Abstract
Background
Brachychiton rupestris and Brachychiton discolor (Malvaceae) are ornamental trees native to Australia. Some members of Brachychiton and its highly related genus, Sterculia, are employed in traditional medicine for itching, dermatitis and other skin diseases. However, scientific studies on these two genera are scarce. Aiming to reveal the scientific basis of the folk medicinal use of these plants, the cytotoxicity, anti-inflammatory and anti-allergic activities of Brachychiton rupestris and Brachychiton discolor leaves extracts and fractions were evaluated. Also, phytochemical investigation of B. rupestris was performed to identify the compounds exerting the biological effect.
Methods
Extracts as well as fractions of Brachychiton rupestris and Brachychiton discolor were tested for their cytotoxicity versus hepatoma HepG2, lung A549, and breast MDA-MB-231 cancer cell lines. Assessment of the anti-allergic activity was done using degranulation assay in RBL-2H3 mast cells. Anti-inflammatory effect was tested by measuring the suppression of superoxide anion production as well as elastase release in fMLF/CB-induced human neutrophils. Phytochemical investigation of the n-hexane, dichloromethane and ethyl acetate fractions of B. rupestris was done using different chromatographic and spectroscopic techniques.
Results
The tested samples showed no cytotoxicity towards the tested cell lines. The nonpolar fractions of both B. rupestris and B. discolor showed potent anti-allergic potency by inhibiting the release of β-hexosaminidase. The dichloromethane fraction of both species exhibited the highest anti-inflammatory activity by suppressing superoxide anion generation and elastase release with IC50 values of 2.99 and 1.98 μg/mL, respectively for B. rupestris, and 0.78 and 1.57 μg/mL, respectively for B. discolor. Phytochemical investigation of various fractions of B. rupestris resulted in the isolation of β-amyrin acetate (1), β-sitosterol (2) and stigmasterol (3) from the n-hexane fraction. Scopoletin (4) and β-sitosterol-3-O-β-D-glucoside (5) were obtained from the dichloromethane fraction. Dihydrodehydrodiconiferyl alcohol 4-O-β-D-glucoside (6) and dihydrodehydrodiconiferyl alcohol 9-O-β-D-glucoside (7) were separated from the ethyl acetate fraction. Scopoletin (4) showed anti-allergic and anti-inflammatory activity.
Conclusions
It was concluded that the nonpolar fractions of both Brachychiton species exhibited anti-allergic and anti-inflammatory activities.
Electronic supplementary material
The online version of this article (10.1186/s12906-018-2359-6) contains supplementary material, which is available to authorized users.
Keywords: Anti-allergic, Anti-inflammatory, Brachychiton discolor, Brachychiton rupestris, Cytotoxicity, Phytochemistry
Background
Allergy is one of the most popular diseases worldwide and its great prevalence makes allergic disorder a growing global concern [1]. Allergic reaction can be defined as the development of signs and symptoms of hypersensitivity reactions upon exposure to certain allergenic substances resulting in massive production of allergen-specific IgE and allergen-specific T-cell populations [2]. Allergic reaction can be a life-threatening condition especially in anaphylaxis and severe asthma or it can be a chronic condition that interferes with the quality of life such as in eczema and allergic rhinitis [3].
Inflammation is another common disorder which is an innate immune response from the host defense mechanism. It consists of a series of complex biological processes aiming to combat infection and tissue injury. These processes lead to accumulation of plasma and blood cells in the tissue in addition to the release of inflammatory mediators aiming to reestablish tissue structures and function [4, 5]. Untreated inflammation can lead to a chronic condition which is characterized as a very long-term inflammation affecting the remodeling of tissue for many weeks and even years. It is considered as a main cause in the development of a various life threatening disorders, such as neurodegenerative diseases and cancers [4].
Non-steroidal anti-inflammatory drugs (NSAIDs) constitute the commonly adopted classes for the alleviation of inflammation and related conditions. Meanwhile, their intolerable side effects represented by gastrointestinal ulcers, and perforation with concomitant bleeding are the main obstacles facing their therapeutic usage [6]. On the contrary, nature continues to serve as a rich and appealing source of novel, safer, and cheaper bioactive molecules in comparison to many synthetic drugs. A plethora of plant extracts, as well as isolated compounds, possess notable anti-allergic and anti-inflammatory activities, as previously reported [5, 7–11].
Malvaceae, the mallows, is a family that comprises more than 200 genera and 2300 species. A great diversity of phytoconstituents such as triterpenes, flavonoids, coumarins, as well as alkaloids was previously reported in the members of this family [12, 13]. Brachychiton (Malvaceae) is a small genus native to Australia comprising of 30 species [14, 15]. Recently, Brachychiton has been considered as a separate genus from Sterculia as proved by the detailed investigation of its follicles, seed coats and embryo [14]. Members of the Brachychiton genus were used as food by Australian Aborigines and some are used as ornamental trees or shrubs [16, 17]. Different members of the genus possess several interesting biological effects such as antioxidant, antibacterial, anti-hyperglycemic, hepatoprotective and anti-schistosomal activities [18–21]. Phytochemical studies of various members of Brachychiton sp. resulted in the identification of various classes of compounds such as flavonoids, coumarins, triterpenes, sterols, and alkaloids [22–24]. Brachychiton rupestris is commonly known as “Queensland bottle tree” because it is native to Queensland and has a bottle shaped trunk. B. discolor (synonym B. luridus) is commonly called the lacebark tree [25–27]. The mucilage and ethyl acetate fraction of B. rupestris leaves were previously investigated for their in vivo anti-hyperglycemic effect and the phytochemical investigation of this species led to the isolation and identification of flavonoid aglycones and glycosides from the leaves [20, 28]. However, no complete phytochemical study was done on this species. Regarding B. discolor, two complete phytochemical studies were reported on this species where many classes of compounds were reported from the leaves, seeds and roots including triterpenes, flavonoids, phenolic acids, coumarins and alkaloids [23, 24].
Tracing current literature, nothing was found regarding the anti-allergic and anti-inflammatory effects of B. rupestris. However, different studies were carried out confirming the anti-allergic and anti-inflammatory activities of several triterpenes such as β-amyrin, oleanolic acid and lupeol [29–33] which were also isolated from B. discolor [23, 24]. Another species (B. populneus) was reported to be effective in relieving pain and skin diseases in folk medicine [34]. Furthermore, many members of the related genus, Sterculia, are popular in folk medicine for alleviating itching, dermatitis, boils, inflammations and other skin diseases [35–40]. Herein, we investigated the anti-allergic and anti-inflammatory activities of the methanol extracts and fractions of both B. rupestris and B. discolor leaves. The cytotoxic effect of B. rupestris and B. discolor leaves extracts and fractions was also evaluated to ascertain their safety. Additionally, the isolation and structural elucidation of the major constituents from the bioactive fractions of B. rupestris was achieved within this work.
Methods
Plant materials
The leaves of B. rupestris (T.Mitch.ex Lindl) K.Schum and B. discolor F.Muell were obtained from El-Orman Botanical Garden, Giza, Egypt, in summer 2014. The plants were generously authenticated by Prof. Dr. Mohamed El-Gibaly, Department of Botany, National Research Center (NRC), Giza, Egypt. Voucher specimens (PHG-P-BR-248 and PHG-P-BL-249) for B. rupestris and B. discolor (B. luridus), respectively were kept at the Pharmacognosy Department, Faculty of Pharmacy, Ain Shams University.
Extraction and fractionation
Total amount of 3.05 kg of B. rupestris air-dried leave were crushed, macerated in 29 L of distilled methanol for three times and filtered. Subsequently, the obtained filtrate was evaporated in vacuo at low temperature (45 °C) till dryness and then subjected to lyophilization to give 333.56 g of the total methanol extract. A portion of the extract (300 g) was successively partitioned with n-hexane (37.9 L), dichloromethane (4.8 L) and ethyl acetate (5.2 L) to give 54.35, 8.54 and 5.91 g, respectively along with the remaining hydromethanolic fraction estimated as 191.5 g.
Similarly, for B. discolor, the crushed air-dried leaves 600 g were macerated in distilled methanol (6 L × 3), filtered, and evaporated at 45 °C under reduced pressure till dryness to yield 36 g of the total methanol extract. Then, 11 g of the total extract were fractionated using 430 mL of n-hexane, 300 mL of dichloromethane and 300 mL of ethyl acetate successively to give 1.3, 1.2, and 0.9 g of the dried residues, respectively.
Biological investigations
In vitro assessment of the cytotoxic activity
Cell culture
The cytotoxicity of B. rupestris and B. discolor total extracts as well as their obtained fractions was examined on A549 (adenocarcinoma human alveolar basal epithelial cells), HepG2 (human liver cancer cell line) and MDA-MB-231 (invasive ductal carcinoma) cells. Cells were preserved in Dulbecco’s modified Eagle’s medium-high glucose powder (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS), 1 mM sodium pyruvate, 100 μg/mL streptomycin, 100 U/mL penicillin, and 2 mM L-glutamine. Cells were cultured in culture dishes (Cellstar) that were kept in a humidified chamber supplied with 5% (v/v) CO2 at 37 °C. Then the cells were maintained as a monolayer culture adopting serial subculturing. Cells growing in the logarithmic phase were employed in all experiments [41].
Cytotoxicity assay
MTT (methylthiazoltetrazolium) assay was employed to evaluate the cytotoxic activity of the tested samples against human cancer cells [42, 43]. Trypsinized cell suspensions were freshly prepared and then planted in a 96-well culture plate followed by overnight incubation. Tested samples were prepared in dimethyl sulfoxide (DMSO) to form stock solutions of 1 mg/mL. Cells were treated with the tested samples using different concentrations (2.5–20 μg/mL) then incubated for 72 h at 37 °C under 5% CO2. After the incubation, and removal of the cells medium, 100 μL of MTT solution was added to each well followed by incubation of the cells for 1 h. The formed formazan crystals were dissolved in DMSO after the removal of the medium to measure absorbance at 550 nm. The percentage of cell viability was calculated by the following formula:
Where O.D = optical density
Cytotoxicity was expressed as % cell inhibition. Doxorubicin was used as the positive control.
In vitro assessment of the anti-allergic activity
Chemicals and reagents
DMEM, dexamethasone, p-nitrophenyl-N-acetyl-D-glucosaminide (p-NAG), MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), penicillin and streptomycin, calcium ionophore A23187, mouse anti-DNP (dinitrophenyl) IgE antibody, and DMSO were purchased from Sigma-Aldrich (St. Louis, MO, USA). Moreover, FBS was obtained from Hyclone (Logan, UT, USA). Dinitrophenyl-conjugated bovine serum albumin (DNP-BSA) was purchased from Merck (Kenilworth, NJ, USA). Additional chemicals as well as reagents were purchased at the highest possible purity.
