Abstract
Background
High flow nasal therapy (HFNT) is a technique in which humidified and heated gas is delivered to the airways through the nose via small nasal prongs at flows that are higher than the rates generally applied during conventional oxygen therapy. The delivered high flow rates combine mixtures of air and oxygen and enable different inspired oxygen fractions ranging from 0.21 to 1. HFNT is increasingly used in critically ill adult patients, especially hypoxemic patients in different clinical settings.
Main body
Noninvasive ventilation delivers positive pressure (end-expiratory and inspiratory pressures or continuous positive airway pressure) via different external interfaces. In contrast, HFNT produces different physiological effects that are only partially linked to the generation of expiratory positive airway pressure. HFNT and noninvasive ventilation (NIV) are interesting non-invasive supports in perioperative medicine. HFNT exhibits some advantages compared to NIV because HFNT is easier to apply and requires a lower nursing workload. Tolerance of HFNT remains a matter of intense debate, and it may be related to selected parameters. Patients receiving HFNT and their respiratory patterns should be closely monitored to avoid delays in intubation despite correct oxygenation parameters.
Conclusion
HFNT seems to be an interesting noninvasive support in perioperative medicine. The present review provides anesthesiologists with an overview of current evidence and practical advice on the application of HFNT in perioperative medicine in adult patients.
Keywords: High flow nasal therapy, Noninvasive ventilation, Acute respiratory failure, Perioperative medicine
Background
High flow nasal therapy (HFNT) is a technique in which humidified and heated gas is delivered to the airways through the nose via nasal prongs, which are larger than conventional nasal prongs, at higher flow rates than are generally applied during conventional oxygen therapy. Inspiratory high flow of humidified air may be used alone or in combination with oxygen to generate different inspiratory oxygen fractions (FiO2) ranging from 0.21 to 1. Noninvasive ventilation (NIV) delivers noninvasive continuous positive airway pressure (CPAP) or noninvasive positive end expiratory pressure (PEEP) plus an inspiratory pressure to the patient’s airway via different external interfaces [1]. HFNT produces different physiological effects that are only partially linked to the generation of an end expiratory airway pressure [2].
HFNT is receiving growing attention as an alternative respiratory support in critical care settings, and it is used in patients with different underlying diseases. [2–8]. Several studies suggest that HFNT decreases a patient’s effort and respiratory rate and eventually reduces the need for more invasive respiratory support in patients with different lung diseases [2–7]. It has been also demonstrated that HFNT may be a potential alternative to NIV, especially in patients with acute hypoxemic respiratory failure who exhibit a ratio of arterial blood and delivered oxygen (PaO2/FiO2) < 200 [9, 10]. Currently, the evidence on the use of NIV in perioperative medicine is growing [6, 11–13]. There are several reasons why HFNT may be an alternative or complementary to NIV in perioperative medicine 1) NIV applies high continuous or intermittent positive pressure [14], and it remains the best noninvasive tool to increase functional residual capacity (FRC) [1, 15]. However, HFNT exhibits some unique physiological mechanisms that are discussed in this review. 2) NIV is not always applicable because of technical aspects, such as the patient’s poor tolerance to the interface during NIV and problems fitting the mask to the patient [16, 17]. 3) Effective application of NIV presupposes specific knowledge and training. However, inadequate use of HFNT may delay intubation, which was observed during the early years of NIV [18, 19].
This review aims to provide anesthesiologists with an overview of current evidence and practical advice on the application of HFNT in perioperative medicine in adult patients.
Main text
What is high flow nasal therapy?
