Abstract
Objectives:
To determine whether relative delays among domains exist in the conversational use of vocabulary, syntax and morphology by children with cochlear implants (CIs) and whether these were differentially affected by age of implantation (AOI) and the audibility of speech.
Methods:
Participants in this short-term longitudinal study were 126 children with AOI 6–38 months and a matched group of 30 children without hearing loss. Language samples of the same children at ages 3.5 and 4.5 were analyzed for breadth of vocabulary and bound morphemes used, and sentence length.
Results:
At both test ages, expressive language domains were delayed equally. Higher performance across domains was independently associated with younger AOI and better pre-implant aided thresholds. No domain was affected differently by very early implantation, but bound morpheme breadth was associated with better CI-aided thresholds. Between 63%−78% of children with 6–11 months AOI scored close to hearing age-mates by 4.5, a level achieved by fewer than 25% of those with AOI of 19–24 months or later ages.
Discussion:
Previous studies indicated greater language delays in the areas of morphology and syntax than vocabulary, with the earliest ages of implantation conferring the greatest benefit to those domains. The current design addressed inconsistency across studies in modes of communication used, presence/absence of other disabilities, and differences in language domains chosen as outcome measures.
Conclusions:
Linguistic domains benefitted equally from early implantation, regardless of duration of auditory stimulation. Better pre-CI aided hearing often compensated for later AOI. Bound morpheme use benefitted from better CI-aided thresholds.
Keywords: deafness, pediatric, cochlear implant, expressive language development, syntax, morphology, vocabulary, audibility
The surgical placement of one or more cochlear implants (CIs) in a young child with severe-profound hearing loss has now become a widespread practice in many countries. Early access to this higher-quality auditory input usually provides very significant benefit, though variability in outcomes across children remains. Further, while most children with CIs make good progress over time in language, it’s unclear whether this development occurs in a uniform fashion across different linguistic domains or whether sub-optimal auditory input affects some domains more than others. In this study, we investigate whether measures of expressive vocabulary, morphology, and syntax show relative strengths and weaknesses in children using CIs compared to their age-mates without hearing loss. We explore the interplay between surgery age, duration of use, audibility of input, and whether these variables affect development of specific language domains.
Development of linguistic domains in different populations
Normally-hearing, typically-developing children.
An extensive literature exists showing that in typically-developing children there is a very strong and stable association between the lexical and morpho-syntactic domains of language in early development. Bates, Dale, and Thal (1995) reviewed data from the norming study of the MacArthur Communicative Development Inventories (Fenson et al., 1993) involving more than 1,800 children. These data consistently showed close lexical-grammatical correspondence in the developmentally-important period between 16–30 months of age. This finding was supported by Dale, Dionne, Eley, and Plomin (2000) who used behavioral genetic techniques to examine evidence for asynchronies in development among domains in 2,898 pairs of typically-developing 2-year-olds. They conclude: “there is little evidence either genetically or phenotypically for a dissociation between vocabulary and grammar within language”. This relationship is also found in studies of other languages (Mariscal & Gallego, 2012) and of lexically-precocious toddlers (McGregor, Sheng, & Smith, 2005). Tomblin and Zhang (2006) approached the same question by administering comprehensive language test batteries to children (longitudinally) at kindergarten, second, fourth, and eighth grades. Participants included both typically-developing children with a wide range of skill levels and children with a diagnosis of language disorder. When individual items from all tests were subjected to a form of exploratory factor analysis the results indicated that a single-dimension (domain) model fit the data equally well as a 2-dimensional model in the younger grades, with the 2-dimensional model (vocabulary-sentences) fitting data better only at the eighth grade test point. Thus, the authors conclude “there is good evidence that these are not independent traits and that the correlation between these aspects of language is high” (p. 1206), suggesting that at least in early childhood the domains develop in tandem.
Children using CIs.
Examination of developmental patterns among domains in children with CIs is important for both theoretical and practical reasons. Determining whether they mirror those of normally-hearing children or are fundamentally different will inform our understanding of the effects of auditory deprivation and subsequent late exposure to spoken language input. From a practical point of view, advance knowledge of relative differences in domain outcomes and the underlying skills supporting them can be helpful in guiding assessments and planning intervention in clinical and educational settings (Houston, Stewart, Moberly, Hollich, & Miyamoto, 2012).
Within the literature on language-learning by deaf children, the findings regarding relative domain strengths and weaknesses have been decidedly mixed, perhaps due to methodological issues. For example, some studies do not clearly separate receptive and expressive modalities when making domain comparisons. Both Duchesne, Sutton, and Bergeron (2009) and Schorr, Roth, and Fox (2008) report no significant differences in linguistic domains of children with CIs, but in each of these expressive vocabulary appears to be compared with a measure of receptive morpho-syntax or a combination of expressive and receptive.
One approach to comparing expressive domains uses different standardized tests and scores are compared to either a matched normally-hearing (NH) comparison group or to test norms (Boons et al., 2013; Caselli, Rinaldi, Varuzza, Giuliani, & Burdo, 2012; Geers, Moog, Biedenstein, Brenner, & Hayes, 2009; Szagun, 2001). These studies generally find that a larger percentage of children score ‘within normal limits’ (at least 1 SD below the mean or better) on expressive vocabulary than on expressive morphology or syntax but have the limitation that the tests used are based on different normative samples and statistical comparisons across domains were not made. Using language quotients, Boons et al. (2013) made direct comparisons among domains and found no significant differences. A second approach is to use a comprehensive language test and make comparisons among domain sub-scale scores, which allows for comparisons involving the same normative sample. Using this method, Spencer (2004) found no domain differences on expressive vocabulary, morphology, and syntax as measured on the CELF-P (Wiig, Secord & Semel, 1992) in a sample of 4–8 year old children implanted between 14–38 months of age. Even with this approach, however, there is a limitation of comparisons in that the subtests present distinctly different task demands, thereby possibly introducing extraneous variability in the spoken language produced. Furthermore, the Spencer study included children with a simultaneous speech and sign communication background, which may complicate interpretations of delays
Ideally studies will compare linguistic domains on a single measure with no interacting task demands. One way to do this is to collect language samples from interactions between familiar conversational partners for analysis of constituent linguistic competencies. Nittrouer (2014) sampled conversations of 21 children using CIs compared to NH age-mates and expressed domain differences between CI and NH children in terms of effect sizes, which in turn may be compared across domains. The effect sizes for vocabulary (1.08) and syntax (1.12) were greater than for bound morpheme use (0.83). Without direct comparisons among domains on a common metric, however, questions remain about domain competency congruence.
Predictors of Language Ability
Across domains, the acquisition of spoken language in severely-profoundly deaf children is affected by the amount and quality of auditory input provided by hearing aids or cochlear implants, the age at which this input is provided (i.e., sensitive period) and the duration of auditory input. These factors do not operate independently and the degree to which language domains are differentially affected by these variables is not completely understood.
Audibility of speech.
Children who receive auditory benefit from a hearing aid prior to cochlear implantation exhibit an advantage in early language development (Dettman et al., 2004; Nicholas & Geers, 2007) and word learning ability (Houston et al., 2012). After CI surgery occurs children differ in their ability to detect and discriminate speech sounds with the device (Geers, Brenner & Davidson, 2003). The ability to detect soft speech sounds is important for perceiving word endings (morphology) and sentences (syntax) as well as vocabulary, especially when language is acquired incidentally, as is often the case in infancy and early childhood (e.g., Davidson, Geers, & Nicholas, 2014),. Audibility through hearing aids has been shown to affect morphological development more than lexical development in children with mild-severe losses (Tomblin et al., 2015). However, the extent to which syntax and morphology are sensitive to differences in the audibility of speech is still an open question for children using CIs.
