adhesion of mussel foot protein-3 to TiO2 surfaces: the effect of pH. Biomacromolecules 14, 1072–1077. ( 10.1021/bm301908y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J, et al. 2013. Adaptive hydrophobic and hydrophilic interactions of mussel foot proteins with organic thin films. Proc. Natl Acad. Sci. USA 110, 15680–15685. ( 10.1073/pnas.1315015110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalsin JL, Hu B-H, Lee BP, Messersmith PB. 2003. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J. Am. Chem. Soc. 125, 4253–4258. ( 10.1021/ja0284963) [DOI] [PubMed] [Google Scholar]
- 5.Lee H, Lee BP, Messersmith PB. 2007. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 448, 338–341. ( 10.1038/nature05968) [DOI] [PubMed] [Google Scholar]
- 6.Holten-Andersen N, Waite J. 2008. Mussel-designed protective coatings for compliant substrates. J. Dent. Res. 87, 701–709. ( 10.1177/154405910808700808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee BP, Messersmith PB, Israelachvili JN, Waite JH. 2011. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 41, 99–132. ( 10.1146/annurev-matsci-062910-100429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett DG, Bushnell GG, Messersmith PB. 2013. Mechanically robust, negative-swelling, mussel-inspired tissue adhesives. Adv. Healthc. Mater. 2, 745–755. ( 10.1002/adhm.201200316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waite H. 1983. Adhesion in byssally attached bivalves. Biol. Rev. 58, 209–231. ( 10.1111/j.1469-185X.1983.tb00387.x) [DOI] [Google Scholar]
- 10.Waite JH, Qin X. 2001. Polyphosphoprotein from the adhesive pads of Mytilus edulis. Biochemistry 40, 2887–2893. ( 10.1021/bi002718x) [DOI] [PubMed] [Google Scholar]
- 11.Zhao H, Robertson NB, Jewhurst SA, Waite JH. 2006. Probing the adhesive footprints of Mytilus californianus byssus. J. Biol. Chem. 281, 11090–11096. ( 10.1074/jbc.M510792200) [DOI] [PubMed] [Google Scholar]
- 12.Waite JH. 1987. Nature's underwater adhesive specialist. Int. J. Adhes. Adhes. 7, 9–14. ( 10.1016/0143-7496(87)90048-0) [DOI] [Google Scholar]
- 13.Waite JH. 1992. The formation of mussel byssus: anatomy of a natural manufacturing process. In Structure, cellular synthesis and assembly of biopolymers (ed. ST Case), pp. 27–54. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 14.Martinez Rodriguez NR, Das S, Kaufman Y, Israelachvili JN, Waite JH. 2015. Interfacial pH during mussel adhesive plaque formation. Biofouling 31, 221–227. ( 10.1080/08927014.2015.1026337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J, Wei W, Danner E, Ashley RK, Israelachvili JN, Waite JH. 2011. Mussel protein adhesion depends on interprotein thiol-mediated redox modulation. Nat. Chem. Biol. 7, 588–590. ( 10.1038/nchembio.630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller DR, Spahn JE, Waite JH. 2015. The staying power of adhesion-associated antioxidant activity in Mytilus californianus. J. R. Soc. Interface 12, 20150614 ( 10.1098/rsif.2015.0614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicklisch SC, Spahn JE, Zhou H, Gruian CM, Waite JH. 2016. Redox capacity of an extracellular matrix protein associated with adhesion in Mytilus californianus. Biochemistry 55, 2022–2030. ( 10.1021/acs.biochem.6b00044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang DS, Zeng H, Srivastava A, Krogstad DV, Tirrell M, Israelachvili JN, Waite JH. 2010. Viscosity and interfacial properties in a mussel-inspired adhesive coacervate. Soft Matter 6, 3232–3236. ( 10.1039/c002632h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei W, Tan Y, Rodriguez NRM, Yu J, Israelachvili JN, Waite JH. 2014. A mussel-derived one component adhesive coacervate. Acta Biomater. 10, 1663–1670. ( 10.1016/j.actbio.2013.09.