Cell culture
The mucosal mast cell-derived rat basophilic leukemia (RBL-2H3) cell line was obtained from the American Type Culture Collection. Cells were grown in DMEM medium accompanied with 10% FBS in addition to 100 U/mL penicillin plus 100 μg/mL streptomycin. Cells were cultured in 10 cm cell culture dishes (Cellstar) at 37 °C with 5% CO2 in air.
Cell viability assay
MTT assay was used to assess the toxic effects of samples on RBL-2H3 cells [44] and was done as previously mentioned [42, 43]. All experiments were done in triplicates. DMSO served as the negative control not affecting the growth of RBL-2H3 cells. Triton X-100 (0.5% solution) was employed as the positive control resulting in the death of all cells in a well.
Degranulation β-hexosaminidase assay induced by A23187
A23187-induced degranulation in RBL-2H3 cells was evaluated by a β-hexosaminidase activity assay as previously reported employing certain modifications [45, 46]. RBL-2H3 cells were seeded into 96-wells plate using a density of 2 × 104 cells/well and were incubated at 37 °C for 5 h in 5% CO2. Cells were washed with PBS (phosphate buffered saline) and then various concentrations of samples or medium (untreated control) were added to each well (100 μL), and the treated cells were incubated at 37 °C in 5% CO2 for 20 h. The cells were stimulated by calcium ionophore A23187 (1 μM) diluted in Tyrode’s buffer (135 mM NaCl, 1.8 mM CaCl2, 5 mM KCl, 1.0 mM MgCl2, 5.6 mM glucose, 20 mM HEPES at pH 7.4), and kept at 37 °C in 5% CO2 for 1 h. For the total amount of β-hexosaminidase release, the unstimulated cells were lysed using 0.5% Triton X-100. Untreated unstimulated cells represented spontaneous β-hexosaminidase release. The control wells were represented by the stimulated untreated cells. The cells supernatants (50 μL) were incubated with equal volume of 1 μM of p-NAG (50 μL), a substrate for β-hexosaminidase, prepared in 0.05 M citrate buffer (pH 4.5) for 1 h at 37 °C. The reaction was stopped by 100 μL of stop buffer (0.1 M Na2/NaHCO3, pH 10.0). Microplate reader was used to measure the absorbance at 405 nm. The inhibition percentage of β-hexosaminidase release from RBL-2H3 cells was calculated using the following equation:
Dexamethasone (10 nM) was employed as the positive control.
Degranulation β-hexosaminidase assay induced by IgE
β-Hexosaminidase release from the activated RBL-2H3 cells was measured as previously reported [45, 47], with some modifications. The inhibition percentage of antigen-induced β-hexosaminidase release from RBL-2H3 cells was assessed in a similar way as described above in the degranulation A23187-induced β-hexosaminidase assay, except of the stimulation process. The cells were sensitized with anti-DNP IgE (0.1 μg/mL) for at least 2 h and then washed with pre-warmed Tyrode’s buffer, followed by stimulation by antigen DNP-BSA (100 ng/mL). Dexamethasone (10 nM) was employed as the positive control.
In vitro assessment of the anti-inflammatory activity
Preparation of human neutrophils
Blood was withdrawn from 20 to 35 years old healthy human donors adopting a protocol approved by the institutional review board at Chang Gung Memorial Hospital. Isolation of neutrophils was done employing a standard method which was previously reported [48].
Measurement of superoxide generation
Ferricytochrome c (0.5 mg/mL) and Ca2 (1 mM) were incubated with neutrophils at 37 °C for 2 min, followed by the treatment with the tested samples for 5 min. Cells activation was done using formyl-methionyl-leucyl-phenylalanine (fMLF, 100 nM)/cytochalasin B (CB, 1 μg/mL) for 10 min. The absorbance was detected at 550 nm in a double-beam spectrophotometer Hitachi U-3010. Superoxide inhibition was determined by lowering ferricytochrome c as reported previously [48, 49]. The differences in absorbance between the measurements in the presence of superoxide (100 U/mL) and its absence divided by the extinction coefficient for the reduction of ferricytochrome c (ε = 21.1/mM/10 mm) were used as the basis for calculations. Genistein was adopted as the positive control [50, 51].
Measurement of elastase release
The release of elastase was determined by assessing the degranulation of azurophilic granules [48, 49]. An elastase substrate MeO-Suc-Ala-Ala-Pro-Val-p-nitroanilide (100 μM) was equilibrated with neutrophils at 37 °C for 2 min, followed by incubation with drugs for 5 min. Activation of the cells was done using 100 nM fMLF and 0.5 μg/mL CB, and then the variations in absorbance were detected at 405 nm. The results are shown as the percentage of the initial rate of elastase release in the fMLF/CB-activated, drug-free control system. Genistein was employed as the positive control [50, 51].
Statistical analysis
Results are represented as mean ± SD value of at least three independent measurements unless otherwise specified. The 50% inhibitory concentration (IC50) was determined using the dose-response curve which was constructed by plotting the percentage of inhibition versus concentrations (linear function, Microsoft Office). Statistical analysis was done using one-way analysis of variance (ANOVA) followed by Dunnet’s test (GraphPad Prism 6.0, GraphPad Software, Sand Diego, CA, USA, anti-allergic assay) or Student’s t-test (Sigma Plot, Systat software, Systat Software Inc., San Jose, CA, USA, anti-inflammatory assay). Values which show *p < 0.05, **p < 0.001 are statistically significant.
Phytochemical investigations
General experimental procedures
1H and 13C (APT) NMR analyses were done using a Bruker Ascend 400/R spectrometer (Burker Avance III, Fallanden Switzerland) at the Center for Drug Discovery, Research and Development, Faculty of Pharmacy, Ain Shams University using 400 and 100 MHz the operating frequencies. Chemical shifts were reported in δ ppm and were related to that of the solvents. Dissolution of the tested samples was done using various deuterated solvents (Sigma Aldrich, Germany) in 3 mm NMR tubes (Bruker). Spectra were recorded at 25 °C; δ ppm relative to tetramethylsilane (Me4Si) as the internal standard. Two-dimensional (2D) NMR experiments (1H, 1H-1H COSY; 1H-13C HSQC; 1H-13C HMBC) were done using the pulse sequences from the Bruker user library. Waters Xevo TQD mass spectrometer supplied with UPLC Acquity mode (Milford, USA) was employed to carry out ESI-MS analysis. Normal phase column chromatography was done using silica gel (Kieselgel 60, 70–230, and 230–400 mesh, Merck KGaA, Darmstadt, Germany). TLC analysis was done utilizing normal phase silica gel precoated plates F254 (Merck, Germany). Detection of TLC spots was done using UV light at 254 nm and 365 nm as well as by spraying with 10% H2SO4 with subsequent heating on a hot plate at 100 °C.
Isolation of the secondary metabolites from the bioactive fractions
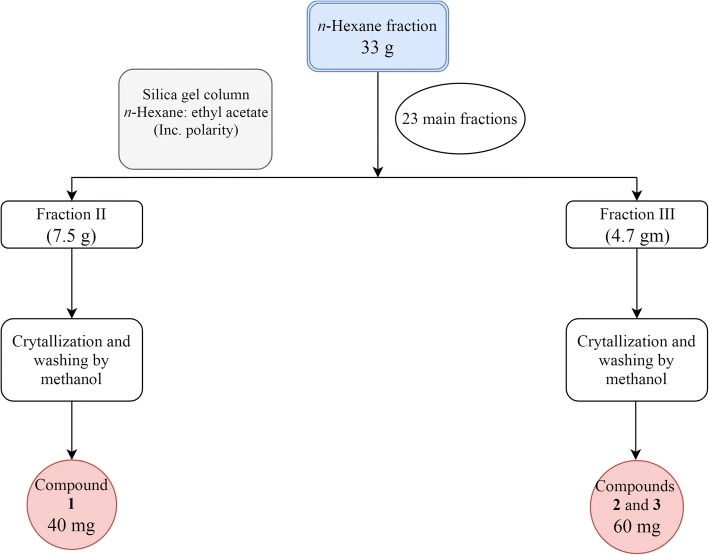
The n-hexane fraction (33 g) of B. rupestris was chromatographed on silica gel (600 g) employing n-hexane:EtOAc with increasing polarity to give 23 major fractions. Fraction II was further eluted with a mixture of n-hexane: EtOAc (9.0:1.0) from which compound 1 (40 mg) was precipitated as a white amorphous powder. A mixture of compounds 2 and 3 (60 mg) was precipitated from fraction III as white crystalline needles using the solvent system n-hexane:EtOAc (9.0:1.0) as illustrated in Fig. 1.
Fig. 1.
Scheme showing the chromatographic fractionation of the n-hexane fraction
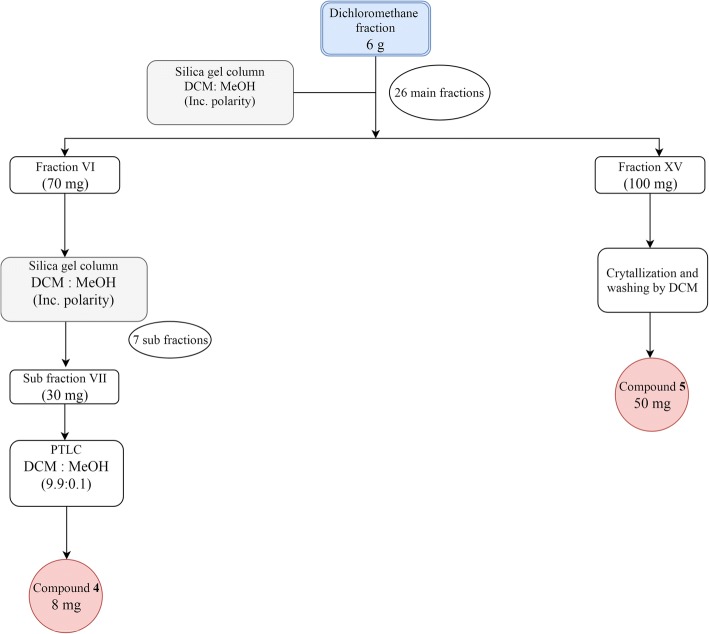
The dichloromethane fraction of B. rupestris (6 g) was chromatographed on silica gel (300 g) using mixtures of CH2Cl2:CH3OH with increasing polarity as eluents to afford 26 major fractions. Fraction VI (70 mg) was further eluted with dichloromethane and was subjected to silica gel column using a mixture of CH2Cl2:CH3OH to give seven subfractions. Subfraction 7 (30 mg) was eluted with a mixture of CH2Cl2:CH3OH (9.9:0.1) and purified over preparative TLC which resulted in the separation of compound 4 (8 mg) that showed strong fluorescent yellow color. Fraction XV was eluted using a mixture of CH2Cl2:CH3OH (9.6:0.4) from which compound 5 (50 mg) was precipitated as a yellow powder as shown in Fig. 2.
