Abstract
A 61-year-old Caucasian man presented with papules on his left forearm and hand three months after liver transplantation: images from physical exam, pathology, and microbiology are presented. Skin biopsy confirmed the presence of fungal elements within the hair shaft, which is consistent with Majocchi granuloma, also known as nodular granulomatous perifolliculitis. A combination of fungal culture, microscopic morphology, and gene sequencing was used to identify the causative organism. The patient recovered with appropriate systemic antifungal therapy.
Keywords: dermatophyte, Majocchi granuloma, nodular granulomatous perifolliculitis, transplant, Trichophyton rubrum
1 |. CASE REPORT
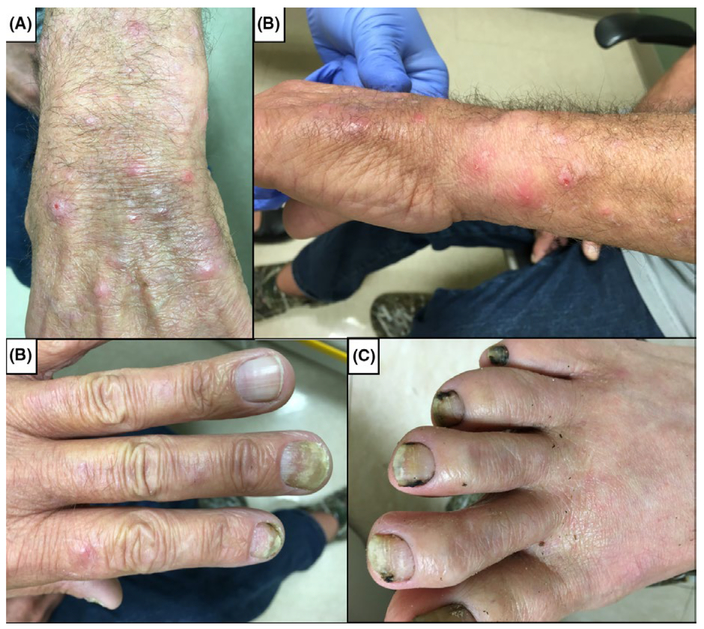
A 61-year-old Caucasian male presented to clinic with a 2-month history of erythematous papules with distribution on his left forearm and dorsal surface of the left hand and digits (Figure 1A–B). The lesions were raised, <1 cm in diameter, and rarely pruritic. Lesions were temporally evolving, with some resolving and new papules emerging at varying locations along the distal left arm and dorsal surface of the left hand. He was afebrile and did not display any other signs of systemic illness. The patient underwent liver transplantation 3 months prior to presentation for cirrhosis secondary to transfusion-related hepatitis C viral infection. The donor was seronegative for cytomegalovirus and seropositive for Epstein-Barr virus, while the recipient was seropositive for both viruses. He received induction immuno-suppression with basiliximab and steroids, followed by maintenance with tacrolimus and mycophenolic acid. Tacrolimus was changed to cyclosporine one month after transplant due to concern for posterior reversible encephalopathy syndrome. The patient was receiving trimethoprim-sulfamethoxazole (TMP/SMX) and valganciclovir as antimicrobial prophylaxis. Two months after transplantation, TMP/SMX was changed to inhaled pentamidine due to headaches and fever. Prior to transplantation, the patient had a history of thick and yellow left fingernails and bilateral toenails, but his toenails became black after transplantation (Figure 1C–D). The patient is a fisherman. He does not garden and has not had any recent insect bites.
FIGURE 1.
(A-B) Erythematous papules found on the patient’s left forearm and wrist, and (C) onychomycosis found on patient’s fingernails and (D) toenails
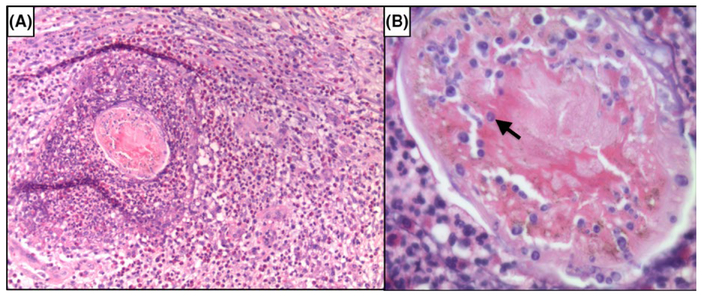
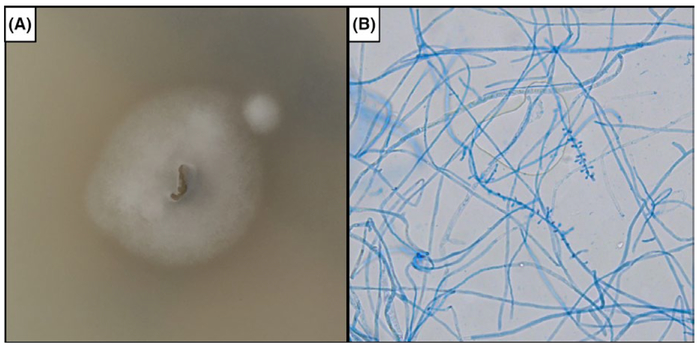
Punch biopsy of the left wrist was obtained for diagnosis. Histologic examination of the patient’s skin punch biopsy demonstrated dermal suppurative and granulomatous inflammation with scattered histiocytes and giant cells (Figure 2A). A high-power cross-sectional view of a hair shaft reveals fungal elements within the hair (arrow), consistent with endothrix (Figure 2B). No pathogen was isolated from culturing the biopsy specimen, but culture of his nail clipping was notable for Trichophyton rubrum, which was identified by colony and microscopic morphology (Figure 3) and by DNA sequencing of the D1/D2 region of the large subunit of the 28s ribosomal RNA gene and the internal transcribed spacer (ITS) region. He was initiated on oral terbinafine and at his 6-week follow up visit he had resolution of his skin lesions and improvement in his onychomycosis with growth of normal proximal nail plate.
