Abstract
A 35-year-old lactating woman with pre-existing polyacrylamide gel (PAAG) implants for 10 years presented on numerous occasions following both her pregnancies with bilateral recurrent breast infection, pain and finally massive breast enlargement with a ruptured galactocoele necessitating surgical intervention. As the safety of PAAG for the breastfeeding baby is not known, breastfeeding with PAAG implants is not recommended.
Keywords: Breast Surgery, Radiology, Infant Nutrition (including Breastfeeding)
Background
Polyacrylamide gel (PAAG) was first used as a biomaterial for ’breast augmentation without surgery' in Ukraine in the late 1980s.1 Since then it has been used in Europe, Russia and parts of Asia including China and Iran for augmentation of the breast, face and lips.2 However, the long-term effects and safety of PAAG implants are of concern as they have not been as well studied as other implants.
A 35-year-old lactating woman with pre-existing PAAG implants for 10 years presented to the emergency room on numerous occasions following both her pregnancies with bilateral recurrent breast enlargement, erythema, pain and, on the last occasion, a ruptured galactocoele necessitating surgical intervention.
Case presentation
A 35-year-old breastfeeding mother (G2P2) presented to the emergency department for the third time within 6 months of the birth of her second child. Over the preceding 10 days she had noted a massive painful increase in the size of her right breast with fever and erythema, all of which were obvious on clinical examination. Her two previous presentations, while feeding her second child, were to another hospital with bilateral recurrent mastitis which was treated with intravenous and oral antibiotics resulting in symptom resolution. The first presentation was with left breast pain and swelling, fever and erythema. The patient continued to breastfeed. The second presentation, 1 week before this episode, was with right breast enlargement, pain and fever. Physical examination revealed a firm, exquisitely tender erythematous right breast without cervical or axillary lymphadenopathy.
The patient had no family history of breast or ovarian cancer. She had emigrated from the Philippines to Australia 2 years before presentation. Her relevant history included receiving PAAG injections for breast augmentation 10 years previously in China. She had had similar presentations of recurrent mastitis 6 years previously when breastfeeding after her first pregnancy.
Investigations
Ultrasound of the left breast at the first presentation (5 weeks after a normal vaginal delivery) showed mild diffuse oedema of the parenchyma with subtle loss of definition of tissue planes in keeping with clinical cellulitis but no evidence of a drainable collection. An injected breast augmentation, not typical of silicone, was noted as a bilateral prepectoral hypoechoic mass with a well-defined capsule. Multiple discrete separate hypoechoic masses, some with internal echogenicity, were noted anterior to the injected material throughout both breasts (figure 1).
Figure 1.
Bilateral breast ultrasound demonstrates bilateral polyacrylamide gel implant (A, B)and a separate well-defined ovoid area of reduced echogenicity with interspersed microcystic areas anterior to the left implant (B).
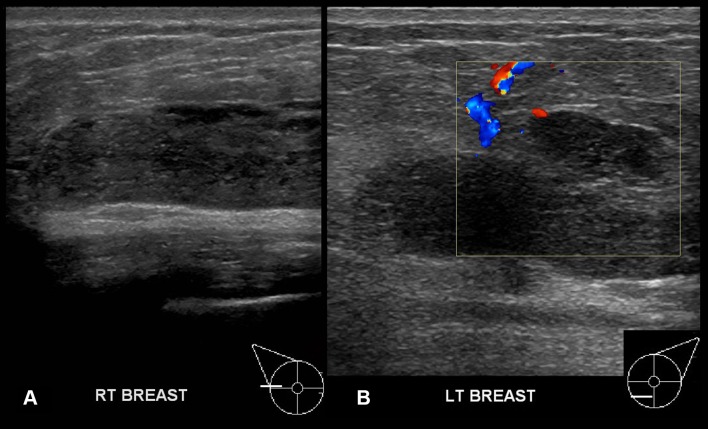
A right breast ultrasound performed on the second presentation (4 months later) showed a small 6×11 mm hypoechoic collection (A)with increased vascularity (B)adjacent to the implant at the 10 o’clock position in the right breast, in addition to extreme tenderness and subcutaneous tissue oedema.
At her third presentation, the patient was unable to elevate her right arm above her head due to breast pain and swelling (figure 2). The prepectoral implant was massively increased in size (6.5 cm AP) on ultrasound and the echogenicity had increased. Reflective particles and microcystic spaces were noted within the echogenic material (figure 3,B). Examination of the left breast showed a shallower implant with similar echogenicity and microcystic change.