Age at cochlear implantation.
Children with CIs experience auditory deprivation during a developmental period that is known to be sensitive to auditory input. Therefore, a well-established predictor of language outcome is a child’s precise age of implantation (AOI), which is shown to affect outcomes in the years immediately following implantation (Bruijnzeel, Ziylan, Stegeman, Topsakal, & Grolman, 2016; Colletti, Mandala, Zoccante, Shannon, & Colletti, 2011; Leigh, Dettman, & Dowell, 2016; May-Mederake, 2012; Nicholas & Geers, 2006, 2007, 2013; Nittrouer et al. 2014; Tobey et al., 2013). This result appears to also hold in most long-term follow-up studies (Castellanos et al., 2014; Geers & Nicholas, 2013; Uziel et al., 2007), though this conclusion is not universal (Dunn et al., 2014). In most of these studies, CI surgery below 18–24 months of age provides a reliably better benefit than surgery after this age.
The first year of life is a particularly sensitive period for language learning (Levine et al, 2016) and several recent reports show an additional advantage for exceptionally early surgery (i.e., < 12 months) on a range of outcome measures. For example, Dettman et al. (2016) reported results from a study of 403 children using CIs who were tested at the beginning and end of the primary school years. Those receiving a CI before 12 months of age scored significantly higher on measures of vocabulary, receptive and expressive language, and speech production than those implanted at 13–18 months or older (see also Nicholas & Geers, 2013).
That very early cochlear implantation might provide special benefit for spoken language development is not surprising but the reasons for this are open to interpretation. Some attribute AOI effects to the existence of sensitive periods while others point out that children who receive a CI in infancy simply have a smaller language delay to overcome at the point of surgery. On the first point, documentation of sensitive periods for auditory input in NH infants is fairly abundant (Kuhl et al., 2006; Rivera-Gaxiola, Silvia-Pereyra, & Kuhl, 2005; Werker & Tees, 1984). These studies suggest that the brains of all infants may be differentially receptive to auditory input at different points in early development and their subsequent ability to organize that input in the efficient learning of spoken language has potential to be compromised (see Teoh, Pisoni, & Miyamoto, 2004). A recent review of the mechanisms underlying all critical/sensitive periods suggests that both maturation of underlying neural circuitry and sufficient sensory input are necessary for initiating the period onset or opening. Molecular “brakes” are being identified which serve to limit plasticity and maintain changes at the close of this period of special receptivity and growth (Werker and Hensch, 2015). These neural activities very likely influence separate, though overlapping and highly related language domain timelines (Harrison, Gordon & Mount, 2005; Knudsen, 2004). Therefore the introduction of better-quality auditory input to deaf children during these special periods of receptivity in infancy and early childhood will be important across all domains in the language system. Optimal timing may be different across language domains or it may be that a domain such as phonology, which appears to be most sensitive from 6 months of fetal life through 12 months after birth, provides the foundational underpinning for subsequent lexical, morphological, and grammatical development to follow and is therefore most determining (Dettman & Dowell, 2010).
Duration of CI use.
Studies examining AOI effects must contend with the confounding fact that as the age is lowered, the duration of use is lengthened, given a particular test age. It is important to distinguish the effects of age at implantation, age at test, and duration of CI experience if possible and several of the studies cited above have this limitation. Spoken language has been shown to improve with increased CI experience on a variety of clinical measures and across a number of different age ranges (Geers & Nicholas, 2013; Nicholas & Geers, 2007; Svirsky, Teoh, & Neuburger, 2004; Tobey et al., 2013; Tomblin, Barker, Spencer, Zhang, & Gantz, 2005). A number of studies suggest that performance is related to years of CI experience rather than chronological age per se (Nicholas & Geers, 2006; Tomblin, Spencer, Flock, Tyler, & Gantz, 1999).
Problems inherent in comparing language progress in young deaf children using CIs include inconsistency across studies in modes of communication used, presence/absence of other disabilities, and differences in language domains chosen as outcome measures. Primarily because of the labor intensity involved in the analysis of language samples, most studies to date have not used these methods. We address the issues of interest in this study by holding several sources of variability constant through sample selection (communication method, early intervention, additional diagnoses, and AOI range) to answer our questions with measures derived from relatively lengthy parent-child conversations.
Questions Addressed in the Present Study
1. Are there relative strengths and weaknesses among expressive language domains and are they differently sensitive to age of implantation?
Based on the limited number of studies in children using CIs to date, we predicted the delays would be greater in the areas of morphology and syntax and that the earliest ages of implantation would confer the greatest benefit to those domains.
2. Which demographic and device-related factors predict the magnitude of language delay present at 4.5 years of age?
Given set test ages, we will first attempt to separate the effects of AOI from duration of use in our analyses. We expect younger AOI will predict better language outcomes in all domains, even when duration of device use is similar. We expect very early placement of the first CI (e.g., before 12 months) to differentially reduce delays in all domains, with greater benefit to morpho-syntax than to vocabulary. Previous reports suggest an added advantage might be seen for good hearing through a hearing aid before receipt of a CI and we will examine that but also determine further benefit provided by differences in post-surgery hearing through the implant. In line with results from hearing children we expected that higher mother’s education level would also predict better language skill.
Methods
Study Design
This was a longitudinal study with testing of children at Age 3.5 years (± 2 months) and follow-up testing one year later, at Age 4.5 years (± 2 months).
Participants
Participants were 126 children with severe-profound hearing loss who used CIs and 30 children with no hearing loss. Inclusion criteria included: severe-profound hearing loss from birth, first CI between ages of 6–38 months, from a primarily English-speaking home, listening and spoken language intervention since time of CI surgery, no conditions other than hearing loss known to affect communication, and no loss of CI use for more than 30 days.
Children with cochlear implants.
Child-parent pairs were recruited for participation from 25+ locations across North America. Letters describing the study were distributed to parents of children enrolled at schools for deaf children and speech-language therapy practices who met the inclusion criteria. A research team member then traveled to perform the data collection at the school or therapist’s office. Child, family, and audiological characteristics of all children are presented in Table 1.
Table 1.
Child, family, and audiological characteristics of all children with cochlear implants.
| Mean | SD | Range | N | |
|---|---|---|---|---|
| Age 1st CI surgery (months) | 19.23 | 8.51 | 6 – 38 | 126 |
| 6–11 mos N=27 | ||||
| 12–18 mos N=42 | ||||
| 19–24 mos N=24 | ||||
| 25–30 mos N=14 | ||||
| 31–38 mos N=22 | ||||
| Duration of CI Use at Age 4.5 (months) | 35.54 | 8.47 | 18 – 48 | 126 |
| Pre-CI aided thresholds | 65.54 | 15.23 | 32 – 98 | 123 |
| CI-aided thresholds | 24.80 | 6.18 | 10 – 38 | 123 |
| Mother’s Educ (years) | 15.74 | 2.24 | 11 – 20 | 125 |
| % Bilateral CIs | 33.3% | 42/126 | ||
| % Female | 47.6% | 60/126 | ||
| % Male | 52.3% | 66/126 | ||
Children without hearing loss.