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson TH, Yu J, Estrada A, Hammer MU, Waite JH, Israelachvili JN. 2010. The contribution of DOPA to substrate–peptide adhesion and internal cohesion of mussel-inspired synthetic peptide films. Adv. Funct. Mater. 20, 4196–4205. ( 10.1002/adfm.201000932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danner EW, Kan Y, Hammer MU, Israelachvili JN, Waite JH. 2012. Adhesion of mussel foot protein Mefp-5 to mica: an underwater superglue. Biochemistry 51, 6511–6518. ( 10.1021/bi3002538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waite JH. 1983. Quinone-tanned scleroproteins. The Mollusca 1, 467–504. [Google Scholar]
- 23.Holten-Andersen N, Harrington MJ, Birkedal H, Lee BP, Messersmith PB, Lee KYC, Waite JH. 2011. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proc. Natl Acad. Sci. USA 108, 2651–2655. ( 10.1073/pnas.1015862108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor SW, Chase DB, Emptage MH, Nelson MJ, Waite JH. 1996. Ferric ion complexes of a DOPA-containing adhesive protein from Mytilus edulis. Inorg. Chem. 35, 7572–7577. ( 10.1021/ic960514s) [DOI] [Google Scholar]
- 25.Xu Z. 2013. Mechanics of metal–catecholate complexes: the roles of coordination state and metal types. Sci. Rep. 3, 2914 ( 10.1038/srep02914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George MN, Carrington E. 2018. Environmental post-processing increases the adhesion strength of mussel byssus adhesive. Biofouling 34, 388–397. ( 10.1080/08927014.2018.1453927) [DOI] [PubMed] [Google Scholar]
- 27.Haemers S, Koper GJ, Frens G. 2003. Effect of oxidation rate on cross-linking of mussel adhesive proteins. Biomacromolecules 4, 632–640. ( 10.1021/bm025707n) [DOI] [PubMed] [Google Scholar]
- 28.Baumann H, Wallace RB, Tagliaferri T, Gobler CJ. 2014. Large natural pH, CO2 and O2 fluctuations in a temperate tidal salt marsh on diel, seasonal, and interannual time scales. Estuaries Coasts 38, 220–231. [Google Scholar]
- 29.Reum JC, Alin SR, Feely RA, Newton J, Warner M, McElhany P. 2014. Seasonal carbonate chemistry covariation with temperature, oxygen, and salinity in a fjord estuary: implications for the design of ocean acidification experiments. PLoS ONE 9, e89619 ( 10.1371/journal.pone.0089619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGowan JA, Cayan DR, Dorman LM. 1998. Climate-ocean variability and ecosystem response in the northeast pacific. Science 281, 210 ( 10.1126/science.281.5374.210) [DOI] [PubMed] [Google Scholar]
- 31.Feely RA, Alin SR, Newton J, Sabine CL, Warner M, Devol A, Krembs C, Maloy C. 2010. The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Estuar. Coast. Shelf Sci. 88, 442–449. ( 10.1016/j.ecss.2010.05.004) [DOI] [Google Scholar]
- 32.Filippidi E, DeMartini DG, Malo de Molina P, Danner EW, Kim J, Helgeson ME, Waite JH, Valentine MT. 2015. The microscopic network structure of mussel (Mytilus) adhesive plaques. J. R. Soc. Interface 12, 20150827 ( 10.1098/rsif.2015.0827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibragimova GT, Wade RC. 1998. Importance of explicit salt ions for protein stability in molecular dynamics simulation. Biophys. J. 74, 2906–2911. ( 10.1016/S0006-3495(98)77997-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Formaneck MS, Ma L, Cui Q. 2006. Effects of temperature and salt concentration on the structural stability of human lymphotactin: insights from molecular simulations. J. Am. Chem. Soc. 128, 9506–9517. ( 10.1021/ja061620o) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun C, Vaccaro E, Waite JH. 2001. Oxidative stress and the mechanical properties of naturally occurring chimeric collagen-containing fibers. Biophys. J. 81, 3590–3595. ( 10.1016/S0006-3495(01)75989-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haemers S, van der Leeden MC, Koper GJ, Frens G. 2002. Cross-linking and multilayer adsorption of mussel adhesive proteins. Langmuir 18, 4903–4907. ( 10.1021/la025626c) [DOI] [Google Scholar]
