Abstract
OBJECTIVE:
We compared bone mineral density (BMD) changes and their correlates, between men and women participating in two randomized trials of initial (ART) regimens, with or without tenofovir disoproxil fumarate (TDF).
METHODS:
Covariates in linear regression models of 48-week hip and spine %BMD changes, by dual energy X-ray absorptiometry, included baseline and 48-week changes in plasma viral load (pVL), CD4 cells, plasma C-terminal telopeptide, procollagen 1 N–terminal propeptide and glomerular filtration rates; and the 48-week area under the curve of fractional excretion of phosphate (FEP-AUC).
RESULTS:
Despite overall hip and spine BMD declines of 2.8% and 2.9%, respectively, pVL suppression to <50 vs. ≥50 copies/mL was associated 1.0% (P=0.02) and 0.8% (P=0.01) less BMD decline. Women had lower baseline spine (P=0.04; n=59 women, 418 men) and hip BMD (P=0.01) in adjusted models, with 1.7% more hip decline on ART than men (P=0.001). Serum phosphate was positively associated with baseline spine BMD in women (P=0.03) but not men, and FEP-AUC was negatively associated with spine BMD changes, particularly in women randomized to TDF-regimens (P=0.03 & 0.054 for interactions by sex, and randomization to TDF vs. non TDF-regimens, respectively; n= 44 women, 326 men). Women also had 0.6% (P=0.004) more hip BMD decline than men associated with each 100 CD4 cell/μL increase on ART (P=0.02; n=49 women, 379 men).
CONCLUSIONS:
Women randomized to TDF-containing ART had accentuated spine loss associated with phosphaturia, and accentuated hip loss associated with CD4 restoration, regardless of TDF-exposure. Viral load suppression reduced bone loss.
INTRODUCTION
Persons living with HIV (PLWH) on antiretroviral therapy (ART) have a higher prevalence of osteoporosis and an increased fracture risk compared to the general population (1–4). Average bone mineral density (BMD) declines of 2 to 6% develop predominately during the first year of ART, and the largest of these are associated with regimens that include tenofovir disoproxil fumarate (TDF) or protease inhibitors (5–8). In a large sample of PLWH in the US, the incidence of fracture increased from 1.78 per 100 person-years (py) in those with normal BMD, to 2.10 and 7.62 per 100 py, in those with osteopenia and osteoporosis, respectively (9). Among U.S. Veterans with HIV, increasing cumulative exposure to TDF or protease inhibitors was associated with higher incidence of fracture (10). PLWH also have an over-representation of traditional risk factors for bone loss, which combined with the detrimental effects on bone by ART and other HIV-specific factors, also contribute to lower BMD and increased fracture risk (10–13).
Among PLWH, women have faster rates of bone loss, maintain lower BMD and have higher rates of fracture than men (3, 14, 15). It is not known whether, or to what extent the detrimental effects of ART on bone, or other HIV-specific correlates of bone loss, differ between men and women. To examine this question, we compared hip and spine BMD changes, and their correlates, between men and women during the first 48 weeks of initial ART in participants of two multicenter AIDS Clinical Trials Group randomized trials (ACTG studies A5224s and A5303).
METHODS
Study Design and Treatment Regimen
A5224s is a metabolic substudy of a larger trial that compared TDF–emtricitabine vs. abacavir–lamivudine, combined with either efavirenz or atazanavir plus ritonavir as initial ART (5, 16). A5303 compared initial ART with TDF vs. maraviroc, each combined with emtricitabine and darunavir plus ritonavir (17). ART-naïve participants with plasma VL >1,000 copies/mL, who were ≥16 years for A5224s, or ≥18 years for A5303, were eligible to participate. Eligibility for A5224s was additionally restricted to participants who did not use medications for osteoporosis, and eligibility for A5303 was restricted to participants with R5 tropism. Additional information can be found about these studies at ClinicalTrials.gov: NCT 00118898 and 01400412.
The Institutional Review Board of each study site approved the protocol. All participants provided written informed consent.
Measurements of BMD, Phosphaturia and Bone Turnover
Dual energy X-ray absorptiometry (DXA) scans of the left hip and lumbar spine (L1-L4) were performed within 4 weeks prior to randomization, using either a Lunar [GE Healthcare, Fairfield, Connecticut] or Hologic [Hologic Incorporated, New Bedford, Massachusetts] DXA scanner. A second scan was performed at week-48 (±4 weeks) using the same scanning system. All DXA scans were read centrally at the Body Composition Analysis Center at Tufts University. The European Spine Phantom was used for cross-calibration of DXA machines and quality assurance at each study site.
Phosphaturia was measured as the week 0 to 48 area under the curve of the fractional excretion of phosphate (FEP-AUC) from urine and serum specimens collected at weeks 0 and 48, calculated using the trapezoidal rule (18, 19).
Bone turnover was measured in A5303 participants only, at weeks 0, 24 and 48 in plasma as C-terminal telopeptides of type I collagen (CTx), and procollagen 1 intact N–terminal propeptide (P1NP). CTx was measured by ELISA (Immunodiagnostic Systems, Scottsdale, Arizona) with inter- and intra-assay variabilities of 9.7% and 1.7%, respectively (normal range: 0.142–1.351 pg/mL for postmenopausal women). P1NP was measured by radioimmunoassay (Immunodiagnostic Systems, Scottsdale, Arizona) with inter- and intra-assay variabilities of 8.3 and 6.5%, respectively (normal range 16–96 pg/mL for postmenopausal women).