Children were recruited for the NH comparison group from the local community (Midwestern US city). An attempt was made to roughly match this group on age, gender and maternal education to the children with CIs. There were no significant differences between the deaf and hearing children (or between the different AOI groups) on proportions of boys and girls or chronological age at test. All children in the NH comparison group passed a hearing screening and none scored below the normal range on the Peabody Picture Vocabulary Test-III (Dunn & Dunn, 1997).
Data Collection/Language Sampling
Each participant was videotaped in a 30-minute play session with his or her own parent in a quiet room once at Age 3.5 and again at Age 4.5. These sessions took place in the child’s school, therapist’s office, or cochlear implant center. The parent was instructed to communicate with the child as they would in everyday interactions and to simply play with their child. Four sets of toys, one set introduced by the examiner approximately every 7–8 minutes, were provided for purposes of stimulating conversation. As described in the following section, one measure was derived from the full 30-minute sessions and two measures from the first 100 utterances of the sessions.
Study Variables
Independent variables.
Demographic characteristics were collected through a questionnaire and included maternal education level, gender and age at first CI surgery. Audiological characteristics were collected from the child’s audiologist and included pre- and post-implantation aided thresholds and whether or not the child had 1 or 2 CIs.
Dependent variables.
Three measures of spoken language were derived from the language sample and were used to represent three different linguistic domains. They were counted using the CLAN software programs provided by the CHILDES project (MacWhinney, 2000). Number of Different Root Words (NDRW), Mean Length of Utterance in Words (MLU-w), and Number of Different Bound Morphemes (NDBM) were counted from the transcripts to represent lexical, syntactic, and morphological performance, respectively. The NDRW is a measure of the breadth of the child’s vocabulary. The Mean Length of Utterance (in words; MLU-w) is included as a broad estimate of syntactic development. The Number of Different Bound Morphemes indicates the breadth of the child’s mastery of bound morpheme types. These measures are commonly used in the analysis of language samples in the field of language disorders and in the analysis of spoken language samples of children with hearing loss (Dollaghan et al., 1999; Geers, Nicholas, & Sedey, 2003; Klee, 1992; Nittrouer et al., 2014; Pan, 1994; Paul, 2001; Watkins, Kelly, Harbers, & Hollis, 1995). All of the children were learning spoken language exclusively; only spoken language was included in the dependent variable counts, and all references to a word in this report refer to a spoken word.
Transcript Preparation and Reliability
Transcription procedures.
Experienced teachers of deaf children were trained to transcribe all intelligible spoken words produced by children and parents from high-quality video-recordings. When a transcriber questioned whether an utterance was an intelligible representation of a potential target word, the following criteria were required: same number of syllables, match on at least one vowel, and match on at least one consonant. A second transcriber reviewed each of the videotapes with its transcript and made any necessary corrections due to omission or error. The transcription procedures follow the CHAT format of the Child Language Data Exchange System (CHILDES; MacWhinney, 2000) and follow the same procedures as previous studies in our laboratory (Nicholas & Geers, 2007). Utterance segmentation was determined by syntactic cues, prosodic cues (intonation contour), pauses of 2–3 seconds and changes in conversational turn. More than 2 independent clauses joined by conjunctions led to an utterance division before the third independent clause to avoid artificial lengthening by run-on clausal chaining. Total number of utterances for the children using CIs ranged from 36 to 466 at Age 3.5 and from 78 to 502 at Age 4.5. For the NH children, total number of utterances ranged from 154 to 411 at Age 3.5 and from 105 to 411 at 4.5.
In the calculation of NDRW, words that contain a single free (root) morpheme, such as look (root of look-s, look-ed, look-ing) were counted as a single vocabulary word, thereby decreasing the possibility of over- or underestimating the breadth of the child’s base lexicon on the basis of high or low usage of bound morphemes. In order to control for possible effects of overall productivity of the language sample, this measure was computed only from the child’s first 100 utterances.2 There were 5 children in this sample who did not produce 100 utterances in one or another of their language samples. Their total utterances in those samples were 36, 77, and 96 (in the 3.5 samples) and 78 and 99 (in the Age 4.5 samples). The decision was made to exclude from analysis those children who produced fewer than 95 utterances in either sample and to retain those who produced 96 and 99. Therefore 126 children with CIs were included in the analyses.
In the calculation of MLU-w, it is our practice to exclude repetitions, false starts, and abandoned utterances in the calculation of this measure, consistent with other researchers. Parker and Brorson (2005) showed MLU-w and MLU-m (in morphemes) to be nearly perfectly correlated (r = .998) in 40 preschool language samples of 100 utterances. Similarly, Rice et al. (2010) found an MLU-w/MLU-m correlation of .994 and noted “…the MLU in words or morphemes yields reliable and age-validated estimates of children’s language growth”. MLU-w was chosen for the present study for efficiency and greater reliability of transcription. It was calculated from the entire 30-minute language transcript. When transcribing bound morphemes, we included word-final inflectional suffixes, such as –s, –es, –’s, –ing, –ed, --er (e.g., bigger)- as well as contractions, such as –’s (is), –’nt (not), –ll (will), –’re (are), –’m (am), and –’us (us). This count was taken from the child’s first 100 utterances in the language sample, repeating the exclusion of the 3 children mentioned above who produced fewer than 96 utterances.
Transcription reliability.
We employ extensive and detailed training procedures to establish good inter-transcriber reliability in our studies (Nicholas & Geers, 2006). A single person was utilized as the primary transcriber for all language samples in this study, with 3 different persons serving as verifiers. For the purposes of examining transcriber reliability, fifteen 30-minute language samples were independently transcribed in their entirety by the primary transcriber and a verifier. Mean values for each of the language variables produced were as follows: Number of Different Root Words (47, 51), Number of Different Bound Morphemes (6.2, 6.0), and MLU-w (0.82, 0.85). Correlations were calculated to determine the degree of correspondence between the two transcribers for each dependent variable and the mean correlation was .97.
Converting Raw Scores into Z-Scores
For the initial analyses that follow, we utilize raw scores, which are based on counts within the language transcripts. However, as the analyses proceed to questions that involve comparison of performance within language domains a common metric is required. For this reason, z-scores scores were created for each variable based on the values obtained from the normally-hearing comparison group (at the same test age). A value of “0” in this metric represents the mean value of the NH group at that test age and a value of “1.0” represents one standard deviation in the NH group. These scores will be referred to as “NHz scores” to reflect the idea that they operate like z-scores but were derived in this case from the NH sample. Use of these scores will allow direct comparison among the different linguistic domain variables based on the extent of language delay relative to NH age-mates.
Statistical analysis
Standard descriptive statistics were used to describe the children’s characteristics in each of the groups, the distribution of scores for the dependent variables, and the NHz scores. Histograms and Kolmogorov–Smirnof tests were used to test the assumption of normality for all continuous variables. A linear mixed-model analysis with within- and between- subjects factors (using the Proc Mixed procedure in SAS v. 9.4) was employed to explore the change in the scores for each of the dependent variables at the 2 test ages (time points) and also as separate analyses at each of the two test ages. Further, we compared changes (or outcome levels in the separate analyses) between groups and between the three domains. Estimated marginal means were used to explore the estimated differences and evaluate their clinical importance. All statistical tests were 2-tailed and evaluated at the alpha level of 0.05.
Human Subjects (IRB) Approval
The study protocol was approved by the Human Research Protection Office of the first author’s university.
