Abstract
The relationship between biodiversity and functional redundancy has remained ambiguous for over a half-century, likely due to an inability to distinguish between positivist and apophatic (that which is missing) properties of ecosystems. Apophases are best addressed by mathematics that is predicated upon absence, such as information theory. More than 40 years ago, the conditional entropy of a flow network was proposed as a formulaic way to quantify trophic functional redundancy, an advance that has remained relatively unappreciated. When applied to a collection of 25 fully quantified trophic networks, this authoritative index correlates only poorly and transitively with conventional Hill numbers used to represent biodiversity. Despite such a weak connection, the underlying biomass distribution remains useful in conjunction with the qualitative diets of system components for providing a quick and satisfactory emulation of a system's functional redundancy. Furthermore, an information-theoretic cognate of the Wigner Semicircle Rule can be formulated using network conditional entropy to provide clues to the relative stability of any ecosystem under study. The necessity for a balance between positivist and apophatic attributes pertains to the functioning of a host of other living ensemble systems.
Keywords: apophasis, biodiversity, functional redundancy, information theory, system stability, trophic networks
1. Background
The intuitive relationship between biodiversity and ecosystem stability or persistence has remained an unresolved theme of ecosystems science for almost seven decades now. The widely shared consensus is that a positive relationship does exist between the two attributes, and significant resources are being devoted globally towards the conservation of biodiversity [1]. Theoretical results that firmly support this hypothesis remain, at best, ambiguous [2–4].
The notion that trophic functional redundancy might contribute to ecosystem persistence harkens back at least to the 1950s when Odum [5] suggested that interruption to any given trophic pathway could be compensated by increased flows along parallel routes—a manifestation of system reliability. That is, if A → M → B is a two-step pathway between autotroph A and carnivore B, and A → N → B is a parallel pathway between the same two endpoints, then any dysfunction in the link A → M might be compensated by an increase in flow over the link A → N and vice-versa. The links A → M and A → N are thus said to be functionally redundant with respect to flows from A to B. MacArthur [6] proposed using the then inchoate field of information theory (IT) to estimate such functional redundancy. In particular, he employed the Shannon–Weaver index of diversity to quantify the variety, D, among the trophic exchanges in an ecosystem:
| 1.1 |
where pi is the relative proportion of total system flow constituted by a particular exchange, i.
It is important to note that both Odum and MacArthur focused upon system processes (exchanges) thereby maintaining the emphasis in ecology upon relationships over objects. For some, however, this focus was problematic, both empirically and philosophically. Measuring exchanges (flows) in the field is far more difficult than is counting numbers of specimens or estimating the biomass of populations. Furthermore, the convention in approaching problems, even in biology, has long been to focus on objects and to investigate how they move according to universal laws, that is, to follow the protocols of physics, which regard flows and processes as secondary and derivative.
Perhaps inevitably then, focus regressed early in the 1960s from processes back towards population numbers and stocks of species, and the quantities pi came to represent the proportions of population sizes within the system, while D came to be called the system ‘biodiversity’.
Although other measures of diversity were explored (e.g. the Hill numbers [7]), the preoccupation for almost the entire decade of the 1960s became to elucidate a definitive connection between biodiversity and system stability. This ambitious search was brought to a sudden end when a noted physicist employed linear stability analysis to demonstrate that more species actually confer less internal stability to a system [8]. Because IT had played a significant role in the diversity/stability initiative, its reputation among ecologists suffered collateral damage and scant attention was paid to the discipline over the next few decades.
2. Reconsiderations
While a number have questioned the assumptions made in the argument against diversity → stability [9], no analytical demonstration that increased biodiversity contributes to ecosystem persistence has been universally accepted. One is justified in asking why not?
Somewhat surprisingly, it appears that a deficiency in the discipline of physics is to blame. It was remarked in the late 1960s by anthropologist/philosopher Gregory Bateson [10] that physics deals almost exclusively with positivist notions. That is, it treats entities that are palpable and only in very few instances (e.g. the Pauli Exclusion Principle) does physics consider what is missing. That which does not exist is not key to the foundational dynamics of physics and is marginal at best to the formulation of physical models. That which is lacking can, however, make a major difference in ecosystems. (For example, loss of predator or prey species will usually result in significant differences in system dynamics.)
Here that which is absent will be referred to as apophasis (cf. [11]), and in particular, the focus will be upon the lack of constraint (which is key to the concept of entropy). While it may initially sound nonsensical to measure something that does not exist, its magnitude can always be reckoned in relation to that which does [12]. (Consider, for example, a glass that is half empty.) Furthermore, a very propitious tool for quantifying apophasis is the discipline of IT, because the very starting point for IT was Shannon's estimate of the lack of certainty. The intuitive (positivist) concept of information thereby becomes a double negative (i.e. any decrease in uncertainty; ch. 5 in [13]).
That MacArthur applied Shannon's formula to the distribution of flows reveals that he implicitly was regarding the diversity of flows as an apophasis. The same inference carried over into the definition of biodiversity. Little wonder, then, that positivist models were ill-suited for dealing with biodiversity. If one wishes to treat apophases, it is only reasonable to start with tools, such as IT, that incorporate that ontological category into their very constitution.
Fortunately, the sudden decline in interest in diversity/stability studies did not discourage everyone from continuing to apply IT to ecology. For example, a team of electrical engineers from Oklahoma interpreted MacArthur's scenario using subsequent developments in IT [14]. They retained MacArthur's emphasis on ecosystem relationships, and networks of material and energetic exchanges in ecosystems. Their genius was to regard trophic networks not simply as representing the constraints that bind the nodes (as the vast majority of network investigators still do), but also as portraying the (apophatic) indeterminacy inhering in the multiplicity of routes over which material or energy could possibly flow, i.e. trophic functional redundancy. That both constraint and redundancy reside in almost all networks is almost self-evident: If an entity is at a given node (population) in the web, it is very rarely the case that the given node connects with all the other nodes in the structure. It is constrained to interact directly with only a subset of nodes. At the same time, several possibilities usually exist for the next destination and the one that transpires cannot be determined in advance. As Claudia Pahl-Wostl [15] proclaimed, ‘Networks are amalgams of determinism and chaos [apophasis]’.
With this background, we now embark on a quantitative investigation of how well biodiversity serves as an indicator of system functional redundancy. The first task is to establish an unequivocal measure for the apophatic notion of trophic functional redundancy. We then seek data on networks of ecosystem trophic transfers with which to compare biodiversity and the selected measure of functional redundancy within the assembled systems. If these indexes do not correlate well, we then examine other indexes related to the functional redundancy in a search for a reliable surrogate that can estimate functional redundancy as readily as the quick-to-estimate biodiversity. Finally, we seek some threshold among the examined indexes which might provide a clue as to whether a given system is likely to be persistent.
3. Quantifying functional redundancy
Rutledge et al. applied newly formulated measures in IT that employed conditional probabilities to parse out how much of total network activity is constrained along certain pathways and how much remains free to flow in an indeterminate manner.1 To follow this derivation, one may denote Tij as the magnitude of a flow from compartment i to compartment j. A dot in the place of an index will signify the sum over all values of that index. Thus, Ti. denotes the partial sum of all flows leaving i, and T.j the aggregate of all flows into compartment j. Finally, T.. will represent the sum of all flows over all origins i and all destinations j, and is commonly referred to as the total systems throughput [17].