Figure 2.
Clinical image shows breast asymmetry.
Figure 3.
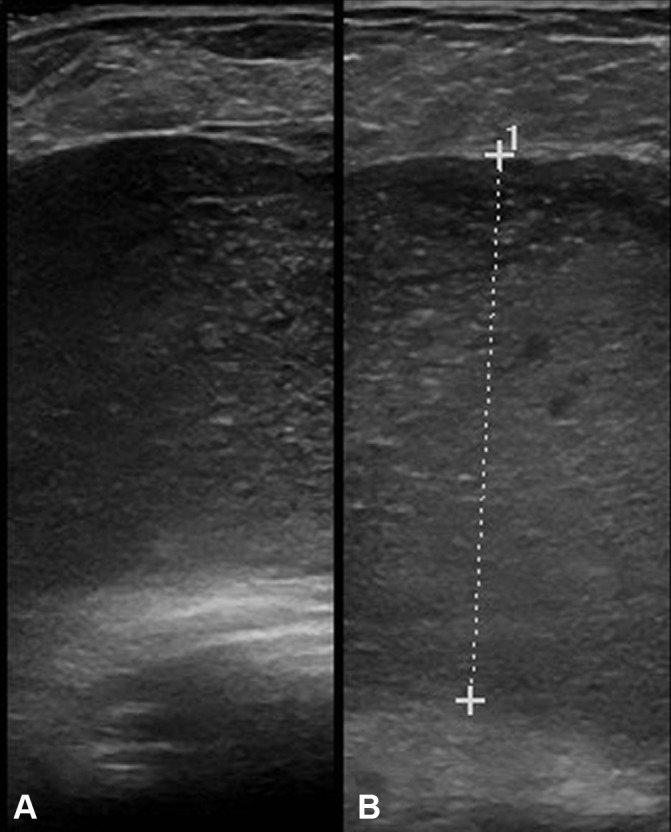
Ultrasound of the right breast shows a large, well-defined, hypoechoic polyacrylamide gel implant which is echogenic and contains hyperechoic flecks and microcysts.
Bilateral single view-mediolateral oblique (MLO) projection mammography showed asymmetry and a marked but uniform increase in the density of the right breast. The bilateral implants were partially defined superiorly but poorly defined inferiorly (figure 4). 2 mm benign-appearing micro calcifications were identified in the left retro-areolar region. No malignant features were observed in the overlying parenchyma.
Figure 4.
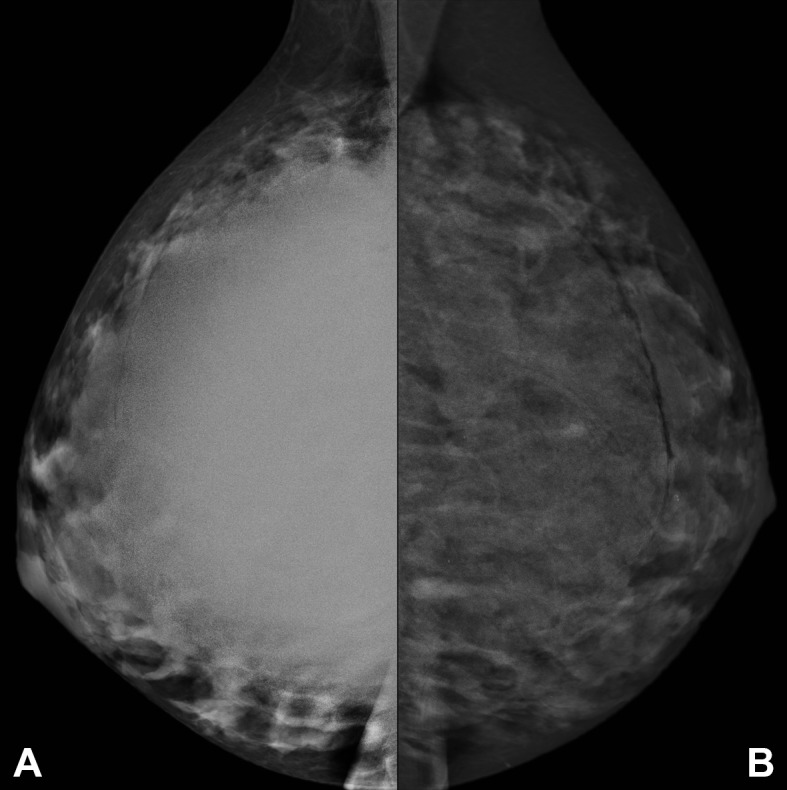
Right (A) and left (B) mediolateral oblique (MLO) mammograms demonstrate large, bilateral, uniformly dense, partially defined, retroglandular masses representing the polyacrylamide gel implants, with smooth superior contours superiorly and less well defined outlines inferiorly. The mass on the right is more dense than that on the left. Benign calcifications are noted on the left. There are no suspicious mammographic features.