Fig. 2.
Scheme showing the chromatographic fractionation of the dichloromethane fraction
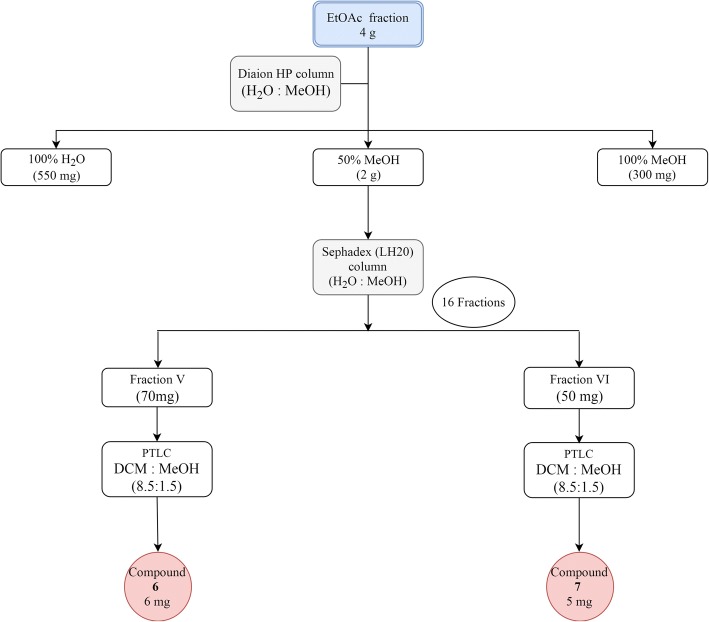
The EtOAc fraction (4 g) was applied on the top of 150 g Diaion HP column using water, 50% methanol, 100% methanol as the mobile phases. The 50% methanol fraction (2 g) was the most promising fraction after comparing its TLC with the other fractions and was applied on the top of 40 g Sephadex® LH 20 and eluted using water and methanol of decreasing polarity to give 16 fractions. Fraction V (70 mg) and fraction VI (50 mg) were eluted using water 100% and were purified over preparative TLC using CH2Cl2:CH3OH (8.5:1.5) as the mobile phase to separate compounds 6 (6 mg) and 7 (5 mg), respectively (Fig. 3).
Fig. 3.
Scheme showing the chromatographic fractionation of the ethyl acetate fraction
Spectroscopic data of compounds 1–7
β-Amyrin acetate (1)
It was isolated as a white amorphous powder; with Rf = 0.530 in n-hexane:EtOAc (9.5:0.5). 1H NMR (400 MHz, CDCl3), 13C NMR (100 MHz, CDCl3) and 2D NMR spectroscopic data are displayed in the Additional file 1: Figure S1).
β-Sitosterol (2) and Stigmasterol (3)
They were isolated as white crystalline needles; showing Rf = 0.206 in n-hexane:EtOAc (9:1). 1H-NMR (400 MHz, CDCl3), 13C NMR (100 MHz, CDCl3) and 2D NMR spectral data are displayed in the Additional file 1: Figure S2.
Scopoletin (4)
It was obtained as a yellow powder; with Rf = 0.630 in CH2Cl2:CH3OH (9.9:0.1). 1H-NMR (400 MHz, CD3OD) (δ ppm): 7.75 (1H, d, J = 9.1, H-4), 6.80 (1H, s, H-5), 6.45 (1H, s, H-8), 5.85 (1H, d, J = 9.1 Hz, H-3), 3.81 (3H, s, 6-OCH3). 13C NMR data (100 MHz, CD3OD) (δ ppm): 166.26 (C-2), 153.86 (C-7), 151.4 (C-6), 146.99 (C-4), 107.65 (C-5), 105.50 (C-3), 104.59, (C-8), 56.03 (6-OCH3). It exhibited a deprotonated molecular ion peak at m/z 190.8 [M-H]− in ESI-MS negative ion mode, corresponding to the molecular formula C10H8O4 (Additional file 1: Figure S3).
β-Sitosterol-3-O-β-D-glucoside (5)
It was isolated as a buff amorphous powder; with Rf = 0.630 in CH2Cl2:CH3OH (9.2:0.8). 1H-NMR (400 MHz, DMSO-d6), 13C NMR (100 MHz, DMSO-d6) and 2D NMR spectroscopic data are displayed in the Additional file 1: Figure S4.
Dihydrodehydrodiconiferyl alcohol 4-O-β-D-glucoside (6)
It was obtained as a yellowish white amorphous powder; with Rf = 0.259 in CH2Cl2:CH3OH (8.5:1.5). 1H-NMR (400 MHz, CD3OD), 13C NMR data (100 MHz, CD3OD) are illustrated in Table 4, (Additional file 1: Figure S5).
Table 4.
1H- and 13C-NMR spectroscopic data for 6 and 7
| Dihydrodehydrodiconiferyl alcohol 4-O-β-D-glucoside (6) | Dihydrodehydrodiconiferyl alcohol 9-O-β-D-glucoside (7) | |||
|---|---|---|---|---|
| δ C | δH (Mult, Int), J in Hz | δ C | δH (Mult, Int), J in Hz | |
| 1 | 137.09 | 134.67 | ||
| 2 | 111.19 | 7.03 (d, 1H), 1.84 | 110.71 | 7.02 (d, 1H), 2.01 |
| 3 | 150.9 | 149.05 | ||
| 4 | 147.6 | 147.48 | ||
| 5 | 117.95 | 7.14 (d, 1H), 8.43 | 116.08 | 6.78 (d, 1H), 8.09 |
| 6 | 119.37 | 6.93 (dd, 1H), 8.31, 2.03 | 119.72 | 6.89 (dd, 1H), 8.28, 2 |
| 7 | 88.48 | 5.56 (d, 1H), 5.85 | 88.98 | 5.62 (d, 1H), 6.21 |
| 8 | 55.68 | 3.44 (m, 1H) | 53.28 | 3.69 (m, 1H) |
| 9 | 65.07 | 3.80, 3.75 (m, m, 2H) | 72.46 | 4.23, 3.79 (dd, m, 2H) |
| 3-OCH3 | 56.79 | 3.87 (s, 3H) | 56.44 | 3.85 (s, 3H) |
| 1′ | 138.37 | 136.64 | ||
| 2′ | 114.19 | 6.73 (s, 1H) | 114.19 | 6.75 (s, 1H) |
| 3′ | 145.24 | 145.21 | ||
| 4′ | 147.6 | 147.48 | ||
| 5′ | 129.58 | 129.56 | ||
| 6′ | 118.04 | 6.72 (s, 1H) | 118.21 | 6.80 (s, 1H) |
| 7′ | 32.89 | 2.63 (t, 2H) | 32.89 | 2.65 (t, 2H) |
| 8′ | 35.84 | 1.82 (m, 2H) | 35.82 | 1.84 (m, 2H) |
| 9′ | 62.22 | 3.57 (t, 2H) | 62.23 | 3.59 (t, 2H) |
| 3′-OCH3 | 56.71 | 3.83 (s, 3H) | 56.77 | 3.88 (s, 3H) |
| 1″ | 102.78 | 4.88 (covered by solvent, 1H) | 104.57 | 4.38 (d, 1H), 7.79 |
| 2″ | 74.90 | 3.48 (m, 1H) | 75.18 | 3.25 (m, 1H) |
| 3″ | 77.84 | 3.39 (m, 1H) | 78.07 | 3.31 (m, 1H) |
| 4″ | 71.34 | 3.39 (m, 1H) | 71.66 | 3.31 (m, 1H) |
| 5″ | 78.19 | 3.39 (m, 1H) | 78.26 | 3.31 (m, 1H) |
| 6″ | 62.51 | 3.85, 3.69 (m, 2H) | 62.81 | 3.88, 3.70 (m 2H) |
NMR data (δ) were measured 1H-NMR (400 MHz, CD3OH) and 13C-NMR data (100 MHz, CD3OH)
Dihydrodehydrodiconiferyl alcohol 9-O-β-D-glucoside (7)
It was obtained as a yellowish white amorphous powder; with Rf = 0.304 in CH2Cl2:CH3OH (8.5:1.5). 1H-NMR (400 MHz, CD3OD), 13C NMR (100 MHz, CD3OD) and 2D NMR spectroscopic data are displayed in Table 4 and the Additional file 1: Figure S6.
Results
In vitro assessment of the cytotoxic activity of B. rupestris and B. discolor
The cytotoxicity of the total methanol extracts and fractions of both B. rupestris and B. discolor was evaluated versus HepG2, A549 and MDA-MB-231 cancer cells using doxorubicin as the positive control. Extracts and fractions of both species at 20 μg/mL exhibited no cytotoxic activity against any of the tested cell lines. Noteworthy to mention that doxorubicin showed 91.28, 97.69 and 98.05% cell growth inhibition against HepG2, MDA-MB-231 and A549, respectively at 2 μg/mL. The results are illustrated in Table 1. Together with the nontoxic effects of all samples towards RBL-2H3 mast cells (see the following section, and Table 2) the results suggested that both species extracts and fractions exhibited no cytotoxicity against the tested cancer cell lines.
Table 1.
In vitro cytotoxicity of different extracts and fractions of B. rupestris and B. discolor against HepG2, MDA-MB-231 and A549 cell lines
| Cell line | BRT | BRH | BRD | BRE | BRR | BDT | BDH | BDD | BDE | doxorubicin |
|---|---|---|---|---|---|---|---|---|---|---|
| HepG2 | 1.28 | −2.078 | −8.02 | −8.43 | −13.74 | 1.66 | −7.43 | −16.18 | −6.62 | 91.28 ± 0.3 |
| MDA-MB-231 | −15.86 | − 9.70 | −18.83 | −25.52 | − 23.30 | − 6.19 | − 6.73 | 13.17 | −20.64 | 97.69 ± 0.4 |
| A549 | 0.44 | −0.81 | 7.85 | −8.37 | −0.89 | 12.59 | 8.18 | 14.99 | −6.52 | 98.05 ± 0.0 |
Results are presented as growth inhibition percentage at concentration of 20 μg/mL, mean (n = 1). Doxorubicin (2 μg/mL) was used as the reference drug, mean ± SD (n = 2). BRT: B. rupestris total methanol extract; BHT: B. rupestris n-hexane fraction; BRD: B. rupestris dichloromethane fraction; BRE: B. rupestris ethyl acetate fraction; BRR: B. rupestris remaining MeOH(aq) fraction; BDT: B. discolor total methanol extract; BDH: B. discolor n-hexane fraction; BDD: B. discolor dichloromethane fraction; BDE: B. discolor ethyl acetate fraction
Table 2.