Statistical Analysis
Baseline, and 48-week changes from baseline, in time-varying covariates were compared by sex, and by randomization to TDF vs. non TDF-containing regimens, using the Wilcoxon rank-sum test for continuous, and the Pearson chi-square test for categorical variables. Baseline for all covariates except BMD was defined as the randomization date (designated as week 0); baseline for BMD measurements were defined as up to 4 weeks before randomization. The exploratory covariates included baseline serum phosphate and 48-week FEP-AUC, as well as baseline and 48-week changes from baseline in: CD4 cell counts, glomerular filtration rates (using the CKD-EPI equation), CTx and P1NP.
Separate multivariable linear regression models tested the independent contributions to baseline hip and spine BMD, and to the 48-week changes from baseline of BMD by each exploratory variable. Adjusting variables that were retained in the models regardless of significance included age, sex, race (as black, not Hispanic vs. white, Hispanic regardless of race, and other races/ethinicities), baseline hip or spine BMD, randomization status to TDF vs. non TDF-containing regimens, baseline plasma viral load (pVL), and pVL at week-48 (as <50 vs. ≥50 copies HIV-RNA/mL). Models of hip BMD were additionally adjusted for baseline body mass index (BMI).
These models were extended to test interactions by sex and randomization status, where significance was defined by P<0.10. Stratified, unadjusted models by sex and randomization status explored any significant interactions that were detected. All analyses used SAS version 9.4 [SAS Institute, Cary, North Carolina].
RESULTS
Included in this analysis were 499 participants, 433 of whom had baseline and week-48 hip or spine BMD measurements [Table 1]. Complete CD4 cell and phosphaturia data was available in 429 and 374 of these 433 participants, respectively. Turnover markers were measured only in A5303 participants (n=220). Data from participants who represented extreme outliers in the distributions of hip BMD changes (n=3), or of the 48-week area under the curve of FEP-AUC (n=4) was excluded for the linear regression models.
Table 1:
Baseline Characteristics by randomization to TDF vs. non TDF-containing regimens, and by sex
| Randomization-status | Sex | |||||
|---|---|---|---|---|---|---|
| ART regimen n (%) | TDF+ (n=245) | TDF− (n=254) | Women (n=61) | Men (n=438) | ||
| TDF/FTC/PI-r | 176 (72%) | – | 16 (26%) | 160 (37%) | ||
| TDF/FTC/EFV | 69 (28%) | – | 11 (18%) | 58 (13%) | ||
| ABC/3TC/PI-r | – | 65 (26%) | 6 (10%) | 59 (13%) | ||
| MVC/FTC/PI-r | – | 119 (47%) | 14 (23%) | 105 (24%) | ||
| ABC/3TC/EFV | – | 70 (27%) | 14 (23%) | 56 (11%) | ||
| P | P | |||||
| Age years mean (±S.D.) | 37 ±10.6 | 37 ±10.4 | 0.92 | 40 ±10.8 | 37 ±10.4 | 0.02 |
| Female n (%) | 27 ±11% | 34 ±13% | 0.70 | |||
| Race/Ethnicity n (%) | 0.34 | <.0001 | ||||
| White | 114 (47%) | 114 (45%) | 17 (28%) | 211 (48%) | ||
| Black, Not Hispanic | 72 (29%) | 90 (35%) | 36 (59%) | 126 (29%) | ||
| Hispanic | 51 (21%) | 43 (17%) | 8 (13%) | 86 (20%) | ||
| Other | 8 (3%) | 7 (3%) | 0 (0%) | 15 (3%) | ||
| BMI kg/m2 mean (±S.D.) | 25.8 ±4.7 | 25.8 ±4.8 | 0.96 | 27.4 ±5.7 | 25.6 ±4.5 | 0.03 |
| log10 VL copies/mL mean (±S.D.) | 4.6 ±0.7 | 4.6 ±0.7 | 0.60 | 4.3 ±0.6 | 4.6 ±0.7 | 0.01 |
| Week 0 CD4 cells/μL mean (±S.D.) | 320 ±207 | 317 ±200 | 0.88 | 314 ±198 | 319 ±205 | 0.68 |
| Week 0 BMD g/cm2 mean (±S.D.) | ||||||
| Spine | 1.14 ±0.19 | 1.14 ±0.18 | 0.57 | 1.17 ±0.21 | 1.14 ±0.18 | 0.13 |
| Hip | 1.05 ±0.16 | 1.06 ±0.17 | 0.30 | 1.02 ±0.15 | 1.06 ±0.16 | 0.27 |
| Serum PHOS mg/dL mean (±S.D.) | 3.5 ±0.6 | 3.4 ±0.6 | 0.31 | 3.6 ±0.5 | 3.4 ±0.6 | 0.001 |
| FEP% mean (±S.D.) | 10.8 ±10.3 | 9.5 ±4.9 | 0.26 | 9.6 ±5.0 | 10.2 ±8.4 | 0.69 |
| GFR ml/min*1.72m2 mean (±S.D.) | 106.46 ±17.38 | 108.49 ±18.40 | 0.16 | 106.89 ±19.41 | 107.59 ±17.72 | 0.68 |
FTC= emtricitabine, PI-r=ritonavir boosted protease inhibitor, EFV=efavirenz, ABC=abacavir, MVC=maraviroc
Participants randomized to TDF vs. non TDF-containing regimens had significantly more hip (3.6 % vs. 2.4%, P<.0001; Table 2) and spine BMD decline (3.6 % vs. 1.9%, P<.0001). They also had significantly larger increases in bone turnover markers (P<.0001, for CTx and P1NP), greater 48-week FEP-AUC (P=0.01) and more GFR decline (4.33 vs. 0.54 ml/min/1.73m2, P=0.004).