Devices that offer high flows of totally humidified gases (37 °C, 100% relative humidity (RH), 44 mg H2O/L) from 20 to 60 L/min through specific nasal cannulae are commonly called HFNT systems. A heated humidifier connected to a heated insulated single-limb circuit provides active humidification. High flow may be generated in different ways:
Using a Venturi system driven by high oxygen pressure. The predetermined FiO2 is obtained from a blender. The lowest applicable FiO2 is 0.3 because the system is driven only by oxygen;
Using two high pressure sources, namely, high pressure air and oxygen. The predetermined FiO2 is obtained according to the total amount of oxygen divided by the total delivered flow (i.e., HFNT 60 L/min: 50 L/min of air and 10 L/min of oxygen; 50 L/min of air contains 21% of oxygen equal to approximately 10 L/min; if this 10 L/min extrapolated from the 50 L/min of air are added to 10 L/min of oxygen, the total amount of oxygen will be approximately 20 L/min; if the overall flow is 60 L/min (10 plus 50 L/min) the FiO2 will be 20 × 100/60, equal to a FiO2 close to 0.33);
Using a turbine system. A turbine allows the delivery of heated and humidified gases up to 60 L/min. FiO2 is predetermined only if the turbine has an internal blender. Alternatively, a low-pressure oxygen inlet allows the addition of oxygen up to 60 L/min with a dedicated flow meter. An oxygen cell determines the FiO2;
Using a conventional compressed air or turbine-driven mechanical ventilator with a dedicated HFNT system. FiO2 is predetermined by the ventilator blender [20].
Several mechanisms explain how HFNT reduces dyspnea and improves arterial blood gases and patient comfort [2, 21]:
-
A)
Improvement in the oxygen pharyngeal concentration in patients undergoing treatment with oxygen.
Oxygen administration is the first-line supportive treatment in patients with acute hypoxemic respiratory failure. The maintenance of adequate oxygenation depends on the management of FiO2. Oxygen is generally administered via facial masks or nasal prongs at a flow that generally does not exceed 15 L/min. FiO2 pharyngeal values are unstable using conventional oxygen therapy in the presence of patients with high inspiratory flows and respiratory rates [14]. By limiting air entrainment, HFNT might maintain high FiO2 levels via the application of high flow rates above a patient’s inspiratory requirements [2, 22, 23]. Chanques et al. did not find significant differences in FiO2 between mouth closed and open when HFNT was applied at 45 L/min at FiO2 1 [14]. In a bench study, administration of oxygen through an HFNT system showed no lack of performance at different inspiratory tidal volume and respiratory rates [23].
-
B)
Less metabolic cost for gas conditioning.
Inspired air is heated to 37 °C and humidified to 100% RH during normal breathing [24]. This process involves an energy cost [25] that can be reduced by the use of HFNT or an optimal humidification system during NIV [25]. Theoretically, each liter of inspired air requires 107.5 joules (26 cal) to increase the temperature and humidity of room air, based on ambient air at room temperature (21 °C) and 50% humidity [25]. A normal adult breathing a 500-ml tidal volume for each breath at a respiratory frequency of 12 breaths/min requires approximately 156 cal/min for the heating and humidifying of inspired gas [25]. The metabolic cost of heating inspiratory gases increases in patients who generate higher minute ventilation due to acute respiratory failure (ARF). HFNT may also affect carbon dioxide (CO2) production by decreasing the energy expenditure for conditioning gas [25].
-
C)
Reduction of inspiratory resistances.
During inspiration, the negative pressure generated by the respiratory muscles may cause a retraction of the nasopharynx [26] that may increase inspiratory resistance of the upper airway. HFNT can reduce respiratory resistance by 1) meeting or exceeding the patient’s peak inspiratory flow by supplying gas at a high flow; 2) “splinting” the nostrils by activating the nasal muscles [27, 28]; and 3) reducing bronchoconstriction from the nasal inhalation of cold air [28, 29].
-
D)
Improvement in mucociliary clearance.
Practice guidelines recommend conditioning oxygen when its delivery exceeds 4 L/min, but patients undergoing oxygen therapy usually receive very low conditioned gas because optimal humidification cannot be reached by the nasal mucosa [30]. Critically ill patients may benefit from HFNT because it improves the humidification of inspired gases to decrease the symptoms of dryness and eventually improve comfort [31]. Breathing cold and low conditioned gas may also modify the mucociliary transport system [32]. HFNT can also improves airway humidification and possibly improves mucus clearance retention [33].