One begins by rewriting MacArthur's index of the diversity of flows (1.1) in terms of all origins and destinations as
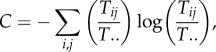 |
3.1 |
where Tij/T.. is now the joint probability that a unit of medium (mass or energy) flows from i to j. If one focuses on only the origins of flows, the diversity of their marginal probabilities will be
where it can be proved that C ≥ Co ≥ 0. One can similarly aggregate all inputs into j and their ensuing diversity as
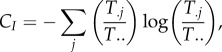 |
where, similarly, C ≥ CI ≥ 0.
Now, the combined diversities (CI + Co) always exceeds (or equals) the joint diversity, C, because the constraints that order how the components are connected (their topology, Tij) lowers how much indeterminacy is possible without such information. Thus, the difference (CI + Co − C) measures the overall constraint in the system topology. Call this difference A, and a little algebra shows that
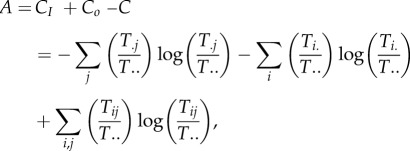 |
Or, collecting terms,
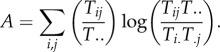 |
3.2 |
It can be proved that C ≥ A ≥ 0, and A is referred to in IT as the ‘average mutual information’ between inputs and outputs. When A is multiplied by the total system throughput, T.., the product has come to be known as the system's network ascendency—a key measure of system performance [18]. The quotient A/C varies between 0 and 1 and can serve as a convenient index of system organization, or its degree of constraint [19].
Defining A, important as it has since become, was for Rutledge et al. only an intermediate step towards their intended goal, which was to improve upon D as a measure of network functional redundancy. That measure, or the residual freedom, (C − A), has subsequently been termed the system overhead and is denoted by Φ [16]. A little algebra shows it can be written as
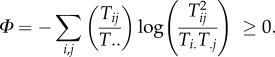 |
3.3 |
To date, Φ appears to be the most accurate estimate of pathway redundancy possible and provides a reference against which to compare other all other putative assessments of trophic functional redundancy, like D [20]. Φ is related to the logarithmically weighted average number of arcs into and out of a typical node of the network, m, calculated as m = 2ϕ/2 [19,21].
That (C − A) is known in IT as the ‘conditional entropy’ reveals its nature as an apophasis and a substantial contributor to system indeterminacy, just like D. Because Φ and D share the same ontology, they may be legitimately compared as ‘apples with apples’—in contrast to the futile pursuit of searching for positivist descriptions in terms of species numbers and/or biomasses (apples versus oranges).
4. A short-cut to assessment
Comparison of Φ with D is complicated by two differences: (i) Φ is formulated in terms of process relationships. Although MacArthur used D to describe the variety of flows, its later and more conventional form is reckoned via stocks of mass, energy or some other medium and (ii) the topology of interconnections is woven into Φ, whereas it is completely missing from the conventional formula for D. Furthermore, the data needed to evaluate Φ are precisely of the same high difficulty as it was to assemble MacArthur's original diversity of flows. How, then, to proceed?
Chemical reaction theory holds that reactions rates (processes) between two gases can be assumed proportional to the frequencies of the encounter between molecules of the two types. These collisions in turn are described in mass-action theory as proportional to the product of their respective concentrations (stocks) [22]. This assumption is usually represented as r = k[ci][cj], where r is the estimated frequency of collision between molecules of the two types, [ci] and [cj] are the concentrations of the two species of interest, i and j, and k is an empirical constant. The constant of proportionality, k, also converts units of (squared) concentrations (which do not bear any dimension of time) into rates of the encounter (as measured on a per-unit-time basis).
Certainly, animals roaming over a habitat bear only loose analogy to a mixture of gases, but the idea that rates of animal encounters might be proportional to the product of their densities has been assumed since the earliest days of ecosystem modelling—the Lotka–Volterra model of encounters between fish populations being perhaps the most famous example [23]. When applied to ecological scenarios, the constant of proportionality will vary according to other factors, such as the probability of capture by the predator and the degree of avoidance on the part of the prey.
In the formula for biodiversity, D, the relative densities of species appear as their biomass frequencies—the pi. Under the Lotka–Volterra assumptions, the probability of encounter between i and j in the system would be proportional to the product pipj. If one knew nothing more about the nature of the interactions, these products can be arranged as the dyadic product2 between the relative distribution vectors pi and pj, which for a four-species ensemble looks like table 1.
Table 1.
The dyadic product of the components of the normalized biomass partition vector (p1, p2, p3, p4) along with their column and row sums.
| species | 1 | 2 | 3 | 4 | row sum |
|---|---|---|---|---|---|
| 1 | p1p1 | p1p2 | p1p3 | p1p4 | p1 |
| 2 | p2p1 | p2p2 | p2p3 | p2p4 | p2 |
| 3 | p3p1 | p3p2 | p3p3 | p3p4 | p3 |
| 4 | p4p1 | p4p2 | p4p3 | p4p4 | p4 |
| col. sum | p1 | p2 | p3 | p4 | 1.0 |
For example, if the four-component system possesses biomasses in the proportions, B1 = 50, B2 = 15, B3 = 30 and B4 = 53, then the dyadic product would look like table 2.
Table 2.
The dyadic product of the hypothetical biomass partition (0.50, 0.15, 0.30, 0.05).
| species | 1 | 2 | 3 | 4 | row sum |
|---|---|---|---|---|---|
| 1 | 0.25 | 0.075 | 0.15 | 0.025 | 0.50 |
| 2 | 0.075 | 0.0225 | 0.045 | 0.0075 | 0.15 |
| 3 | 0.15 | 0.045 | 0.09 | 0.015 | 0.30 |
| 4 | 0.025 | 0.0075 | 0.015 | 0.0025 | 0.05 |
| col. sum | 0.50 | 0.15 | 0.30 | 0.05 | 1.0 |
Now the constants, kij, linking each biomass product, pipj with its conjugate flow, Tij, are usually not known. In addition, the lack of any information about the magnitude of the flows forces one to assume that all kij are equal, resulting in a ‘back of the envelope’ estimation of the flows. (This particular estimation technique has resulted in flows that usually pass conventional 5% statistics [24].) Because (3.3) deals entirely with quotients of flows divided by their sums, a uniform constant, k, would cancel from all calculations. Whence, substituting the elements of the dyadic product into (3.3) yields Φ = 3.295 bits (which is exactly twice the value of the biodiversity index D when logarithms are taken to base 2).
Functional redundancies emulated in this manner are almost always overestimates, however, because the network of direct interactions was assumed to be totally connected by equiponderant flows, which is virtually never the case with natural ecosystems.4 Rather, many exchanges are simply missing (often greater than 80%). The actual topology of network connection is easy to retrieve, because the diets of most species are usually tabulated and widely available. Knowing the diets of each compartment, allows one to set non-existent transfers to zero.
