Abstract
A 46-year-old previously healthy man presented with 1 week of headache, nausea, vomiting and dizziness. He was found to have cranial nerve deficits, his cerebrospinal fluid (CSF) demonstrated a lymphocytic pleocytosis and brain MRI suggested rhombencephalitis. Although Gram stains and cultures of his CSF did not identify a pathogen, Listeria monocytogenes DNA was detected by the FilmArray Meningitis/Encephalitis panel within 2 hours of performing a lumbar puncture. He was treated with ampicillin and gentamicin and had a near-complete recovery. This case highlights the importance of recognising L. monocytogenes infection as a cause of acute cranial nerve impairment with MRI findings suggestive of brainstem encephalitis. It also highlights the frequently atypical CSF profile and low yield of culture in L. monocytogenes rhombencephalitis and the value of multiplex PCR testing of CSF to rapidly identify this pathogen and permit targeted therapy.
Keywords: foodborne infections, meningitis
Background
To our knowledge, this is the first published report of diagnosing Listeria monocytogenes rhombencephalitis using the FilmArray Meningitis/Encephalitis PCR assay. Listeria rhombencephalitis differs from other forms of central nervous system (CNS) listeriosis in important ways that are illustrated by this case, including the limited diagnostic sensitivity of blood and cerebrospinal fluid (CSF) cultures. Rapidly obtaining the microbiological diagnosis in this case by multiplexed PCR permitted optimisation of therapy for listeriosis and discontinuation of multiple unnecessary antimicrobial agents. This case highlights the tremendous potential advantage of multiplexed PCR-based assays in rapidly diagnosing aetiologies of encephalitis. Also, L. monocytogenes surveillance definitions should be revised to incorporate commercially available PCR-based diagnostic methods so that culture-negative/PCR-positive cases as described here are captured and investigated.
Case presentation
A 46-year-old man with no medical history presented to a New Jersey hospital in March with 1 week of worsening headache, nausea, vomiting, dizziness and low-grade fever. Brain MRI showed abnormalities in the right medulla and pons and CSF obtained by lumbar puncture (LP) demonstrated 0.0360×109/L white blood cells (wbc) (63% neutrophils), a normal glucose level and 106 mg/dL of protein. Antimicrobial therapy was initiated with vancomycin (1 g every 12 hours), ceftriaxone (2 g daily) and acyclovir (10 mg/kg every 8 hours), but subsequently he developed photophobia, ataxia, vertigo and was intubated for airway protection and transferred to our hospital.
On arrival, he was afebrile and haemodynamically stable. On physical examination, he was alert and oriented, despite being intubated. However, he had left ptosis, vertical and horizontal nystagmus when gazing to the right, and right-sided dysmetria. His motor strength and sensory input were intact and the rest of his examination was unremarkable. CT of the brain revealed no acute abnormalities. However, a repeat brain MRI that was performed 3 days after the initial MRI at the other hospital showed a 10×6×11 mm peripherally enhancing collection that involved the right dorsal lateral medulla (figure 1), as well as increased signal of inferior pons, medulla and cerebellar peduncles (figure 2). A repeat LP that was performed 2 days after the first LP revealed 0.31×109/L wbc (81% lymphocytes), a normal glucose level and 53 mg/dL of protein. Gram stain of CSF showed no organisms.
Figure 1.
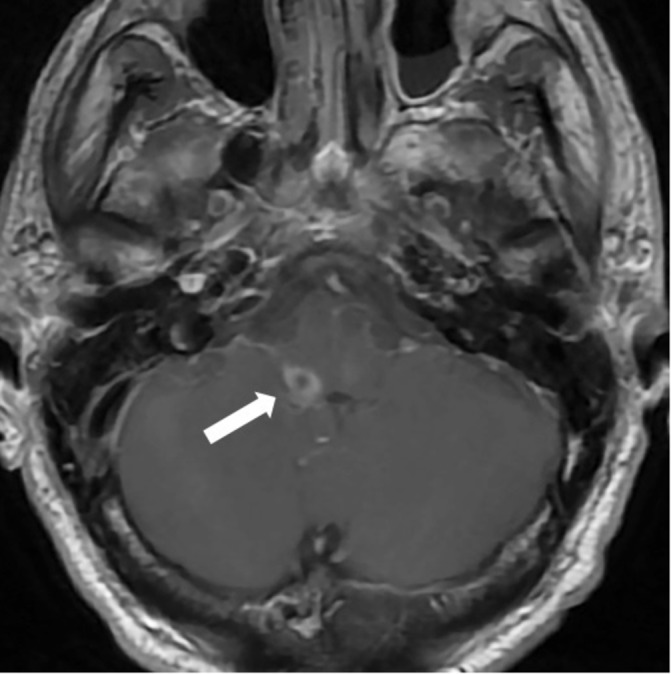
Axial T1 image following gadolinium administration is notable for peripheral enhancement around small abscess in right dorsal lateral medulla (arrow).
Figure 2.
