Abstract
Older adults suffer a disproportionate burden of influenza-related morbidity and mortality typically attributed to defects in the aging immune system collectively known as immunosenescence. While the age-related decline in the adaptive immune system has been well characterized, little is known about how aging affects the principal site of influenza infection—the nasal epithelium. In human nasal epithelial cell cultures (hNECs) from older adults, we found similar or increased levels of cytokines during influenza infection compared with hNECs from younger individuals. However, hNECs from older individuals demonstrated decreased mRNA expression for several key proteins that affect clearance of infected cells, including MHC-I and transporter associated with antigen presentation (TAP). These findings were confirmed at the level of protein expression. In vivo studies corroborated the in vitro differences in MHC-I and TAP gene expression and also revealed important decreases in the expression of key influenza-specific antiviral mediators MX1 and IFITM1. Furthermore, epithelial cell-cytotoxic T lymphocyte co-cultures demonstrate that CTL cytotoxic activity is dose-dependent on MHC-I antigen presentation. Taken together, these results indicate that aging is associated with important changes in the nasal epithelium, including antigen presentation and antiviral pathways, which may contribute to increased severity of disease in older adults through impaired clearance of infected cells.
Keywords: Respiratory epithelial cell, Antigen processing and presentation, Influenza, Infection, Immune function, Inflammation
Despite widespread vaccination efforts, greater than 90% of influenza-related mortality occurs in adults over the age of 65 (1,2). It is well established that aging is associated with changes to the immune system, known as immunosenescence, that limit the capacity to mount an effective immune response to infectious agents resulting in more severe disease (3,4). While immunosenescence affects both the innate and adaptive arms of the immune response, little is known about the effect of aging on the nasal epithelium. Nasal epithelial cells are the primary target of influenza viruses and are responsible for initiating and coordinating an effective immune response (5,6). Upon infection, the nasal epithelial cell response works to control virus replication through the production of interferons and key antiviral mediators including Interferon-induced transmembrane-1 (IFITM1) and Interferon-induced GTP-binding protein MX1 (MX1). In addition, the nasal epithelial cells release chemokines to recruit adaptive immune effector cells such as cytotoxic T lymphocytes (CTLs) that function to limit the spread of influenza through the removal of infected cells (6,7).
The removal of an infected respiratory epithelial cell by CTLs is a complex process centered around the presentation of viral peptides by MHC class I proteins (MHC-I) on the surface of the infected cell (8). MHC-I complexes, composed of a heavy chain (HLA-A, HLA-B, or HLA-C) and a light chain (ß2-microglobulin), are universally expressed on the surface of nucleated cells and specifically present intracellular peptides. Viral proteins produced in the infected cell are degraded by cytosolic and nuclear immunoproteasomes, including those composed of interferon-gamma induced 20S proteasome submit beta-1i (PSMB9), 2i (PSMB10), and 5i (PSMB8), and the resulting peptides are transported to the endoplasmic reticulum by transporter associated with antigen presentation (TAP) proteins. The delivery of viral peptides into the endoplasmic reticulum is coupled with loading of the peptide onto the MHC-I molecule with the help of a protein known as tapasin or TAP-binding protein (TAPBP). A fully assembled MHC-I molecule then leaves the endoplasmic reticulum and presents the viral peptide on the cell surface alerting CTLs that the cell has become infected and requires clearance. Interference with the MHC-I pathway facilitates evasion of CTL clearance (9–12).
In this study, we evaluated the effect of aging on nasal epithelial cells and their ability to respond to infection with influenza virus. Using a primary differentiated in vitro human nasal epithelial cell (hNEC) culture system, we compared production of cytokines and chemokines in hNECs from young (20–27 years of age) and older individuals (>55 years of age) followed by an in-depth comparison of epithelial immune gene expression in vitro and in vivo. While hNECs from older adults produced similar or greater levels of cytokines and chemokines during the first 24 h of influenza infection compared with hNECs from younger subjects, nasal epithelial cells from older adults also produced significantly less MHC-I and TAP both in vitro and in vivo, which may limit the clearance of influenza-infected respiratory epithelial cells that is dependent on antigen presentation by MHC-I. In addition, we found decreased MX1 and IFITM1 mRNA expression in vivo, which suggests important age-related deficiencies that could impair the epithelial antiviral response. Collectively, a decreased ability to clear infected respiratory epithelial cells combined with similar or increased levels of pro-inflammatory cytokine expression may contribute to prolonged viral replication, sustained inflammation, and more severe disease in older individuals.
Methods
Human Nasal Epithelial Cell Procurement and In Vitro Culture
Primary differentiated human nasal epithelial cells (hNECs) were obtained, cultured, and differentiated at air–liquid interface (ALI) as previously described (13,14). hNECs used for culture were obtained from two groups of healthy volunteers between the ages of 20–27 (young; n = 6) and over 55 years of age (old; n = 7) without a diagnosis of asthma or smoking-related disorder. Subjects who smoke, people with immunodeficiency and those who chronically use oral or nasal steroids were excluded. This protocol was approved by the Institutional Review Board for Biomedical Research of the University of North Carolina at Chapel Hill School of Medicine (IRBs No 09-0716 and 13–2660). Separate nasal biopsies from young (n = 6) and older (n = 5) subjects (with the same exclusion criteria mentioned above) were collected, lysed in Trizol (Life Technologies), and stored at −80°C until RNA extraction.
Influenza Virus
Influenza A H3N2 virus (Influenza A/Washington/897/90) was kindly provided by Kanta Subbarao (NIAID/NIH). Viruses were propagated in MDCK cells in Infection Medium: Dulbecco’s Modified Eagle Medium (DMEM, Sigma, St. Louis, MO) containing 100 U/mL of penicillin, 100 ug/mL of streptomycin (Sigma), 2mM Glutamine (Sigma), and 0.3% bovine serum albumin (BSA; Sigma); 4 µg/mL N-acetyltrypsin (Sigma) was included in the medium to ensure infection.
In vitro Influenza Virus Infections
hNEC cultures were incubated at 33°C (to faithfully represent the temperature of the human nasal epithelium) for 24 h prior to infection (hpi). The apical surface of hNECs was washed with dulbecco’s phosphate-buffered saline then infected with Influenza virus at an MOI of 10 TCID50/cell in Infection Medium for 1 h at room temperature. The inoculum was then aspirated, the apical and basolateral chambers were washed twice with dulbecco’s phosphate-buffered saline, and fresh media was added to the basolateral chamber.
Sample Collection
Following infection, hNECs were incubated at 33°C for 24 h. Infection Medium was added to the apical surface of hNECs (apical wash) and collected after a 10-min incubation at 33°C for virus titration and cytokine quantification. Basolateral media was collected for cytokine quantification and LDH measurements. Cells were lysed with Trizol. Samples were stored at −80°C until analysis or RNA extraction.