Anti-allergic activity of B. rupestris and B. discolor extracts and fractions
| Sample | % viability, RBL-2H3a | % inhibition of A23187-induced β-hexosaminidase releaseb | |
|---|---|---|---|
| 100 μg/mL | 10 μg/mL | 100 μg/mL | |
| BRT | 99.0 ± 1.7 | 3.0 ± 5.2 | 25.7 ± 2.1** |
| BRH | 96.7 ± 4.0 | 3.3 ± 5.8 | 39.0 ± 13.1** |
| BRD | 95.3 ± 8.1 | 4.3 ± 7.5 | 19.0 ± 4.4* |
| BRE | 97.7 ± 4.0 | 2.0 ± 3.5 | 7.0 ± 5.2 |
| BRR | 99.0 ± 1.7 | 4.3 ± 5.1 | 3.7 ± 6.4 |
| BDT | 99.0 ± 1.7 | 3.7 ± 6.4 | 16.0 ± 5.0* |
| BDH | 99.7 ± 0.6 | 4.3 ± 7.5 | 30.3 ± 3.1** |
| BDD | 100.0 ± 0.0 | 0.0 ± 0.0 | 44.0 ± 7.8** |
| BDE | 100.0 ± 0.0 | 1.7 ± 2.9 | 0.3 ± 0.6 |
aThe cytotoxicity of samples towards RBL-2H3 cells was evaluated using MTT viability assay and none of the samples showed any toxicity; results are presented as mean ± SD (n = 3)
bDexamethasone (10 nM) was used as the positive control and inhibited 62.0 ± 9.5%** of A23187-induced β-hexosaminidase release in RBL-2H3 cells. Results are presented as mean ± SD (n = 3); *p < 0.05, **p < 0.001 compared with the control value (A23187 only)
BRT B. rupestris total methanol extract, BRH B. rupestris n-hexane fraction, BRD B. rupestris dichloromethane fraction, BRE B. rupestris ethyl acetate fraction, BRR B. rupestris remaining MeOH(aq) fraction, BDT B. discolor total methanol extract, BDH B. discolor n-hexane fraction, BDD B. discolor dichloromethane fraction, BDE B. discolor ethyl acetate fraction
In vitro assessment of the anti-allergic activity of B. rupestris and B. discolor
The anti-allergic activity of the total methanol extracts and fractions of both B. rupestris and B. discolor was assessed using degranulation assay in RBL-2H3 mast cell model and the results are presented in Table 2. Initially, the cytotoxic effect of all samples was tested against RBL-2H3 cells using MTT viability assay. All samples were found to be nontoxic at 100 μg/mL. The samples were subjected to the anti-allergic assay by evaluating their inhibitory effect on β-hexosaminidase release in RBL-2H3 cells induced by calcium ionophore, A23187. According to our results, B. rupestris and B. discolor crude methanol extracts (BRT 25.7% and BDT 16.0% inhibition) and nonpolar n-hexane (BRH 39.0%, BDH 30.3% inhibition) and dichloromethane fractions (BRD 19.0%; and BDD 44.0% inhibition) exhibited significant inhibition of β-hexosaminidase release in A23187-induced degranulation assay at 100 μg/mL (Table 2). Dexamethasone, a positive control, showed 62.0% inhibition of β-hexosaminidase release at 10 nM.
In vitro assessment of the anti-inflammatory activity of B. rupestris and B. discolor
Similarly, the anti-inflammatory activity was determined for the total methanol extracts and fractions of B. rupestris and B. discolor and the results are presented in Table 3. Both Brachychiton species exhibited a promising inhibitory activity on superoxide anion production as well as elastase release in fMLF-activated human neutrophils indicating their potential applications for the alleviation of both acute and chronic inflammatory disorders. All samples inhibited superoxide anion generation showing IC50 values between 0.78 and 6.25 μg/mL in addition to inhibition of elastase release showing IC50 values ranging from 1.57 to > 10 μg/mL (Table 3). The most potent fractions, dichloromethane fractions of B. rupestris (BRD) and B. discolor (BDD) inhibited superoxide anion generation with IC50 values 2.99 μg/mL (BRD) and 0.78 μg/mL (BDD), and inhibited elastase release with IC50 values 1.98 μg/mL (BRD) and 1.57 μg/mL (BDD). Such activities indicated comparable or even better inhibitory potential than that of genistein (superoxide IC50 0.41 μg/mL and elastase IC50 4.41 μg/mL), a known anti-inflammatory natural product [50, 51]. The dichloromethane fractions (BRD and BDD) were capable of almost completely abolishing oxidative burst and degranulation in fMLF-activated human neutrophils at 10 μg/mL (data not shown). Meanwhile, the ethyl acetate fraction of both species showed anti-inflammatory activity by inhibiting elastase release showing IC50 values of 2.71 μg/mL for B. rupestris (BRE) and 2.95 μg/mL for B. discolor (BDE).
Table 3.
Effect of the total extracts and fractions of B. rupestris and B. discolor on superoxide anion generation and elastase release in fMLF/CB-induced human neutrophils
| Sample | Superoxide anion generationa | Elastase releasea |
|---|---|---|
| IC50 (μg/mL)b | IC50 (μg/mL)b | |
| BRT | 4.92 ± 1.47 | 3.82 ± 0.55 |
| BRH | 5.69 ± 0.80 | 3.73 ± 1.16 |
| BRD | 2.99 ± 0.73 | 1.98 ± 1.54 |
| BRE | 6.25 ± 3.10 | 2.71 ± 0.79 |
| BRR | 3.01 ± 1.91 | > 10c |
| BDT | 4.73 ± 0.97 | 5.37 ± 1.23 |
| BDH | 6.25 ± 2.18 | 6.04 ± 2.32 |
| BDD | 0.78 ± 0.29 | 1.57 ± 0.84 |
| BDE | 5.22 ± 1.35 | 2.95 ± 1.08 |
| genistein | 0.41 ± 0.09 | 4.41 ± 1.99 |
aIC50 values, results are presented as mean ± SD (n = 3), compared with the control value (formyl-methionyl-leucyl-phenylalanine/cytochalasin B, fMLF/CB)
bConcentration necessary for 50% inhibition (IC50)
cBRR exerted significant inhibitory activity in superoxide anion generation (49.6 ± 2.9%, **p < 0.001) at 10 μg/mL. BRT B. rupestris total methanol extract, BRH B. rupestris n-hexane fraction, BRD B. rupestris dichloromethane fraction, BRE B. rupestris ethyl acetate fraction, BRR B. rupestris remaining MeOH(aq) fraction, BDT B. discolor total methanol extract, BDH B. discolor n-hexane fraction, BDD B. discolor dichloromethane fraction, BDE B. discolor ethyl acetate fraction
Phytochemical investigations
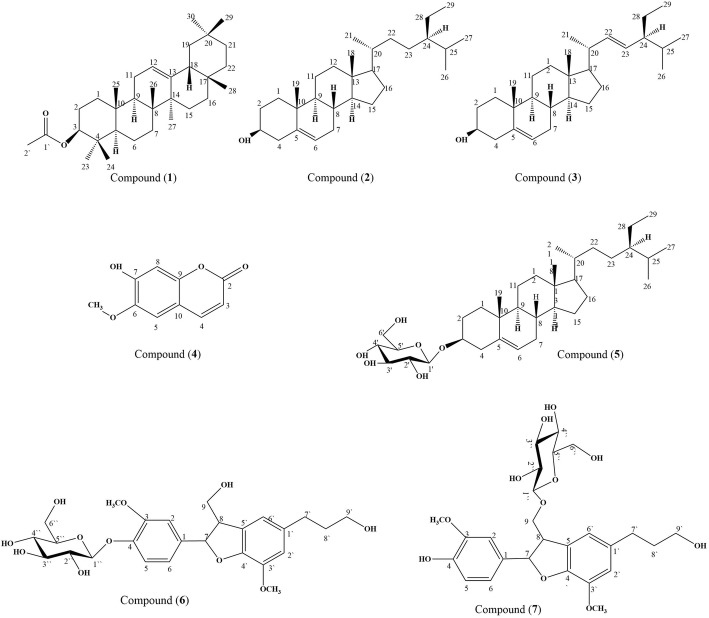
In-depth phytochemical investigation was performed on the most bioactive fractions of B. rupestris leaves including the n-hexane, the dichloromethane and ethyl acetate fractions that showed the highest anti-allergic and anti-inflammatory activities. Three compounds were isolated and structurally elucidated from the n-hexane fraction which were β-amyrin acetate (1) [29], β-sitosterol (2) [52], stigmasterol (3) [52]. Meanwhile, two compounds were obtained from the dichloromethane fraction including scopoletin (4) [53, 54] and β-sitosterol-3-O-β-D-glucoside (5) [55] which were isolated for the first time from B. rupestris leaves. Furthermore, two neolignans were obtained from the ethyl acetate fraction, dihydrodehydrodiconiferyl alcohol 4-O-β-D-glucoside (6) [56] and dihydrodehydrodiconiferyl alcohol 9-O-β-D-glucoside (7) [57] which were isolated for the first time from the genus (Fig. 4). Their structures were fully elucidated using 1D and 2D NMR techniques and they were further ascertained by comparing their data with previously reported data in literature.
Fig. 4.