-
E)
Increased expiratory resistance.
During physiological inspiration the pressure inside the nostrils becomes negative, but the pressure at the beginning and during most of inspiration remains above atmospheric pressure using HFNT because of an increase in expiratory resistances. Two major factors may play a role in increasing expiratory resistance and pressure, primarily a given EPAP level [34]:
1) An increase in expiratory resistance per se. Expiratory resistance is generated by the in-going jet flow against exhaled expiratory air and the size of the cannula [34]. Nasal cannula size plays an important role in controlling EPAP levels during HFNT because EPAP level depends on the number of leaks around the small prongs. The size of the nasal cannula is appropriate when it occupies approximately 2/3 of the surface of the nostrils [35];
2) Mouth closed or mouth open. A significant difference in generated EPAPs between mouth open and mouth closed was demonstrated [14]. Opening the mouth, independently from the inspiratory flow decreased mean airway pressure from 2 to 0.6 cmH2O [14]. This decrease is a crucial point because most patients with acute respiratory failure breathe with their mouth open. Furthermore, an increase in expiratory resistance may decrease respiratory rate via an increase in expiratory time (inspiratory time remains unchanged), which is similar to the respiratory model of the pursued lips breathing (PLB) that is often adopted by patients with chronic obstructive pulmonary disease (COPD) [36, 37].
It is important to stress that HFNT does not provide CPAP [38]. Nevertheless, although the pressure inside the pharynx may be lower than during CPAP because the interface used during HFNT is not tightly fitted to the nostrils, [14], this pressure may be sufficient to increase the end-expiratory lung volume (EELV) [39].
-
F)
Nasopharyngeal dead space washout.
At the beginning of inspiration, nasopharyngeal dead space encloses the end-expiratory gases. The dead space contributes to the heating and optimal humidification of the inspired air and decreases the efficiency of gas exchange. By reducing the dead space inside the nasopharynx via the insufflation of fresh gas, HFNT decreased the overall dead space and improves alveolar ventilation [37, 40]. In a bench study, HFNT, delivered at 30 L/min, eliminated CO2 contained in the dead space of the nose model during the very beginning of expiration and swapped it with fresh air [40]. Therefore, patients with COPD or lung fibrosis may require a lower increase in alveolar ventilation to reduce CO2 [41–43].
Interestingly, although HFNT is often termed high flow oxygen therapy, most of its beneficial effects can be achieved at FiO2 0.21, as observed during CPAP.
Flow and FIO2 should be titrated separately during HFNT application. FiO2 titration is important in patients with COPD because over-oxygenation may cause hypercapnia. Flow should be set as high as possible, up to 60 L/min, to match the patient’s inspiratory flow [44]. Temperature is also a very important issue. HFNT temperature seems to significantly impact the comfort of patients with hypoxemic ARF. Mauri et al. found that, for unchanged flow, a lower temperature seemed to be associated with better comfort [45]. Table 1 shows the practical indications of HFNT use in the perioperative setting.
Table 1.
Suggestions for high flow nasal therapy (HFNT) parameters (flow, FiO2, temperature) for preintubation oxygenation and postoperative setting
| HFNT | Preintubation | Postoperative setting |
|---|---|---|
| Flow | 50–60 l min − 1 | 30–40 l min-1 and increase to match patient’s demand |
| FiO2 | 1.0 | Increase the FiO2 until satisfactory SpO2 is achieved |
| Temperature | 37° | Titrated to best patient comfort |
Rationale for using HFNT in perioperative medicine
Oxygen is usually provided by a face mask or nasal prongs up to 15 L/min. However, using standard oxygenation methods, as mentioned above, FiO2 pharyngeal values may be unstable [23]. Different from conventional oxygen treatments, HFNT may limit air entrainment, enhancing pharyngeal FiO2 values [23, 46]. Several studies reported HFNT as an effective first-line support for mild to moderate acute hypoxemic respiratory failure [4, 6, 8, 47, 48]. Moreover, HFNT decreased breathing frequency and improved oxygenation [46, 49].