Virus Titers
Infectious virus titers were determined using a TCID50/mL assay as previously described using MDCK cells at 33°C (15).
Epithelial Cell Cytokine Expression
Cytokine expression in apical and basolateral compartments were determined using Meso Scale multiplex kits (TNF-α, Eotaxin, Eotaxin-3, MCP-1, MCP-4, MIP-1α, MIP-1β, IL-8, and TARC; Mesoscale Diagnostics, Rockville, MD) and commercially available ELISA kits (IP-10; BD Biosciences). For Meso Scale kits, lower limits of detection (pg/mL) were as follows, for basal and apical samples, respectively: TNF-α: 0.068, 0.066; Eotaxin: 4.53, 1.61; Eotaxin-3: 1.38, 1.42: MCP-1: 2.44, 0.185; MCP-4: 1.43, 0.914; MIP-1a: 3.67, 2.61; MIP-1b: 0.746, 0.725; IL-8: 45.7, 27.6; TARC: 1.87, 0.47. IP-10 was measured by the OptEIA Human IP-10 ELISA Set (BD Biosciences); lower limit of detection is 7.8 pg/mL.
Cell Death
Relative levels of cell death in hNEC cultures and CTL–epithelial cell co-cultures were determined by measuring the level of lactate dehydrogenase (LDH) in the basolateral compartment by colorimetric enzyme assay (Takara) on a CLARIOStar plate reader (BMG Labtech).
RNA Extraction
Total RNA was extracted from hNEC cultures and nasal biopsies using the RNeasy Mini Kit (Qiagen) according to manufacturer’s instructions except that Trizol was used in the initial extracting steps rather than Qiazol. The extracted RNA was stored at −80°C until analysis. The organic phase of the hNEC samples from the initial extracting steps was stored at −80°C until protein extraction.
Gene expression by Nanostring Assay
Relative gene expression levels between epithelial cells from young and older hosts were determined by Nanostring nCounter assay, using the GX Human Immunology v2 Kit (Nanostring), which detects 579 immune-related RNA transcripts. While 15 internal reference (housekeeping) genes are detected by this kit, we determined that several of these genes showed significant or near-significant differences between hNECs from old and young hosts, which could skew the relative expression results. For a more conservative analysis, data were normalized to the geometric mean of all detected genes to account for overall differences in signal between samples. Normalization to housekeeping genes was used for in vivo nasal biopsy studies as there was no difference in expression between samples from young and old subjects. Differential expression was assessed between young and older subjects within each experimental group (Mock or H3N2) by calculating a t statistic for each gene. For analysis of hNEC cultures, a gene had to be greater than background in at least 1 of 24 samples whereas for biopsy analysis, a gene had to be greater than background in at least 1 of 11 samples. Background threshold was set as the mean+2*standard deviation of the negative controls. p-values from the t-test were transformed to q-values to determine the false discovery rate of the resulting candidates (16). Heatmaps were generated in R by hierarchical clustering with average linkage clustering and Spearman correlation. Nanostring raw signal data can be accessed at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE89882.
Host Cell Protein Analysis by Western Blot
Protein was extracted from hNEC lysates in Trizol used previously for RNA extraction according to the Trizol DNA and protein extraction protocols.
hNEC protein extract concentrations were measured by Biorad DC Protein Assay (Biorad). About 5 and 10 µg of each extract were separated by SDS PAGE using 10% Acrylamide gels (Biorad) and transferred to Immobilon PVDF membranes (EMD Millipore). Blots were cut slightly above the 50 kDa marker, blocked, and probed with anti-human HLA-A,B,C (≤50 kDa section, 5 µg protein, 1:5000; MBL International, D226-3), TAP2 (>50 kDa section, 10 µg protein, 1:400; MBL International, K0137-3), and TAPBP (≤50 kDa section, 10 µg protein, 1:400; Santa Cruz Biotechnology, Sc-80647), followed by Goat anti-mouse IgG HRP (1:1000, Santa Cruz Biotechnology, Sc-2005). Blocking buffer, 5% Nonfat Dry Milk in TBST, was used for all antibody dilutions. Immediately after incubation with SuperSignal West Pico Chemiluminescent Substrate (ThermoFisher), blots were visualized using the Fuji LAS3000 imager. All blot sections were extensively washed in TBST and stained with Amido Black for use in normalizing signals to total protein (17); images were taken with the Fuji LAS3000 imager. Chemiluminescent and Amido Black signals were measured with Image Studio Lite (LI-COR Biosciences). Amido Black signals were measured in each lane in each section, adding signals from the >50 kDa and ≤50 kDa sections. The signal from each protein of interest was divided by the absolute value of the Amido Black signal. No differences in Amido Black signals were detected between young and older groups (data not shown).
HLA-A2+ Flu A-Peptide-Specific Cytotoxic Lymphocyte (CTL) Generation
All recombinant human growth factors were obtained from Peprotech, with the exception of GM-CSF (Leukine sargamostim, Sanofi) and IFNα (Schering). Peripheral blood mononuclear cells were isolated from human buffy coats (Gulf Coast Regional Blood Center) by Ficoll Paque gradient (GE Healthcare) and presence of HLA-A2 determined by flow cytometry using anti-human HLA-A2 APC (Biolegend) on a BD LSRII flow cytometer. To generate dendritic cells (DCs), the adherent fraction of peripheral blood mononuclear cells were isolated and cultured in AIM V Medium (Gibco) with heat-inactivated Human AB Serum (Gemini Bioproducts) (AIMV-HS) for 9 days with the following recombinant human growth factors: day 1–9: IL-4 and GM-CSF; day 4–9: TNFα, day 7–9: IL-6 and IFNα. Mature DCs were cryopreserved on day 9 as previously described (18,19).
CD8+ cells were selected from the nonadherent fraction of the HLA-A2+ peripheral blood mononuclear cells using CD8 Microbeads (Miltenyi Biotec). The selected cells were cryopreserved until use.
To generate CTLs, HLA-A2+ DCs were thawed and cultured with the Influenza A M158-66 peptide GILGFVFTL (Peptide 2.0) at 2µg/mL overnight. Autologous CD8+ cells were thawed and cultured with the peptide-loaded DCs in AIMV-HS. rhIL-7 and rhIL-15 were included throughout the CTL culture, with rhIL-21 on the day of stimulation only, and rhIL-2 starting 16 days after culture initiation. CTLs were stimulated a second time with M1 peptide-loaded DCs at 14 days. Subsequent stimulations were carried out every 2 weeks with irradiated M1 peptide-loaded T2 lymphoblasts (American Type Tissue Collection, ATCC).