- 37.Fan C, Fu J, Zhu W, Wang D-A. 2016. A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use. Acta Biomater. 33, 51–63. ( 10.1016/j.actbio.2016.02.003) [DOI] [PubMed] [Google Scholar]
- 38.Carrington E. 2002. Seasonal variation in the attachment strength of blue mussels: causes and consequences. Limnol. Oceanogr. 47, 1723–1733. ( 10.4319/lo.2002.47.6.1723) [DOI] [Google Scholar]
- 39.Moeser GM, Leba H, Carrington E. 2006. Seasonal influence of wave action on thread production in Mytilus edulis. J. Exp. Biol. 209, 881–890. ( 10.1242/jeb.02050) [DOI] [PubMed] [Google Scholar]
- 40.Baumann H, Smith EM. 2017. Quantifying metabolically driven pH and oxygen fluctuations in US nearshore habitats at diel to interannual time scales. Estuaries Coasts 41, 1102–1117. ( 10.1007/s12237-017-0321-3) [DOI] [Google Scholar]
- 41.Booth JAT, McPhee-Shaw EE, Chua P, Kingsley E, Denny M, Phillips R, Bograd SJ, Zeidberg LD, Gilly WF. 2012. Natural intrusions of hypoxic, low pH water into nearshore marine environments on the California coast. Cont. Shelf Res. 45, 108–115. ( 10.1016/j.csr.2012.06.009) [DOI] [Google Scholar]
- 42.Martz TR, Connery JG, Johnson KS. 2010. Testing the Honeywell Durafet® for seawater pH applications. Limnol. Oceanogr. Methods 8, 172–184. ( 10.4319/lom.2010.8.172) [DOI] [Google Scholar]
- 43.Burkett JR, Wojtas JL, Cloud JL, Wilker JJ. 2009. A method for measuring the adhesion strength of marine mussels. J. Adhes. 85, 601–615. ( 10.1080/00218460902996903) [DOI] [Google Scholar]
- 44.Young GA, Crisp D. 1982. Marine animals and adhesion. In Adhesion (ed. Allend KW.), pp. 19–39. Barking, England: Applied Science Publishers, Ltd. [Google Scholar]
- 45.Young TJ, Monclus MA, Burnett TL, Broughton WR, Ogin SL, Smith PA. 2011. The use of the PeakForce™ quantitative nanomechanical mapping AFM-based method for high-resolution Young's modulus measurement of polymers. Meas. Sci. Technol. 22, 125703 ( 10.1088/0957-0233/22/12/125703) [DOI] [Google Scholar]
- 46.Bogner K, Pappenberger F, Cloke HL. 2012. The normal quantile transformation and its application in a flood forecasting system. Hydrol. Earth Syst. Sci. 16, 1085–1094. ( 10.5194/hess-16-1085-2012) [DOI] [Google Scholar]
- 47.Buckley BA, Owen M-E, Hofmann GE. 2001. Adjusting the thermostat: the threshold induction temperature for the heat-shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J. Exp. Biol. 204, 3571–3579. [DOI] [PubMed] [Google Scholar]
- 48.Newcomb LA. 2015. Elevated temperature and ocean acidification alter mechanics of mussel attachment. PhD thesis. University of Washington, Seattle, WA. [Google Scholar]
- 49.Hamer B, et al. 2008. Effect of hypoosmotic stress by low salinity acclimation of Mediterranean mussels Mytilus galloprovincialis on biological parameters used for pollution assessment. Aquat. Toxicol. 89, 137–151. ( 10.1016/j.aquatox.2008.06.015) [DOI] [PubMed] [Google Scholar]
- 50.Gray JS, Wu RS, Or YY. 2002. Effects of hypoxia and organic enrichment on the coastal marine environment. Mar. Ecol. Prog. Ser. 238, 249–279. ( 10.3354/meps238249) [DOI] [Google Scholar]
- 51.Dries R-R, Theede H. 1974. Sauerstoffmangelresistenz mariner Bodenvertebraten aus der westlichen Ostsee. Mar. Biol. 25, 327–333. ( 10.1007/BF00404975) [DOI] [Google Scholar]
- 52.Rosenberg R. 1972. Benthic faunal recovery in a Swedish fjord following the closure of a sulphite pulp mill. Oikos 23, 92–108. ( 10.2307/3543930) [DOI] [Google Scholar]
- 53.Ryan JA, Ulrich JM.2017. quantmod: Quantitative Financial Modelling Framework. See https://CRAN.R-project.org/package=quantmod .