Schwabbauer et al. [50] compared HFNT to NIV and conventional oxygen treatment in functional and subjective respiratory parameters in patients with ARF of hypoxemic origin. They found that HFNT offered an effective compromise between oxygenation and patient comfort and seemed better tolerated than other treatments.
Mauri et al. [21] used a prospective randomized crossover study to evaluate HFNT set at 40 L/min vs. standard therapy (same FiO2) for 20-min trials in 15 hypoxemic ARF patients (PaO2/FiO2 130 ± 35 mmHg). They assessed the effects of HFNT on arterial blood gases, minute volume (Ve), EELV, patient’s inspiratory effort, ventilation homogeneity and dynamic compliance of the respiratory system. The authors found that HFNT produced several physiological effects in this patient population, including a reduction in inspiratory effort, improved lung volume and dynamic compliance.
The same authors [44] performed a prospective randomized crossover study that compared a standard facial mask with HFNT delivered at different flow rates (30, 45 and 60 L/min) in 17 hypoxemic non-intubated patients with PaO2/FiO2 ≤ 300 mmHg. They found that increasing the HFNT flow rate to 60 L/min progressively improved lung aeration, dynamic compliance and oxygenation and decreasing inspiratory effort. Interestingly, most of the physiological effects, such as CO2 clearance, were already found at the lowest flow rate.
The effects of CPAP (5 cmH2O) and HFNT (60 L/min) compared to conventional oxygen therapy were evaluated in 12 ICU patients with hypoxemic ARF [38]. Data were collected during randomly assigned periods of HFNT and CPAP. Each trial lasted approximately 20 min. The authors found no differences in inspiratory effort and in the respiratory rate between HFNT and CPAP. PaO2/FiO2increased significantly with HFNT compared to conventional oxygen therapy (167.2 [157.2–183.8) vs. 155.8 [109.6–171.3], p < 0.01). However, the PaO2/FiO2 ratio was significantly greater during CPAP than during HFNT. Dyspnea improved with HFNT and CPAP but not significantly. Interestingly, when the authors evaluated patient comfort, they did not find any difference in the trials.
HFNT use for endotracheal intubation
Endotracheal intubation (ETI) is a potentially life-threatening procedure in the operating room, especially in critically ill patients who require surgery. ETI may expose these patients to the risk of severe desaturation, eventually resulting in cardiac arrest and death [51–53].
Pre-oxygenation prior ETI is a mandatory and crucial step that extends apnea time and delays the eventual desaturation. Oxygenation through a facial mask is generally recommended in patients with healthy lungs prior to general anesthesia [46]. However, hypoxemic patients are prone to severe oxygen desaturation due to their underlying diseases or clinical conditions, which reduce oxygen stores and increase oxygen consumption [52] (e.g., patients with severe obesity, COPD, idiopathic pulmonary fibrosis, neuromuscular diseases, pregnancy). Alone or in combination with HFNT, NIV has been shown to improve oxygenation and to prolong apnea time without desaturation before intubation [15, 54]. The effect of oxygen administration before ETI is to increase body oxygen stores by replacing nitrogen in the FRC. Oxygen administration during apnea after the induction of general anesthesia may theoretically prolong apnea without desaturation. The mass flow of oxygen delivered to the upper airway is driven to alveoli by the pressure gradient generated by continuous oxygen uptake [55]. However, some patients undergoing ETI for general anesthesia may benefit from respiratory support to reduce intubation-related oxyhemoglobin desaturation or in the attempt to delay it [56, 57].