Results
First, we will confirm whether our sample of children with CIs exhibits language delays across domains in comparison with NH age-mates, a finding that has been very consistent in the literature. Based on published studies we expect the magnitude of any delays found to decrease over the one-year period between our test ages. Next we will address the questions that are the focus of this study:
Performance in Children with CIs relative to NH age-mates
Table 2 shows the raw score means, SDs, and ranges for both groups. The mixed-model analysis of NDBM and MLU data showed that independent of hearing-status group the children’s NDBM scores were 1.25 higher (greater number of different bound morphemes; 95% confidence interval: 0.84–1.66) and MLU scores were 0.52 higher (more words/utterance; 95% confidence interval: 0.42–0.62) at 4.5 as compared to scores at 3.5 years of age. Independent of the age of testing, NH children had significantly higher scores on NDBM by 2.87 points (95% confidence interval: 1.94 −3.79) and MLU by 1.04 points (95% confidence interval: 0.76–1.31) than the scores of children with CIs. For these two measures, the pattern of change in scores was not significantly different between the 2 groups (no statistically significant interaction effects). There was a significant interaction effect between test age and hearing status group for the NDRW, indicating that the increase in NDRW from age 3.5 to age 4.5 was significantly larger in the group of CI children (74.2 to 95.1) as compared to the group of NH children (111.7 to 124.5) with the NH children having higher scores overall.
Table 2.
Means, SDs, and ranges of raw scores for mean Number of Different Root Words (NDRW), Number of Different Bound Morphemes (NDBM) and Mean Length of Utterance in Words (MLUw) at both test ages. The number of children with CIs is 126 and with normal hearing (NH) is 30.
| CI | NH | |
|---|---|---|
| Age at Testing (in years) | Mean (SD) | Mean (SD) |
| NDRW* | ||
| 3.5 | 74.16 (25.49) | 111.67 (17.13) |
| 4.5 | 95.08 (27.08) | 124.53 (15.76) |
| NDBM* | ||
| 3.5 | 4.38 (2.66) | 7.63 (1.67) |
| 4.5 | 6.02 (2.73) | 8.50 (1.43) |
| MLUw + | ||
| 3.5 | 2.04 (.70) | 3.15 (.54) |
| 4.5 | 2.63 (.83) | 3.60 (.58) |
calculated on 100-utterances
calculated on entire 30-minute language sample
Primary study questions:
1. Are there relative strengths and weaknesses among expressive language domains and are they differently sensitive to age at implantation?
Pearson correlations were computed among domain raw scores at both test ages. Strong, positive correlations at both test ages suggest that these linguistic skills developed in tandem in children with CIs (.82 – .89 at Age 3.5; .78 – .89 at Age 4.5).
Standardized scores were used to allow for comparison of the three outcome measures. There was no significant difference in the estimated marginal means of the three domains at either 3.5 or 4.5 years of age. As expected, at both assessments the standardized scores of the CI children were lower than the standardized scores of NH children. The mean NHz-scores at both test ages were at least 1.7 below those for the NH comparison group in all domains.
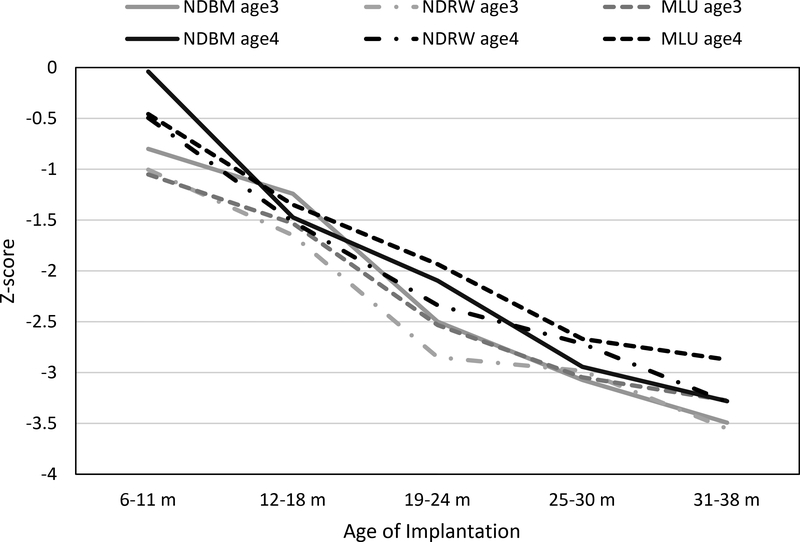
We further explored the differences between the three domains (within the group of CI children) after controlling for the impact of age at CI. Age at CI was categorized into 5 groups as described in Table 1. The mixed-model analysis showed that, independent of test age, there was no statistically significant difference between the 3 domains after controlling for the effect of age at CI. Age at CI was significantly associated with the domain scores at both test ages. The younger the age of implantation, the smaller the delay reflected in the standardized domain scores, i.e., the closer to the NH children’s scores (see Fig. 1). There was no significant interaction effect, indicating that very early cochlear implantation did not differentially benefit any one linguistic domain over another. The same pattern of results were obtained when the model was configured to consider change in scores as when considering each test age separately.
Figure 1.
Magnitude of domain delays by age of implantation group, measured in z-scores derived from the NH comparison group. Higher points on the ordinate (smaller NHz-score values) represent smaller delays.
The percentages of CI children in the 5 AOI groups performing within normal limits (WNL; NHz-score > −1.0) relative to NH age-mates at each test age are displayed in Table 3. A much larger proportion of the group with AOI 6–11 months scored WNL than in all other AOI groups in all domains. By age 4.5 the proportion scoring WNL in the 6–11 month AOI group is 30–40% higher than for those implanted just a few months later (12–18 months). Between 63% and 78% of children in this very early-implanted group scored WNL by the age of 4.5. By age 4.5 normal language levels were achieved by only about one-third of those implanted between 12–18 months and fewer than one-quarter of those implanted in the 19–24 month range. For those implanted between 25–38 months the percentages of the groups performing WNL remains very low at both test ages (ranging from 0–20%), with performance on the syntax measure having the smallest percentages (< 10%) at both test ages.
Table 3.
Percentage of children producing conversational language within normal limits (defined as NHz-scores above −1.0), by AOI group. Groups of children with similar durations of CI use (expressed in months) but different ages of implantation (in months) may be compared. For example, direct comparison can be made between children with 33 months of CI use at Test age 3.5 and those with 34 months of CI use at Test Age 4.5. Appropriate sets of numbers are bolded for ease of comparison. The linguistic variables considered are Number of Different Root Words (NDRW), Number of Different Bound Morphemes (NDBM), and Mean Length of Utterance in Words (MLUw).
| AOI group | Age at Test = 3.5 years | Age at Test = 4.5 years | ||||||
|---|---|---|---|---|---|---|---|---|
| NDRW | NDBM | MLUw | Mean mos. duration of CI use at test | NDRW | NDBM | MLUw | Mean mos. duration of CI use at test | |
| 6–11 | 44 % | 59 % | 44 % | 33 | 67 % | 78 % | 63 % | 45 |
| 12–18 | 37 % | 56 % | 32 % | 28 | 34 % | 37 % | 34 % | 40 |
| 19–24 | 4 % | 17 % | 0 % | 22 | 21 % | 21 % | 13 % | 34 |
| 25–30 | 7 % | 14 % | 0 % | 15 | 14 % | 0 % | 7 % | 27 |
| 31–38 | 0 % | 20 % | 0 % | 9 | 10 % | 10 % | 10 % | 22 |
2. Which demographic or device-related factors predict the magnitude of language delay present at 4.5 years of age?
In the following analyses we begin by addressing the potential multicollinearity issue between AOI and duration of CI use. Because we have two different test ages, we also have the opportunity to compare performance of children with different ages of implantation but same durations of use. Table 3 shows that between 44% and 59% of those implanted between 6–11 months of age scored on par with hearing age-mates after 33 months of device use (age 3.5), while only 13% to 21% of those with AOI between 19–24 months did so after 34 months of use (age 4.5). Similarly, after an average of 28 months of CI experience, scores reached the normal range for 32% to 56% of children implanted at 12–18 months of age, but only 0% to 14% of those implanted at 25–30 months of age reached the NH range after 27 months of CI experience. The advantage declined at older ages of implantation. Fewer than 20% of children implanted after 18 months of age scored WNL at 22 months of use, regardless of AOI.