Table 2:
Baseline to Week-48 Changes by Randomization Arm and Sex
| All | Randomization Status | Sex | |||||
|---|---|---|---|---|---|---|---|
| TDF+ | TDF− | P | Women | Men | P | ||
| Baseline to Week 48 %ΔBMD: mean ±S.D. | |||||||
| Spine | −2.8 ±4.4 | −3.6 ±4.3 | −1.9 ±4.3 | <.0001 | −3.0 ±4.0 | −2.7 ±3.6 | 0.03 |
| Hip | −2.9 ±3.9 | −3.6 ±4.2 | −2.4 ±4.2 | <.0001 | −4.6 ±5.8 | −2.7 ±3.6 | 0.54 |
| Week-48 Plasma Viral Load: (n, %) | |||||||
| ≤ 200 copies/mL | 446 (94%) | 222 (96%) | 224 (93%) | 0.11 | 53 (91%) | 393 (95%) | 0.36 |
| ≤ 50 copies/mL | 214 (45%) | 103 (45%) | 111 (46%) | 0.78 | 20 (34%) | 194 (47%) | 0.09 |
| Baseline to Week 48 ΔCD4 cells/μL mean ±S.D. | 199 ±161 | 186 ±154 | 214 ±171 | 0.06 | 186 ±176 | 201 ±159 | 0.25 |
| Baseline to Week 24 ΔCTx pg/mL | 0.12 ±0.25 | 0.21 ±0.26 | 0.04 ±0.21 | <.0001 | 0.16 ±0.24 | 0.12 ±0.25 | 0.77 |
| Baseline to Week 48 ΔCTx pg/mL mean ±S.D. | 0.12 ±0.26 | 0.19 ±0.28 | 0.05 ±0.21 | <.0001 | 0.18 ±0.28 | 0.11 ±0.25 | 0.53 |
| Baseline to Week 48 ΔP1NP pg/mL mean ±S.D. | 13.7 ±23.1 | 22.0 ±24.8 | 6.1 ±18.6 | <.0001 | 19.3 ±20.6 | 13.2 ±23.3 | 0.10 |
| Baseline to Week 48 FEP-AUC mean ±S.D. | 510 ±250 | 550 ±306 | 474 ±179 | 0.01 | 449 ±153.6 | 519 ±260 | 0.03 |
| Baseline to Week 48 ΔGFR mg/mL*1.73 m2 mean ±S.D. | −2.37 ±12.99 | −4.33 ±12.22 | −0.54 (13.43) | 0.004 | −3.24 (12.13) | −2.20 (13.02) | 0.90 |
Sixty-one (12%) participants were women, who were significantly older (40 vs. 37 years, P=0.02) and more likely to be black than the men (28% vs. 48%, P<.0001). They also had significantly higher baseline BMI (P=0.03) and serum phosphate concentrations (P=0.001), significantly lower FEP-AUC (P=0.03), and a trend towards larger increases in the bone formation marker P1NP (P=0.07).
Correlates of Baseline Spine BMD
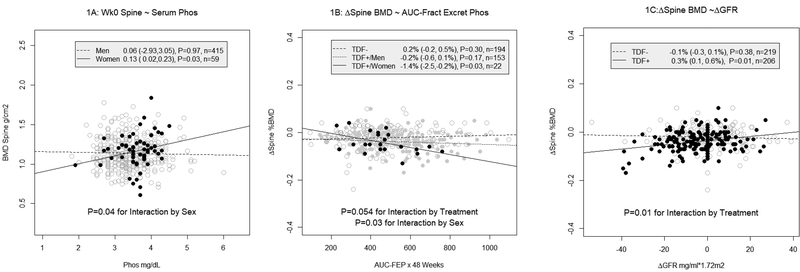
Women had a 0.39 g/cm2 (P=0.04) lower average baseline spine BMD than men in adjusted models. Baseline BMI (P<.0001) and serum phosphate concentrations were positively associated with baseline spine BMD, but the latter association depended on sex (P=0.04 for the interaction by sex; n=49 women, 427 men; Figure 1A & Supplementary Table). In stratified analyses, serum phosphate was positively associated with BMD in women (P=0.03) but not men (P=0.97).
Figure 1:
Scatter plots of baseline BMD and 48-week changes from baseline BMD with serum phosphate (1A), 48-week AUC-fractional excretion of phosphate (1B) and 48-week changes from baseline in GFR.