- 54.Waite JH. 2017. Mussel adhesion—essential footwork. J. Exp. Biol. 220, 517–530. ( 10.1242/jeb.134056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin Q, Gourdon D, Sun C, Holten-Andersen N, Anderson TH, Waite JH, Israelachvili JN. 2007. Adhesion mechanisms of the mussel foot proteins mfp-1 and mfp-3. Proc. Natl Acad. Sci. USA 104, 3782–3786. ( 10.1073/pnas.0607852104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu M, Hwang J, Deming TJ. 1999. Role of l-3,4-dihydroxyphenylalanine in mussel adhesive proteins. J. Am. Chem. Soc. 121, 5825–5826. ( 10.1021/ja990469y) [DOI] [Google Scholar]
- 57.Yu M, Deming T. 1998. Synthetic polypeptide mimics of marine adhesives. Macromolecules 31, 4739–4745. ( 10.1021/ma980268z) [DOI] [PubMed] [Google Scholar]
- 58.Desmond KW, Zacchia NA, Waite JH, Valentine MT. 2015. Dynamics of mussel plaque detachment. Soft Matter 11, 6832–6839. ( 10.1039/C5SM01072A) [DOI] [PubMed] [Google Scholar]
- 59.Holten-Andersen N, Fantner GE, Hohlbauch S, Waite JH, Zok FW. 2007. Protective coatings on extensible biofibres. Nat. Mater. 6, 669 ( 10.1038/nmat1956) [DOI] [PubMed] [Google Scholar]
- 60.Harrington MJ, Masic A, Holten-Andersen N, Waite JH, Fratzl P. 2010. Iron-clad fibers: a metal-based biological strategy for hard flexible coatings. Science 328, 216–220. ( 10.1126/science.1181044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harrington MJ, Waite JH. 2007. Holdfast heroics: comparing the molecular and mechanical properties of Mytilus californianus byssal threads. J. Exp. Biol. 210, 4307–4318. ( 10.1242/jeb.009753) [DOI] [PubMed] [Google Scholar]
- 62.Vaccaro E, Waite JH. 2001. Yield and post-yield behavior of mussel byssal thread: a self-healing biomolecular material. Biomacromolecules 2, 906–911. ( 10.1021/bm0100514) [DOI] [PubMed] [Google Scholar]
- 63.George SG, Pirie BJS, Coombs TL. 1976. The kinetics of accumulation and excretion of ferric hydroxide in Mytilus edulis (L.) and its distribution in the tissues. J. Exp. Mar. Biol. Ecol. 23, 71–84. ( 10.1016/0022-0981(76)90086-1) [DOI] [Google Scholar]
- 64.Benedict CV, Waite JH. 1986. Composition and ultrastructure of the byssus of Mytilus edulis. J. Morphol. 189, 261–270. ( 10.1002/jmor.1051890305) [DOI] [PubMed] [Google Scholar]
- 65.Bouhlel Z, et al. 2017. Interspecies comparison of the mechanical properties and biochemical composition of byssal threads. J. Exp. Biol. 220, 984–994. ( 10.1242/jeb.141440) [DOI] [PubMed] [Google Scholar]
- 66.Floriolli RY, von Langen J, Waite JH. 2000. Marine surfaces and the expression of specific byssal adhesive protein variants in Mytilus. Mar. Biotechnol. 2, 352–363. [DOI] [PubMed] [Google Scholar]
- 67.Babarro JM, Reiriz MJF. 2010. Secretion of byssal threads in Mytilus galloprovincialis: quantitative and qualitative values after spawning stress. J. Comp. Physiol. B 180, 95–104. ( 10.1007/s00360-009-0392-y) [DOI] [PubMed] [Google Scholar]
- 68.Waite JH, Qin X-X, Coyne KJ. 1998. The peculiar collagens of mussel byssus. Matrix. Biol. 17, 93–106. ( 10.1016/S0945-053X(98)90023-3) [DOI] [PubMed] [Google Scholar]
- 69.Miserez A, Rubin D, Waite JH. 2010. Cross-linking chemistry of squid beak. J. Biol. Chem. 285, 38115–38124. ( 10.1074/jbc.M110.161174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao H, Waite JH. 2006. Proteins in load-bearing junctions: the histidine-rich metal-binding protein of mussel byssus. Biochemistry 45, 14 223–14 231. ( 10.1021/bi061677n) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin X, Waite JH. 1995. Exotic collagen gradients in the byssus of the mussel Mytilus edulis. J. Exp. Biol. 198, 633–644. [DOI] [PubMed] [Google Scholar]