For example, the dietary exchange network among the four species of the hypothetical system might appear as in figure 1.
Figure 1.
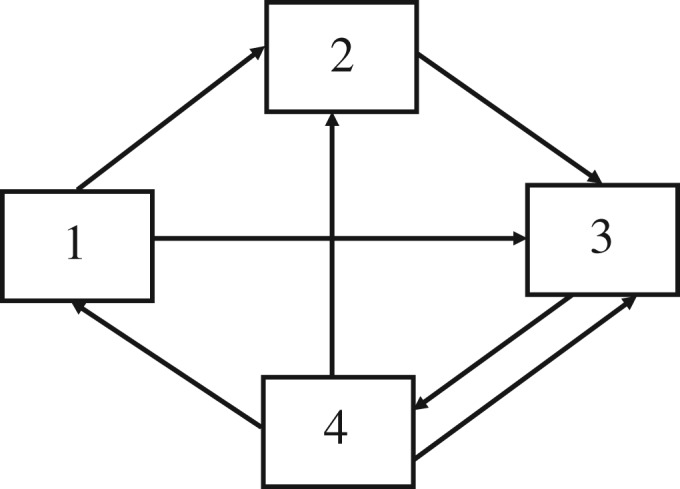
Hypothetical qualitative topology among four components.
In such case, only seven of the possible 16 interactions are realized. The surviving interactions in this case would look like table 3.
Table 3.
| species | 1 | 2 | 3 | 4 | row sum |
|---|---|---|---|---|---|
| 1 | 0. | 0.075 | 0.15 | 0. | 0.225 |
| 2 | 0. | 0. | 0.045 | 0. | 0.045 |
| 3 | 0. | 0. | 0. | 0.015 | 0.015 |
| 4 | 0.025 | 0.0075 | 0.015 | 0. | 0.0425 |
| col. sum | 0.025 | 0.0825 | 0.21 | 0.015 | 0.3325 |
Which can be normalized to yield table 4.
Table 4.
Normalized version of table 3.
| species | 1 | 2 | 3 | 4 | row sum |
|---|---|---|---|---|---|
| 1 | 0. | 0.227 | 0.450 | 0. | 0.677 |
| 2 | 0. | 0. | 0.135 | 0. | 0.135 |
| 3 | 0. | 0. | 0. | 0.045 | 0.045 |
| 4 | 0.075 | 0.023 | 0.045 | 0. | 0.143 |
| col.sum | 0.075 | 0.250 | 0.630 | 0.045 | 1.0 |
When the fractions from table 4 are substituted into equation (3.3), the result will be referred to as the ‘emulated’ functional diversity, Φe, where Φe = 1.6291 bits.
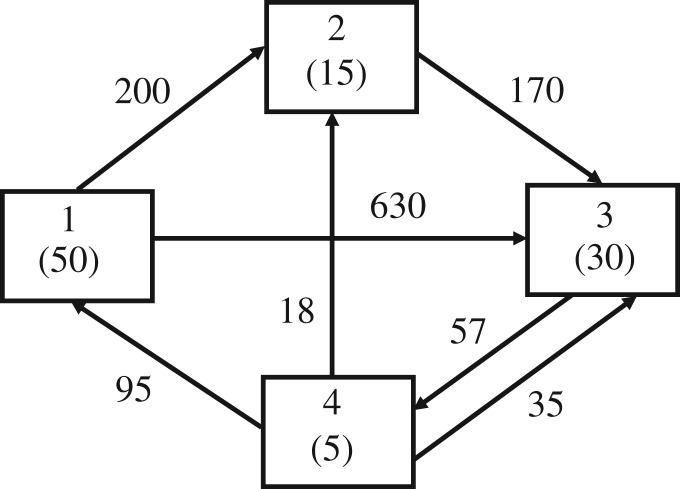
Now, for the sake of demonstration, one supposes that field data reveal that the observed flows per unit time are figure 2.
Figure 2.
Magnitudes of hypothetical flows among the four components in figure 1.
Inserting these values into (3.3) yields an ‘observed’ functional redundancy, call it Φo, where Φo = 1.45 bits.
Both Φe and Φo are always less than or equal to the value of Φ when all existing flows are equal in magnitude. Denoting this upper bound by Φ*, the topology in figure 1 yields Φ* = 5.6147. Still further, Φ has a least upper bound when all possible flows are present and equal in magnitude. This value, Φmax = 2 log(n), where n is the number of nodes.
The various values of Φ are thus ordered as
| 4.1 |
That is, the largest possible value of Φ, or Φmax, is reduced by the amount (Φmax − Φ*) once one knows the topology of the system (82%). Additional data on the magnitudes of the flows reduce Φ further by the amount (Φ* − Φ), or another 3%. The differences in (4.1) allow one to gauge the relative amounts of information that are gained by ascertaining the topology and by estimating the flow weightings, respectively. The emulated Φe and the observed value, Φo, bear no fixed analytical relationship to one another.
5. Key questions about the emulation
Given as how Φo is the most faithful estimate one can make of trophic functional redundancy, several questions arise:
-
—
How well does D represent functional redundancy? That is, do D and Φo correlate when applied to real, natural systems?
-
—
To what extent does Φe emulate Φo? Because Φe is far easier to estimate than Φo, can the former serve as a better surrogate for functional redundancy than D?
-
—
What are the relative contributions of system topology versus flow magnitudes towards estimating functional redundancy?
-
—
What, if anything do any of these indexes reveal about system persistence (the contemporary focus in lieu of ‘stability’)?
6. Description of data
To address these important issues, a suite of 25 quantified trophic flow networks have been assembled from a number of habitats and sources. For each of these systems, all internal trophic exchanges have been estimated as well as the magnitudes of all exogenous inputs and outputs. Only networks with 12 or more components were considered because networks consisting of fewer compartments cannot be trusted to adequately represent ecosystem properties [25].
The 25 systems are summarized in table 5, along with their dimensions and literature citations.
Table 5.
Ecosystem networks, number of components and the literature references.