FIGURE 2.
(A) Neutrophils and macrophages surround a fragmentof hair shaft in the dermis at 10× magnification. (B) The hair shaft has fungal elements within it (arrow) viewed at 40× magnification
FIGURE 3.
Identification of Trichophyton rubrum from a nail clipping. Subculture of organism on potato flake agar (A) produced white, fluffy colony morphology. Tape preparation using lactophenol cotton blue staining imaged using phase contrast microscopy at 20× magnification (B) revealed single tear-shaped microconidia forming along all sides of the hyphae
2 |. DISCUSSION
Majocchi granuloma (MG), also known as nodular granulomatous perifolliculitis, is a deep inflammation of the hair follicle and surrounding dermis caused by a dermatophyte.1 The most common causative agent is Trichophyton rubrum; however, other fungi including T. violaceum, T. mentagrophytes, T. tonsurans, and Epidermophyton floccosum have also been identified in MG.1 Although T. rubrum was recovered from the patient’s nail clipping, we cannot say with certainty that the same organism caused MG given the negative biopsy culture and the commonness of T. rubrum onychomycosis.
Although superficial cutaneous dermatophyte infections are frequent, the deeper involvement of MG is rare and more likely to occur in immunocompromised patients and those undergoing topical glucocorticoid therapy.1 Patients typically present with solitary or multiple non-pruritic papulopustules or plaques. In transplant patients, MG may clinically mimic Kaposi sarcoma, with blue-red papules and nodules.2 Two forms of MG, follicular and subcutaneous nodular, have been described. While the follicular type is more commonly found in individuals who experience frequent leg trauma and follicular occlusion from leg shaving, the subcutaneous nodular type is more common in immunocompromised patients including those undergoing solid organ transplantation.1 Recurrent MG has been reported secondary to chemotherapy-induced neutropenia.3 Using topical steroids on preexisting tinea may also predispose patients to MG.4 While no strong association between specific immunosuppressive drugs and MG exists, one study found that 90% of patients who underwent solid organ transplantation and developed MG had steroids as part of their immunosuppressive regimen.5 Although scarring and alopecia are the most common complications of MG, wide-spread involvement or severe disease requiring surgery is possible in immunocompromised patients. Although disseminated dermatophyte infections are quite rare even in immunosuppressed individuals, there have been several cases reported in the past.6
Diagnosis of MG is based on the histopathology and microbiology of lesions.1 Fungal hyphae are observed using potassium hydroxide (KOH) testing in 76% of patients.1 The diagnosis can be made more definitively by the presence of perifollicular granulomatous inflammation and dermal abscess with the identification of fungal organisms. MG is associated with chronic inflammation with presence of lymphocytes, macrophages, epithelioid cells, and scattered multinucleated giant cells.1 Although detection of fungal elements is difficult on conventional hematoxylin-eosin staining, periodic acid-Schiff and Gomori’s methenamine silver staining can improve detectability.1 The pathogen is typically then identified using culture and/or molecular techniques.
Treatment for all patients, irrespective of their net state of immunosuppression, requires systemic antifungal therapy. Oral terbinafine, itraconazole, and fluconazole have been successfully used to treat MG.1 In a recent retrospective series and literature review, Rouzaud and colleagues reported a complete response in 58% of patients using oral antifungal medications.7 Terbinafine was chosen here for its ability to concentrate in adipose and keratin rich tissue as well as its more moderate interaction with calcineurin inhibitors compared to azole antifungal agents.8 Preventative measures may include avoidance of occlusion, topical corticosteroids and frequent leg shaving. Rouzaud and colleagues also reported that after transplantation 67% of patients developed superficial dermatophytosis, including onychomycosis prior to the development of invasive dermatophytosis.7 Therefore, in patients undergoing organ transplantation, timely treatment of superficial dermatophyte infections should be initiated for the prevention of invasive disease.
REFERENCES
- 1.Ilkit M, Durdu M, Karakas M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol 2012;50:449–457. [DOI] [PubMed] [Google Scholar]
- 2.Brod C, Benedix F, Rocken M, Schaller M. Trichophytic Majocchi granuloma mimicking Kaposi sarcoma. J German Soc Dermatol 2007;5:591–593. [DOI] [PubMed] [Google Scholar]
- 3.Lourdes LS, Mitchell CL, Glavin FL, Schain DC, Kaye FJ. Recurrent dermatophytosis (Majocchi granuloma) associated with chemotherapy-induced neutropenia. J Clin Oncol 2014;32:e92–e94. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs PH. Majocchi’s granuloma (due to therapy with steroid and occlusion). Cutis. 1986;38:23. [PubMed] [Google Scholar]
- 5.Romero FA, Deziel PJ, Razonable RR. Majocchi’s granuloma in solid organ transplant recipients. Transpl Infect Dis 2011;13:424–432. [DOI] [PubMed] [Google Scholar]
- 6.Rinaldi MG. Dermatophytosis: epidemiological and microbiological update. J Am Acad Dermatol. 2000;43:S120–S124. [DOI] [PubMed] [Google Scholar]
- 7.Rouzaud C, Chosidow O, Brocard A., et al. Severe dermatophytosis in solid organ transplant recipients: a French retrospective series and literature review. Transpl Infect Dis 2017;20:e12799. [DOI] [PubMed] [Google Scholar]
- 8.Newland JG, Abdel-Rahman SM. Update on terbinafine with a focus on dermatophytoses. Clin Cosmet Investig Dermatol 2009;2:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]