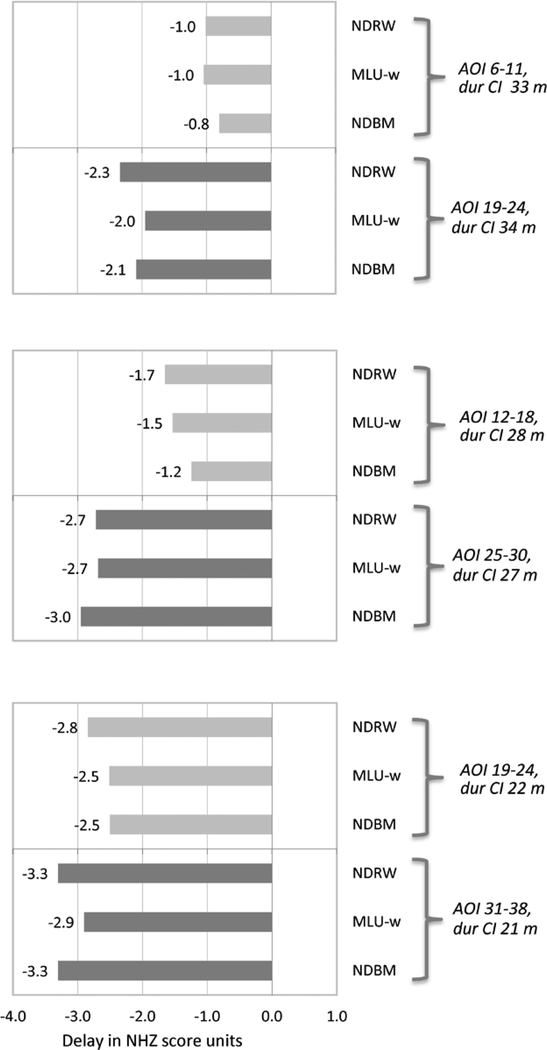
Figure 2 shows mean standardized scores for the three different paired-groups of children who have used a CI for the same amount of time but who have different ages of implantation. Note that the standardized scores used in these comparisons are already corrected for chronological age, since the NHz-score is based on an age-specific comparison/reference group. Also note that within these comparison sets the children tested at younger chronological age (the younger AOI in each comparison pair) would be at the cognitive maturational disadvantage, if one exists. In the top panel, domain scores of 3.5-year-olds who received a first CI between 6–11 months of age are compared with scores of 4.5-year-olds who received their first CI more than a year later (older), at the age of 19–24 months (mean duration of use 33–34 months). Children implanted before their first birthday reached language levels much closer to NH age-mates (within 1 SD) than children implanted a year later in all domains, given the same duration of CI. The middle panel of Figure 2 compares NHz scores for children implanted at 12–18 months with those implanted at 25–30 months with 27–28 months of CI use. Similar to the contrast above, those in the younger-implanted group were closer to NH levels than those implanted later with the same durations of use. The final comparison (bottom panel of Figure 2) was for groups with 21–22 months of CI experience. This comparison resulted in the smallest differences between younger and older AOI groups, but still showed an advantage for those implanted at the somewhat younger age. To examine the effect size of the mean NHz-score differences, Cohen’s d was calculated (with pooled SD) and is presented in Table 4. Very large effect sizes obtained from contrasts at younger surgery ages, indicating substantial benefit from earlier implantation despite same length of CI experience. Effect sizes diminished when surgery age exceeded about 18 months.
Figure 2.
Delays experienced by subsets of children with the same mean durations of CI use but different ages of implantation. Note: The smaller the value of the NHz-score, the better the outcome (closer to NH performance).
Table 4.
Effect size of mean differences in NHz-scores for Number of Different Root Words (NDRW), Number of Different Bound Morphemes (NDBM), and Mean Length of Utterance in Words (MLU-w), between children with different ages of implantation and similar durations of CI use. A Cohen’s D value of greater than 0.8 is considered a large effect size.
| Cohen’s d of Mean Difference | |||||
|---|---|---|---|---|---|
| Age of implantation (in months) | N | Duration of CI use in months | NDRW | MLU-w | NDBM |
| 6–11 | 27 | 33 | 1.02 | 0.88 | 0.92 |
| 19–24 | 24 | 34 | |||
| 12–18 | 41 | 28 | 0.87 | 1.04 | 1.40 |
| 25–30 | 14 | 27 | |||
| 19–24 | 24 | 22 | 0.31 | 0.32 | .48 |
| 31–38 | 20 | 21 | |||
Correlations among candidate characteristics and outcome scores were calculated for the sample of children with CIs. Performance in all three domains was most highly correlated with age at implantation and duration of CI use (absolute value of correlations range from .55 −.61). Age of implantation and duration of CI use were almost perfectly correlated in this sample due to predetermined test ages. Modest though significant correlations were also obtained with mother’s education level (.22 – .25) and use of bilateral implants (r = .26 – .33). Two direct measures of audibility were related to single domain scores: CI-aided thresholds were related to MLU-w (−.18) and Pre-CI aided PTA was related to vocabulary (−.18). Gender was unrelated to domain outcomes. Several predictor variables were significantly inter-related. Children with younger age at implantation were more likely to have bilateral CIs (−.39) and had more highly educated mothers (−.27), poorer pre-CI aided thresholds (−.29), and better CI-aided thresholds post surgery (.26).
Multiple regression analysis was used to examine the proportion of independent variance in language outcomes at age 4.5 accounted for by selected characteristics. We included AOI, Pre-CI aided PTA, post-CI aided PTA and mothers’ education level1. The outcome variables were entered into the model as z-scores. Table 5 summarizes the results of multiple linear regression, exploring factors associated with each of the dependent variables. Beta coefficients reflect the strength of the relationship. 95% confidence intervals around the beta coefficients indicate that only Pre-CI better-ear PTA and Age at 1st CI are associated with better outcomes for vocabulary and sentence length. The same characteristics, plus CI-PTA at Age 3.5, predict better outcomes for the morphology measure.
Table 5.