| # | system | n | reference |
|---|---|---|---|
| 1 | Crystal River Creek (control) | 21 | Ulanowicz [13] |
| 2 | Crystal River Creek (ΔT) | 21 | Ulanowicz [13] |
| 3 | mesohaline Chesapeake Bay | 36 | Baird & Ulanowicz [26] |
| 4 | mesohaline Chesapeake Bay | 15 | Wulff & Ulanowicz [27] |
| 5 | Baltic Sea | 15 | Wulff & Ulanowicz [27] |
| 6 | Florida Bay | 125 | Ulanowicz et al. [28] |
| 7 | gramminoid Everglades | 66 | Ulanowicz et al. [29] |
| 8 | Mondego Estuary | 43 | Patricio [30] |
| 9 | Narragansett Bay | 32 | Monaco [31] |
| 10 | St Marks River | 51 | Baird et al. [32] |
| 11 | polyhaline Chesapeake Bay | 34 | Hagy [33] |
| 12 | southeast Chukchi Sea | 51 | in preparation [34] |
| 13 | northeast Chukchi Sea | 51 | in preparation [34] |
| 14 | Everglades cypress wetlands | 68 | Ulanowicz et al. [35] |
| 15 | Ems Estuary | 15 | Baird & Ulanowicz [26] |
| 16 | Kromme Estuary | 16 | Baird & Ulanowicz [26] |
| 17 | Okefenokee Marsh | 24 | Patten et al. [36] |
| 18 | Swartzkops Estuary | 15 | Baird & Ulanowicz [26] |
| 19 | Ythan Estuary | 14 | Baird & Ulanowicz [26] |
| 20 | Twin Cays fringe mangroves | 87 | Scharler et al. [37] |
| 21 | Twin Cays transition mangroves | 74 | Scharler et al. [37] |
| 22 | Broad Creek | 36 | Egnotovich [38] |
| 23 | N. Benguela Current | 24 | Heymans & Baird [39] |
| 24 | Delaware Bay | 34 | Monaco [31] |
| 25 | Lake Michigan | 36 | AE Krause & DM Mason (2003, personal communication) see also [40] |
Full data on each of the 25 networks are presented in the electronic supplementary material, file <25NETS.CSV>.
7. Results
The values of D, Φmax, Φ*, Φe and Φo were calculated for exchanges among the nl living compartments and are compared in table 6. Additionally, internal flows among all compartments (living and non-living) were included in the calculations and thirdly, all endogenous and exogenous flows were accounted in the indexes. The values for these expanded networks can be found in the electronic supplementary material, file <RESULTS.XLS>.
Table 6.
The values of biodiversity (D) and variations of redundancy measures (in bits) corresponding to the 25 sample ecosystems (as calculated using only feeding relationships).
| no. | system | nl | D | Ømax | Ø* | Øe | Øo |
|---|---|---|---|---|---|---|---|
| 1 | Crystal River Creek (control) | 20 | 0.6325 | 8.643856 | 4.2486 | 2.9381 | 1.7047 |
| 2 | Crystal River Creek (ΔT) | 20 | 0.6527 | 8.643856 | 3.6831 | 2.0873 | 1.4267 |
| 3 | mesohaline Chesapeake Bay | 33 | 0.2624 | 10.08879 | 3.7166 | 2.2461 | 1.6214 |
| 4 | mesohaline Chesapeake Bay | 12 | 0.2219 | 7.169925 | 2.6607 | 1.5866 | 1.8062 |
| 5 | Baltic Sea | 12 | 0.0859 | 7.169925 | 2.6560 | 1.5289 | 1.7770 |
| 6 | Florida Bay | 122 | 0.7622 | 13.86147 | 8.8322 | 3.9478 | 3.1524 |
| 7 | gramminoid Everglades | 63 | 0.4842 | 11.95456 | 7.7819 | 4.225 | 3.6117 |
| 8 | Mondego Estuary | 42 | 1.1525 | 10.78463 | 6.9377 | 1.5797 | 1.5597 |
| 9 | Narragansett Bay | 31 | 0.5006 | 9.908392 | 4.7735 | 3.8524 | 2.5843 |
| 10 | St Marks River | 48 | 0.2526 | 11.16992 | 5.5349 | 3.6705 | 3.6501 |
| 11 | polyhaline Chesapeake Bay | 31 | 1.0280 | 9.908392 | 3.5408 | 2.6913 | 2.4193 |
| 12 | southeast Chukchi Sea | 48 | 0.8718 | 11.16992 | 6.7296 | 2.8137 | 2.9091 |
| 13 | northeast Chukchi Sea | 48 | 0.6063 | 11.16992 | 6.5981 | 2.9224 | 2.1940 |
| 14 | Everglades cypress wetlands | 65 | 0.9258 | 12.04474 | 7.1215 | 1.0381 | 2.0265 |
| 15 | Ems Estuary | 12 | 0.0143 | 7.169925 | 2.8245 | 2.5431 | 2.2603 |
| 16 | Kromme Estuary | 13 | 0.5100 | 7.400879 | 2.4747 | 2.0086 | 1.1358 |
| 17 | Okefenokee Marsh | 21 | 0.1774 | 8.784635 | 4.4187 | 2.6368 | 1.6246 |
| 18 | Swartzkops Estuary | 12 | 0.6805 | 7.169925 | 2.1044 | 1.4123 | 1.0838 |
| 19 | Ythan Estuary | 11 | 0.6652 | 6.918863 | 2.2831 | 1.1329 | 1.6830 |
| 20 | Twin Cays fringe mangroves | 72 | 0.6901 | 12.33985 | 6.2230 | 2.0118 | 1.6940 |
| 21 | Twin Cays transition mangroves | 58 | 0.7372 | 11.71596 | 6.2276 | 2.3545 | 2.6064 |
| 22 | Broad Creek | 31 | 0.4476 | 9.908392 | 4.4787 | 2.4904 | 2.1427 |
| 23 | Northern Benguela Current | 22 | 0.5753 | 8.918863 | 5.3494 | 2.7800 | 1.4480 |
| 24 | Delaware Bay | 33 | 0.5018 | 10.08879 | 5.1920 | 3.5565 | 2.6970 |
| 25 | Lake Michigan | 35 | 2.6230 | 10.25857 | 5.1918 | 3.3972 | 3.0268 |
One can immediately compare the columns for D and Φo in table 6 to ascertain that biodiversity among feeding species correlate poorly with functional redundancy (R = 0.198, p = 0.342). That is, biodiversity is not a good indicator of trophic functional redundancy among active feeders. The correlation between D and the emulated redundancy, Φe, is even worse (R = 0.092, p = 0.663). Significant alone is the correlation between the observed redundancy, Φo, and that emulated by the dyadic matrix of biomass densities as supplemented by dietary topology, Φe (R = 0.760, p = 0.00001). Because most estimates of biodiversity are reckoned among only living ecosystem compartments, this result suggests that a rough estimate of functional redundancy is possible using only the distribution of biomass and the dietary topology among feeding compartments.
The values for the indexes based on only consumers and producers are presented in table 6. Some maintain that detrital pathways contribute to system stability [41], so that these flows were added to the system and the full ensemble of exchanges among both living and non-living compartments, was examined and results can be found. (See electronic supplementary material, file <RESULTS.XLS>.) One expects that the correlation between Φe and Φo for all internal flows will be weaker than those reckoned on feeding flows alone, because the dyadic assumption is likely to be less effective when one of the actors is passive and non-living. Indeed, the correlation among indexes incorporating all internal flows is less (R = 0.556, p = 0.004). The correlation between D and Φo remains insignificant (R = 0.243, p = 0.313), and that between Φe and D improves only marginally (R = 0.384, p = 0.058).
Calculation of the measures using the entire suite of endogenous and exogenous flows becomes problematic in that one must assign values to biomasses associated with the origins and destinations of exogenous flows, and it is not obvious how to accomplish this. One possible assumption is to assign a biomass to the origin of the exogenous inputs that roughly corresponds to the aggregate biomasses of all the primary producers. In order to maintain rough conservation of medium across the system, one can show that the biomass of the destination of all exports and respirations should be roughly the same as that for the origin of all the inputs. Values of all indexes under these assumptions can also be viewed in the electronic supplementary material, file <RESULTS.XLS>.