Baseline & Time-varying Correlates of 48-Week Spine BMD Changes
Participants randomized to TDF vs. non TDF-containing regimens had a 1.8% larger average spine BMD decline (P<0.0001) in adjusted models [Supplementary Table]. BMD change was positively associated with baseline spine BMD (P=0.0002), negatively associated with baseline pVL (1.3% greater decline per every 1 log10 greater HIV RNA copies/mL; P<.0001) and positively associated with viral suppression at week-48 (1.0% less BMD decline with suppression to <50 vs. ≥50 copies/mL; P=0.02). Women had a 0.6% larger average BMD decline than men, but this difference was not significant (P=0.35).
Phosphaturia, baseline and ΔCD4 cells, ΔGFR and bone turnover markers were significantly associated with 48-week spine BMD changes in separate, adjusted models [Supplementary Table]. Sex modified associations with phosphaturia; and randomization status modified associations with phosphaturia and ΔGFR.
Each 2% increased FEP-AUC was associated with a 0.8% (P=0.03) larger average spine BMD decline in women vs. men, and with a 0.4% (P=0.054) larger average decline in participants randomized to TDF vs. non TDF-containing regimens [Supplementary Table]. In analyses stratified by sex among participants randomized to TDF-regimens, this magnitude of phosphaturia was associated with a 1.4% average decline in women (P=0.03, Figure 1B), but was not significantly associated with BMD changes in men (P=0.17). Phosphaturia was not associated with BMD spine changes in participants randomized to non TDF-containing regimens, regardless of sex.
Associations between ΔGFR and spine BMD decline also depended on randomization status. A 5 ml/min*1.73 m2 GFR decline over 48 weeks was associated with a 0.4% larger average BMD decline in participants randomized to TDF vs. non TDF-regimens (P=0.01 for the interaction by randomization status; Figure 1C & Supplementary Table). In stratified analyses, this association was also only significant among participants randomized to TDF-regimens (P=0.01).
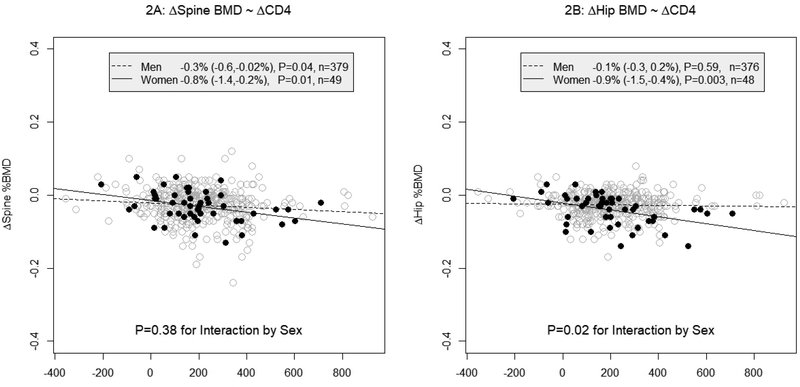
Changes in spine BMD on ART was negatively associated with ΔCD4, with an average BMD decline of 0.3% for every 100 CD4 cells/μL increase (P=0.02). Although the effect size associated with this CD4 cell increase was larger in women (0.8%, P=0.01) than men (0.3%, P=0.04), these differences were not significant (P=0.38 for the interaction by sex; n=49 women and 379 men; Figure 2A & Supplementary Table).
Figure 2:
Scatter plots of 48-week changes from baseline of BMD of spine (2A) and hip (2B) with 48-week changes from baseline of CD4 cell counts.
Each 1 S.D. increased CTx over 24 weeks, or increased P1NP over 48 weeks, was associated with average spine BMD declines of 0.7% (P=0.02) and 1.1% (P<.0001; Supplementary Table), respectively. The small number of women with turnover markers (n=19) precluded reliable comparisons by sex. If bone resorption was instead estimated by 48-week changes from baseline in CTx, the strength of association fell to a 0.5% average BMD decline (P=0.08). Plasma CTx and absolute CD4 cell counts were weakly correlated (r=0.16, P=0.01; r=0.12, P=0.06; and r=0.11, P=0.09 at weeks 0, 24 and 48, respectively).
Correlates of Baseline Hip BMD
Women had a 0.05 g/cm2 (P=0.01) lower average baseline hip BMD than men in adjusted models. Baseline hip BMD was negatively associated with age (P<.0001), positively associated with baseline BMI (P<.0001), and black participants had larger baseline hip BMD than non-blacks (P=0.01). In contrast to the spine, serum phosphate was not associated with baseline hip BMD.
Baseline & Time-Varying Correlates of 48-Week Hip BMD Change
Participants randomized to TDF vs. non TDF-containing regimens had a 1.5% (P<.0001) larger average hip BMD decline in adjusted models, and women had a 1.7% (P=0.001) larger decline than men. Hip BMD changes were positively associated with baseline hip BMD (P=0.001), negatively associated with baseline pVL (0.6% per each 1 log10 greater HIV-RNA copies/mL; P=0.003), and positively associated with pVL suppression to < 50 vs. ≥ 50 copies/mL at week-48 (0.8% less BMD decline, P=0.01; Supplementary Table).