- 72.Benedict CV, Waite JH. 1986. Location and analysis of byssal structural proteins of Mytilus edulis. J. Morphol. 189, 171–181. ( 10.1002/jmor.1051890207) [DOI] [PubMed] [Google Scholar]
- 73.Waite JH. 1990. The phylogeny and chemical diversity of quinone-tanned glues and varnishes. Comp. Biochem. Physiol. Part B Comp. Biochem. 97, 19–29. ( 10.1016/0305-0491(90)90172-P) [DOI] [PubMed] [Google Scholar]
- 74.Waite JH. 1992. The DOPA ephemera: a recurrent motif in invertebrates. Biol. Bull. 183, 178–184. ( 10.2307/1542421) [DOI] [PubMed] [Google Scholar]
- 75.Waite JH. 1985. Catechol oxidase in the byssus of the common mussel, Mytilus edulis L. J. Mar. Biol. Assoc. U. K. 65, 359–371. ( 10.1017/S0025315400050487) [DOI] [Google Scholar]
- 76.Burzio LA. 1996. Catechol oxidases associated with byssus formation in the blue mussel, Mytilus edulis. Master's thesis Newark University of Delaware. [Google Scholar]
- 77.Holten-Andersen N, Zhao H, Waite JH. 2009. Stiff coatings on compliant biofibers: the cuticle of Mytilus californianus byssal threads. Biochemistry 48, 2752–2759. ( 10.1021/bi900018m) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waite JH. 1983. Evidence for a repeating 3,4-dihydroxyphenylalanine- and hydroxyproline-containing decapeptide in the adhesive protein of the mussel, Mytilus edulis L. J. Biol. Chem. 258, 2911–2915. [PubMed] [Google Scholar]
- 79.Inoue K, Waite JH, Matsuoka M, Odo S, Harayama S. 1995. Interspecific variations in adhesive protein sequences of Mytilus edulis, M. galloprovincialis, and M. trossulus. Biol. Bull. 189, 370–375. ( 10.2307/1542155) [DOI] [PubMed] [Google Scholar]
- 80.Fant C, Sott K, Elwing H, Hook F. 2000. Adsorption behavior and enzymatically or chemically induced cross-linking of a mussel adhesive protein. Biofouling 16, 119–132. ( 10.1080/08927010009378437) [DOI] [Google Scholar]
- 81.Fant C, Elwing H, Höök F. 2002. The influence of cross-linking on protein–protein interactions in a marine adhesive: the case of two byssus plaque proteins from the blue mussel. Biomacromolecules 3, 732–741. ( 10.1021/bm025506j) [DOI] [PubMed] [Google Scholar]
- 82.Blanco J, Zapata M, Moroño A. 1996. Some aspects of the water flow through mussel rafts. Sci. Mar. 60, 275–282. [Google Scholar]
- 83.Strohmeier T, Aure J, Duinker A, Castberg T, Svardal A, Strand Ø. 2005. Flow reduction, seston depletion, meat content and distribution of diarrhetic shellfish toxins in a long-line blue mussel (Mytilus edulis) farm. J. Shellfish Res. 24, 15–23. ( 10.2983/0730-8000(2005)24%5B15:FRSDMC%5D2.0.CO;2) [DOI] [Google Scholar]
- 84.Grant J, Bacher C. 2001. A numerical model of flow modification induced by suspended aquaculture in a Chinese bay. Can. J. Fish. Aquat. Sci. 58, 1003–1011. ( 10.1139/f01-027) [DOI] [Google Scholar]
- 85.Paine RT, Levin SA. 1981. Intertidal landscapes: disturbance and the dynamics of pattern. Ecol. Monogr. 51, 145–178. ( 10.2307/2937261) [DOI] [Google Scholar]
- 86.Carrington E, Moeser GM, Thompson SB, Coutts LC, Craig CA. 2008. Mussel attachment on rocky shores: the effect of flow on byssus production. Integr. Comp. Biol. 48, 801–807. ( 10.1093/icb/icn078) [DOI] [PubMed] [Google Scholar]
- 87.Bell E, Gosline J. 1997. Strategies for life in flow: tenacity, morphometry, and probability of dislodgment of two Mytilus species. Mar. Ecol. Prog. Ser. 159, 197–208. ( 10.3354/meps159197) [DOI] [Google Scholar]
- 88.Witman JD, Suchanek TH. 1984. Mussels in flow: drag and dislodgement by epizoans. Mar. Ecol. Prog. Ser. 16, 259–268. ( 10.3354/meps016259) [DOI] [Google Scholar]