Beta weights and their 95% confidence intervals for NHz-scores (at Age 4.5) for three language domains: Number of Different Root Words, Mean Length of Utterance in Words, and Number of Different Bound Morphemes. Statistically significant beta weights are bolded and are detected by confidence intervals that do not include “0” (the null value).
| Number of Different Root Words | MLU-words | Number of Different Bound Morphemes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | Lw 95% Conf Int | Up 95% Conf Int | B | Lw 95% Conf Int | Up 95% Conf Int | B | Lw 95% Conf Int | Up 95% Conf Int | |
| (Constant) | 1.813 | −1.11 | 4.74 | 0.72 | −1.76 | 3.20 | .796 | −2.377 | 3.968 |
| Mother’s Education | .062 | −0.06 | 0.18 | 0.06 | −0.05 | 0.16 | .104 | −.028 | .237 |
| Pre-CI Better Ear PTA | −.044 | −0.06 | −0.03 | −0.02 | −0.04 | −0.01 | −.041 | −.059 | −.023 |
| Age at 1st CI | −.138 | −0.17 | −0.11 | −0.11 | −0.14 | −0.08 | −.156 | −.191 | −.122 |
| CI PTA at Age 3.5 | .033 | −0.01 | 0.07 | 0.02 | −0.02 | 0.05 | .055 | .012 | .098 |
Note: PTA = Pure-tone average, CI = Cochlear implant
Lw = Lower Up = Upper, Conf Int = Confidence Interval
Discussion
This study had two primary study questions. The first sought information about whether any linguistic domains showed larger deficits than others, potentially requiring earlier or more focused intervention. As an ancillary question related to this issue, we examined the relative benefits of younger AOI and longer duration of device experience. For the second of the primary study questions we compared demographic and audiological characteristics of performance in each domain (gender, maternal education, pre-implant aided thresholds, age at first CI, and CI-aided thresholds) for their effect on the various domain outcomes in the preschool years. Results of the present study inform our thinking about sensitive periods for development in different domains and the effects of audibility of speech both before and after cochlear implantation.
In all three expressive domains that we considered, children using CIs showed significant delays compared to NH age-mates when assessed from samples of casual conversation at 3.5 and 4.5 years of age. This finding complements the existing literature analyzing scores on standardized language tests. It extends our knowledge by revealing that these delays persist through preschool for the majority of children in all but the very youngest AOI group (6–11 months). Significant growth in these domain skills was seen over the year between test ages, for both the children using CIs and their NH counterparts. Improvement rates were similar for sentence length and bound morpheme use, indicating that both NH children and those using CIs continue to improve during this developmental period. In the expansion of vocabulary, children with CIs grew at a faster rate than their NH age-mates. The initial question of this investigation was whether domains differed in extent of relative delay compared to NH age-mates. We examined this by scaling scores on a common metric to allow for direct comparisons. This methodological aspect of our study appears to be unique among studies using language sampling. Previous reports of domain differences that utilized formal language testing and focused more heavily on receptive language hinted at relative grammatical delays. Our analyses revealed no relative delays in any particular domain but rather a fairly even pattern of substantial delay at both test ages. Nevertheless, about 1/3 of children in the combined CI group had scores that were similar to their hearing counterparts by age 4.5.
Next we looked a little deeper into age at implantation effects. Given results from previous studies our expectation was that we would find earlier AOI to be predictive of language outcomes, but it was unclear whether effects would differ across language domains. We found, in fact, that they did not. While there was a steady and dramatic effect for earlier implantation (see Figure 1 and Table 3), this effect appeared equally across all expressive linguistic domains measured. The one domain that appears to show a trend toward differential sensitivity (see Figure 1) is bound morpheme use by those implanted at 6–11 months. The Pearson correlations showed that children implanted at younger ages achieved significantly better post-CI aided thresholds. One might speculate that an improved ability to hear less audible word endings during a period of special receptivity might lead to a differentially better result a few years later in the use of these morphemes in spontaneous speech. Focused study with multiple methodologies might be required to fully investigate this possibility.
Another goal was to identify the relative importance of several predictors of outcome at age 4.5. The significant correlations between language scores and age at first implant, mother’s education, bilateral CI use, and CI-aided thresholds are consistent with findings from studies that use formal language testing. When the strongest of these were considered simultaneously using regression analysis, significant independent variance across all domains was predicted by age at CI surgery. Surprisingly, pre-implant aided hearing, which did not exhibit a significant correlation with two out of three language outcomes, predicted significant added variance in all three domains when combined with surgery age in a regression analysis. This result reveals that children who experience better audibility through hearing aids can tolerate somewhat later ages at implantation and still achieve language outcomes that are comparable to those with more profound hearing loss who receive their CI at younger ages. This is consistent with the findings of Houston et al. (2012) and others for foundational word-learning abilities in a similar population. Maternal education level did not account for significant added variance once age at implant surgery and pre-implant hearing were included in the regression. CI-aided thresholds accounted for significant added variance only for bound morphemes, where audibility of soft sounds is most critical. This result is consistent with reports that audibility through hearing aids affects morphological development more than lexical development in children with mild-severe losses (Tomblin et al., 2015). Very few studies of language outcomes have included post-surgery CI-aided hearing thresholds as a predictor but recognition of its importance is emerging (Davidson et al., 2014). Achieving the lowest possible thresholds in CI programming may help to raise the audibility of unstressed word endings that characterize bound morphemes.
Contrasting the effects of AOI and duration of CI use is possible to some degree in this longitudinal study. With this study design we were able to compare groups with the same duration of use and different surgery ages. Of course, this meant that the third variable of the time relationship (chronological age at test) would differ. In this case the child’s chronological test age was always older for the group that had the later/older AOIs. Therefore, if any benefit of older chronological age would be conferred (a developmental cognitive advantage, for instance) it would go to the group with later AOIs. Despite this possible disadvantage for earlier-implanted groups, we found that in all three of these contrasts (see Figure 2), they in fact had notably smaller delays relative to NH age-mates. In other words, children implanted at younger ages approached normal levels of language production more rapidly than those who received their device just one year later even when equated for duration of CI use. Language level is not just a function of “hearing age” as determined by how long a child has received improved access to the auditory speech signal. Rather, children appear to receive more language benefit from auditory stimulation if it occurs earlier rather than later in development. The younger the age at initial stimulation, the smaller the initial language delay, and the stronger the positive influence on future language acquisition.
These results add to our understanding of development in the expressive language domain in several ways. We have seen that significant language delays exist in spontaneous conversations between children with CIs and their parents, even for those with the very youngest CI surgery ages. The relative delays were similar across domains, a finding that is consistent with the finding of our previous reports on older children (ages 9–12), in which we’d found no differences or only slight vocabulary advantages in the expressive language domain on standardized tests (Geers & Nicholas, 2013).
One of the strengths of this study is the ability to focus directly on variables of interest by reducing the effects of confounding influences through sample selection. While this allows for greater precision in making the comparisons of interest, it also necessarily reduces the generalizability of the findings. These conclusions are relevant for those children who receive a CI by the age of 38 months, who take a listening and spoken-language approach to early intervention, and who do not have other conditions which are known to interfere with communication. We believe, however, that these characteristics apply to the majority of children in this country who are receiving cochlear implant today as an intervention for severe-profound hearing loss.
Clearly there is a clinical need to intensify intervention for children implanted after their second birthday. Relatively small language changes between 3.5 and 4.5 years of age were observed for later-implanted children with profound pre-implant thresholds, suggesting they may not catch up with hearing age-mates without extended intervention. Given the recent finding that early expressive language skills in this population predict language, executive functioning, and academic skills up to 16 years later (Castellanos, Pisoni, Kronenberger, & Beer, 2016), future research should focus on identifying parent and child characteristics that protect later-implanted children from long-term language delay and early intervention characteristics that accelerate the rate of language development and promote successful educational integration with hearing age-mates.
Acknowledgments
As a report of a longitudinal study, this manuscript includes some data also used in a previously published report. This work was supported by the NIH-NIDCD under grant # R01-DC004168 (Nicholas, PI). Special thanks to Julia Biedenstein and Sarah Fessenden for data collection, transcription and verification of language samples and to Christine Brenner, Dorina Kallogjeri, and Michael Strube for data management and statistical analyses. We thank the families that participated in the study and the schools, therapists, and implant centers assisting in recruitment.