As with the internal flows, correlations between D and Φo and between Φe and Φo for all flows are marginal (R = 0.118, p = 0.583, and R = 0.055, p = 0.798, respectively). Surprisingly, the correlation between D and Φe was borderline significant (R = 0.600, p = 0.002). It remains unknown whether this significant connection is an artefact of the rather arbitrary assumptions made to assign biomasses to the exogenous flows, or whether it constitutes a true natural correlation. The latter remains possible, because if the products in the arguments of the logarithms in (3.3) are expanded as the sum of the logarithms of the factors, the resulting formula resembles an expression that is twice the value of C, and (3.1) in its turn formally resembles expression (1.1) for D.
If this connection happens to be real, then a tentative, transitive relationship has been established between D and Φe. That is, D is weakly correlated to Φe and Φe is marginally correlated to Φo, so that D is related to Φo in transitive fashion. One still should ask why such a transitive connection was not perceived when dealing with internal flows?
As to the relative contributions of topology versus flow magnitudes of the values of Φ, one observes in table 6 that the drop from the median Φmax to the median Φ* attributable to the flow topology is 51.8%, while the decrease from Φmax to Φo caused by unequal flow magnitudes resolves another 27.8% of the original ambiguity. When all internal flows enter the calculations, the topology resolves 39.5% and the flow magnitudes eliminate another 40% of the original uncertainty. The respective declines when all flows are used become 42.9% and 31.0%. It seems likely that topology in most instances will account for more information than flow magnitudes, but at times comparable amounts will be resolved by both attributes.
8. Connections with system persistence
It remains to explore what, if anything, these indexes have to do with systems persistence, in light of the fact that none contains any explicit reference to system dynamics? To repeat, considerable effort was invested in developing (mostly dynamical) models in the attempt to associate greater biodiversity with increased systems ‘stability’. The project suffered significantly when linear stability analysis revealed exactly the opposite—that more biodiversity implies less internal stability [8].
It is worth noting that in these attempts stability was taken to mean that the forces causing the system to cohere were on average greater than the tendency for the ensemble to fall apart. In other words, the spotlight was on ‘internal’ cohesion. The goal was to identify a single upper threshold beyond which the system can no longer maintain its dynamical structure. Such is no longer the picture, once attention is broadened from stability to the more general notion of ‘persistence’. Open dynamical systems that are tightly bound tend to be efficient, but ‘brittle’ [42]. Their internal cohesion is high, but with rigid constraint, they become inflexible and less capable of adapting to novel external perturbations. Might a complementary threshold on freedom and flexibility also exist, below which systems become prone to break down under external stress?
Some have suggested these two thresholds coincide and that living systems always reside on the ‘edge of chaos’ [43]. This edge hypothesis was developed, however, using mechanical and algorithmic models that are capable of creating sharp, rigid distinctions, when in fact the very notion of flexibility implies freedom and a degree of indeterminacy. Empirical data on existing trophic networks show that systems tend to cluster within a narrow range of mutual information, demarcating what has been called the ‘window of vitality’ [44]. A collection of 17 trophic networks (including some used here) exhibited normalized (dimensionless) values of ascendency (A/C) that grouped around a value of 0.40 [45].
Presumably, the upper threshold identifies when the system possesses insufficient internal cohesion to maintain itself without spontaneously falling apart. Robert May [8] identified his limit with the Wigner Semicircle Criterion,5 which was based on studies of randomly assembled matrices [47]. May deduced that, on average, any network for which α < (nc)–1/2 will possess local linear stability, where α is the average strength of interaction (cohesion), n is the number of components and c is the topological connectivity (the number of non-zero links divided by the maximum possible).
Ulanowicz developed an information-theoretic analogue to the Wigner Criterion using dimensional reasoning [44]. He noticed that the product nc is the link-density, or the effective number of flows per node, which Ulanowicz & Wolff ([21], appendix A) had shown to be m = 2Φ/2. As for the effective strength of interaction, Ulanowicz defined it as that constant, a, by which each flow in Φ* must be multiplied in order to yield the same value as Φ. This provided a relationship between Φ and Φ* as
or equivalently, between m and m*,
One can now eliminate a between this latter relationship, and the ersatz Wigner statement, a < (m)−1/2, to yield the erzatz criterion purely in terms of m and m*,
That is, whenever the observed number of effective links per node is no greater than the expression on the right-hand side, the network is likely to persist.
Using the values of Φ*, Φe and Φo from table 6, the values of m*, me, mo and the Wigner Criterion (mmax) are displayed in table 7.
Table 7.
Values of the effective link densities (# of links per node) m*, me and mo, as compared with the Wigner Criterion, mmax.
| no. | system | nl | m* | me | mo | mmax |
|---|---|---|---|---|---|---|
| 1 | Crystal River Creek (control) | 20 | 4.359915 | 2.768395 | 1.805439 | 2.879958 |
| 2 | Crystal River Creek (ΔT) | 20 | 3.583949 | 2.061436 | 1.639607 | 2.749424 |
| 3 | mesohaline Chesapeake Bay | 33 | 3.625802 | 2.178070 | 1.754062 | 2.75849 |
| 4 | mesohaline Chesapeake Bay | 12 | 2.514637 | 1.733034 | 1.870080 | 2.392307 |
| 5 | Baltic Sea | 12 | 2.510544 | 1.698722 | 1.851250 | 2.390319 |
| 6 | Florida Bay | 122 | 21.34905 | 3.928286 | 2.981834 | 2.701279 |
| 7 | gramminoid Everglades | 63 | 14.83517 | 4.324400 | 3.496351 | 2.858596 |
| 8 | Mondego Estuary | 42 | 11.07205 | 1.728895 | 1.716952 | 2.956179 |
| 9 | Narragansett Bay | 31 | 5.229779 | 3.800528 | 2.448927 | 2.959826 |
| 10 | St Marks River | 48 | 6.809033 | 3.568332 | 3.543193 | 3.012261 |
| 11 | polyhaline Chesapeake Bay | 31 | 3.411485 | 2.541447 | 2.312815 | 2.709027 |
| 12 | southeast Chukchi Sea | 48 | 10.30162 | 2.651576 | 2.740711 | 2.974249 |
| 13 | northeast Chukchi Sea | 48 | 9.842672 | 2.753373 | 2.139094 | 2.984179 |
| 14 | Everglades cypress wetlands | 65 | 11.80029 | 1.433011 | 2.018453 | 2.938017 |
| 15 | Ems Estuary | 12 | 2.661519 | 2.414208 | 2.188815 | 2.459710 |
| 16 | Kromme Estuary | 13 | 2.357651 | 2.005970 | 1.482364 | 2.311407 |
| 17 | Okefenokee Marsh | 21 | 4.624669 | 2.493894 | 1.756009 | 2.910053 |
| 18 | Swartzkops Estuary | 12 | 2.073690 | 1.631445 | 1.455889 | 2.137639 |
| 19 | Ythan Estuary | 11 | 2.206179 | 1.480875 | 1.791912 | 2.223498 |
| 20 | Twin Cays fringe mangroves | 72 | 8.642807 | 2.008196 | 1.798757 | 3.005433 |
| 21 | Twin Cays transition mangroves | 58 | 8.656597 | 2.261453 | 2.467756 | 3.005240 |
| 22 | Broad Creek | 31 | 4.721843 | 2.370514 | 2.101399 | 2.919689 |
| 23 | Northern Benguela Current | 22 | 6.385059 | 2.620787 | 1.651755 | 3.005815 |
| 24 | Delaware Bay | 33 | 6.046080 | 3.430098 | 2.546472 | 2.997271 |
| 25 | Lake Michigan | 35 | 6.045661 | 3.245858 | 2.854820 | 2.997258 |
One see from table 7 that the Wigner Criterion, mmax, is exceeded three times by values of mo and six times by me (numbers in italics). Given that mmax is only a dimensional interpretation of the Wigner Criterion and not an analytical mapping, it is not too surprising that some mo occasionally surpass that estimate. Such violation does not imply that the system in that case is inherently unstable, but it should caution managers to explore it more thoroughly using sensitivity analysis to identify its most vulnerable species and links [48]. Furthermore, because in most instances me > mo, one should expect that me > mmax more frequently than mo > mmax. (This follows from the observation above that Φe tends to be higher than Φo.) In the large majority of cases, however, the ersatz Wigner Criterion was satisfied by both observation and emulation.