Simple supplemental oxygen administration prior to and/or during ETI cannot be considered a form of ventilator support. NIV reduces alveolar collapse and the likelihood of atelectasis formation, which are responsible for hypoventilation, increased perfusion-ventilation mismatch and eventual hypoxemia [58]. NIV may be used for pre-oxygenation [15], but it must be discontinued during laryngoscopy. Therefore, NIV cannot completely prevent desaturation during tracheal intubation. Owing to the use of dedicated nasal cannulae, HFNT does not interfere with laryngoscopy (Fig. 1). Therefore, HFNT can be used to supply oxygen during peri-intubation apnea [53].
Fig. 1.
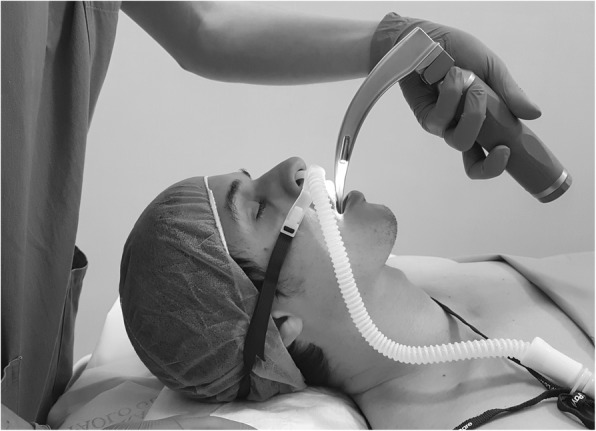
Example of HNFT use for peri-intubation apneic oxygenation. Authors’ own figure
HFNT was also evaluated in 50 patients who were at risk of difficult intubation during awake fiber-optic intubation. HFNT use was safe, well tolerated, and improved oxygenation while avoiding hypercapnia [59]. Heinrich et al. found that HFNT was an effective and safe pre-oxygenation method in a randomized controlled study in 33 obese patients undergoing bariatric surgery [60]. Three pre-oxygenation techniques were tested before rapid sequence intubation (RSI): 1) HFNT set at 50 L/min at a FiO2 of 1; 2) CPAP set at 7 cmH2O and FiO2 of 1; and 3) traditional facial mask at 12 L/min with an FiO2 of 1. The primary outcome was PaO2 values at defined time points during pre-oxygenation (1, 3, 5, 7, and 8 min). HFNT significantly improved oxygenation during the pre-oxygenation at 5 and 7 min prior to RSI compared to traditional oxygen therapy, but no significant difference in oxygenation compared to CPAP was observed.
Although CPAP should increase EELV by recruiting collapsed alveoli, its beneficial effect might not be associated with an increase in oxygenation [57]. Thus, alveolar recruitment may not be associated with functional recruitment, as defined by improved gas exchange [61].
Patel et al. [62] showed that a transnasal humidified oxygen supply system [transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) technique], which is an analogue of HFNT, can prolong apnea time in patients with anticipated difficult airways who were scheduled for general anesthesia. Patients with obstructive sleep apnea (OSA) undergoing surgery for laryngotracheal stenosis or vocal cord pathology were treated with THRIVE at 70 L/min for 10 min before anesthesia induction and laryngoscopy. THRIVE at 70 L/min was maintained for the entire maneuver until a safe airway was eventually achieved regardless of the number of intubation attempts or the presence of expected or unexpected difficult intubation. The authors found that in patients at risk of difficult airway management, THRIVE increased the apnea time to an average time of 17 min without SpO2 levels below 90%. No patients presented adverse events related to CO2 toxicity, such as episodes of cardiac arrhythmias.
Raineri et al. assessed the efficacy and safety of HFNT in 45 patients receiving RSI for urgent abdominal surgery. HFNT at 60 L/min and FiO2 of 1 was used for 4 min prior to induction and maintained until ETI [53]. Heart rate, SpO2, and mean arterial pressure were assessed at baseline (T0), after 4 min on HFNT (T1), during laryngoscopy (T2) and at the time of ETI (T3). Episodes of SpO2 values < 3% from baseline were recorded. SpO2 increased significantly at T1, T2 and T3 compared to T0. Minimal SpO2 was 96%. No episodes of SpO2 values < 3% from baseline were found in any patient. Maximum apnea time was 12 min. Mean end-tidal CO2 (ETCO2) at the time of ETI was 36 mmHg.