Changes in hip BMD were associated with baseline and ΔCD4 cells and turnover markers [Supplementary Table], but neither phosphaturia nor ΔGFR were associated with hip BMD changes. Each 100 fewer baseline CD4 cells/μL was associated with a 0.1% larger average BMD decline (P=0.02), while sex modified associations with ΔCD4 so that women had an average 0.6% more BMD decline than men for every 100 CD4 cell/μL increase (P=0.02 for the interaction by sex; n=48 women, 376 men; Figure 2B). In stratified analyses, this magnitude of CD4 increase was associated with an average BMD decline of 0.9% (P=0.003) in women, but ΔCD4 was not associated with BMD changes in men (P=0.59).
Each 1 S.D. increased CTx and P1NP over 48 weeks was associated with a 0.5% (P=0.02), and 0.7% (P=0.001) average hip BMD decline, respectively. In contrast to the spine, the strength of association between CTx fell to 0.3% (P=0.14) if bone resorption was instead estimated by 24-week changes from baseline in this marker.
DISCUSSION
In this pooled analysis of 2 randomized trials comparing 48-week BMD changes with the initiation of TDF vs. non-TDF regimens in HIV-infected ART naïve persons, we observed significant differences in the correlates of bone loss that depended on bone site (spine vs. hip), sex and TDF-exposure. Participants randomized to TDF-containing regimens had accentuated spine BMD loss associated with phosphaturia, particularly among women. Women also had significantly more hip loss than men associated with CD4 restoration, regardless of TDF-exposure. Despite overall spine and hip BMD declines of 2.8% and 2.9%, respectively, with initial ART, pVL suppression to < 50 vs. ≥ 50 copies/mL was associated with 1.0% and 0.8% less BMD loss.
Bone turnover is a synchronized process of osteoclast-derived resorption, osteoblast-derived collagen matrix deposition and mineralization. Plasma CTx, a marker of bone resorption, increases within 2 weeks after initiating ART, reaching peak levels by 24 weeks (20). Plasma P1NP, a marker of new bone formation involving type I collagen matrix deposition, reaches peak levels approximately one year after ART initiation (21). In the present study, the differential associations of spine vs. hip ΔBMD with ΔCTx when comparing 24- and 48-week changes from baseline, suggests earlier resorption of spine (predominately composed of trabecular bone) than hip (predominately cortical bone), a dynamic that is also evident with perimenopausal bone loss (22).
The mineralization of collagen fibrils by osteoblasts incorporates carbonated hydroxyapatite, whose source of calcium and phosphate is derived from serum (23). Serum phosphate is tightly regulated by parathyroid hormone and fibroblast growth factor-23 via intestinal phosphate absorption, and by phosphate reabsorption in the proximal renal tubules (24). As in the present study, menopausal women retain phosphate, maintaining higher serum concentrations with lower urinary phosphate excretion than men or pre-menopausal women (25). The positive association between serum phosphate and baseline spine BMD among women in the present study also is consistent with a similar previous association in postmenopausal women (26). These observations implicate contributions by menopausal bone loss to the differential associations by sex, between markers of phosphate homeostasis and changes in spine BMD. Because bone mineralization is a late step in remodeling, the differential associations with phosphaturia between spine and hip similarly implies earlier remodeling of the spine.
Consistent with previous studies, participants randomized to TDF-containing regimens had significantly larger increases in bone turnover markers, which were associated with BMD declines (6, 13, 20, 27, 28). TDF also may induce a proximal renal tubulopathy leading to urinary phosphate wasting, with or without GFR reductions, by engaging drug transporters in the renal tubular membrane (29–31). The risk for tubulopathy may be accentuated by concomitant protease inhibitor-use, including ritonavir, which also may directly engage these renal drug transporters, or enhance TDF exposure via drug-drug interactions (32, 33). This tubulopathy has been postulated as a mechanism of BMD decline with TDF (27, 34), but evidence for this effect is conflicting.
A previous analysis of A5224s participants did not detect an association between phosphaturia (as FEP) and hip or spine BMD changes (35). In this subset, the association between FEP-AUC and spine BMD decline was not significant among women (r=0.13, P=0.52, n=27), or in those randomized to TDF-containing regimens (r=0.14, P=0.18, n=90) probably because of the smaller sample size. In a second study of men with HIV receiving TDF, the urinary retinol-binding protein to creatinine ratio (a marker of renal tubule dysfunction), but not FEP, was negatively associated with spine BMD (36). In a third longitudinal study of mostly men with HIV who received TDF, phosphaturia (as FEP) was negatively associated with femoral neck BMD decline, a site with approximately equal proportions of trabecular and cortical bone (37). Tenofovir alafenamide (TAF) achieves higher intracellular accumulation, with lower plasma concentrations, and is associated with significantly less tubulopathy and BMD decline than TDF (38). In adults with low BMD, switching from TDF to TAF was associated with increased hip BMD, which was predicted by higher FEP (39). Although it is difficult to reconcile these disparate observations, the significant associations between phosphaturia as well as GFR changes, with early spine BMD loss in the present study supports an interpretation of TDF-mediated renal tubulopathy leading to impaired trabecular bone mineralization, particularly affecting women.