The correlations between all pairs of network indices are given in table 8. With the exception of the correlations between Φe and me with Φo, all other highly significant connections represent functional relationships between definitional formulae. Correlations between D and all the other definitions are uniformly poor. Corresponding correlations pertaining to the internal flows and the full complement of flows are presented in electronic supplementary material, file <RESULTS.XLS>.
Table 8.
Correlations among all pairs of network indices. R-values appear in the upper-right triangular matrix (row < column). Probabilities are given in the lower-left triangle (column c < row). Highly significant correlations (p < 0.1%) are indicated in italics.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| D | Ømax | Ø* | m* | Øe | me | Øo | mo | mmax | ||
| 1 | D | 1 | 0.301 | 0.284 | 0.221 | 0.0917 | 0.0929 | 0.198 | 0.183 | 0.282 |
| 2 | Ømax | 0.144 | 1 | 0.931 | 0.864 | 0.475 | 0.496 | 0.61 | 0.595 | 0.719 |
| 3 | Ø* | 0.168 | <0.00001 | 1 | 0.942 | 0.483 | 0.514 | 0.583 | 0.573 | 0.720 |
| 4 | m* | 0.289 | <0.00001 | <0.00001 | 1 | 0.431 | 0.489 | 0.551 | 0.553 | 0.461 |
| 5 | Øe | 0.663 | 0.0164 | 0.0144 | 0.0313 | 1 | 0.987 | 0.760 | 0.766 | 0.518 |
| 6 | me | 0.659 | 0.0117 | 0.00852 | 0.0132 | <0.00001 | 1 | 0.793 | 0.805 | 0.473 |
| 7 | Øo | 0.342 | 0.00121 | 0.00223 | 0.00432 | <0.001 | <0.00001 | 1 | 0.992 | 0.456 |
| 8 | mo | 0.381 | 0.0017 | 0.00273 | 0.00417 | <0.00001 | <0.00001 | <0.00001 | 1 | 0.422 |
| 9 | mmax | 0.172 | <0.001 | <0.001 | 0.0203 | 0.008 | 0.017 | 0.0218 | 0.0357 | 1 |
As discussed above, the Wigner Criterion is likely only one of two thresholds delimiting persistence. There is almost certainly a minimum m, mmin, above which mo must remain, lest it become too brittle to persist in a noisy environment. (The smallest value of mo in all the networks examined was 1.396 links per node.) The conditions that defined mmax were concerned with the homogeneous notion of attraction among compartments, rendering it amenable to analytical considerations. It is likely, however, that the arbitrary and very heterogeneous events associated with the collapse of brittle systems will make it difficult to determine mmin in precise analytical fashion.
9. Macarthur reconsidered
As a postscript, it is interesting to compare how MacArthur's original suggestion (3.1) relates to the Rutledge's authoritative formula for functional diversity (3.3). It happens that (3.1) correlates very well with (3.3) (R = 0.873, p = 0.129 × 10−7 for the feeding relationships). Such correspondence is not unexpected, because (3.1) serves as a least upper bound on (3.3), i.e. C ≥ Φ, and the ratio of C to A does not vary much. It appears in retrospect that MacArthur's index is more reliable than the later switch to D. Unfortunately, determining C requires exactly the same data as the calculation of Φ, and thus demands far more effort than assessing D. The comparisons just presented suggest, however, that expending the moderate extra work to assemble the dietary topology and calculate Φe will yield a far more reliable gauge of functional diversity than is possible with conventional biodiversity. In retrospect, the preoccupation by ecologists with objects and laws has cost the discipline much wasted effort and confusion, so that progress is best resumed by a return to process ecology.
10. Summary and recommendations
Acknowledging and quantifying the role of the apophatic in ecosystem dynamics substantially changes how ecosystem stability and persistence are conceived. No longer is stability simply a matter of cohesion, but becomes instead a balance between the mutually exclusive traits of internal cohesion versus reliability when confronted by novel external disturbances. No longer will a single threshold suffice to characterize system stability, but rather two limits become necessary to demarcate the window of vitality within which ecosystems remain sustainable.
System resilience to external stresses derives principally from the freedom that apophasis allows within the system—mainly in the form of functional redundancy. Such a necessity for parallel pathways constrains the system from channelling excessive resources along its most efficient pathways. Redundancy in trophic networks is accurately characterized by system overhead, Φ, which is expressed and measured using the concept of conditional entropy from IT. Shannon-based IT is eminently suited to quantifying both apophatic freedom and positivist system performance using the same algebraic rubrics.
Rutledge et al. [14] suggested the network conditional entropy as an improvement upon MacArthur's simple entropy of system flows, and because it incorporates trophic topology, it can faithfully capture functional redundancy. Statistical comparison of the Rutledge measure with the later index of biodiversity (based solely on biomass or population distributions) reveals the latter to be a poor index of functional diversity. Biodiversity is considerably easier to measure than system flows, however, and the quantity speaks to the need for a quick evaluation of ecosystem status.
Fortunately, a compromise between realism and ease of evaluation is possible that requires only moderate additional effort to quantify. Data on qualitative dietary items (feeding topology) can be combined with an assessment of biomass distribution to calculate an index that statistically emulates the Rutledge index. It is recommended, therefore, that those lacking the time or resources to quantify all transfers comprising a system undertake the moderate extra effort to compute the ersatz functional redundancy, Φe, as a substantial improvement upon conventional indexes for biodiversity.