Indeed, in critically ill patients there are discordant results on the efficacy of HFNT before intubation [63–66]. The reasons for these results may be the different indications for ETI and the patient’s baseline oxygen value [63].
A recent randomized controlled trial (OPTINIV) evaluated the combination of HFNT at 60 L/min and FiO2 1 coupled with NIV compared to NIV alone as a pre-oxygenation method to reduce desaturation prior to ETI in ICU patients with severe hypoxemic ARF [54]. The primary outcome was the lowest SpO2 during ETI. The lowest SpO2 values during ETI were significantly higher [100 (95–100)%] in the HFNT + NIV group compared to the NIV alone group [96 (92–99)] (p = 0.029).
However, all these data were collected from ICU patients. Extrapolation to patients in the operating room is not certain.
HFNT in the postoperative setting
General anesthesia, surgery duration and postoperative pain may determine important modifications in the respiratory system. General anesthesia and neuromuscular paralysis induce muscle relaxation with a cranial shift of the diaphragm. This shift reduces pulmonary volumes and induces atelectasis [67, 68]. Modification of lung volume is mainly caused by the reduction of the FRC. Its reduction increases as the surgical site approaches the diaphragm (chest and upper abdomen) [69]. Typically, atelectasis occurs in the sloping portions of the lung next to the diaphragm and may involve nearly 10% of the lung and persist up to 24–48 h after major surgery [67, 70]. After formation, atelectasis may persist up to 24–48 h with use of neuromuscular blocking agents, even in patients undergoing general anesthesia with normal lungs.
Oxygen administration is by far the most common therapy after extubation to correct hypoxemia. During the postoperative period, NIV use may be difficult due to the lack of a proper setting or resources (e.g., in general surgical wards) or because the patient cannot tolerate the interface [71]. Because of these multiple effects, HFNT would seem to be an interesting method to contrast hypoxemia and possibly prevent post-extubation atelectasis, though this remains to be confirmed [72–74]. HFNT may offer a less traumatic interface and potentially offers fewer burdens for the patient and caregiver than NIV. However, the primary limitation of HFNT use is the correction of hypoxemia using high oxygen flow but without correcting substrates, such as atelectasis, due to low pressure levels. HFNT use should find its rationale as a “stand alone” or intermediate level of respiratory support that is in between NIV and standard oxygen therapy or in patients who show intolerance to NIV treatment.
A multicenter randomized trial was performed in 527 mechanically ventilated ICU patients with a low risk of reintubation (251 post-elective or urgent surgery) [75]. The authors randomized patients to receive HFNT (treatment group) or conventional oxygen therapy (control group) after extubation to evaluate the reintubation rate. The study found that HFNT was associated with a reduced risk of reintubation compared to standard oxygen reduced (4.9% versus 12.2%) [75]. However, only half of the included patients were surgical, and it is difficult to reach a conclusive clinical message on this topic.
A further multicenter randomized non-inferiority study compared HFNT and NIV to evaluate the incidence of post-extubation ARF and reintubation in 604 high-risk patients [76]. A total of 232 of the included patients were evaluated after scheduled or urgent surgery. Patients were randomized to receive HFNT or NIV for 24 h after extubation. Sixty-six patients treated with HFNT did not require reintubation (22.8%) vs. 60 (19.1%) treated with NIV. Very interestingly, 42.9% patients in the NIV group vs. none in HFNT group presented adverse effects that required device removal.