Among HIV specific factors, CD4 cell increases, nadir CD4 cell counts, and pre-ART pVL were previously associated with BMD loss after ART-initiation (11, 20). The importance of T-cell restoration in ART-associated bone loss is supported by an animal model simulating ART-associated immune restoration that adoptively transferred T-cells from healthy mice into T-cell receptor knockout mice (40). Consistent with the present study, CTx levels were previously correlated with CD4 cell counts in HIV patients who initiated ART (20). We extend this observation by demonstrating more hip loss, for the same magnitude of CD4 restoration in women than men. Previous studies have demonstrated negative associations between BMD and pVL suppression on ART (41). Although we also observed net detrimental effects by initial ART on BMD, we detected salutary effects by pVL suppression.
This study is limited by a relatively small number of women. Menstrual history and biological markers of menopause also were not captured in women, nor was the history of current or past tobacco or alcohol use among all participants. Bone turnover markers were measured in A5303 participants only, precluding reliable comparisons by sex with these markers. Nevertheless, the prospective study design with uniform measurements of BMD and other exploratory variables, in the context of randomized, initial ART regimens are major strengths of this study.
Upon ART-initiation, women who received TDF had enhanced spine BMD loss compared to men in association with phosphaturia. Women also had larger hip and spine BMD declines for the same magnitude of CD4 cell restoration as men, regardless of TDF exposure. Despite overall BMD declines with initial ART, viral suppression mitigated bone loss. The complex interactions that we observed involving bone site, sex and TDF-exposure may be explained by simultaneous and overlapping effects of perimenopausal bone loss, TDF-associated bone toxicity, adverse bone effects by immune restoration and beneficial effects of viral suppression. Because of the importance of bone health among PLWH, a better understanding of these sex differences in ART-associated BMD loss is warranted.
Interventions that have been shown to reduce early bone loss at the time of ART initiation include high dose vitamin D and calcium supplementation and single dose zoledronic acid (42, 43). BMD also improves when switching from TDF to TAF (38), or to other non-TDF containing regimens, but TDF remains a critical component of ART particularly in low-income countries where the majority of PLWH are women.
Clinicians are encouraged to screen for bone disease, avoid TDF and ritonavir-boosted protease inhibitors in patients at risk for fragility fractures, and to advise patients about preventative measures including lifestyle assessments, and considerations for vitamin D and calcium supplementation (44).
Supplementary Material
Acknowledgments
We are grateful to the participants of this study.
Funding
The project described was supported by Award Number U01AI068636 from the National Institute of Allergy and Infectious Diseases and supported by National Institute of Mental Health (NIMH), National Institute of Dental and Craniofacial Research (NIDC ), the Veterans Administration: VISN10 Geriatric Research Education and Clinical Centers, Louis Stokes Cleveland Veterans Administration Medical Center, and the research sites that participated: [grant numbers UMAI069494, UMAI069432, UM1AI 069471, UM1AI069452, UM1 AI069501, 2UM1AI069432, UL1 TR001082, 1U01AI069477-01, P30AI073961, 5UM1 AI068636, 2UM1AI069503, UM1 AI069471, 2UM1AI069439-08, and UL1 TR000445 from the National Center for Advancing Translational Sciences/NIH AI69439, UM1 AI069496, 5UM1AI069412, UM1 AI069423, 1UL1TR001111, P30 AI50410, 2UMIA1069423-08, 2UM1AI069418-08, 2P30 AI 50409-10, UL1TR000454, AI069501, 5UM1AI069415-10, 2UM1AI069412-08, AI069424, UL1 RR025780, 2UM1 AI069470-08, UM1AI069472, 2UMAI069432, AI 69501, UM1AI069471, UM1A 068636-09, 5 P30 AI- 045008-15, U01AI069447, NO1-HD-3-3345, UMI AI069511, UM1 AI069465, UL1TR001079, UL1 RR024160, UL1 TR000042, and from the Center for AIDS Research AI 036219]. ViiV, Gilead, and AbbVie provided study drugs. ViiV provided funding for DXA scanning. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health
Footnotes
Potential Conflicts of Interest
R.C.K. receives research funding from Gilead Sciences; C.J.F.’s institution receives research funding from Gilead, Pfizer, and Cubist; T.T.B. has received research funding from Gilead Sciences, Bristol-Myers Squibb, Merck, EMD-Serono, and Theratechnologies; B.O.T. has served as a consultant to ViiV, Pfizer, Janssen, GlaxoSmithKline (GSK), and Gilead, and has received research support to Northwestern University from ViiV and Pfizer. G.A.M. has received research funding from Gilead, Merk, Astellas and ViiV. S.K.G. reports advisory fees from Gilead Sciences, GSK/ViiV, and BMS; travel support from Gilead Sciences and BMS; and independent research grant support from Gilead Sciences and GSK/ViiV. All other authors report no conflicts of interest.