Structures of identified compounds from n-hexane, dichloromethane and ethyl acetate fractions of B. rupestris
Dihydrodehydrodiconiferyl alcohol 4-O-β-D-glucoside (6) was isolated as a yellowish white amorphous powder. 1H-NMR of (6) revealed the presence of a 1,2,4-trisubstituted benzene ring with signals at δH 7.03 (d, J = 1.84 Hz), 7.14 (d, J = 8.43 Hz) and 6.93 (dd, J = 8.31, 2.03 Hz) for H-2, H-5 and H-6, respectively, each integrated for one proton. Also, a 1,2,3,5-tetrasubstituted benzene ring was presented by two broad singlet signals at δH 6.73 (H-2′) and 6.72 (H-6′). The spectrum revealed the existence of a hydroxypropyl group showing three signals at δH 2.63 (t, H-7′), 1.82 (m, H-8′), 3.57 (t, H-9′). Furthermore, a methine-methine-methylene group (CH-CH-CH2) appeared at δH 5.56 (d, J = 5.85, H-7), 3.44 (m, H-8), 3.80, 3.75 (m, m, H-9). The presence of β-D-glucose was proposed by the appearance of anomeric proton at δH 4.88 and other sugar protons at 3.39–3.85. Two singlet signals each integrated for three protons at δH 3.87 and 3.83 were attributed to two methoxy groups. 13C-NMR spectrum of compound (6) showed the presence of five aromatic methines and seven quaternary aromatic carbons signals attributed to two benzene rings at δC 111.19, 117.95, 119.37, 114.19, 118.04, 137.09, 150.9, 147.6, 138.37, 145.24, 147.6, 129.58. Downfield shifts of C-3 (150.9), C-4 (147.6), C-3′ (145.24), C-4′ (147.6) indicated their attachment to oxygenated functional groups. A signal at δC 102.78 was attributed to the anomeric carbon of glucose unit and the other sugar carbons appeared at 74.90, 77.84, 71.34, 78.19 and 62.51. The two signals at δC 56.79 and 56.71 represented two methoxy groups. Other aliphatic signals appeared at δC 88.48, 65.07, 62.22, 55.68, 35.84 and 32.89. The HMBC spectrum showed that the methoxy group at δC 56.79 was placed at C-3 (150.9) and the methoxy group at δC 56.71 was placed at C-3′ (145.24). Also, it showed a correlation between C-6, C-2 with H-7; C-6′, C-2′ with H-7′; and C-9′ with H-7′. The correlation between C-4 with H-1″ supported the presence of the sugar at C-4. From the displayed data (Table 4) and through comparison with the previously reported literature [56], compound (6) was identified as dihydrodehydrodiconiferyl alcohol 4-O-β-D-glucoside which was the first time to be reported in the genus.
Dihydrodehydrodiconiferyl alcohol 9-O-β-D-glucoside (7) was isolated as a yellowish white amorphous powder. The 1H-NMR and 13C-NMR data for this compound were similar to compound (6) suggesting the same neolignan nucleus; the two compounds differ in the position of the glucose moiety. The HMBC spectrum revealed the correlation between C-9 with H-1″ which supported the attachment of the sugar at C-9. The downfield shift of C-9 at δC 72.46 also supported the attachment of the sugar at C-9 [57] (Table 4).
In vitro assessment of the cytotoxic activity of the isolated compounds
Additionally, the cytotoxic activity of the compounds obtained from the n-hexane and dichloromethane fractions of B. rupestris was examined using different concentrations (20, 10, 5, 2.5 μg/mL) of these compounds on the same cell lines utilized in the determination of the cytotoxic effect of the total extracts and subsequent fractions. The isolated compounds showed no cytotoxicity against hepatoma HepG2, breast MDA-MB-231 and lung A549 cancer cell lines with growth inhibition below 20%. The results are illustrated in Table 5. Doxorubicin was employed as a positive control and exhibited a strong cytotoxic effect against HepG2 (IC50 0.49 μg/mL), MDA-MB-231 (IC50 0.68 μg/mL) and A549 (IC50 0.13 μg/mL) cells.
Table 5.
Cytotoxic activity of the isolated compounds
| Cell line | Conc. (μg/mL) | % Inhibition | |
|---|---|---|---|
| β-amyrin acetate (1) | Scopoletin (4) | ||
| HepG2 | 20 | 12.1 ± 3.4 | 11.4 ± 2.1 |
| 10 | 16.0 ± 0.3 | 6.5 ± 1.2 | |
| 5 | 20.4 ± 2.3 | 0.9 ± 0.1 | |
| 2.5 | 8.23 ± 0.7 | 5.9 ± 1.2 | |
| MDA-MB-231 | 20 | −19.6 ± 1.2 | 8.2 ± 1.0 |
| 10 | −13.9 ± 1.2 | 6.0 ± 0.3 | |
| 5 | −5.5 ± 1.2 | 10.7 ± 9.8 | |
| 2.5 | 7.0 ± 0.1 | 15.5 ± 0.3 | |
| A549 | 20 | 2.2 ± 0.5 | 7.9 ± 0.4 |
| 10 | 9.0 ± 1.0 | 14.9 ± 0.4 | |
| 5 | 10.8 ± 0.5 | 14.6 ± 0.5 | |
| 2.5 | 0.4 ± 0.7 | 14.3 ± 0.7 | |
Results are presented as cell growth inhibition percentage at concentrations of 2.5 to 20 μg/mL, mean ± SD (n = 3). Doxorubicin was used as the positive control and exerted significant cell viability inhibitory effects against HepG2 (IC50 0.49 μg/mL), MDA-MB-231 (IC50 0.68 μg/mL)
In vitro assessment of the anti-allergic activity of the isolated compounds
To ascertain, whether the isolated compounds might be responsible for the anti-allergic activity observed in Brachychiton sp. crude extracts and nonpolar fractions, the isolated compounds were subjected to degranulation assay in RBL-2H3 mast cell model. The results are presented in Table 6. MTT viability assay was used to evaluate the potential toxic effects against RBL-2H3 cells. A mixture of β-sitosterol (2) and stigmasterol (3) (200 and 100 μg/mL) was considered toxic (viability below 85%). According to our results, scopoletin (4) showed 23.0% inhibition of A23187-induced and 30.0% of antigen-induced degranulation at 500 μM. Dihydrodehydrodiconiferyl alcohol 9-O-β-D-glucoside (7) showed only weak inhibitory effect in the A23187-induced assay (16.3% at 100 μM and 18.0% at 500 μM). Dexamethasone (10 nM) was utilized as the positive control and inhibited β-hexosaminidase release by 93.7%.
Table 6.
Anti-allergic activity of compounds isolated from B. rupestris
| Sample | % viability, RBL-2H3a | % inhibition of A23187-induced β-hexosaminidase releaseb | |||
|---|---|---|---|---|---|
| 100 μM | 500 μM | 10 μM | 100 μM | 500 μM | |
| β-amyrin acetate (1) | 98.3 ± 2.9 | c | 0.3 ± 0.6 | 0.7 ± 1.2 | c |
| scopoletin (4) | 96.0 ± 4.0 | 93.7 ± 6.5 | 3.7 ± 4.7 | 13.7 ± 8.0 | 23.0 ± 8.0**d |
| β-sitosterol-3-O-β-D-glucoside (5) | 96.0 ± 1.0 | c | 5.0 ± 5.6 | 7.7 ± 3.8 | c |
| dihydrodehydrodiconiferyl alcohol 4-O-β-D-glucoside (6) | 97.3 ± 2.5 | e | 7.0 ± 10.4 | 12.7 ± 7.6 | e |
| dihydrodehydrodiconiferyl alcohol 9-O-β-D-glucoside (7) | 97.7 ± 2.1 | 95.3 ± 4.2 | 3.3 ± 4.2 | 16.3 ± 5.5* | 18.0 ± 8.7* |
A mixture of β-sitosterol (2) and stigmasterol (3) was toxic towards RBL-2H3 cells at the concentration of 200 μg/mL (73.7 ± 11.7% viability) and 100 μg/ml (78.7 ± 11.6% viability) and inactive at the concentration of 10 μg/mL (5.0% ± 5.0% inhibition) in A23187-induced degranulation assay
aThe cytotoxicity of samples to RBL-2H3 was evaluated using MTT viability assay; results are presented as mean ± SD (n = 3)
bDexamethasone (10 nM) was used as the positive control and inhibited 93.7 ± 1.5%** of A23187-induced β-hexosaminidase release in RBL-2H3 cells. Results are presented as mean ± SD (n = 3); *p < 0.05, **p < 0.001 compared with the control value (A23187 only)
cPrecipitate was formed upon the addition into the medium at the concentration of 500 μM, therefore the result could not be justified
dScopoletin (500 μM) exerted 30.0 ± 7.1% inhibition of antigen-induced β-hexosaminidase release (mean ± SD, n = 2)
eDihydrodehydrodiconiferyl alcohol 4-O-β-D-glucoside was not tested at the concentration of 500 μM, however, it was nontoxic towards RBL-2H3 cells (96.0 ± 6.9% viability) and inactive in A23187-induced degranulation assay (10.0 ± 4.6% inhibition) at the concentration of 200 μM
In vitro assessment of the anti-inflammatory activity of the isolated compounds
The anti-inflammatory effect of the isolated compounds was determined to understand whether any of these compounds might be accountable for the potent activity of B. rupestris crude extract and its fractions. The results are illustrated in Table 7. According to the results, scopoletin (4) was found to significantly inhibit elastase release in fMLF-induced human neutrophils by 22.8% at 10 μM. Genistein, natural tyrosine kinase inhibitor [50, 51], was used as the positive control and caused significant suppression of superoxide anion generation (IC50 1.16 μM) and elastase release (IC50 21.51 μM).
Table 7.
Effect of pure compounds on superoxide anion generation and elastase release in fMLF/CB-induced human neutrophils
| Sample | Superoxide anion generationa | Elastase releasea |
|---|---|---|
| IC50 (μΜ)b | IC50 (μΜ)b | |
| β-amyrin acetate (1) | > 10 | > 10 |
| scopoletin (4) | > 10 | > 10c |
| β-sitosterol-3-O-β-D-glucoside (5) | > 10 | > 10 |
| dihydrodehydrodiconiferyl alcohol 4-O-β-D-glucoside (6) | > 10 | > 10 |
| dihydrodehydrodiconiferyl alcohol 9-O-β-D-glucoside (7) | >1d | >1d |
| genistein | 1.16 ± 0.12 | 21.51 ± 6.50 |
aIC50 values, results are presented as mean ± SD (n = 3–4), compared with the control value (formyl-methionyl-leucyl-phenylalanine/cytochalasin B, fMLF/CB)
bConcentration necessary for 50% inhibition (IC50)
cScopoletin (4) exerted significant inhibitory activity in elastase release assay (22.8 ± 15.3%, *p < 0.05) at 10 μM
dDihydrodehydrodiconiferyl alcohol 9-O-β-D-glucoside (10) was used at the final concentration of 1 μM due to solubility issues
Discussion
RBL-2H3 are mast cells that greatly affect the development of allergic response [58]. Upon activation by antigen or A23187 (calcium ionophore), mast cells produce histamine in addition to other mediators that immediately initiate hypersensitivity reactions. β-Hexosaminidase represents an important mast cells degranulation marker that is commonly used for the assessment of anti-allergic activity [59].