Stephan et al. performed a multicenter, randomized, non-inferiority trial (BiPOP Study) to determine whether HFNT therapy was inferior to NIV (administered as two levels of pressure - BiPAP) for the prevention or treatment of ARF after cardiothoracic surgery [77]. Patients were randomized (n = 416) to NIV delivered as pressure support ventilation set at 8 cmH2O plus 4 cmH2O of PEEP, FiO2 50% administered via face mask for at least 4 h/day or HFNT (n = 414) 50 L/min with an FiO2 of 50%. HFNT was not inferior to NIV since 21% of patients in the HFNT group failed the treatment vs. 21.9% in the NIV group. ICU mortality was not significantly different between the 2 groups. Interestingly, after 24 h, nasal skin lesions were significantly more present in the NIV group.
Futier et al. performed a multicenter randomized trial in 220 patients scheduled for major abdominal surgery (OPERA STUDY) with a moderate to high risk for developing postoperative pulmonary complications (PPCs) and compared post-extubation HFNT versus standard oxygen [73]. The primary outcome was the risk of hypoxemia. Secondary end points were the presence of PPCs within 7 days after surgery, hospital length of stay, and in-hospital mortality. Sixty-two percent of patients from both groups received recruitment maneuvers during lung-protective mechanical ventilation. At 1 h after extubation, the proportion of patients who presented hypoxemia was similar (21% in the HFNT group vs. 27% in the control group). Moreover, the incidence of PPCs between groups over a 7-day postoperative period was not different. The authors found that early application of HFNT vs. standard oxygen after extubation did not reduce the risk of developing pulmonary complications [73]. Interestingly, HFNT was significantly beneficial in the subgroup of patients with complete protective ventilation (receiving high PEEP, low tidal volume and recruitment maneuvers).
Conclusions
Although most of the evidence on HFNT was collected from ICU studies, HFNT seems to be an interesting noninvasive support in perioperative medicine. HFNT presents some advantages over NIV. HFNT does not require patient cooperation, but it is generally better tolerated and easier to use and requires less equipment and lower nursing workload. On the other hand, since the patient does not need cooperation, surveillance by physicians must be highly reinforced to avoid delays intubation [19]. In addition, HFNT therapy may not the best option for all patients with or who are at risk of postoperative ARF [63]. To date, NIV should be considered the first-line ventilatory support for several perioperative clinical situations because it generates a real assistance on respiratory muscles, as opposed to HFNT therapy. Therefore, NIV should be more indicated in more severe patients, but its role is now discussed in hypoxemic patients [12, 47, 48, 78].
The role of HFNT in postoperative ARF remains an open question with some important issues to be solved, such as which patient will benefit, the correct timing of treatment application and escalation. Further studies are needed to define these important issues in perioperative medicine.
Acknowledgements
None.
Funding
None.
Availability of data and materials
Not applicable.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- ARF
Acute respiratory failure
- ARF
Acute respiratory failure
- CO2
Carbon dioxide
- COPD
Chronic obstructive pulmonary disease
- CPAP
Continuous positive airway pressure
- EELV
End-expiratory lung volume
- EPAP
Expiratory positive airway pressure
- ETCO2
End-tidal carbon dioxide
- ETI
Endotracheal intubation
- FiO2
Fraction of inspired oxygen
- FRC
Functional residual capacity
- HFNT
High flow nasal therapy
- ICU
Intensive care unit
- NIV
Noninvasive ventilation
- OSA
Obstructive sleep apnea
- PEEP
Positive end-expiratory pressure
- PLB
Pursued lips breathing
- PPC
Postoperative pulmonary complication
- RH
Relative humidity
- RSI
Rapid sequence intubation
- SpO2
Peripheral oxygen saturation
- THRIVE
Transnasal humidified rapid-insufflation ventilatory exchange
- Ve
Minute volume
Authors’ contributions
AC conceived the content, wrote the manuscript, and approved the final version. GA wrote the manuscript and approved the final version. SM conceived the content, wrote the manuscript, and approved the final version. AG conceived the content, wrote the manuscript, and approved the final version. CG conceived the content, wrote the manuscript, and approved the final version.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the participants for publication of this article and any accompanying tables/images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
Andrea Cortegiani is an Associate Editor of BMC Anesthesiology. GA, AG, and SM declare no competing interests. CG received travel cost reimbursement from Fisher & Paykel Healthcare (New Zealand).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Andrea Cortegiani, Email: cortegiania@gmail.com.