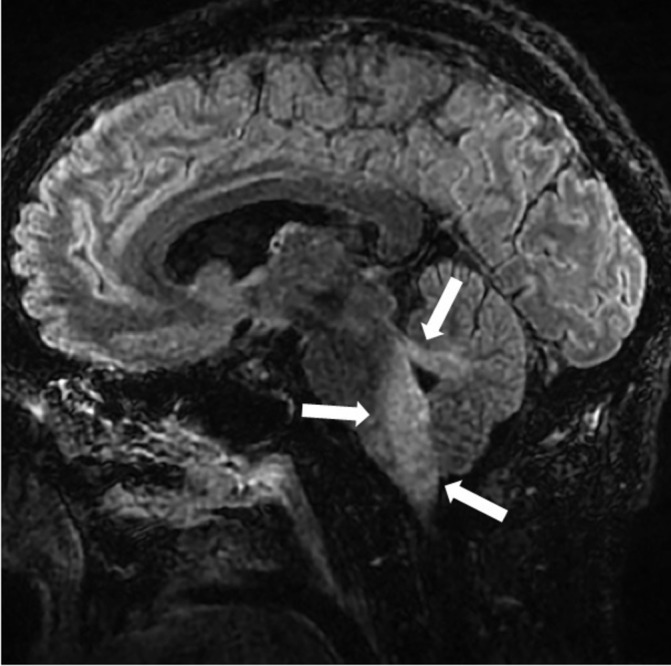
Sagittal T2 fluid-attenuated inversion recovery (FLAIR) image at presentation demonstrates abnormal increased signal of inferior pons, medulla and cerebellar peduncles (arrows).
The patient was originally from South Africa, but had lived in the USA for 25 years, and lived in New Jersey prior to presentation. He worked as a steel trader and had travelled to Turkey 3 weeks prior to the onset of his symptoms. He did not know if he had consumed unpasteurised diary products in Turkey. He denied a history of known tuberculosis exposure or ever having a positive skin or blood test for tuberculosis. He frequently went hiking in the woods near his home, but had not in the last couple of months. He had no history of autoimmune disease or recurrent mouth ulcers and did not use tobacco or illicit drugs.
Investigations
Brain MRI. CSF obtained via LP underwent the following testing: cell count with differential, protein, glucose, Gram stain and bacterial culture, calcofluor potassium hydroxide stain and fungal culture, acid-fast stain and mycobacterial culture, cryptococcal antigen and FilmArray Meningitis/Encephalitis panel (BioFire Diagnostics, Salt Lake City, Utah, USA).
Differential diagnosis
Tuberculosis.
Herpes simplex virus.
Epstein-Barr virus infection.
Lyme disease.
Multiple sclerosis.
Behçet’s disease.
Paraneoplastic syndrome.
Systemic lupus erythematosus.
Treatment
Intravenous ampicillin (2 g every 4 hours) for 6 weeks and intravenous gentamicin (80 mg every 8 hours) for 7 days. After completion of intravenous ampicillin, he was switched to intravenous penicillin for home administration, but developed a rash and subsequently completed a 3-week course of oral trimethoprim–sulfamethoxazole.
Outcome and follow-up
A FilmArray Meningitis/Encephalitis panel (BioFire Diagnostics) was positive for L. monocytogenes within 2 hours of the LP. Antibiotics were optimised to ampicillin and gentamicin on the same day, although gentamicin was stopped 1 week later because of worsening vertigo. Cultures of blood and CSF from both hospitals never yielded bacteria. The patient remained in the medical intensive care unit for 13 days for respiratory failure and inability to control secretions, requiring tracheostomy and percutaneous endoscopic gastrostomy (PEG) tube placement for feeding. He was eventually transferred to an inpatient rehabilitation unit with progressive improvement in functional status, including decannulation of tracheostomy and removal of PEG tube, and was discharged to home on hospital day 43. A brain MRI obtained 6 weeks after presentation showed resolution of the right brainstem abscess and decreased nodular enhancement along his cranial nerves. He was treated with 6 weeks of intravenous ampicillin, followed by 1 week of intravenous penicillin at home, but developed a rash requiring change to oral trimethoprim–sulfamethoxazole for 3 weeks. He was last seen 6 months after his initial presentation and his deficits had largely resolved, except for mild right-sided spasticity.
Discussion
L. monocytogenes is a Gram-positive bacillus that causes a variety of clinical syndromes, including food-borne gastroenteritis, fetal demise, sepsis and CNS infections. CNS listeriosis may manifest as meningoencephalitis, rhombencephalitis (brainstem encephalitis) or brain abscesses.1 In contrast to meningoencephalitis, which most commonly occurs in chronically ill, immunosuppressed or elderly patients, L. monocytogenes rhombencephalitis typically occurs in immunocompetent, healthy, middle-aged patients,2 3 similar to our case. Our patient demonstrated many important and illustrative features of L. monocytogenes rhombencephalitis3: (1) a biphasic course with a prodrome of fever, nausea and vomiting, followed by an abrupt onset of asymmetrical cranial nerve deficits with respiratory failure; (2) a normal CT of the head, but MRI that showed marked unilateral brainstem involvement; (3) a CSF profile that demonstrated mild abnormalities compared with patients with meningoencephalitis, including only modest elevations in wbc counts and protein, a lymphocytic predominance and a normal glucose; (4) negative blood and CSF cultures, which occurs in nearly half of patients with L. monocytogenes rhombencephalitis.