CTL and Epithelial Cell Co-Culture
NCI-H522, a lung adenocarcinoma cell line expressing HLA-A*02:01, and A549, a lung carcinoma cell line lacking HLA-A*02:01 were obtained from ATCC. HLA-A2 phenotype was verified by flow cytometry (data not shown) (20). Cells in a 96-well plate were loaded with M1 peptide at concentrations ranging from 30 to 2.0 µg/mL overnight in their respective media. As a control for the effect of residual peptide on CTLs, empty wells were “loaded” at the same concentrations.
Wells were washed gently but extensively, and M1 peptide-specific CTLs in AIMV-HS were applied at a 4:1 CTL: target cell ratio. An equal number of CTLs was applied to the residual peptide control wells. Cells were co-cultured 24h in a 37°C 5% CO2 incubator, and media sampled for LDH assay.
Statistics
Analysis of the ELISA, Meso Scale, and Western blot results was performed to test hypotheses regarding differential abundance by treatment and age. Non-parametric statistics were selected to alleviate the need for assumptions regarding the distribution of this data. The Wilcoxon matched pairs test was used to identify association between treatment groups (Mock and H3N2) within the young or old samples. The Mann–Whitney test was performed to test for differences between young and old for each treatment group. These tests were performed using GraphPad Prism software. Statistical analysis of Nanostring data is described above.
Results
Similar Levels of Influenza Virus Replication and Cell Death in hNECs from Young and Older Hosts
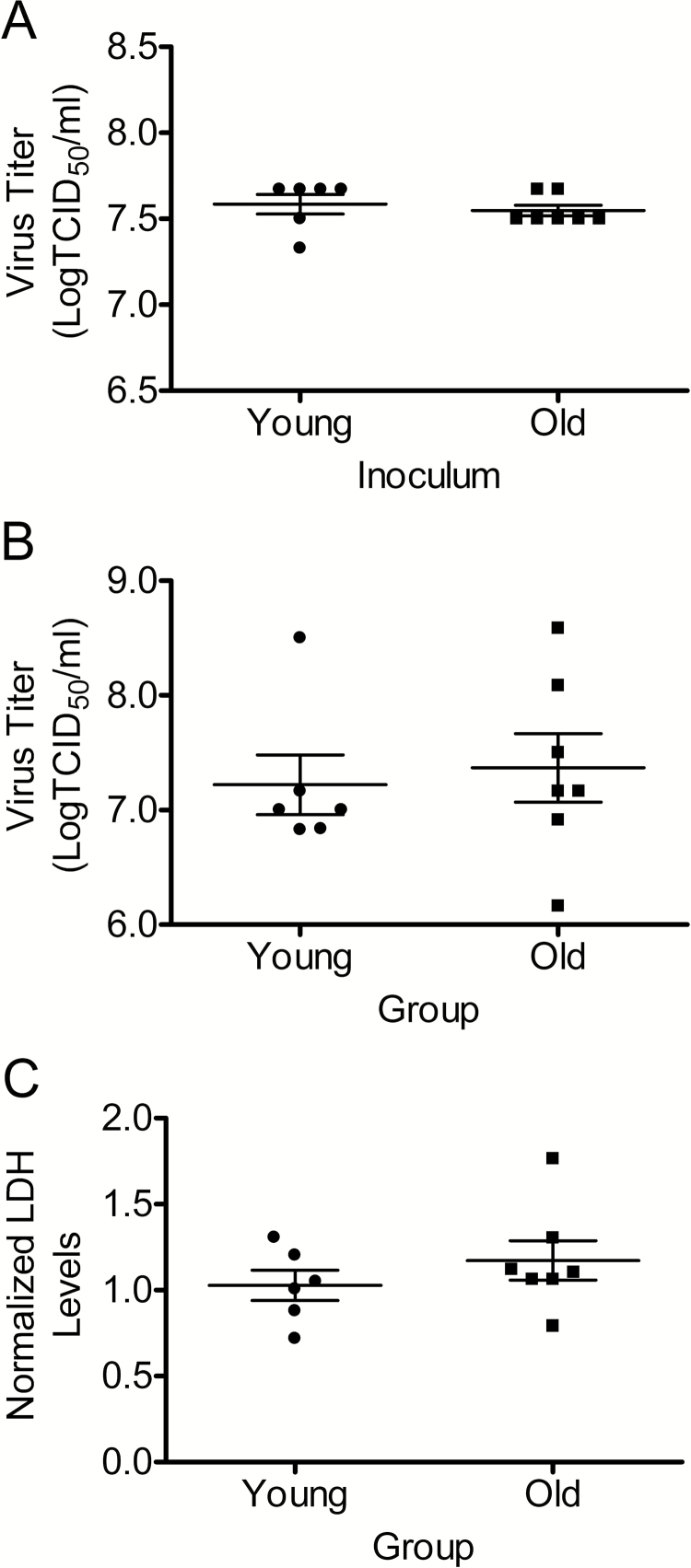
After similar inoculums (Figure 1A), there were no significant differences in virus replication between hNECs from young and older subjects at 24hpi (Figure 1B), and there were no differences in cell death at 24 hpi as measured by basolateral LDH release (Figure 1C).
Figure 1.
Virus inoculum, replication at 24 h postinfection, and cell cytotoxicity. Similar levels of Influenza virus replication and cytotoxicity were found in hNECs from young and older individuals. hNEC cultures were infected with an H3N2 strain of influenza virus at an MOI of 10 TCID50/mL at 33°C. Virus titers in inoculum (A) and apical supernatants at 24 h postinfection (B) were measured by calculating the TCID50/mL in MDCK. Cell death was determined by measuring lactate dehydrogenase (LDH) in the basolateral chamber and normalized to mock-infected hNECs from both young and older individuals (C). n = 6 different donors for young and n = 7 donors for older adults.