The anti-allergic activity of the crude extracts as well as n-hexane and dichloromethane fractions of B. rupestris and B. discolor leaves (Table 2) might be attributed to the presence of many active constituents from their non-polar fractions. Sterols, sterol glycosides, coumarin, and triterpenes were isolated and identified in B. rupestris leaves. Lanosterol, lupeol, β-amyrin, β-amyrin acetate and oleanolic acid were previously reported from B. discolor leaves by Kassem et al. [23]. These triterpenes were reported to exert a potent anti-allergic activity [60–62] including β-amyrin that was previously documented to exhibit mast cell membrane stabilization [30]. The anti-allergic activity of triterpenes might be attributed to the suppression of secretion of histamine and interleukins (IL-2, IL-4) from mast cells [62]. Also, β-sitosterol was reported to possess anti-allergic activity and might have therapeutic potential in allergic asthma [63, 64]. It was suggested that β-sitosterol and its glycoside inhibited the release of IL-4 so it could act as an immune modulator to relieve symptoms associated with seasonal allergic response [65]. However, we did not observe any significant effect of either β-amyrin acetate (1), the mixture of β-sitosterol (2) and stigmasterol (3) or β-sitosterol glycoside (5) in degranulation assay using the RBL-2H3 mast cell model (Table 6). Meanwhile, scopoletin (4) and dihydrodehydrodiconiferyl alcohol 9-O-β-D-glucoside (7) showed inhibitory activity on degranulation in RBL-2H3 cells.
Regarding the in vitro anti-inflammatory activity, neutrophils exert a vital role in host’s defenses versus the attack by microorganisms and in the pathogenesis of various inflammatory diseases [66]. In response to stimuli, such as fMLF, the activated neutrophils secrete a series of inflammatory mediators such as superoxide anion (O2.−) and elastase which are major contributors to the destruction of tissue in inflammatory response [67]. We observed that the crude extracts and fractions of B. rupestris and B. discolor leaves (Table 3) exerted potent anti-inflammatory activity in human neutrophils. Many studies supported the anti-inflammatory activity of sterols and their glycosides [68–70]. They induce immunomodulatory response that affects inflammatory mediators [71, 72]. They were also reported to possess potent in vivo anti-inflammatory activity with the concomitant reduction of edema and inflammation in rats [73]. Various studies confirmed the anti-inflammatory activity of triterpenes including β-amyrin and β-amyrin acetate [29, 74–76]. Coumarins also were found to have anti-inflammatory activity by inhibiting different inflammatory mediators such as cyclooxygenase-2, nitric oxide, tumor necrosis factor-α and interleukins [77–79]. Neolignans were reported to exert anti-inflammatory effects by suppressing superoxide anion generation and elastase release [80], they also exhibited nitric oxide (NO) and tumor necrosis factor-α (TNF-α) inhibitory effects [81]. However, according to our results, only scopoletin (4) exerted mild inhibition of elastase release. All other isolated compounds including dihydrodehydrodiconiferyl alcohol glycosides (6 and 7) were inactive in fMLF-activated human neutrophils.
Conclusions
The total extract and fractions of B. rupestris and B. discolor were nontoxic against hepatoma, breast and lung cancer cell lines. The crude extracts as well as the n-hexane and dichloromethane fractions of B. rupestris and B. discolor exhibited significant anti-allergic as well as anti-inflammatory activities. The phytochemical study of the leaves of B. rupestris resulted in the isolation of compounds from different chemical classes, including triterpene, sterols, sterol glycoside, coumarin and neolignans. All the tested compounds were nontoxic against the tested cancer cell lines. Among the isolated compounds, scopoletin exerted anti-allergic effects and mild anti-inflammatory activity by reducing elastase release in human neutrophils. However, the bioactivity of B. rupestris extracts and fractions was much more potent compared with any of the isolated compounds. Thus, leaves of B. rupestris and B. discolor are worth to be considered for further development and research based on their anti-allergic and anti-inflammatory activities. In vivo evaluation of the anti-allergic and anti-inflammatory activities is highly recommended for the active fractions of both Brachychiton species.
Additional file
Supplementary data contains supplementary figures (Figures S1-S6) showing the spectroscopic data of isolated compounds 1–7 from n-hexane, dichloromethane and ethyl acetate fractions of B. rupestris. (DOCX 2306 kb)
Acknowledgements
We are grateful to the Center for Research Resources and Development, Kaohsiung Medical University for providing instrumentation support and to Ms. Shu-Li Chen for kind assistance in cytotoxicity measurements. Also, we would like to thank the Center for Drug Discovery, Research and Development at Faculty of Pharmacy, Ain Shams University for the analyses of the isolated compounds.
Funding
This research was financial supported by the grants from the Ministry of Science and Technology (MOST 107–2911-I-037-502, MOST 106–2320-B-037-007-MY3, MOST 106–2320-B-255-003-MY3 and MOST 104–2320-B-255-004-MY3), Ministry of Education (EMRPD1G0231), Kaohsiung Medical University (106CM-KMU-02, KMU-DK107003, KMU-M106009), and Chang Gung Memorial Hospital (CMRPF1F0011~ 3, CMRPF1F0061~ 3, CMRPF1G0241~ 3, and BMRP450), Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA
One-way analysis of variance
- APT
Attached proton test
- B
Brachychiton
- BHT
tert-Butyl-1-hydroxytoluene
- BDD
Brachychiton discolor leaves dichloromethane fraction
- BDE
Brachychiton discolor leaves ethyl acetate fraction
- BDH
Brachychiton discolor leaves n-hexane fraction
- BDT
Brachychiton discolor leaves total extract
- BRD
Brachychiton rupestris leaves dichloromethane fraction
- BRE
Brachychiton rupestris leaves ethyl acetate fraction
- BRH
Brachychiton rupestris leaves n-hexane fraction
- BRR
B. rupestris remaining fraction
- BRT
Brachychiton rupestris leaves total extract
- CDCL3
Deuterated chloroform
- CD3OD
Deuterated methanol
- 13C-NMR
Carbon-13 Nuclear Magnetic Resonance
- d
doublet
- dd
doublet of doublet
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
Dimethylsulfoxide
- DMSO-d6
Deuterated dimethylsulfoxide-d6
- 2D-NMR
Two-dimensional nuclear magnetic resonance spectroscopy
- DNP
Dinitrophenyl
- DNP-BSA
Dinitrophenyl-conjugated bovine serum albumin
- ESI-MS
Electro-Spray Ionization Mass Spectrometry
- EtOAc
Ethyl acetate
- FBS
Fetal bovine serum
- fMLF/CB
formyl-methionyl-leucyl-phenylalanine/cytochalasin B
- 1H-1H COSY
1H-1H Correlated spectroscopy
- HMBC
Heteronuclear Multiple-Bond Correlation Spectroscopy
- 1H-NMR
Proton Nuclear Magnetic Resonance
- HSQC
Heteronuclear Single Quantum Coherence
- IC50
The half maximal inhibitory concentration
- m
multiplet
- MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
- p-NAG
p-Nitrophenyl-N-acetyl-D-glucosaminide
- PTLC
Preparative thin layer chromatography
- q
quartet
- Rf
Retardation factor
- s
Singlet
- S.D.
Standard deviation
- SEM
Standard error of mean
- t
Triplet
- TLC
Thin Layer Chromatography
- UPLC
Ultra Performance Liquid Chromatography
Authors’ contributions
AAT and FSY performed extraction, isolation and identification of pure compounds and shared writing the whole manuscript. MK, YCW, and BHC carried out the cytotoxic and anti-allergic studies, interpreted the results and edited the manuscript. ME, FRC and ANBS formulated the research hypothesis, supervised the biological part and shared in critical revision of the manuscript and the whole work. TLH contributed to the anti-inflammatory assays. All authors participated in interpretations of results, read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amany A. Thabet, Email: amany.thabet@pharma.asu.edu.eg
Fadia S. Youssef, Email: fadiayoussef@pharma.asu.edu.eg
Michal Korinek, Email: mickorinek@hotmail.com.
Fang-Rong Chang, Email: aaronfrc@kmu.edu.tw.
Yang-Chang Wu, Email: yachwu@kmu.edu.tw.
Bing-Hung Chen, Email: bhchen@kmu.edu.tw.
Mohamed El-Shazly, Phone: +201-001401091, Email: mohamed.elshazly@pharma.asu.edu.eg.
Abdel Nasser B. Singab, Phone: +201-005036231, Email: dean@pharma.asu.edu.eg
Tsong-Long Hwang, Phone: +886-3-2118800, Email: htl@mail.cgu.edu.tw.