REFERENCES
- 1.Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Lo Y, Klein RS. Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection. AIDS. 2007;21(5):617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansen AB, Gerstoft J, Kronborg G, Larsen CS, Pedersen C, Pedersen G, et al. Incidence of low and high-energy fractures in persons with and without HIV infection: a Danish population-based cohort study. AIDS. 2012;26(3):285–93. [DOI] [PubMed] [Google Scholar]
- 3.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93(9):3499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young B, Dao CN, Buchacz K, Baker R, Brooks JT, Investigators HIVOS. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000–2006. Clin Infect Dis. 2011;52(8):1061–8. [DOI] [PubMed] [Google Scholar]
- 5.McComsey GA, Kitch D, Daar ES, Tierney C, Jahed NC, Tebas P, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203(12):1791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernardino JI, Mocroft A, Mallon PW, Wallet C, Gerstoft J, Russell C, et al. Bone mineral density and inflammatory and bone biomarkers after darunavir-ritonavir combined with either raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults with HIV-1: a substudy of the NEAT001/ANRS143 randomised trial. Lancet HIV. 2015;2(11):e464–73. [DOI] [PubMed] [Google Scholar]
- 7.Duvivier C, Kolta S, Assoumou L, Ghosn J, Rozenberg S, Murphy RL, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS. 2009;23(7):817–24. [DOI] [PubMed] [Google Scholar]
- 8.Stellbrink HJ, Orkin C, Arribas JR, Compston J, Gerstoft J, Van Wijngaerden E, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51(8):963–72. [DOI] [PubMed] [Google Scholar]
- 9.Battalora L, Buchacz K, Armon C, Overton ET, Hammer J, Patel P, et al. Low bone mineral density and risk of incident fracture in HIV-infected adults. Antivir Ther. 2016;21(1):45–54. [DOI] [PubMed] [Google Scholar]
- 10.Bedimo R, Maalouf NM, Zhang S, Drechsler H, Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012;26(7):825–31. [DOI] [PubMed] [Google Scholar]
- 11.Grant PM, Kitch D, McComsey GA, Dube MP, Haubrich R, Huang J, et al. Low baseline CD4+ count is associated with greater bone mineral density loss after antiretroviral therapy initiation. Clin Infect Dis. 2013;57(10):1483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kooij KW, Wit FW, Bisschop PH, Schouten J, Stolte IG, Prins M, et al. Low bone mineral density in patients with well-suppressed HIV infection: association with body weight, smoking, and prior advanced HIV disease. J Infect Dis. 2015;211(4):539–48. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen TA, Jensen D, Tolstrup M, Nielsen US, Erlandsen EJ, Birn H, et al. Comparison of bone and renal effects in HIV-infected adults switching to abacavir or tenofovir based therapy in a randomized trial. PLoS One. 2012;7(3):e32445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotter AG, Sabin CA, Simelane S, Macken A, Kavanagh E, Brady JJ, et al. Relative contribution of HIV infection, demographics and body mass index to bone mineral density. AIDS. 2014;28(14):2051–60. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson DL, Spiegelman D, Knox TK, Wilson IB. Evolution and predictors of change in total bone mineral density over time in HIV-infected men and women in the nutrition for healthy living study. J Acquir Immune Defic Syndr. 2008;49(3):298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sax PE, Tierney C, Collier AC, Daar ES, Mollan K, Budhathoki C, et al. Abacavir/lamivudine versus tenofovir DF/emtricitabine as part of combination regimens for initial treatment of HIV: final results. J Infect Dis. 2011;204(8):1191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taiwo BO, Chan ES, Fichtenbaum CJ, Ribaudo H, Tsibris A, Klingman KL, et al. Less Bone Loss With Maraviroc- Versus Tenofovir-Containing Antiretroviral Therapy in the AIDS Clinical Trials Group A5303 Study. Clin Infect Dis. 2015;61(7):1179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(9):e96–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Psyrogiannis A, Kyriazopoulou V, Symeonidis A, Leotsinidis M, Vagenakis AG. Relative iron “overload” in offspring of patients with type 2 diabetes mellitus: a new component in the conundrum of insulin resistance syndrome? Hormones (Athens). 2003;2(3):161–8. [DOI] [PubMed] [Google Scholar]
- 20.Ofotokun I, Titanji K, Vunnava A, Roser-Page S, Vikulina T, Villinger F, et al. Antiretroviral therapy induces a rapid increase in bone resorption that is positively associated with the magnitude of immune reconstitution in HIV infection. AIDS. 2016;30(3):405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray BF, van Vonderen MG, Mallon PW, Doran P, van Agtmael MA, Danner SA, et al. Changes in bone biomarkers in antiretroviral naive HIV-infected men randomised to nevirapine/lopinavir/ritonavir (NVP/LPV/R) or zidovudine/lamivudine/lopinavir/ritonavir (AZT/3TC/LPV/R) help explain limited loss of bone mineral density over first 12 months. Bone. 2012;50:S167–S. [Google Scholar]
- 22.Seeman E Age- and menopause-related bone loss compromise cortical and trabecular microstructure. J Gerontol A Biol Sci Med Sci. 2013;68(10):1218–25. [DOI] [PubMed] [Google Scholar]