Influenza-Infected hNECs From Older Hosts Release Similar or Greater Levels of Pro-Inflammatory Cytokines and Chemokines
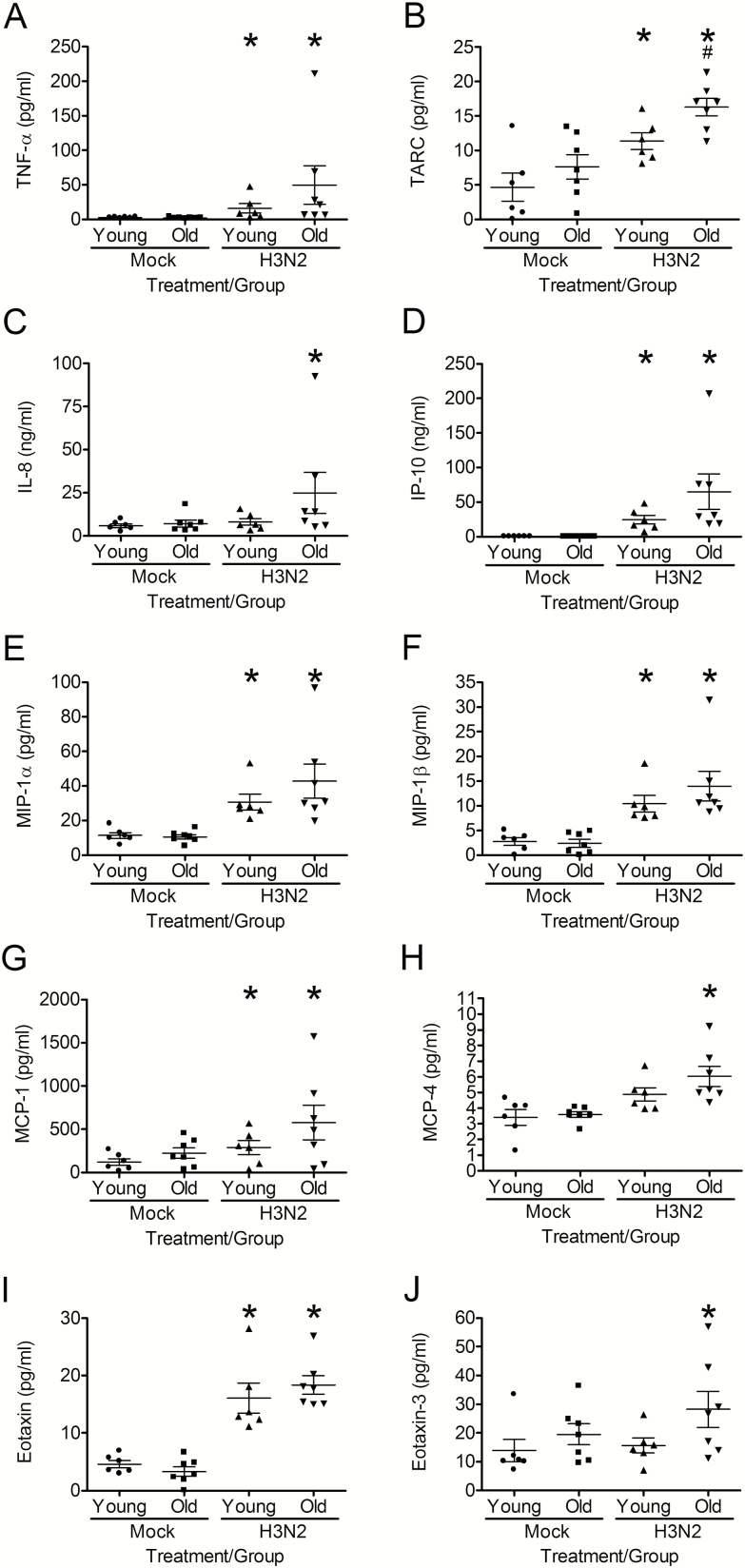
To compare the age-related differences in the nasal epithelial innate immune response to influenza infection, we first measured the bidirectional secretion of cytokines and chemokines. In mock-infected cultures, there was no difference in the apical or basolateral secretion of cytokines in hNECs from young and older hosts (Figure 2 and Supplementary Figure 1). Twenty-four hours postinfluenza infection, hNECs from young and older hosts released significantly greater levels of TNF-α, TARC, IP-10, MIP-1α, MIP-1β, MCP-1, and Eotaxin apically compared with mock infection (Figure 2A, B, D, E, F, G and I). Influenza-infected hNECs, from older adults released significantly greater levels of IL-8, MCP-4, and Eotaxin-3 apically compared with uninfected hNECs, but this was not seen in hNECs from younger adults (Figure 2C, H and J). While there was increased release of cytokines apically by influenza-infected hNECs from older compared with younger adults, only TARC was statistically significance (Figure 2B).
Figure 2.
hNECs from older individuals release similar or greater levels of apical pro-inflammatory cytokines in response to H3N2 influenza infection as compared to hNECs from young individuals at 24 h postinfection. Cytokines were measured by Meso Scale multiplex kits and IP-10 ELISA in apical washes collected 24 h postinfection. *p < .05 compared with mock-infected cultures. #p < .05 compared with hNECs from young individuals infected with H3N2 influenza (n = 6 different donors for young and n = 7 donors for older adults).
The basolateral release of cytokines during influenza infection was similar to that seen in the apical chamber. Influenza-infected hNECs from both young and older subjects released significantly greater levels of TARC, IP-10, MIP-1β, and MCP-1 compared with mock-infected hNECs (Supplementary Figure 1B, D, F, and G). Although greater levels of cytokines were released in influenza-infected hNECs from older compared with younger adults, the differences did not reach statistical significance (Supplementary Figure 1B, D, E, F, and I). Significantly greater levels of TNF-α, IL-8, MIP-1α, MCP-4, and Eotaxin were released from influenza-infected hNECs from older adults compared with un-infected cultures, a difference that was not seen in hNECs from younger subjects (Supplementary Figure 1A, C, E, H, and I).
Taken together, these results suggest that aging is not associated with an attenuated epithelial cell-specific cytokine or chemokine response to influenza infection and in most cases hNECs from older adults release greater levels of pro-inflammatory chemokines compared with hNECs from younger adults.
Aging Results in a Differential Nasal Epithelial Cell Immune Gene Expression Profile at Baseline and During Influenza Infection
Next, we examined the effect of aging on epithelial cell immune gene expression. Of the 579 genes profiled by the Nanostring analysis, the expression of 23 genes was significantly different between mock-infected hNECs from young and older subjects (q > 0.73) (Supplementary Figure 2), and 77 genes were differentially expressed during influenza infection (q = 0.05–0.30) (Supplementary Figure 3). The differentially expressed genes at baseline (mock) and following influenza infection of hNECs from young and older subjects are notable for enrichment in antigen processing and presentation pathways.