Precisely what the observed redundancy, Φo, and its emulated substitute, Φe, tell one about the persistence of a system remains to be fully explored. It is known that an ecosystem requires adequate amounts of mutually exclusive flexibility and performance to persist. Moreover, the balance between emulated performance (Ae) and reliability (Φe) can inform a manager whether or not the community is functioning within normal bounds. Further assessment using the ersatz Wigner stability criterion can possibly reveal whether the given system requires additional sensitivity analysis to identify potentially vulnerable compartments and links.
Biodiversity, functional redundancy and system persistence are all entwined, albeit some relationships are more subtle than others. Biodiversity, for example, is only very weakly and transitively related to functional redundancy. The latter, however, is key to maintaining system persistence and can be assessed directly with only moderate additional effort. Both biodiversity and functional redundancy contribute to system persistence—the former only subtly; the latter more significantly. Further accumulation of network data and more inventive use of IT and other quantitative tools for treating apophasis in systems should eventually render these connections more definitive and lead to more effective ecosystem management.
Finally, this emended scenario of ecosystem development should give pause to those theorists who see evolution purely as a variational (min/max) game. Certainly, organisms and systems continue to strive towards greater performance, but achieving greater efficiency can at times seriously jeopardize a system's resilience. The same caution applies to those who study other process-based ensembles, such as those in economics [49], industry [50], society and ontogeny [51]. To continue to ignore the role of the apophatic in natural systems is to pursue science with one eye shut [52].
Supplementary Material
Supplementary Material
Acknowledgements
The author wishes to thank Michael Barfield for helpful guidance on statistical analysis and formatting. Stefano Allesina and Brian Fath and several anonymous reviewers also provided critical comments helpful to the revision of the text. Contribution no. 5505 of the University of Maryland Center for Environmental Science.
Endnotes
The following derivation is not the one used by Rutledge et al., but rather follows Ulanowicz & Norden [16].
See https://en.wikipedia.org/wiki/Dyadics (alternatively called the ‘outer product’).
This particular mass distribution was chosen to sum to 100 to make the ensuing probabilities immediately normalized.
The same cannot be said of indirect connections, but they are not germane to Φ.
Formerly known as the Wigner Semicircle Conjecture, which has subsequently been proved analytically [46].
Data accessibility
All data and tabulated results are available in the electronic supplementary materials created to accompany this article.
Competing interests
I declare I have no competing interests.
Funding
All work on this contribution was performed using the personal resources of the author. No external funding was available.
References
- 1.Guruswamy LD, McNeely JA (eds). 1998. Protection of global biodiversity: converging strategies. Durham, NC: Duke University Press. [Google Scholar]
- 2.McCann KS. 2000. The diversity–stability debate. Nature 405, 228 ( 10.1038/35012234) [DOI] [PubMed] [Google Scholar]
- 3.Loreau M, et al. 2001. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294, 804–808. ( 10.1126/science.1064088) [DOI] [PubMed] [Google Scholar]
- 4.Loreau M, Mazancourt C. 2013. Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecol. Lett. 16, 106–115. ( 10.1111/ele.12073) [DOI] [PubMed] [Google Scholar]
- 5.Odum EP, Odum HT, Andrews J. 1953. Fundamentals of ecology. Philadelphia, PA: Saunders. [Google Scholar]
- 6.MacArthur R. 1955. Fluctuations of animal populations and a measure of community stability. Ecology 36, 533–536. ( 10.2307/1929601) [DOI] [Google Scholar]
- 7.Hill MO. 1973. Diversity and evenness: a unifying notation and its consequences. Ecology 54, 427–432. ( 10.2307/1934352) [DOI] [Google Scholar]
- 8.May RM. 1972. Will a large complex system be stable? Nature 238, 413–414. ( 10.1038/238413a0) [DOI] [PubMed] [Google Scholar]
- 9.Cohen JE, Charles MN. 1985. When will a large complex system be stable? J. Theor. Biol. 113, 153–156. ( 10.1016/S0022-5193(85)80081-3) [DOI] [Google Scholar]
- 10.Bateson G. 1972. Steps to an ecology of mind: collected essays in anthropology, psychiatry, evolution, and epistemology. Chicago, IL: University of Chicago Press. [Google Scholar]
- 11.Deacon TW. 2006. Emergence: the hole at the wheel’s hub. In The re-emergence of emergence (eds P Clayton, P Davies), pp. 111–150. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Ulanowicz RE. 2014. Reckoning the nonexistent: putting the science right. Ecol. Modell 293, 22–30. ( 10.1016/j.ecolmodel.2014.03.014) [DOI] [Google Scholar]
- 13.Ulanowicz RE. 1986. Growth and development: ecosystems phenomenology. Berlin, Germany: Springer. [Google Scholar]
- 14.Rutledge RW, Basore BL, Mulholland RJ. 1976. Ecological stability: an information theory viewpoint. J. Theor. Biol. 57, 355–371. ( 10.1016/0022-5193(76)90007-2) [DOI] [PubMed] [Google Scholar]
- 15.Pahl-Wostl C. 1995. The dynamic nature of ecosystems: chaos and order entwined. New York, NY: John Wiley & Sons Incorporated. [Google Scholar]
- 16.Ulanowicz RE, Norden JS. 1990. Symmetrical overhead in flow networks. Int. J. Syst. Sci. 21, 429–437. ( 10.1080/00207729008910372) [DOI] [Google Scholar]
- 17.Finn JT. 1976. Measures of ecosystem structure and function derived from analysis of flows. J. Theor. Biol. 56, 363–380. ( 10.1016/S0022-5193(76)80080-X) [DOI] [PubMed] [Google Scholar]
- 18.Ulanowicz RE. 1997. Ecology, the ascendent perspective: Robert E. Ulanowicz. New York, NY: Columbia University Press. [Google Scholar]
- 19.Ulanowicz RE, Goerner SJ, Lietaer B, Gomez R. 2009. Quantifying sustainability: resilience, efficiency and the return of information theory. Ecol. Complexity 6, 27–36. ( 10.1016/j.ecocom.2008.10.005) [DOI] [Google Scholar]
- 20.Farnsworth KD, Albantakis L, Caruso T. 2017. Unifying concepts of biological function from molecules to ecosystems. Oikos, 126, 1367–1376. [Google Scholar]
- 21.Ulanowicz RE, Wolff WF. 1991. Ecosystem flow networks: loaded dice? Math. Biosci. 103, 45–68. ( 10.1016/0025-5564(91)90090-6) [DOI] [PubMed] [Google Scholar]