References
- 1.Kawai M, Hirano T, Higa S, Arimitsu J, Maruta M, Kuwahara Y, et al. Flavonoids and related compounds as anti-allergic substances. Allergol Int. 2007;56:113–123. doi: 10.2332/allergolint.R-06-135. [DOI] [PubMed] [Google Scholar]
- 2.Galli Stephen J., Tsai Mindy, Piliponsky Adrian M. The development of allergic inflammation. Nature. 2008;454(7203):445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broide DH. Immunomodulation of allergic disease. Annu Rev Med. 2009;60:279–291. doi: 10.1146/annurev.med.60.041807.123524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baek K-S, Yi Y-S, Son Y-J, Yoo S, Sung NY, Kim Y, et al. In vitro and in vivo anti-inflammatory activities of Korean red ginseng-derived components. J Ginseng Res. 2016;40:437–444. doi: 10.1016/j.jgr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y-T, Zhu L, Zeng D, Long W, Zhu S-M. Chemical composition and anti-inflammatory activities of essential oil from Trachydium roylei. J Food Drug Anal. 2016;24:602–609. doi: 10.1016/j.jfda.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaikh RU, Pund MM, Gacche RN. Evaluation of anti-inflammatory activity of selected medicinal plants used in Indian traditional medication system in vitro as well as in vivo. J Tradit Complement Med. 2016;6:355–361. doi: 10.1016/j.jtcme.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andhare RN, Raut MK, Naik SR. Evaluation of antiallergic and anti-anaphylactic activity of ethanolic extract of Sanseveiria trifasciata leaves (EEST) in rodents. J Ethnopharmacol. 2012;142:627–633. doi: 10.1016/j.jep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Sato A, Zhang T, Yonekura L, Tamura H. Antiallergic activities of eleven onions (Allium cepa) were attributed to quercetin 4′-glucoside using QuEChERS method and Pearson's correlation coefficient. J Funct Foods. 2015;14:581–589. doi: 10.1016/j.jff.2015.02.029. [DOI] [Google Scholar]
- 9.Shi Y-H, Zhu S, Ge Y-W, He Y-M, Kazuma K, Wang Z, et al. Monoterpene derivatives with anti-allergic activity from red peony root, the root of Paeonia lactiflora. Fitoterapia. 2016;108:55–61. doi: 10.1016/j.fitote.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Singh B, Nadkarni JR, Vishwakarma RA, Bharate SB, Nivsarkar M, Anandjiwala S. The hydroalcoholic extract of Cassia alata (Linn.) leaves and its major compound rhein exhibits antiallergic activity via mast cell stabilization and lipoxygenase inhibition. J Ethnopharmacol. 2012;141:469–473. doi: 10.1016/j.jep.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Tian J, Che H, Ha D, Wei Y, Zheng S. Characterization and anti-allergic effect of a polysaccharide from the flower buds of Lonicera japonica. Carbohydr Polym. 2012;90:1642–1647. doi: 10.1016/j.carbpol.2012.07.044. [DOI] [PubMed] [Google Scholar]
- 12.Silva FV, Oliveira IS, Figueiredo KA, Melo Júnior FB, Costa DA, Chaves MH, et al. Anti-inflammatory and antinociceptive effects of Sterculia striata a. St.-Hil. & Naudin (Malvaceae) in rodents. J Med Food. 2014;17:694–700. doi: 10.1089/jmf.2013.0062. [DOI] [PubMed] [Google Scholar]
- 13.Dai Y, Harinantenaina L, Brodie PJ, Callmander MW, Randrianasolo S, Rakotobe E, et al. Isolation and synthesis of two antiproliferative calamenene-type sesquiterpenoids from Sterculia tavia from the Madagascar rain forest. Bioorg Med Chem. 2012;20:6940–6944. doi: 10.1016/j.bmc.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guymer GP. A taxonomic revision of Brachychiton (Sterculiaceae) Aust Syst Bot. 1988;1:199–323. doi: 10.1071/SB9880199. [DOI] [Google Scholar]
- 15.Wilkie P, Clark A, Pennington RT, Cheek M, Bayer C, Wilcock CC. Phylogenetic relationships within the subfamily Sterculioideae (Malvaceae/Sterculiaceae-Sterculieae) using the chloroplast gene ndhF. Syst Bot. 2006;31:160–170. doi: 10.1600/036364406775971714. [DOI] [Google Scholar]
- 16.Salem MZM, Ali HM, Mansour MM. Fatty acid methyl esters from air-dried wood, bark, and leaves of Brachychiton diversifolius R. Br: antibacterial, antifungal, and antioxidant activities. Bioresources. 2014;9:3835–3845. [Google Scholar]
- 17.Rao KS. Characteristics and fatty acid composition of Brachychiton species seeds and the oils (Sterculiaceae) J Agric Food Chem. 1991;39:881–882. doi: 10.1021/jf00005a014. [DOI] [Google Scholar]
- 18.Abdel-Megeed A, Salem MZ, Ali HM, Gohar YM. Brachychiton diversifolius as a source of natural products: antibacterial and antioxidant evaluation of extracts of wood branches. J Pure Appl Microbiol. 2013;7:1843–1850. [Google Scholar]
- 19.Yousif F, Hifnawy MS, Soliman G, Boulos L, Labib T, Mahmoud S, et al. Large-scale in vitro screening of Egyptian native and cultivated plants for schistosomicidal activity. Pharm Biol. 2007;45:501–510. doi: 10.1080/13880200701389425. [DOI] [Google Scholar]
- 20.Thabet AA, Youssef FS, El-Shazly M, El-Beshbishy HA, Singab ANB. Validation of the antihyperglycaemic and hepatoprotective activity of the flavonoid rich fraction of Brachychiton rupestris using in vivo experimental models and molecular modelling. Food Chem Toxicol. 2018;114:302–310. doi: 10.1016/j.fct.2018.02.054. [DOI] [PubMed] [Google Scholar]
- 21.Kassem HA, Eid HH, Abdel-Latif HA. Phytochemical and hypoglycemic studies of the leaves of Brachychiton australis (Schott & Endl.) a. Terrac.Grown in Egypt. Bull Fac Pharm Cairo Univ. 2001;40:85–91. [Google Scholar]
- 22.De Laurentis N, Armenise D, Milillo M, Matrella R. Chemical investigation of Sterculia acerifolia leaves. Riv Ital EPPOS. 2003;13:21–30. [Google Scholar]
- 23.Kassem HA. Study of further phytoconstituents of Brachychiton discolor F.J. Muell. cultivated in Egypt. Bull Fac Pharm Cairo Univ. 2007;45:155–160. [Google Scholar]
- 24.Kassem HA, Aziz WM. A Pharmacognostical study of Brachychiton discolor F.J.Muell. Cultivated in Egypt. Az Pharm Sci. 2002;29:196–219. [Google Scholar]
- 25.Chapman AD. Australian plant name index: Australian Biological Resources Study Canberra. 1991. [Google Scholar]
- 26.The International Plant Names Index. 2012. http://www.ipni.org. Accessed 24 Sept 2018.
- 27.The Plant List. Version 1. 2010. http://www.theplantlist.org. Accessed 24 Sept 2018.
- 28.Desoky E, Youssef S. Hypoglycaemic effect of Sterculia rupestris and a comparative study of its flavonoids with Sterculia diversifolia. Bull Fac Pharm Cairo Univ. 1997;35:257–261. [Google Scholar]
- 29.Okoye NN, Ajaghaku DL, Okeke HN, Ilodigwe EE, Nworu CS, Okoye FB. Beta-Amyrin and alpha-amyrin acetate isolated from the stem bark of Alstonia boonei display profound anti-inflammatory activity. Pharm Biol. 2014;52:1478–1486. doi: 10.3109/13880209.2014.898078. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira FA, Lima-Junior RCP, Cordeiro WM, Vieira-Júnior GM, Chaves MH, Almeida FRC, et al. Pentacyclic triterpenoids, α, β-amyrins, suppress the scratching behavior in a mouse model of pruritus. Pharmacol Biochem Behav. 2004;78:719–725. doi: 10.1016/j.pbb.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Ayeleso TB, Matumba MG, Mukwevho E. Oleanolic acid and its derivatives: biological activities and therapeutic potential in chronic diseases. Molecules. 2017;22:1915. doi: 10.3390/molecules22111915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Córdova C, Gutiérrez B, Martínez-García C, Martín R, Gallego-Muñoz P, Hernández M, et al. Oleanolic acid controls allergic and inflammatory responses in experimental allergic conjunctivitis. PLoS One. 2014;9:e91282. doi: 10.1371/journal.pone.0091282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geetha T, Varalakshmi P. Anti-inflammatory activity of lupeol and lupeol linoleate in rats. J Ethnopharmacol. 2001;76:77–80. doi: 10.1016/S0378-8741(01)00175-1. [DOI] [PubMed] [Google Scholar]
- 34.Khan Aisha Saleem. Medicinally Important Trees. Cham: Springer International Publishing; 2017. Antipyretic and Analgesic Activities of Some Economically Important Woody Plants; pp. 159–185. [Google Scholar]
- 35.Mujumdar AM, Naik DG, Waghole RJ, Kulkarni DK, Kumbhojka MS. Pharmacological studies on Sterculia foetida leaves. Pharm Biol. 2000;38:13–17. doi: 10.1076/1388-0209(200001)3811-BFT013. [DOI] [PubMed] [Google Scholar]
- 36.Raja T. Evaluation of anticonvulsant effect of Sterculia foetida (Pinari) in pentylenetetrazole (PTZ) and MES induced convulsions in albino rats. World J Pharm Pharm Sci. 2014;3:1898–1907. [Google Scholar]
- 37.Babalola IT, Adelakun EA, Wang Y, Shode FO. Anti-TB activity of Sterculia setigera Del., leaves (Sterculiaceae). J Pharmacogn. Phytochemistry. 2012;1:19–26. [Google Scholar]
- 38.Tor-Anyiin T, Akpuaka M, Oluma H. Phytochemical and antimicrobial studies on stem bark extract of Sterculia setigera, Del. Afr J Biotechnol. 2011;10:11011–11015. doi: 10.5897/AJB10.1493. [DOI] [Google Scholar]
- 39.Hossain MM, AIH E, Akbar MA, Ganguly A, Rahman SA. Evaluation of analgesic activity of Sterculia villosa Roxb.(Sterculiaceae) bark in swiss-albino mice. Dhaka Univ J Pharm Sci. 2013;12:125–129. [Google Scholar]
- 40.Hossain MF, Talukder B, Rana MN, Tasnim R, Nipun TS, Uddin SN, et al. In vivo sedative activity of methanolic extract of Sterculia villosa Roxb. Leaves. BMC Complement Altern Med. 2016;16:398. doi: 10.1186/s12906-016-1374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sobeh M, Mamadalieva NZ, Mohamed T, Krstin S, Youssef FS, Ashour ML, et al. Chemical profiling of Phlomis thapsoides (Lamiaceae) and in vitro testing of its biological activities. Med Chem Res. 2016;25:2304–2315. doi: 10.1007/s00044-016-1677-9. [DOI] [Google Scholar]
- 42.Van de Loosdrecht AA, Nennie E, Ossenkoppele GJ, Beelen RH, Langenhuijsen MM. Cell mediated cytotoxicity against U 937 cells by human monocytes and macrophages in a modified colorimetric MTT assay: a methodological study. J Immunol Methods. 1991;141:15–22. doi: 10.1016/0022-1759(91)90205-T. [DOI] [PubMed] [Google Scholar]
- 43.Marks DC, Belov L, Davey MW, Davey RA, Kidman AD. The MTT cell viability assay for cytotoxicity testing in multidrug-resistant human leukemic cells. Leuk Res. 1992;16:1165–1173. doi: 10.1016/0145-2126(92)90114-M. [DOI] [PubMed] [Google Scholar]