Aging is Associated with a Decrease in mRNA and Proteins Required for Antigen Processing and presentation In Vitro
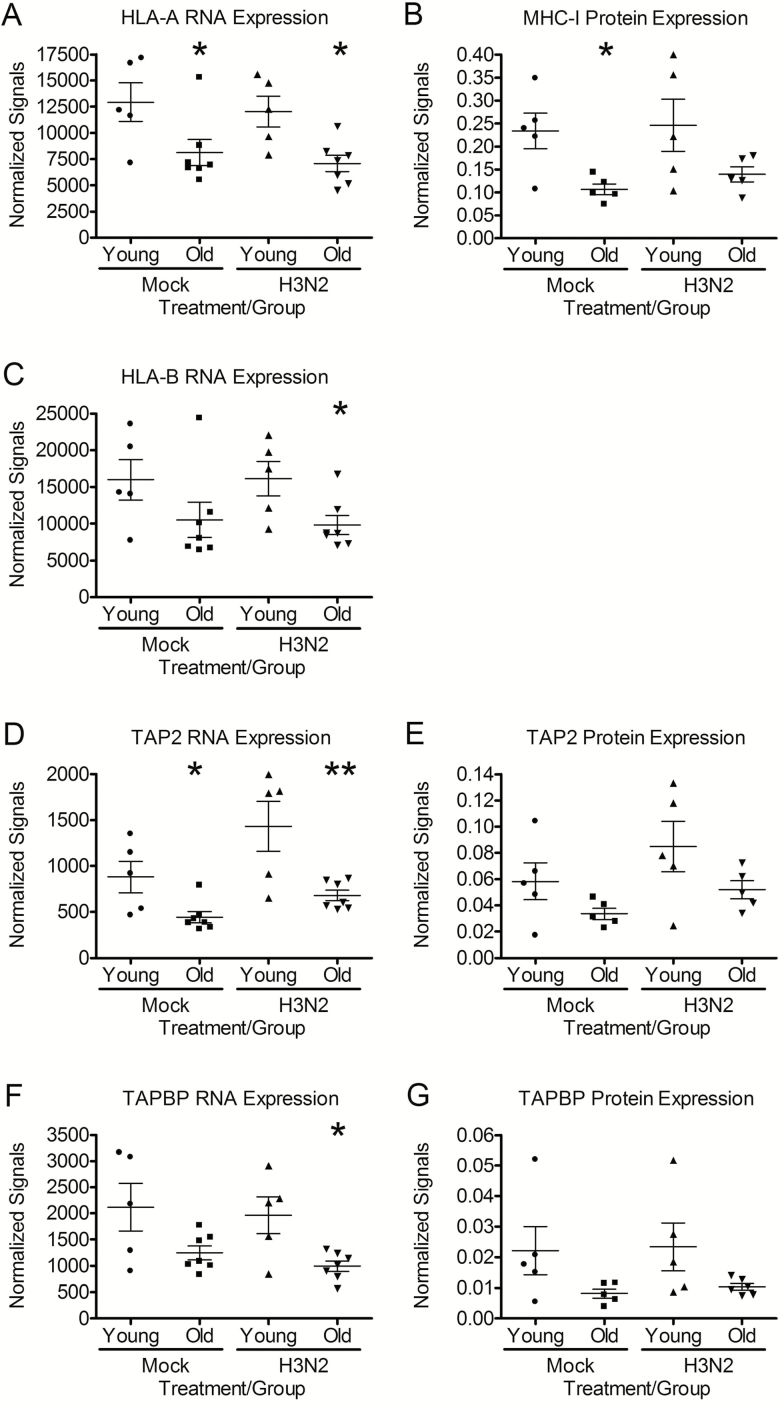
HLA-A and HLA-B, two of the three classical MHC-I paralogs, and TAP2 and TAPBP, genes involved in peptide processing and MHC assembly, were differentially expressed at the RNA level between hNECs from young and old subjects. HLA-A, and TAP2 were reduced significantly in hNECs from older hosts both at baseline (mock) and after 24 h of influenza infection (Figure 3A and D), while HLA-B and TAPBP were significantly reduced in influenza-infected cells alone but showed a trend toward reduced expression at baseline (p = .10 and 0.07, respectively) (Figure 3C and F).
Figure 3.
hNECs from older subjects express significantly less MHC-I and antigen-processing mRNA and protein. (A, C, D, and F) mRNA was compared in mock and influenza-infected hNECs 24 h postinfection using Nanostring nCounter assay (GX Human Immunology v2 kit) (n = 5 different donors for young and n = 7 donors for older adults). (B, E, and G) Protein in mock and influenza-infected hNECs was analyzed by western blot. Protein bands were quantified using chemiluminesence and normalized to amido black staining. n = 5 different donors for young and n = 5 donors for older adults. *p < .05 and **p < .01 compared with hNECs from young individuals as determined by t-test.
To assess whether the differences in the expression of these genes at the RNA level translate into differences in protein expression, we compared cellular protein in hNECs from young and older adults by western blot. In mock-infected hNECs, there was significantly lower levels of classical MHC-I (HLA-A, B, and C) protein expression in hNECs from older as compared with younger subjects (Figure 3B) consistent with the mRNA expression levels detailed above (Figure 3A and C). A similar trend was seen after 24 h of influenza infection but was no longer statistically significant. Likewise, hNECs from older hosts also expressed lower levels of TAP2 (Figure 3E) and TAPBP proteins (Figure 3G) during mock and influenza infection. While the difference in TAP2 did not reach statistical significance, the difference in TAPBP expression between hNECs from young and old subjects trended towards statistical significance (p = .09). Collectively, this data highlights novel age-related deficiencies in the mRNA and proteins necessary for processing intracellular antigens and MHC-I molecules.