Footnotes
The authors have no conflicts of interest to report. No financial interest or benefit has been provided to the authors as a result of this research.
The Number of CIs was significantly correlated with CI-aided thresholds. Separate regression analyses were conducted with each of these post-CI audibility variables included in the models, but the model with Number of CIs is not included here as that variable did not predict significant independent variance.
It should be noted here that our measure of vocabulary diversity is fairly conservative in that we focus on ‘root words’, so caution should be exercised when raw score counts are compared across studies.
Contributor Information
Johanna G. Nicholas, Dept of Otolaryngology, Box 8115, Washington University School of Medicine, 660 S. Euclid Ave., St. Louis, MO 63130, USA, JNicholas@wustl.edu, phone: (314)747-7172.
Ann E. Geers, School of Behavioral and Brain Sciences, The University of Texas at Dallas, GR41, 800 West Campbell Rd., Richardson, TX 75080 phone: (972)883-2357, AGeers@utdallas.edu
References
- Bates E, Dale P, Thal D 1995. Individual differences and their implications for theories of language development (pp. 96–151). In Fletcher P & MacWhinney B (Eds.), The Handbook of Child Language. Cambridge, MA: Blackwell. [Google Scholar]
- Boons T, DeRaeve L, Langereis M, Peeraer L, Wouters J, VanWieringen A (2013). Expressive vocabulary, morphology, syntax, and narrative skills in profoundly deaf children after early cochlear implantation. Research in Developmental Disabilities, 34: 2008–2022. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel H, Ziylan F, Stegeman I, Topsakal V, Grolman W 2016. A systematic review to define the speech and language benefit of early (<12 months) pediatric cochlear implantation. Audiology & Neurotology, 21: 113–126. [DOI] [PubMed] [Google Scholar]
- Caselli MC, Rinaldi P, Varuzza C, Giuliani A, Burdo S 2012. Cochlear implant in the second year of life: Lexical and grammatical outcomes. Journal of Speech, Language and Hearing Research, 55: 382–394. doi: 10.1044/1092-4388(2011/10-0248) [DOI] [PubMed] [Google Scholar]
- Castellanos I, Kronenberger WG, Beer J, Henning SC, Colson BG, Pisoni DB 2014. Preschool speech intelligibility and vocabulary skills predict long-term speech and language outcomes following cochlear implantation in early childhood. Cochlear Implants International, 15: 200–210. doi: 10.1179/1754762813Y.0000000043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos I, Pisoni DB, Kronenberger WG, Beer J 2016. Early expressive language skills predict long-term neurocognitive outcomes in cochlear implant users: Evidence from the MacArthur-Bates Communicative Development Inventories. American Journal of Speech-Language Pathology, 25: 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colletti L, Mandala M, Zoccante L, Shannon RV, Colletti V 2011. Infants versus older children fitted with cochlear implants: Performance over 10 years. International Journal of Pediatric Otorhinolaryngology, 75: 504–509. doi: 10.1016/j.ijporl.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Dale PS, Dionne G, Eley TC, Plomin R 2000. Lexical and grammatical development: A behavioural genetic perspective. Journal of Child Language, 27: 619–642. doi: n/a [DOI] [PubMed] [Google Scholar]
- Davidson LS, Geers AE, Nicholas JG 2014. The effects of audibility and novel word learning ability on vocabulary level in children with cochlear implants. Cochlear Implants International, 15: 211–221. doi: 10.1179/1754762813Y.0000000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettman SJ, D’Costa WA, Dowell RC, Winton EJ, Hill KL, Williams SS 2004. Cochlear implants for children with significant residual hearing. Archives of Otolaryngology: Head and Neck Surgery, 130: 612–618. [DOI] [PubMed] [Google Scholar]
- Dettman S & Dowell R 2010. Language acquisition and critical periods for children using cochlear implants In Marschark M & Spencer PE (Eds.), The Oxford Handbook of Deaf Studies, Language, and Education, Vol. 2 Oxford, UK: Oxford University Press. [Google Scholar]
- Dettman SJ, Dowell RC, Choo D, Arnott W, Abrahams Y, Davis A, Dornan D, Leigh J, Constantinescu G, Cowan R, Briggs RJ 2016. Long-term communication outcomes for children receiving cochlear implants younger than 12 months: A multicenter study. Otology & Neurotology, 37: e82–e95. [DOI] [PubMed] [Google Scholar]
- Dollaghan CA, Campbell TF, Paradise JL, Feldman HM, Janosky JE, Pitcairn DN & Kurs-Lasky M 1999. Maternal education and measures of early speech and language. Journal of Speech Language and Hearing Research, 42: 1432–1443. doi: 1092-4388/99/4206-1432 [DOI] [PubMed] [Google Scholar]
- Duchesne L, Sutton A, Bergeron F 2009. Language achievement in children who received cochlear implants between 1 and 2 years of age: Group trends and individual patterns. Journal of Deaf Studies and Deaf Education, 14: 465–485. doi: 10.1093/deafed/enp010 [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM 1997. The Peabody Picture Vocabulary Test – 3rd edition. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Dunn CC, Walker EA, Oleson J, Kenworthy M, Van Voorst T, Tomblin JB……Gantz B 2014. Longitudinal speech perception and language performance in pediatric cochlear implant users: The effect of age at implantation, Ear and Hearing, 35: 148–160. doi: 0196/0202/14/352-0148/0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenson L, Dale PS, Reznick JS, Thal D, Bates E, Hartung J….Reilly J 1993. The MacArthur Communicative Development Inventories: User’s Guide and Technical Manual. San Diego, CA: Singular Press. [Google Scholar]
- Geers A, Brenner C, Davidson L 2003. Factors associated with development of speech perception skills in children implanted by age five. Ear and Hearing, 24: S24–S35. doi: 0196/0202/03/241S-0024S/0 [DOI] [PubMed] [Google Scholar]
- Geers AE, Moog JS, Biedenstein J, Brenner C, Hayes H 2009. Spoken language scores of children using cochlear implants compared to hearing age-mates at school entry. Journal of Deaf Studies and Deaf Education, 14: 371–385. [DOI] [PubMed] [Google Scholar]
- Geers AE, Nicholas JG 2013. Enduring advantages of early cochlear implantation for spoken language development. Journal of Speech, Language, and Hearing Research, 56: 643–653. doi: 10.1044/1092-4388(2012/11-0347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers AE, Nicholas JG, Sedey AL 2003. Language skills of children with early cochlear implantation. Ear and Hearing, 24: 46S–58S. doi: 0196/0202/03/241S-0046S/0 [DOI] [PubMed] [Google Scholar]
- Harrison RV, Gordon KA, Mount RJ 2005. Is there a critical period for cochlear implantation in congenitally deaf children? Analyses of hearing and speech perception performance after implantation. Developmental Psychobiology, 46: 252–261. [DOI] [PubMed] [Google Scholar]
- Houston DM, Stewart J, Moberly A, Hollich G, Miyamoto RT 2012. Word learning in deaf children with cochlear implants: Effects of early auditory experience. Developmental Science, 15: 448–461. doi: 10.1111/j.1467-7687.2012.01140.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee T 1992. Developmental and diagnostic characteristics of quantitative measures of children’s language production. Topics in Language Disorders, 12: 28–41. [Google Scholar]