A non-coring needle aspiration of the right-sided collection revealed creamy fluid, histopathology of which showed inflammatory tissue with granular material consistent with PAAG and a foreign body reaction. There was no bacterial growth.
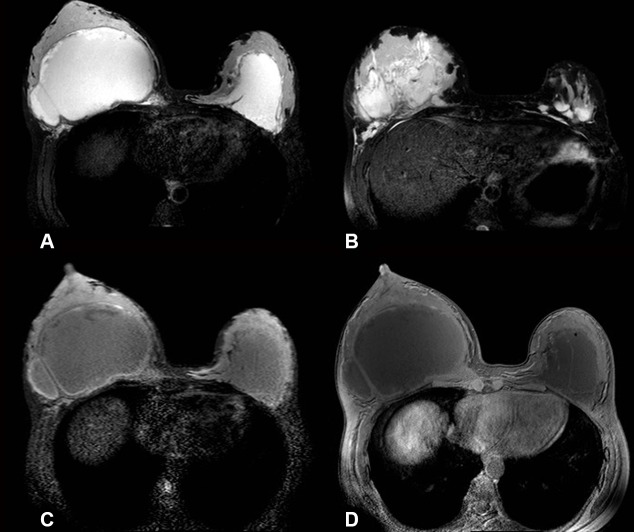
MRI was performed. High signal PAAG was noted on non-contrast T2-weighted images with and without fat suppression/silicone sequences (figure 5 A,B). The right breast was at least double the size of the left with a pseudo-capsule (figure 5 C,D)
Figure 5.
Breast MRI T2 SPAIR images show bilateral asymmetrical hyperintense fluid collections (right larger than left) with some septations on the right (A). More inferiorly (B) multiple loculations are evident. Both silicone only (C) and silicone suppressed (D) sequences show low signal intensity within the implants.
Treatment
The patient continued to have a painful enlarged right breast without evidence of infection for 2 months at follow-up outpatient clinic review so she underwent surgical drainage of the collection. Histology of the 1.3 L of turbid fluid removed during the operation was benign and showed granular and mucoid material consistent with injected PAAG on an inflammatory background. Microscopy revealed small Gram-positive cocci and leucocytes, but there was no growth on culture.
Outcome and follow-up
A follow-up MRI was performed 2 months after surgery due to the persistence of symptoms of pain and swelling in the right breast. The right breast showed a significant decrease in the extent of PAAG (figure 6 A) but residual PAAG was noted on both non-contrast T2-weighted images with and without fat suppression/silicone sequences. The gel was partly located within a fibrous capsule but there was some extension beyond the capsule into the glandular tissue, towards the nipple and into the anterior chest wall musculature (figure 6 A, B axial views, C, D coronal views). The difficulty of removing all the gel was explained to the patient so she elected to follow a conservative ’watch and see' approach.
Figure 6.
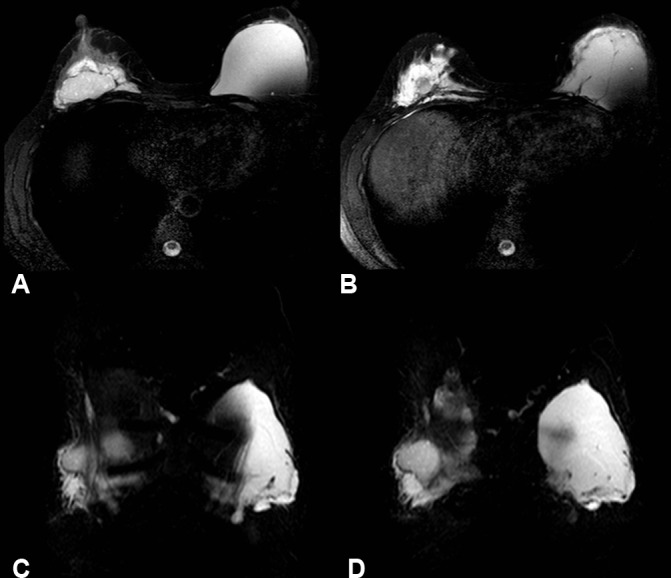
T2 SPAIR images show residual polyacrylamide gel postoperatively on the right. Infiltration into the musculature of the anterior thoracic wall is demonstrated on both axial (A, B) and coronal views (C, D).