- 89.Dolmer P, Svane I. 1994. Attachment and orientation of Mytilus edulis L. in flowing water. Ophelia 40, 63–74. ( 10.1080/00785326.1994.10429551) [DOI] [Google Scholar]
- 90.McDowell LM, Burzio LA, Waite JH, Schaefer J. 1999. Rotational echo double resonance detection of cross-links formed in mussel byssus under high-flow stress. J. Biol. Chem. 274, 20293–20295. ( 10.1074/jbc.274.29.20293) [DOI] [PubMed] [Google Scholar]
- 91.Moeser GM, Carrington E. 2006. Seasonal variation in mussel byssal thread mechanics. J. Exp. Biol. 209, 1996–2003. ( 10.1242/jeb.02234) [DOI] [PubMed] [Google Scholar]
- 92.García-March JR, Solsona MÁS, García-Carrascosa AM. 2008. Shell gaping behaviour of Pinna nobilis L., 1758: circadian and circalunar rhythms revealed by in situ monitoring. Mar. Biol. 153, 689–698. ( 10.1007/s00227-007-0842-6) [DOI] [Google Scholar]
- 93.Robson A, Wilson R, de Leaniz CG. 2007. Mussels flexing their muscles: a new method for quantifying bivalve behaviour. Mar. Biol. 151, 1195–1204. ( 10.1007/s00227-006-0566-z) [DOI] [Google Scholar]
- 94.Woźniak SB, Stramski D, Stramska M, Reynolds RA, Wright VM, Miksic EY, Cichocka M, Cieplak AM. 2010. Optical variability of seawater in relation to particle concentration, composition, and size distribution in the nearshore marine environment at Imperial Beach, California. J. Geophys. Res. Oceans 115 ( 10.1029/2009JC005554) [DOI] [Google Scholar]
- 95.Waite JH. 2008. Surface chemistry: mussel power. Nat. Mater. 7, 8–9. ( 10.1038/nmat2087) [DOI] [PubMed] [Google Scholar]
- 96.Krogsgaard M, Behrens MA, Pedersen JS, Birkedal H. 2013. Self-healing mussel-inspired multi-pH-responsive hydrogels. Biomacromolecules 14, 297–301. ( 10.1021/bm301844u) [DOI] [PubMed] [Google Scholar]
- 97.Lee BP, Dalsin JL, Messersmith PB. 2002. Synthesis and gelation of DOPA-modified poly(ethylene glycol) hydrogels. Biomacromolecules 3, 1038–1047. ( 10.1021/bm025546n) [DOI] [PubMed] [Google Scholar]
- 98.Lee BP, Huang K, Nunalee FN, Shull KR, Messersmith PB. 2004. Synthesis of 3,4-dihydroxyphenylalanine (DOPA) containing monomers and their co-polymerization with PEG-diacrylate to form hydrogels. J. Biomater. Sci. Polym. Ed. 15, 449–464. ( 10.1163/156856204323005307) [DOI] [PubMed] [Google Scholar]
- 99.Zhao Q, Lee DW, Ahn BK, Seo S, Kaufman Y, Israelachvili JN, Waite JH. 2016. Underwater contact adhesion and microarchitecture in polyelectrolyte complexes actuated by solvent exchange. Nat. Mater. 15, 407–412. ( 10.1038/nmat4539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu H, Nishida J, Ma W, Wu H, Kobayashi M, Otsuka H, Takahara A. 2012. Competition between oxidation and coordination in cross-linking of polystyrene copolymer containing catechol groups. ACS Macro Lett. 1, 457–460. ( 10.1021/mz200217d) [DOI] [PubMed] [Google Scholar]
- 101.García-Fernández L, et al. 2013. Antibacterial strategies from the sea: polymer-bound Cl-catechols for prevention of biofilm formation. Adv. Mater. 25, 529–533. ( 10.1002/adma.201203362) [DOI] [PubMed] [Google Scholar]
- 102.Menyo MS, Hawker CJ, Waite JH. 2013. Versatile tuning of supramolecular hydrogels through metal complexation of oxidation-resistant catechol-inspired ligands. Soft Matter 9, 10314–10323. ( 10.1039/c3sm51824h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shafiq Z, Cui J, Pastor-Pérez L, San Miguel V, Gropeanu RA, Serrano C, del Campo A. 2012. Bioinspired underwater bonding and debonding on demand. Angew. Chem. 124, 4408–4411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are archived under project no. 2250 at www.bco-dmo.org.