- 44.Chen B-H, Wu P-Y, Chen K-M, Fu T-F, Wang H-M, Chen C-Y. Antiallergic potential on RBL-2H3 cells of some phenolic constituents of Zingiber officinale (ginger) J Nat Prod. 2009;72:950–953. doi: 10.1021/np800555y. [DOI] [PubMed] [Google Scholar]
- 45.Korinek M, Tsai YH, El-Shazly M, Lai KH, Backlund A, Wu SF, et al. Anti-allergic hydroxy fatty acids from Typhonium blumei explored through ChemGPS-NP. Front Pharmacol. 2017;8:356. doi: 10.3389/fphar.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuda H, Tewtrakul S, Morikawa T, Nakamura A, Yoshikawa M. Anti-allergic principles from Thai zedoary: structural requirements of curcuminoids for inhibition of degranulation and effect on the release of TNF-α and IL-4 in RBL-2H3 cells. Bioorg Med Chem. 2004;12:5891–5898. doi: 10.1016/j.bmc.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 47.Chen B-H, Hung M-H, Chen JY-F, Chang H-W, Yu M-L, Wan L, et al. Anti-allergic activity of grapeseed extract (GSE) on RBL-2H3 mast cells. Food Chem. 2012;132:968–974. doi: 10.1016/j.foodchem.2011.11.079. [DOI] [Google Scholar]
- 48.Chung Y-M, Chang F-R, Tseng T-F, Hwang T-L, Chen L-C, Wu S-F, et al. A novel alkaloid, aristopyridinone a and anti-inflammatory phenanthrenes isolated from Aristolochia manshuriensis. Bioorg Med Chem Lett. 2011;21:1792–1794. doi: 10.1016/j.bmcl.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 49.Yang SC, Chung PJ, Ho CM, Kuo CY, Hung MF, Huang YT, et al. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J Immunol. 2013;190:6511–6519. doi: 10.4049/jimmunol.1202215. [DOI] [PubMed] [Google Scholar]
- 50.Mócsai A, Jakus Z, Tibor Vántus T, Berton G, Lowell GA, Ligeti E. Kinase pathways in chemoattractant-induced degranulation of neutrophils: the role of p38 mitogen-activated protein kinase activated by Src family kinases. J Immunol. 2000;164:4321–4331. doi: 10.4049/jimmunol.164.8.4321. [DOI] [PubMed] [Google Scholar]
- 51.Liou JR, El-Shazly M, Du YC, Tseng CN, Hwang TL, Chuang YL, et al. 1,5-Diphenylpent-3-en-1-ynes and methyl naphthalene carboxylates from Lawsonia inermis and their anti-inflammatory activity. Phytochemistry. 2013;88:67–73. doi: 10.1016/j.phytochem.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Youssef FS, Ashour ML, Sobeh M, El-Beshbishy HA, Singab ANB, Wink M. Eremophila maculata- isolation of a rare naturally-occurring lignan glycoside and the hepatoprotective activity of the leaf extract. Phytomedicine. 2016;23:1484–1493. doi: 10.1016/j.phymed.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Dubey H, Ticari J. Flavonoids and other constituents of Sterculia genus. J Indian Chem Soc. 1991;68:426–427. [Google Scholar]
- 54.Anjaneyulu A, Raju S. Terpenoids and phenolics from the bark and heartwood of Sterculiaurens ROXB. J Indian Chem Soc. 1987;64:323–324. [Google Scholar]
- 55.Khan NMU, Hossain MS. Scopoletin and ß-sitosterol glucoside from roots of Ipomoea digitata. J Pharmacogn. Phytochemistry. 2015;4:05–07. [Google Scholar]
- 56.H-x K, Y-g X, B-y Y, Wang Q-h, S-w L. Lignan constituents from Chloranthus japonicus Sieb. Arch Pharm Res. 2009;32:329–334. doi: 10.1007/s12272-009-1303-1. [DOI] [PubMed] [Google Scholar]
- 57.Lee S, Song I-H, Lee J-H, Yang W-Y, Oh K-B, Shin J. Sortase a inhibitory metabolites from the roots of Pulsatilla koreana. Bioorg Med Chem Lett. 2014;24:44–48. doi: 10.1016/j.bmcl.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 58.De Souza Santos M, Jonis Andrioli W, Freire de Morais Del Lama MP, Kenupp Bastos J, NPD N, Zumstein Georgetto Naal RM. In vitro anti-allergic activity of the fungal metabolite pyridovericin. Int Immunopharmacol. 2013;15:532–538. doi: 10.1016/j.intimp.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 59.Korinek M, Chen KM, Jiang YH, El-Shazly M, Stocker J, Chou CK, et al. Anti-allergic potential of Typhonium blumei: inhibition of degranulation via suppression of PI3K/PLCgamma2 phosphorylation and calcium influx. Phytomedicine. 2016;23:1706–1715. doi: 10.1016/j.phymed.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 60.Ryu SY, Oak MH, Yoon SK, Cho DI, Yoo GS, Kim TS, et al. Anti-allergic and anti-inflammatory triterpenes from the herb of Prunella vulgaris. Planta Med. 2000;66:358–360. doi: 10.1055/s-2000-8531. [DOI] [PubMed] [Google Scholar]
- 61.Yoshikawa M, Nakamura S, Kato Y, Matsuhira K, Matsuda H. Medicinal flowers. XIV. New acylated oleanane-type triterpene oligoglycosides with antiallergic activity from flower buds of chinese tea plant (Camellia sinensis) Chem Pharm Bull. 2007;55:598–605. doi: 10.1248/cpb.55.598. [DOI] [PubMed] [Google Scholar]
- 62.Chen M-L, Hsieh C-C, Chiang B-L, Lin B-F. Triterpenoids and polysaccharide fractions of Ganoderma tsugae exert different effects on antiallergic activities. Evid Based Complement Altern Med. 2015;2015:1–10. doi: 10.1155/2015/754836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nirmal SA, Patel AP, Bhawar SB, Pattan SR. Antihistaminic and antiallergic actions of extracts of Solanum nigrum berries: possible role in the treatment of asthma. J Ethnopharmacol. 2012;142:91–97. doi: 10.1016/j.jep.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 64.Mahajan SG, Mehta AA. Suppression of ovalbumin-induced Th2-driven airway inflammation by beta-sitosterol in a Guinea pig model of asthma. Eur J Pharmacol. 2011;650:458–464. doi: 10.1016/j.ejphar.2010.09.075. [DOI] [PubMed] [Google Scholar]
- 65.Bouic P, Lamprecht JH. Plant sterols and sterolins: a review of their immune-modulating properties. Altern Med Rev. 1999;4:170–177. [PubMed] [Google Scholar]
- 66.Hwang TL, Yeh SH, Leu YL, Chern CY, Hsu HC. Inhibition of superoxide anion and elastase release in human neutrophils by 3′-isopropoxychalcone via a cAMP-dependent pathway. Br J Pharmacol. 2006;148:78–87. doi: 10.1038/sj.bjp.0706712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 68.Kim JA, Son JH, Song SB, Yang SY, Kim YH. Sterols isolated from seeds of Panax ginseng and their antiinflammatory activities. Pharmacogn Mag. 2013;9:182. doi: 10.4103/0973-1296.111288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaith BS, Kaith NS, Chauhan NS. Anti-inflammatory effect of Arnebia euchroma root extracts in rats. J Ethnopharmacol. 1996;55:77–80. doi: 10.1016/S0378-8741(96)01477-8. [DOI] [PubMed] [Google Scholar]
- 70.Vassallo A, De Tommasi N, Merfort I, Sanogo R, Severino L, Pelin M, et al. Steroids with anti-inflammatory activity from Vernonia nigritiana Oliv. & Hiern. Phytochemistry. 2013;96:288–298. doi: 10.1016/j.phytochem.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 71.Bouic PJ. The role of phytosterols and phytosterolins in immune modulation: a review of the past 10 years. Curr Opin Clin Nutr Metab Care. 2001;4:471–475. doi: 10.1097/00075197-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 72.Lee J-H, Lee JY, Park JH, Jung HS, Kim JS, Kang SS, et al. Immunoregulatory activity by daucosterol, a β-sitosterol glycoside, induces protective Th1 immune response against disseminated candidiasis in mice. Vaccine. 2007;25:3834–3840. doi: 10.1016/j.vaccine.2007.01.108. [DOI] [PubMed] [Google Scholar]
- 73.Correa G, Abreu VDC, Martins D, Takahashi JA, Fontoura H, Cara DC, et al. Antiinflamatory and antimicrobial activities of steroids and triterpenes isolated from aerial parts of Justicia acuminatissima (Acanthaceae) Int J Pharm Pharm Sci. 2014;6:75–81. [Google Scholar]
- 74.de Almeida PD, Boleti AP, Rudiger AL, Lourenco GA, da Veiga Junior VF, Lima ES. Anti-inflammatory activity of triterpenes isolated from Protium paniculatum oil-resins. Evid Based Complement Altern Med. 2015;2015:293768. doi: 10.1155/2015/293768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Romero-Estrada A, Maldonado-Magaña A, González-Christen J, Bahena SM, Garduño-Ramírez ML, Rodríguez-López V, et al. Anti-inflammatory and antioxidative effects of six pentacyclic triterpenes isolated from the Mexican copal resin of Bursera copallifera. BMC Complement Altern Med. 2016;16:422. doi: 10.1186/s12906-016-1397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thirupathi A, Silveira PC, Nesi RT, Pinho RA. Beta-Amyrin, a pentacyclic triterpene, exhibits anti-fibrotic, anti-inflammatory, and anti-apoptotic effects on dimethyl nitrosamine-induced hepatic fibrosis in male rats. Hum Exp Toxicol. 2017;36:113–122. doi: 10.1177/0960327116638727. [DOI] [PubMed] [Google Scholar]
- 77.Wei W, Wu X-W, Deng G-G, Yang X-W. Anti-inflammatory coumarins with short- and long-chain hydrophobic groups from roots of Angelica dahurica cv. Hangbaizhi. Phytochemistry. 2016;123:58–68. doi: 10.1016/j.phytochem.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 78.Azelmat J, Fiorito S, Taddeo VA, Genovese S, Epifano F, Grenier D. Synthesis and evaluation of antibacterial and anti-inflammatory properties of naturally occurring coumarins. Phytochem Lett. 2015;13:399–405. doi: 10.1016/j.phytol.2015.08.008. [DOI] [Google Scholar]
- 79.Kang K-H, Kong C-S, Seo Y, Kim M-M, Kim S-K. Anti-inflammatory effect of coumarins isolated from Corydalis heterocarpa in HT-29 human colon carcinoma cells. Food Chem Toxicol. 2009;47:2129–2134. doi: 10.1016/j.fct.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 80.Shih H-C, Kuo P-C, Wu S-J, Hwang T-L, Hung H-Y, Shen D-Y, et al. Anti-inflammatory neolignans from the roots of Magnolia officinalis. Bioorg Med Chem. 2016;24:1439–1445. doi: 10.1016/j.bmc.2016.01.049. [DOI] [PubMed] [Google Scholar]
- 81.Peng Y, Lou L-L, Liu S-F, Zhou L, Huang X-X, Song S-J. Antioxidant and anti-inflammatory neolignans from the seeds of hawthorn. Bioorg Med Chem Lett. 2016;26:5501–5506. doi: 10.1016/j.bmcl.2016.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data contains supplementary figures (Figures S1-S6) showing the spectroscopic data of isolated compounds 1–7 from n-hexane, dichloromethane and ethyl acetate fractions of B. rupestris. (DOCX 2306 kb)
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.