Clinically, the patient’s surgical wound healed well. Since 2011 she has had no further children or symptoms.
Discussion
Polyacrylamide is a jelly-like medical hydrogel which is hydrophilic, non-toxic, non-teratogenic and non-mutagenic.3 4 Although it has been used for breast implants for decades, its use has been discouraged due to concerns which include complications and a lack of adequate literature. PAAG was temporarily banned in China in 1999. A report in 20065 by the Hong Kong Medical Device Control Office (Department of Health) recommended against its use in breast augmentation. The manufacturer suggested retraining surgeons regarding correct procedure6 and postulated improper surgical procedures or post-operative care contributed to complications rather than the toxicity of PAAG itself.7 In Australia, although there are no official guidelines, caution in the use of PAAG is advised (Australian Society of Plastic Surgeons, personal communication). Complications range from breast induration and lumps, recurrent infections, gel leakage and implant migration8 to haematoma, galactocoele, potential misdiagnosis in malignancy screening2 and even possible breast cancer.9 The mean time from injection to complications was 6.1 years in a review of 106 patients in Ukraine who had operations for PAAG complications.1
In one of the few studies examining the long-term effects of PAAG implants, Wang et al2 found that the risk of infection in breastfeeding mothers with PAAG implants was greater than 50% in a study of 58 patients. Qiao et al6 found that in a series of 30 patients (not all actively breastfeeding), nearly all had complications between 3 and 36 months post-operatively. Conversely, Cheng et al10 found that the incidence of late seroma, galactocoele or haematoma was significantly rarer in patients with PAAG implants compared to silicone implants. Trauma and breastfeeding were contributing factors to developing complications.10
It is hypothesised that post-operative fibrosis and blockage of mammary ducts after augmentation mammoplasty may be the cause of infection and galactocoele rupture.11
A mammogram in this case showed the PAAG/galactocoele collection as a water-dense material. A galactocoele might be expected to show a fat fluid level or be lucent depending on the nature of the milk content.
Ultrasound examination of PAAG implants may show diffuse, irregular, hypo-, hyper- or anechoic areas or cystic/microcystic lesions.12 PAAG differs from silicone in that residues of raw material (acrylamide monomer) remain in the product.5 While PAAG is said to be atoxic and biocompatible,6 the acrylamide monomer is a known genetic, reproductive and neural toxicant and carcinogen.5 Hence, it is important to distinguish between silicone and PAAG implants as there are safety implications for continued breastfeeding. Ultrasound is a simple method of differentiating between the two. The appearance of the silicone ’snowstorm' differs from the hypoechoic PAAG appearance (see figures 1 and 2).
Silicone implants have complications similar to PAAG.10 However, due to a wider evidence base, it has been established that silicone confers no additional risks on the breastfeeding mother or baby, irrespective of whether the implants have ruptured.13
PAAG implants may pose a diagnostic dilemma by obscuring the breast parenchyma on imaging and may mask a cancer. Huch et al identified that breast implants obscure a significant amount of breast tissue and that post-therapeutic scarring can make mammographic interpretation difficult; it is suggested that MRI may be the modality of choice for diagnosing breast implant complications.12 Due to the high water content in PAAG implants, sagittal and axial T2-weighted imaging has been suggested to detect complications.14 MR imaging has the advantage of showing PAAG infiltration into the thoracic wall15 as was the case with our patient. In assessing silicone implants and complications, silicon saturation techniques can differentiate a milk signal from silicone in patients who are actively breastfeeding.15
Histology results in complicated implants usually indicate inflammatory cell infiltration, fibrosis and foreign body reaction,16 as was the case with our patient.
It is suggested PAAG is not used for breast augmentation and that women who already have PAAG injections be informed that they should not breastfeed.
Learning points.
Women of reproductive age may present with PAAG-related complications and so medical professionals should be aware of this condition.
Patients undergoing breast augmentation should be informed of the implant material to be used and the potential complications.
PAAG implants may give rise to a range of complications some of which may require surgical intervention.
It is important to distinguish between PAAG and silicone implants as the former has safety implications for the health of mothers and their breast-fed babies; women with PAAG implants should not breastfeed.