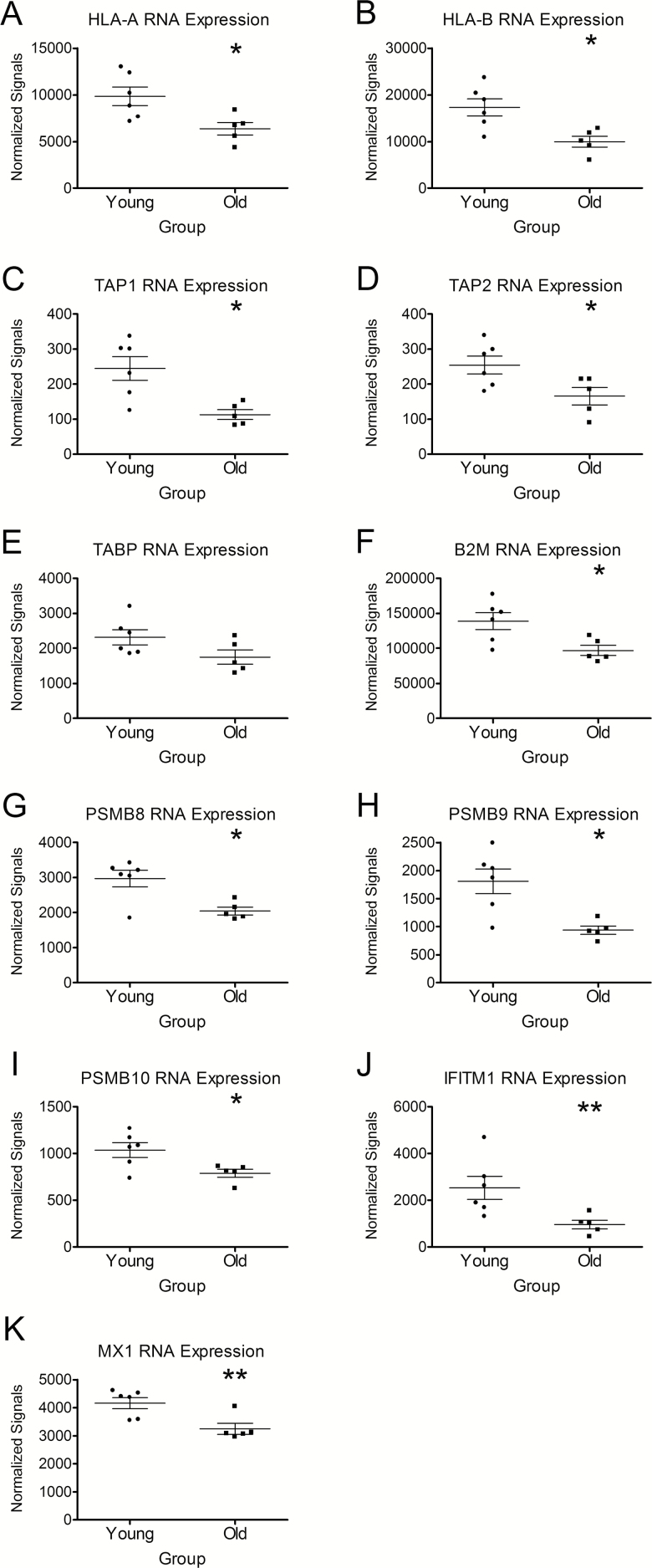
In Vivo Nasal Epithelial Cells From Older Adults Express Significantly Less MHC-I, TAP1, and TAP2 mRNA
To confirm the novel age-related disparities in MHC-I, TAP, and TAPBP expression in vivo, RNA from nasal biopsies from young and older hosts were profiled using the same Nanostring panel used for hNECs. Global expression patterns were significantly different in nasal epithelial cells from young compared with older subjects with 60 genes found to be differentially expressed (p < .05, q = 0.28–0.39) between young and older subjects (Supplementary Figure 4). Similar to our in vitro data (Supplementary Figures 2 and 3; Figure 3), we found the differentially expressed genes in vivo also enriched for MHC-I and antigen-processing pathways. Specifically, HLA-A and HLA-B are expressed 1.5- and 1.7-fold lower, respectively, in nasal epithelial biopsies from older compared with young subjects (Figure 4A and B). TAP1 and TAP2 mRNA were expressed at 2.1- and 1.6-fold lower levels in nasal epithelial cell biopsies from older compared with young subjects, respectively (Figure 4C and D). TAPBP mRNA expression was 1.3-fold lower in nasal epithelial biopsies from older compared with young subjects but this difference did not reach statistical significance (Figure 4E). ß2-microglobulin, which plays a key role in the peptide-loading complex (composed of MHC-I, TAPBP, TAP, and ERp57) and is responsible for stabilizing the MHC-I molecule, was also significantly reduced by 1.4-fold in nasal epithelial cells from older compared with young subjects (Figure 4F). Additionally, the mRNA for the proteasome subunits PSMB8, 9, and 10 were significantly reduced at 1.4-, 1.9, and 1.3-fold lower, respectively, in nasal epithelial cells from older compared with young adults (Figure 4G, H, and I). Collectively, the in vivo findings confirm our in vitro baseline data and highlight important age-associated differences in the expression of key genes involved in the antigen processing and presentation pathway.
Figure 4.
Nasal epithelial cells from older individuals express significantly less mRNA for proteins important in antigen presentation and viral evasion. To confirm and extend our in vitro findings, immune gene profiles of nasal epithelial biopsies were performed using Nanostring nCounter assay (GX Human Immunology v2 kit). In older hosts (A, B) MHC-I (HLA-A and HLA-B), (C, D) TAP associated protein (TAP1, TAP2), (E) TAP Binding Protein (TAPBP), (F) ß2-microglobulin (B2M), (G, H, I) proteasome subunits PSMB8, 9, and 10, and (J, K) the antiviral IFITM1 and MX1, were reduced compared with nasal epithelial cells from young hosts in vivo. *p < .05 and **p < .01 compared with hNECs from young individuals as determined by t-test. n = 6 different donors for young and n = 5 donors for older adults.
Cytotoxic T Lymphocyte (CTL) Clearance of Epithelial Cells is Dependent on the Level of MHC-I Antigen Presentation
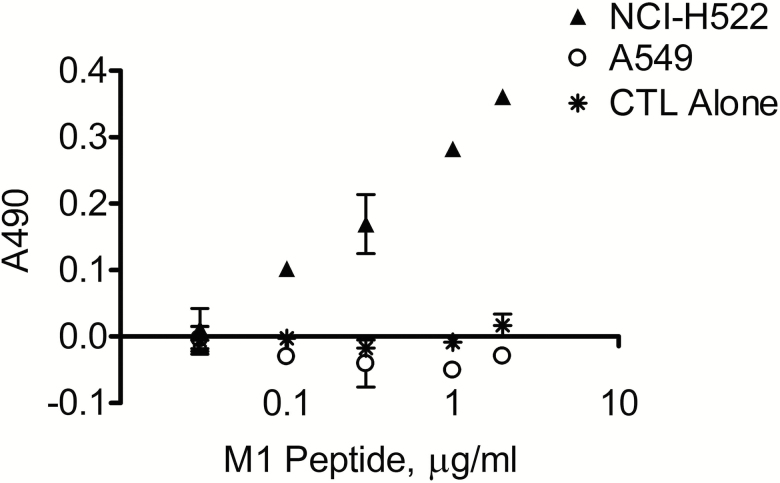
In order to determine the functional effect of a reduction in HLA-A and other proteins relevant to antigen presentation in the airway epithelium, we developed an epithelial cell–CTL co-culture model using Influenza A M158-66 (M1) peptide-specific HLA-A2+ CTLs and two airway epithelial cell lines, NCI-H522 and A549. Both cell lines revealed cell-surface expression of MHC-I by flow cytometry (data not shown). NCI-H522 expresses HLA-A2 (20), while A549, lacking HLA-A2, was used as a negative control. NCI-H522 and A549 cells, loaded overnight with increasing levels of M1 peptide (30 ng/mL–2 µg/mL), were co-cultured for 24 h with the M1 peptide-specific CTLs. Media LDH levels, measured to compare cytotoxicity between NCI-H522-CTL and A549-CTL co-cultures, demonstrate clear antigen concentration-dependent cytotoxicity above vehicle control in NCI-H522 but not A549, with increasing cell death as peptide concentration increases (Figure 5). These findings indicate that the cytotoxic activity of CTLs is dependent on the concentration of the peptide–MHC-I complex on the target cell surface and support the idea that reduced expression of MHC-I or antigen-processing proteins will impair CTL clearance of influenza-infected airway epithelial cells.
Figure 5.