This limited diagnostic sensitivity of blood and CSF cultures presents a major clinical challenge, particularly given that L. monocytogenes rhombencephalitis is uniformly fatal if untreated3; empirical therapy for meningitis in young healthy adults does not adequately treat L. monocytogenes, and the differential diagnosis for rhombencephalitis also includes tuberculosis, herpes viruses, Lyme disease, multiple sclerosis, Behçet’s disease, paraneoplastic syndrome and systemic lupus erythematosus.4 Thus, a rapid, culture-independent method to diagnose L. monocytogenes rhombencephalitis would represent a major advance. To our knowledge, this is the first published report of diagnosing L. monocytogenes rhombencephalitis using the FilmArray Meningitis/Encephalitis panel. This assay detects 14 common pathogens associated with CNS infections directly from the CSF within 2 hours, and is designed to diagnose infectious aetiologies of meningitis and encephalitis.5–7 This case highlights the potential advantage of multiplex PCR-based assays in rapidly diagnosing aetiologies of rhombencephalitis, but additional clinical studies are warranted to better understand the accuracy and utility of this assay for this indication. Although L. monocytogenes rhombencephalitis was suspected based on clinical and radiographic findings, rapidly obtaining the microbiological diagnosis permitted discontinuation of multiple antimicrobial agents and led to the addition of gentamicin, an intervention that is associated with improved survival in CNS listeriosis.8 Furthermore, despite PCR evidence of infection, this case did not meet the current European and US Centers for Disease Control and Prevention case definition of listeriosis, which requires isolation of L. monocytogenes from a normally sterile site.9 10 Public health agencies may want to consider incorporating commercially available PCR-based diagnostic methods into L. monocytogenes surveillance definitions so that culture-negative/PCR-positive cases as described here are captured and investigated.
Learning points.
The importance of recognising Listeria monocytogenes as a cause of acute cranial nerve impairment with MRI findings suggestive of brainstem encephalitis.
Critical features of L. monocytogenes rhombencephalitis differ from other presentations of central nervous system listeriosis, including its predilection for healthy patients, mild cerebrospinal fluid (CSF) abnormalities and the limited diagnostic sensitivity of blood and CSF cultures.
This case demonstrates the tremendous clinical and public health value of multiplex PCR methods to rapidly diagnose this frequently fatal disease.
Acknowledgments
The authors thank Lars F Westblade for his contribution to establishing the microbiological diagnosis.
Footnotes
Contributors: All authors served important roles in the drafting and editing of the manuscript. RJR, MSS, LL and MS contributed to direct patient care. SGJ established the microbiological diagnosis and CDP provided the radiographic diagnosis.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lorber B. Listeria monocytogenes : Mandell GL, Bennett JE, Dolin R, Principles and Practice of Infectious Diseases. 7th edn Philadelphia, PA: Churchill Livingstone, 2010:2707–14. [Google Scholar]
- 2.Eck H. Encephalomyelitis listeriaca apostematosa. Schweiz Med Wochenschr 1957;87:210–4. [PubMed] [Google Scholar]
- 3.Armstrong RW, Fung PC. Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes: case report and review. Clin Infect Dis 1993;16:689–702. 10.1093/clind/16.5.689 [DOI] [PubMed] [Google Scholar]
- 4.Moragas M, Martínez-Yélamos S, Majós C, et al. . Rhombencephalitis: a series of 97 patients. Medicine 2011;90:256–61. 10.1097/MD.0b013e318224b5af [DOI] [PubMed] [Google Scholar]
- 5.Dien Bard J, Naccache SN, Bender JM. Use of a molecular panel to aid in diagnosis of culture-negative meningitis. J Clin Microbiol 2016;54:3069–70. 10.1128/JCM.01957-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anand V, Holmen J, Neely M, et al. . The brief case: neonatal meningitis caused by Listeria monocytogenes diagnosed by multiplex molecular panel. J Clin Microbiol 2016;54:2846–9. 10.1128/JCM.01159-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leber AL, Everhart K, Balada-Llasat JM, et al. . Multicenter evaluation of biofire filmarray meningitis/encephalitis panel for detection of bacteria, viruses, and yeast in cerebrospinal fluid specimens. J Clin Microbiol 2016;54:2251–61. 10.1128/JCM.00730-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charlier C, Perrodeau É, Leclercq A, et al. . Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infect Dis 2017;17:510–9. 10.1016/S1473-3099(16)30521-7 [DOI] [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control. EU case definitions. Food- and waterborne diseases and diseases of environmental origin: listeriosis. https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions (Accessed 22 Mar 2018).
- 10.Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System (NNDSS): listeriosis. https://wwwn.cdc.gov/nndss/conditions/listeriosis/case-definition/2000/ (Accessed 21 Nov 2017).