- 22.Chapman S, Cowling TG. 1970. The mathematical theory of non-uniform gases: an account of the kinetic theory of viscosity, thermal conduction and diffusion in gases. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.Lotka AJ. 1910. Contribution to the theory of periodic reactions. J. Phys. Chem. 14, 271–274. ( 10.1021/j150111a004) [DOI] [Google Scholar]
- 24.Ulanowicz RE. 2008. Scharler UM. Least-inference methods for constructing networks of trophic flows. Ecol. Modell. 210, 278–286. ( 10.1016/j.ecolmodel.2007.08.001) [DOI] [Google Scholar]
- 25.Bersier LF, Sugihara G. 1997. Scaling regions for food web properties. Proc. Natl Acad. Sci. USA 94, 1247–1251. ( 10.1073/pnas.94.4.1247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baird D, Ulanowicz RE. 1993. Comparative study on the trophic structure, cycling and ecosystem properties of four tidal estuaries. Mar. Ecol. Progress Series. 99, 221–237. ( 10.3354/meps099221) [DOI] [Google Scholar]
- 27.Wulff F, Ulanowicz RE. 1989. A comparative anatomy of the Baltic Sea and Chesapeake Bay ecosystems. In Network analysis in marine ecology, pp. 232–256. Berlin, Germany: Springer. [Google Scholar]
- 28.Ulanowicz RE, Bondavalli C, Egnotovich MS. 1998. Network analysis of trophic dynamics in South Florida ecosystems, FY 97: the Florida Bay ecosystem. Report to USGS, Coral Gables. Ref. no. [UMCES]CBL 98–123. Solomons, MD: Chesapeake Biological Laboratory. [Google Scholar]
- 29.Ulanowicz RE, Heymans JJ, Egnotovich MS. 2000. Network analysis of trophic dynamics in South Florida ecosystems, FY 99: the gramminoid ecosystem. Report to USGS, Coral Gables. Ref. no. [UMCES] CBL 00-0176 Solomons, MD: Chesapeake Biological Laboratory. [Google Scholar]
- 30.Patrício J, Marques JC. 2006. Mass balanced models of the food web in three areas along a gradient of eutrophication symptoms in the south arm of the Mondego estuary (Portugal). Ecol. Modell 197, 21–34. ( 10.1016/j.ecolmodel.2006.03.008) [DOI] [Google Scholar]
- 31.Monaco ME. 1995. Comparative analysis of estuarine biophysical charactenstics and trophic structure: defining ecosystem function to fishes. PhD thesis University of Maryland, College Park. [Google Scholar]
- 32.Baird D, Luczkovich J, Christian RR. 1998. Assessment of spatial and temporal variability in ecosystem attributes of the St Marks National Wildlife Refuge, Apalachee Bay, Florida. Estuar. Coast. Shelf Sci. 47, 329–349. ( 10.1006/ecss.1998.0360) [DOI] [Google Scholar]
- 33.Hagy JD. 2002. Eutrophication, hypoxia and trophic transfer efficiency in Chesapeake Bay. PhD thesis University of Maryland Center for Environmental Science, Solomons, MD, USA. [Google Scholar]
- 34.Ulanowicz RE, Grebmeier JM, Bluhm B, Carmack E. In preparation. Comparative trophic structure of two regions of the Chukchi Sea.
- 35.Ulanowicz RE, Bondavalli C, Egnotovich MS. 1997. Network analysis of trophic dynamics in South Florida ecosystems, FY 96: the Cypress Wetland. To: USGS, Coral Gables, Ref. no. [UMCES]CBL 97-075 Solomons, MD: Chesapeake Biological Laboratory. [Google Scholar]
- 36.Patten BC, Higashi M, Burns TP. 1989. Network trophic dynamics: the food web of an Okefenokee Swamp aquatic bed marsh. In Freshwater wetlands and wildlife. CONF-8603101 (DE90005384), US Dept. Energy (DOE) Sympos. Ser 1989 (No. 61, pp. 401-424).
- 37.Scharler UM, et al. 2015. Variable nutrient stoichiometry (carbon: nitrogen: phosphorus) across trophic levels determines community and ecosystem properties in an oligotrophic mangrove system. Oecologia 179, 863–876. ( 10.1007/s00442-015-3379-2) [DOI] [PubMed] [Google Scholar]
- 38.Egnotovich MS. 2006. Eutrophication and coastal wetlands: linking nutrient enrichment to tidal freshwater marsh ecosystem structure and function. PhD thesis University of Maryland, College Park, MD, USA. [Google Scholar]
- 39.Heymans JJ, Baird D. 2000. Network analysis of the northern Benguela ecosystem by means of NETWRK and ECOPATH. Ecol. Modell 131, 97–119. ( 10.1016/S0304-3800(00)00275-1) [DOI] [Google Scholar]
- 40.Krause AE, Frank KA, Mason DM, Ulanowicz RE, Taylor WW. 2003. Compartments revealed in food-web structure. Nature 426, 282 ( 10.1038/nature02115) [DOI] [PubMed] [Google Scholar]
- 41.Moore JC, et al. 2004. Detritus, trophic dynamics and biodiversity. Ecol. Lett. 7, 584–600. ( 10.1111/j.1461-0248.2004.00606.x) [DOI] [Google Scholar]
- 42.Holling CS. 1986. The resilience of terrestrial ecosystems: local surprise and global change. Sustain. Dev. Biosph. 14, 292–317. [Google Scholar]
- 43.Langton CG. 1990. Computation at the edge of chaos: phase transitions and emergent computation. Physica D 42, 12–37. ( 10.1016/0167-2789(90)90064-V) [DOI] [Google Scholar]
- 44.Ulanowicz RE. 2002. The balance between adaptability and adaptation. Biosystems 64, 13–22. ( 10.1016/S0303-2647(01)00170-8) [DOI] [PubMed] [Google Scholar]
- 45.Ulanowicz RE. 2009. The dual nature of ecosystem dynamics. Ecol. Modell. 220, 1886–1892. ( 10.1016/j.ecolmodel.2009.04.015) [DOI] [Google Scholar]
- 46.Tao T, Vu V. 2010. Random matrices: universality of ESDs and the circular law. The Annals of Probability. 38, 2023–2065. ( 10.1214/10-AOP534) [DOI] [Google Scholar]
- 47.Wigner EP. 1959. Statistical properties of real symmetric matrices with many dimensions. In Proceedings of the Fourth Canadian Mathematical Congress (ed. MacPhail MS.), pp. 174–184. Toronto, Canada: University of Toronto Press. [Google Scholar]
- 48.Ulanowicz RE, Baird D. 1999. Nutrient controls on ecosystem dynamics: the Chesapeake mesohaline community. J. Mar. Syst. 19, 159–172. ( 10.1016/S0924-7963(98)90017-3) [DOI] [Google Scholar]
- 49.Goerner SJ, Lietaer B, Ulanowicz RE. 2009. Quantifying economic sustainability: implications for free-enterprise theory, policy and practice. Ecol. Econ. 69, 76–81. ( 10.1016/j.ecolecon.2009.07.018) [DOI] [Google Scholar]
- 50.Korhonen J, Seager TP. 2008. Beyond eco-efficiency: a resilience perspective. Business Strategy Environ. 17, 411–419. ( 10.1002/bse.635) [DOI] [Google Scholar]
- 51.Martins MJ, Puckett TM, Lockwood R, Swaddle JP, Hunt G. 2018. High male sexual investment as a driver of extinction in fossil ostracods. Nature 11, 1 ( 10.1038/s41586-018-0020-7) [DOI] [PubMed] [Google Scholar]
- 52.Ulanowicz RE. 2011. Towards quantifying a wider reality: Shannon exonerata. Information 2, 624–634. ( 10.3390/info2040624) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and tabulated results are available in the electronic supplementary materials created to accompany this article.