CTL clearance of airway epithelial cells is dependent upon concentration of antigen presentation. NCI-H522, a lung adenocarcinoma cell line expressing HLA-A*02:01, and A549, a lung carcinoma cell line without HLA-A*02:01 expression, were loaded with a dose–response of Influenza M1 peptide (30.0 ng/mL to 2.0 µg/mL) then co-cultured with M1 peptide-specific HLA-A2+ CTLs at a 4:1 CTL:target cell ratio for 24 h at 37°C. Cell death was measured by lactate dehydrogenase (LDH) levels in media.
Critical Antiviral Mediators Are Reduced in Nasal Epithelial Cells From Older Compared With Young Adult Subjects In Vivo
Antiviral mediators including IFITM1 and MX1 have recently been shown to play important roles in establishing an antiviral state against influenza infection. In human nasal epithelial cells from older individuals, IFITM1 and MX1 expression were significantly reduced 2.6- and 1.3-fold, respectively, compared with nasal epithelial cells from younger adults in vivo suggesting that the antiviral state of the primary cells infected with influenza is reduced in older adults at baseline (Figure 4J and K).
Discussion
Influenza infection in older adults is associated with a more severe clinical course compared to younger adults. To better understand the effect of aging on respiratory epithelial cells and their antiviral response, we compared viral replication and cell death, cytokine release, and immune gene expression in primary differentiated human nasal epithelial cells in vitro at baseline and during infection with H3N2 Influenza A virus. Baseline differences were then confirmed in nasal biopsies of young and older subjects in vivo. Our results indicate that nasal epithelial cells in older adults manifest a dysregulated immune response to influenza infection compared with younger hosts in that there is an equivalent and often greater cytokine production but that this is accompanied by a decrease in the expression of specific antiviral mediators (MX1 and IFITM1) as well as genes responsible for antigen processing and presentation (MHC-I, TAP2, and TAPBP).
Nasal epithelial cells, the first cells infected in influenza, play a critical role in initiating the innate immune response and in modulating the adaptive immune response through the expression of cytokines, chemokines, and growth factors that inhibit virus replication and recruitment of an organized cellular response to influenza infection (5,6). The role of epithelial cells in alerting CTLs to viral infection, through viral antigen presentation on MHC-I proteins, is pivotal to containing infection. Although aging is a risk factor for severe influenza, this is the first study to combine an in vitro and in vivo approach to systematically evaluate how aging affects the immune response to influenza at the primary site of infection.
Comparison of the nasal epithelial cell immune gene profile between young and older hosts reveals important differences in the expression of MHC-I, TAP, and TAPBP, which play pivotal roles in alerting adaptive immune cells that the cell is infected. Following infection, a complex process ensues requiring a number of host cell proteins to process (PSMB8, 9, and 10) and deliver cytosolic peptides into the endoplasmic reticulum (TAP1 and TAP2), load the viral peptide onto MHC-I (TAPBP) resulting in presentation of the MHC-I–viral antigen complex on the cell surface. Viral replication and inflammation are controlled by the clearance of infected cells by CTLs mediated by the recognition of viral antigen on MHC-I proteins (21). Cells deficient in MHC-I have been shown to be less susceptible to CTLs and NK cells and mice lacking MHC-I and functional CD8+ T lymphocytes experience delayed viral clearance and increased mortality after influenza viral challenge (9–11). Similarly, the level of MHC-I (HLA-A2) expression has been shown to directly correlate with susceptibility to cellular lysis by CTLs (22). We have provided further evidence that CTL cytotoxic activity decreases in a dose-dependent manner with decreasing Influenza antigen presentation on MHC-I (HLA-A2) in an airway epithelial cell line (Figure 5).
Here, we demonstrate that the expression of MHC-I (specifically HLA-A) and TAP2 mRNA is decreased in hNECs from older adults at baseline and after infection with influenza in vitro and baseline differences in MHC-I (HLA-A and HLA-B) and TAP2 are confirmed in nasal biopsies in vivo. Nasal biopsies from older compared with younger adults also revealed significantly decreased levels of TAP2, ß2-microglobulin and proteosome subunits PSMB8, 9, and 10 mRNA, all of which are critical to viral antigen presentation. We also demonstrate that hNECs from older hosts produce significantly less HLA-B and TAPBP compared with hNECs from younger hosts after infection with influenza. To our knowledge, this is the first report that aging is associated with a decreased expression of MHC-I and TAP-associated proteins at the primary site of influenza infection and replication.
Decreased clearance of influenza-infected respiratory epithelial cells, in the setting of persistent cytokine and chemokine production, will likely contribute to sustained and/or increased mucosal inflammation and may ultimately explain the increase in disease severity in older adults. The significantly increased thymus and activation regulated chemokine (TARC) and TNF-α, as well as relative increases in the expression of Eotaxin, MCP-1, MCP-4, MIP 1α, MIP 1β, and IL-8 levels likely direct a distinct immune cell recruitment to the respiratory epithelium. TARC is a high affinity ligand, which binds to and induces chemotaxis in Th2 cells via interaction with the CCR4 receptor (23). Previous bronchoscopic studies evaluating the effect of aging on lung immunity discovered an elevated CD4/CD8 cell ratio in bronchoalveolar lavage fluid potentially reflecting a shift in the respiratory mucosa towards a Th2-mediated milieu in older adults rather than the classic antiviral Th1 response (24,25). Additionally, the relative increase in IL-8 expression apically and basolaterally in hNECs from older compared with younger subjects also may contribute to increased airway pathology through the induction of neutrophil chemotaxis.
Comparison of immune gene expression profiles in vivo also revealed that nasal epithelial cells from older adults express significantly less MX1 and IFITM1, two key antiviral mediators. MX proteins are GTPases that are induced by type I and type III interferons and are thought to interfere with the function of the influenza RNA-dependent RNA polymerase. Mice with a functional Mx1 locus are naturally resistant to influenza, suggesting an important antiviral role for MX proteins (26). Additionally, our findings of reduced MX1 expression in nasal epithelial biopsies of older compared with younger adults are supported by the recent work demonstrating reduced MXA (MX1) gene expression in monocytes from older adults (27). Similarly, IFITM proteins have also been found to display antiviral activity with ectopic production of IFITM1 associated with markedly reduced influenza A virus replication. Thus, a decrease in expression of MX1 or IFITM1 by respiratory epithelial cells from older adults may explain the increased susceptibility to influenza infection among this high-risk population.