Footnotes
Contributors: AGB and CJ both contributed to the concept and manuscript writing.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Unukovych D, Khrapach V, Wickman M, et al. Polyacrylamide gel injections for breast augmentation: management of complications in 106 patients, a multicenter study. World J Surg 2012;36:695–701. 10.1007/s00268-011-1273-6 [DOI] [PubMed] [Google Scholar]
- 2.Wang Z-X, Luo D-L, Dai X, et al. Polyacrylamide hydrogel injection for augmentation mammaplasty. Ann Plast Surg 2012;69:123–8. 10.1097/SAP.0b013e318225931c [DOI] [PubMed] [Google Scholar]
- 3.Margolis NE, Bassiri-Tehrani B, Chhor C, et al. Polyacrylamide gel breast augmentation: report of two cases and review of the literature. Clin Imaging 2015;39:339–43. 10.1016/j.clinimag.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 4.Cheng NX, Wang YL, Wang JH, et al. Complications of breast augmentation with injected hydrophilic polyacrylamide gel. Aesthetic Plast Surg 2002;26:375–82. 10.1007/s00266-002-2052-4 [DOI] [PubMed] [Google Scholar]
- 5.Medical Device Control Office, Department of Health. Risks of using polyacrylamide gel (PAAG) for breast augmentation. http://www.mdco.gov.hk/english/emp/emp_gp/files/Risks%20of%20Using%20Polyacrylamide%20Gel%20(PAAG)%20for%20Breast%20Augmentation.pdf (accessed Apr 2016)
- 6.Qiao Q, Wang X, Sun J, et al. Management for postoperative complications of breast augmentation by injected polyacrylamide hydrogel. Aesthetic Plast Surg 2005;29:156–61. 10.1007/s00266-004-0099-0 [DOI] [PubMed] [Google Scholar]
- 7.Evstatiev D. Late complications after injections of hydrogel in the breast. Plast Reconstr Surg 2004;113:1878 10.1097/01.PRS.0000119877.63517.8B [DOI] [PubMed] [Google Scholar]
- 8.Cheng NX, Xu SL, Deng H, et al. Migration of implants: a problem with injectable polyacrylamide gel in aesthetic plastic surgery. Aesthetic Plast Surg 2006;30:215–25. 10.1007/s00266-005-0081-5 [DOI] [PubMed] [Google Scholar]
- 9.Cheng NX, Liu LG, Hui L, et al. Breast cancer following augmentation mammaplasty with polyacrylamide hydrogel (PAAG) injection. Aesthetic Plast Surg 2009;33:563–9. 10.1007/s00266-008-9298-4 [DOI] [PubMed] [Google Scholar]
- 10.Cheng N-X, Zhang Y-L, Luo S-K, et al. Late hematoma, seroma, and galactocele in breasts injected with polyacrylamide gel. Aesthetic Plast Surg 2011;35:365–72. 10.1007/s00266-010-9617-4 [DOI] [PubMed] [Google Scholar]
- 11.Acartürk S, Gencel E, Tuncer I, et al. An uncommon complication of secondary augmentation mammoplasty: bilaterally massive engorgement of breasts after pregnancy attributable to postinfection and blockage of mammary ducts. Aesthetic Plast Surg 2005;29:274–9. 10.1007/s00266-005-1093-x [DOI] [PubMed] [Google Scholar]
- 12.Huch RA, Künzi W, Debatin JF, et al. MR imaging of the augmented breast. Eur Radiol 1998;8:371–6. 10.1007/s003300050397 [DOI] [PubMed] [Google Scholar]
- 13.Professor Chris Baggoley (Chief Medical Officer). PIP breast implants and breast feeding advice from the Chief Medical Officer. http://www.health.gov.au/internet/main/publishing.nsf/Content/PIP-breast-implants-breastfeeding.htm
- 14.Lui CY, Ho CM, Lu PP, et al. Evaluation of MRI findings after polyacrylamide gel injection for breast augmentation. AJR Am J Roentgenol 2008;191:677–88. 10.2214/AJR.07.2733 [DOI] [PubMed] [Google Scholar]
- 15.Lin WC, Hsu GC, Hsu YC, et al. A late complication of augmentation mammoplasty by polyacrylamide hydrogel injection: ultrasound and magnetic resonance imaging findings of huge galactocele formation in a puerperal woman with pathological correlation. Breast J 2008;14:584–7. 10.1111/j.1524-4741.2008.00652.x [DOI] [PubMed] [Google Scholar]
- 16.Leung KM, Yeoh G, Chan KW, et al. Breast pathology in complications associated with polyacrylamide hydrogel (PAAG) mammoplasty. Hong Kong Med J 2007;12:137–40. [PubMed] [Google Scholar]