- 23.Clarke B Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3 Suppl 3:S131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lederer E Regulation of serum phosphate. J Physiol. 2014;592(18):3985–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bansal N, Katz R, de Boer IH, Kestenbaum B, Siscovick DS, Hoofnagle AN, et al. Influence of estrogen therapy on calcium, phosphorus, and other regulatory hormones in postmenopausal women: the MESA study. J Clin Endocrinol Metab. 2013;98(12):4890–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins D, Jasani C, Fogelman I, Swaminathan R. Vitamin D and bone mineral density. Osteoporos Int. 1998;8(2):110–4. [DOI] [PubMed] [Google Scholar]
- 27.Calmy A, Fux CA, Norris R, Vallier N, Delhumeau C, Samaras K, et al. Low bone mineral density, renal dysfunction, and fracture risk in HIV infection: a cross-sectional study. J Infect Dis. 2009;200(11):1746–54. [DOI] [PubMed] [Google Scholar]
- 28.Haskelberg H, Hoy JF, Amin J, Ebeling PR, Emery S, Carr A, et al. Changes in bone turnover and bone loss in HIV-infected patients changing treatment to tenofovir-emtricitabine or abacavir-lamivudine. PLoS One. 2012;7(6):e38377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutierrez F, Fulladosa X, Barril G, Domingo P. Renal tubular transporter-mediated interactions of HIV drugs: implications for patient management. AIDS Rev. 2014;16(4):199–212. [PubMed] [Google Scholar]
- 30.Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D’Agati VD, Markowitz GS. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 2010;78(11):1171–7. [DOI] [PubMed] [Google Scholar]
- 31.Gupta SK, Anderson AM, Ebrahimi R, Fralich T, Graham H, Scharen-Guivel V, et al. Fanconi syndrome accompanied by renal function decline with tenofovir disoproxil fumarate: a prospective, case-control study of predictors and resolution in HIV-infected patients. PLoS One. 2014;9(3):e92717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lapadula G, Bernasconi DP, Casari S, Maggiolo F, Cauda R, Di Pietro M, et al. Risk of Chronic Kidney Disease among Patients Developing Mild Renal Impairment during Tenofovir-Containing Antiretroviral Treatment. PLoS One. 2016;11(9):e0162320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kearney BP, Mathias A, Mittan A, Sayre J, Ebrahimi R, Cheng AK. Pharmacokinetics and safety of tenofovir disoproxil fumarate on coadministration with lopinavir/ritonavir. J Acquir Immune Defic Syndr. 2006;43(3):278–83. [DOI] [PubMed] [Google Scholar]
- 34.Borderi M, Gibellini D, Vescini F, De Crignis E, Cimatti L, Biagetti C, et al. Metabolic bone disease in HIV infection. AIDS. 2009;23(11):1297–310. [DOI] [PubMed] [Google Scholar]
- 35.Gupta SK, Yeh E, Kitch DW, Brown TT, Venuto CS, Morse GD, et al. Bone mineral density reductions after tenofovir disoproxil fumarate initiation and changes in phosphaturia: a secondary analysis of ACTG A5224s. J Antimicrob Chemother. 2017;72(7):2042–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamzah L, Samarawickrama A, Campbell L, Pope M, Burling K, Fisher M, et al. Effects of renal tubular dysfunction on bone in tenofovir-exposed HIV-positive patients. AIDS. 2015;29(14):1785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casado JL, Santiuste C, Vazquez M, Banon S, Rosillo M, Gomez A, et al. Bone mineral density decline according to renal tubular dysfunction and phosphaturia in tenofovir-exposed HIV-infected patients. AIDS. 2016;30(9):1423–31. [DOI] [PubMed] [Google Scholar]
- 38.Sax PE, Wohl D, Yin MT, Post F, DeJesus E, Saag M, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–15. [DOI] [PubMed] [Google Scholar]
- 39.Brown T, Yin MT, Gupta S, Katalama C, Lazzarin A, Melborne K, et al. Switching from TDF to TAF in HIV-infected adults with low BMD: A pooled analysis. Conference on Retroviruses and Opportunistic Infections, Seattle WA Feb 13–16, 2017:Abstract #683. [Google Scholar]
- 40.Ofotokun I, Titanji K, Vikulina T, Roser-Page S, Yamaguchi M, Zayzafoon M, et al. Role of T-cell reconstitution in HIV-1 antiretroviral therapy-induced bone loss. Nat Commun. 2015;6:8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cazanave C, Dupon M, Lavignolle-Aurillac V, Barthe N, Lawson-Ayayi S, Mehsen N, et al. Reduced bone mineral density in HIV-infected patients: prevalence and associated factors. AIDS. 2008;22(3):395–402. [DOI] [PubMed] [Google Scholar]
- 42.Ofotokun I, Titanji K, Lahiri CD, Vunnava A, Foster A, Sanford SE, et al. A Single-dose Zoledronic Acid Infusion Prevents Antiretroviral Therapy-induced Bone Loss in Treatment-naive HIV-infected Patients: A Phase IIb Trial. Clin Infect Dis. 2016;63(5):663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Overton ET, Chan ES, Brown TT, Tebas P, McComsey GA, Melbourne KM, et al. Vitamin D and Calcium Attenuate Bone Loss With Antiretroviral Therapy Initiation: A Randomized Trial. Ann Intern Med. 2015;162(12):815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown TT, Hoy J, Borderi M, Guaraldi G, Renjifo B, Vescini F, et al. Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis. 2015;60(8):1242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.