In addition to the small number of young and older adult subjects enrolled in this study, which limit the power to detect differences in epithelial cell chemokine and cytokine expression, there are other limitations that should be considered when interpreting these results. While CD4+ T helper cells also play a critical role in the response to influenza infection, given the differences found in MHC-I expression between nasal epithelial cells from young and older adults, we focused on the respiratory epithelial–CTL interaction. The epithelial cell–CTL co-culture experiment (Figure 5) demonstrates that reduced antigen presentation on the surface of respiratory epithelial cells leads to decreased antigen-specific CTL-induced cell death, suggesting that clearance of target cells is dose-dependent on the level of influenza antigen presentation. However, further experiments are needed to evaluate the relative contributions of decreased MHC-I and TAP associated protein expression on antigen processing and presentation and ultimately on CTL-induced killing of infected primary nasal epithelial cells.
The findings of age-associated decreased gene and protein expression related to antigen processing and presentation have profound implications beyond that of viral infections including other inflammatory conditions such as obstructive lung disease, cancer, and other respiratory pathogens. The presence of these defects in both our in vivo and in vitro systems suggest that these changes are conserved features of the epithelium and thus the effect of aging on the respiratory epithelium in other respiratory disease conditions warrants further evaluation.
Funding
The work was supported by funds from National Institutes of Health, National Institute on Aging (NIA) and National Institute of Allergy and Infectious Diseases [grant numbers: R03AG045088 and K23AI121516 (WF)], and an Infectious Disease Society of America (IDSA) Young Investigator Award in Geriatrics. The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
Supplementary Material
Acknowledgments
Conception and design: WF, KC, SH, and PA; Experiments KC, MB, WF; Analysis and interpretation: KC, JP, WF, and IJ; Drafting the manuscript for important intellectual content: WF, SS.
Conflict of interest statement
None declared.
References
- 1. Sprenger MJ, Mulder PG, Beyer WE, Van Strik R, Masurel N. Impact of influenza on mortality in relation to age and underlying disease, 1967–1989. Int J Epidemiol. 1993;22:334–40. [DOI] [PubMed] [Google Scholar]
- 2. Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi:10.1001/jama.289.2.179 [DOI] [PubMed] [Google Scholar]
- 3. Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol. 2008;43:718–728. doi: 10.1016/j.exger.2008.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen WH, Kozlovsky BF, Effros RB, Grubeck-Loebenstein B, Edelman R, Sztein MB. Vaccination in the elderly: an immunological perspective. Trends Immunol. 2009;30:351–359. doi: 10.1016/j.it.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12:295–305. doi: 10.1038/nri3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oslund KL, Baumgarth N. Influenza-induced innate immunity: regulators of viral replication, respiratory tract pathology & adaptive immunity. Future Virol. 2011;6:951–962. doi: 10.2217/fvl.11.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bender BS, Johnson MP, Small PA. Influenza in senescent mice: impaired cytotoxic T-lymphocyte activity is correlated with prolonged infection. Immunology. 1991;72:514–519. [PMC free article] [PubMed] [Google Scholar]
- 8. Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11:823–836. doi: 10.1038/nri3084 [DOI] [PubMed] [Google Scholar]
- 9. Kaufman DS, Schoon RA, Leibson PJ. Role for major histocompatibility complex class I in regulating natural killer cell-mediated killing of virus-infected cells. Proc Natl Acad Sci USA. 1992;89:8337–8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hewitt EW. The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology. 2003;110:163–169. doi:10.1046/j.1365-2567.2003.01738.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bender BS, Croghan T, Zhang L, Small PA Jr. Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992;175:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eichelberger M, Allan W, Zijlstra M, Jaenisch R, Doherty PC. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J Exp Med. 1991;174:875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fischer WA 2nd, Chason KD, Brighton M, Jaspers I. Live attenuated influenza vaccine strains elicit a greater innate immune response than antigenically-matched seasonal influenza viruses during infection of human nasal epithelial cell cultures. Vaccine. 2014;32:1761–1767. doi: 10.1016/j.vaccine.2013.12.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muller L, Brighton LE, Carson JL, Fischer WA 2nd, Jaspers I. Culturing of human nasal epithelial cells at the air liquid interface. JoVE. 2013. doi: 10.3791/50646.PubMedPMID:24145828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCown MF, Pekosz A. The influenza A virus M2 cytoplasmic tail is required for infectious virus production and efficient genome packaging. J Virol. 2005;79:3595–3605. doi: 10.1128/JVI.79.6.3595-3605.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods. 2008;172:250–254. doi: 10.1016/j.jneumeth.2008.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Attayek PJ, Hunsucker SA, Sims CE, Allbritton NL, Armistead PM. Identification and isolation of antigen-specific cytotoxic T lymphocytes with an automated microraft sorting system. Integr Biol (Camb). 2016;8:1208–1220. doi: 10.1039/c6ib00168h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castiello L, Sabatino M, Jin P, et al. Monocyte-derived DC maturation strategies and related pathways: a transcriptional view. Cancer Immunol Immunother. 2011;60:457–466. doi: 10.1007/s00262-010-0954-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adams S, Robbins FM, Chen D, et al. HLA class I and II genotype of the NCI-60 cell lines. J Transl Med. 2005;3:11. doi: 10.1186/1479-5876-3-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yeh CY, Yeh TH, Jung CJ, Chen PL, Lien HT, Chia JS. Activated human nasal epithelial cells modulate specific antibody response against bacterial or viral antigens. PLoS One. 2013;8:e55472. doi: 10.1371/journal.pone.0055472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rivoltini L, Barracchini KC, Viggiano V, et al. Quantitative correlation between HLA class I allele expression and recognition of melanoma cells by antigen-specific cytotoxic T lymphocytes. Cancer Res. 1995;55:3149–3157. [PMC free article] [PubMed] [Google Scholar]
- 23. Panina-Bordignon P, Papi A, Mariani M, et al. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest. 2001;107:1357–1364. doi: 10.1172/JCI12655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meyer KC, Ershler W, Rosenthal NS, Lu XG, Peterson K. Immune dysregulation in the aging human lung. Am J Respir Crit Care Med. 1996;153:1072–1079. doi: 10.1164/ajrccm.153.3.8630547 [DOI] [PubMed] [Google Scholar]
- 25. Meyer KC, Soergel P. Variation of bronchoalveolar lymphocyte phenotypes with age in the physiologically normal human lung. Thorax. 1999;54:697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verhelst J, Parthoens E, Schepens B, Fiers W, Saelens X. Interferon-inducible protein Mx1 inhibits influenza virus by interfering with functional viral ribonucleoprotein complex assembly. J Virol. 2012;86:13445–13455. doi: 10.1128/JVI.01682-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pillai PS, Molony RD, Martinod K, et al. Mx1 reveals innate pathways to antiviral resistance and lethal influenza disease. Science. 2016;352:463–466. doi: 10.1126/science.aaf3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang YH, Terabe M, Pendleton CD, et al. Identification and enhancement of HLA-A2.1-restricted CTL epitopes in a new human cancer antigen-POTE. PLoS One. 2013;8:e64365. doi: 10.1371/journal.pone.0064365 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.