- Knudsen EI 2004. Sensitive periods in the development of brain and behavior. Journal of Cognitive Neuroscience, 16: 1412–1425. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Stevens E, Hayashi A, Deguchi T, Kiritani S, Iverson P 2006. Infants show facilitation for native language phonetic perception between 6 and 12 months. Developmental Science, 9: F13–F21. [DOI] [PubMed] [Google Scholar]
- Leigh JR, Dettman SJ, Dowell RC 2016. Evidence-based guidelines for recommending cochlear implantation for young children: Audiological criteria and optimizing age at implantation. International Journal of Audiology, 55: S9–S18. [DOI] [PubMed] [Google Scholar]
- MacWhinney B 2000. The CHILDES Project: Tools for Analyzing Talk (3rd ed.). Mahwah, NJ: Erlbaum. [Google Scholar]
- Mariscal S, Gallego C 2012. The relationship between early lexical and grammatical development in Spanish: Evidence in children with different linguistic levels. Spanish Journal of Psychology, 15: 112–123. doi: 10.5209/rev_SJOP.2012.v15.n1.37293 [DOI] [PubMed] [Google Scholar]
- May-Mederake B 2012. Early intervention and assessment of speech and language development in young children with cochlear implants. International Journal of Pediatric Otorhinolaryngology, 76: 939–946. doi: 10.1016/j.ijporl.2012.02.051 [DOI] [PubMed] [Google Scholar]
- McGregor KK, Sheng L, Smith B 2005. The precocious two-year-old: status of the lexicon and links to the grammar. Journal of Child Language, 32: 563–585. [DOI] [PubMed] [Google Scholar]
- Newport EL 2006. Critical periods in language development In Nadel L (Ed.), Encyclopedia of Cognitive Science. London: Macmillan Publishers Ltd/Nature Publishing Group. [Google Scholar]
- Nicholas JG, Geers AE 2006. Effects of early auditory experience on the spoken language of deaf children at 3 years of age. Ear & Hearing, 27: 286–298. doi: 0196/0202/06/2703-0286/0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas JG, Geers AE 2007. Will they catch up? The role of age at cochlear implantation in the spoken language development of children with severe-profound hearing loss. Journal of Speech, Language and Hearing Research, 50: 1048–1062. doi: 1092-4388/07/5004-1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas JG, Geers AE 2013. Spoken language benefits of extending cochlear implant candidacy below 12 months of age. Otology & Neurotology, 34: 532–538. doi: 10.1097/MAO.0b013e318281e215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nittrouer S, Sansom E, Low K, Rice C, Caldwell-Tarr A 2014. Language structures used by kindergartners with cochlear implants: Relationship to phonological awareness, lexical knowledge and hearing loss. Ear and Hearing, 35: 506–518. doi: 0196/0202/2014/355-0506/0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BA 1994. Basic measures of child language (pp. 26–49). In Sokolov JL & Snow CE (Eds.), Handbook of Research in Language Development Using CHILDES. Hillsdale, NJ: Lawrence Earlbaum. [Google Scholar]
- Parker MD, Brorson K 2005. A comparative study between mean length of utterance in morphemes (MLUm) and mean length of utterance in words (MLUw). First Language, 25: 365–376. doi: 10.1177/0142723705059114 [DOI] [Google Scholar]
- Paul R 2001. Language Disorders: From Infancy Through Adolescence (2nd ed.). St. Louis, MO: Mosby-Yearbook. [Google Scholar]
- Rice ML, Smolik F, Perpich D, Thompson T, Rytting N, Blossom M 2010. Mean length of utterance levels in 6-month intervals for children 3 to 9 years with and without language impairments. Journal of Speech, Language, and Hearing Research, 53: 333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Gaxiola M, Silvia-Pereyra J, Kuhl PK 2005. Brain potentials to native and non-native speech contrasts in 7- and 11-month-old American infants. Developmental Science, 8: 162–172. [DOI] [PubMed] [Google Scholar]
- Schorr EA, Roth FP, Fox NA 2008. A comparison of the speech and language skills of children with cochlear implants and children with normal hearing. Communication Disorders Quarterly, 29: 195–210. doi: 10.1177/1525740108321217 [DOI] [Google Scholar]
- Spencer PE 2000. Individual differences in language performance after cochlear implantation at one to three years of age: Child, family, and linguistic factors. Journal of Deaf Studies and Deaf Education, 9: 395–412. doi: 10.1093/deafed/enh033 [DOI] [PubMed] [Google Scholar]
- Svirsky MA, Teoh S-W, Neuburger H 2004. Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiology & Neurotology, 9: 224–223. doi: 10.1159/000078392 [DOI] [PubMed] [Google Scholar]
- Szagun G 2001. Language acquisition in young German speaking children with cochlear implants: Individual differences and implications for conceptions of a ‘sensitive phase’. Audiology & Neurotology, 6: 288–297. doi: 1420-3030/01/0065-0288 [DOI] [PubMed] [Google Scholar]
- Teoh SW, Pisoni DB, Miyamoto RT 2000. Cochlear implantation in adults with prelingual deafness. Part II. Underlying constraints that affect audiological outcomes. Laryngoscope, 114: 1714–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey EA, Thal D, Niparko JK, Eisenberg LS, Quittner AL, Wang N-Y, CDaCI Investigative Team. 2013. Influence of implantation age on school-age language performance in pediatric cochlear implant users. International Journal of Audiology, 52: 219–229. doi: 10.3109/14992027.2012.759666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Barker BA, Spencer LJ, Zhang X, Gantz BJ 2005. The effect of age at cochlear implant initial stimulation on expressive language growth in infants and toddlers. Journal of Speech, Language, and Hearing Research, 48: 854–867. doi: 1092-4388/05/4804-0853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Harrison M, Ambrose SE, Walker EA, Oleson JJ, Moeller MP 2015. Language outcomes in young children with mild to severe hearing loss. Ear & Hearing, 36: 76S–91S. doi: 0196/0202/2015/36Supplement1-076S/0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Spencer L, Flock S, Tyler R, Gantz B 1999. A comparison of language achievement in children with cochlear implants and children with hearing aids. Journal of Speech, Language, and Hearing Research, 42: 497–511. doi: 1092-4388/99/4202-0497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Zhang X 2006. The dimensionality of language ability in school-age children. Journal of Speech, Language, and Hearing Research, 49: 1193–1208. doi: 1092-4388/06/4906-1193 [DOI] [PubMed] [Google Scholar]
- Uziel AS, Sillon M, Vieu A, Artieres F, Piron J-P, Daures J-P, Mondain M 2007. Ten-year follow-up of a consecutive series of children with multichannel cochlear implants. Otology & Neurotology, 28: 615–628. doi: 10.1097/01.mao.0000281802.59444.02 [DOI] [PubMed] [Google Scholar]
- Watkins RV, Kelly DJ, Harbers HM, Hollis W 1995. Measuring children’s lexical diversity: Differentiating typical and impaired language learners. Journal of Speech and Hearing Research, 38: 1349–1355. doi: 0022-4685/95/3806-1349 [DOI] [PubMed] [Google Scholar]
- Werker JF, Hensch TK 2015. Critical periods in speech perception: New directions. Annual Review of Psychology, 66: 173–196. [DOI] [PubMed] [Google Scholar]
- Werker JF, Tees RC 1984. Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behavior and Development, 7: 49–63. [Google Scholar]
- Wiig E, Secord W, Semel E 1992. Clinical Evaluation of Language Fundamentals - Preschool. San Antonio, TX: Psychological Corporation, Harcourt Brace. [Google Scholar]