Giuseppe Accurso, Email: giuseppe.accurso86@gmail.com.
Sebastiano Mercadante, Email: terapiadeldolore@lamaddalenanet.it.
Antonino Giarratano, Email: antonino.giarratano@unipa.it.
Cesare Gregoretti, Phone: +390916552730, Email: c.gregoretti@gmail.com.
References
- 1.Gregoretti C, Pisani L, Cortegiani A, Ranieri VM. Noninvasive ventilation in critically ill patients. Crit Care Clin. 2015;31:435–457. doi: 10.1016/j.ccc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Spoletini G, Alotaibi M, Blasi F, Hill NS. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest. 2015;148:253–261. doi: 10.1378/chest.14-2871. [DOI] [PubMed] [Google Scholar]
- 3.Del Sorbo L, Vendramin A, Mehta S. High flow oxygen therapy in adult critically ill patients. Minerva Anestesiol. 2017;83:402–411. doi: 10.23736/S0375-9393.16.11608-6. [DOI] [PubMed] [Google Scholar]
- 4.Sztrymf B, Messika J, Bertrand F, Hurel D, Leon R, Dreyfuss D, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med. 2011;37:1780–1786. doi: 10.1007/s00134-011-2354-6. [DOI] [PubMed] [Google Scholar]
- 5.Roca O, Masclans JR. High-flow nasal cannula oxygen therapy: innovative strategies for traditional procedures. Crit Care Med. 2015;43:707–708. doi: 10.1097/CCM.0000000000000796. [DOI] [PubMed] [Google Scholar]
- 6.Frat J-P, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 7.Lee MK, Choi J, Park B, Kim B, Lee SJ, Kim S-H, et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018;12(6):2046–2056. doi: 10.1111/crj.12772. [DOI] [PubMed] [Google Scholar]
- 8.Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55:408–413. [PubMed] [Google Scholar]
- 9.Jaber S, Chanques G, Jung B. Postoperative noninvasive ventilation. Anesthesiology. 2010;112:453–461. doi: 10.1097/ALN.0b013e3181c5e5f2. [DOI] [PubMed] [Google Scholar]
- 10.Renda T, Corrado A, Iskandar G, Pelaia G, Abdalla K, Navalesi P. High-flow nasal oxygen therapy in intensive care and anaesthesia. Br J Anaesth. 2018;120:18–27. doi: 10.1016/j.bja.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50:1602426. doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 12.Cortegiani A, Russotto V, Antonelli M, Azoulay E, Carlucci A, Conti G, et al. Ten important articles on noninvasive ventilation in critically ill patients and insights for the future: a report of expert opinions. BMC Anesthesiol. 2017;17:122. doi: 10.1186/s12871-017-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregoretti C, Cortegiani A, Raineri SM, Giarrjatano A. Noninvasive ventilation in hypoxemic patients: an ongoing soccer game or a lost one? Turk J Anaesth Reanim. 2018;45:329–331. doi: 10.5152/TJAR.2017.241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chanques G, Riboulet F, Molinari N, Carr J, Jung B, Prades A, et al. Comparison of three high flow oxygen therapy delivery devices: a clinical physiological cross-over study. Minerva Anestesiol. 2013;79:1344–1355. [PubMed] [Google Scholar]
- 15.Russotto V, Cortegiani A, Raineri SM, Gregoretti C, Giarratano A. Respiratory support techniques to avoid desaturation in critically ill patients requiring endotracheal intubation: a systematic review and meta-analysis. J Crit Care. 2017;41:98–106. doi: 10.1016/j.jcrc.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Racca F, Appendini L, Berta G, Barberis L, Vittone F, Gregoretti C, et al. Helmet ventilation for acute respiratory failure and nasal skin breakdown in neuromuscular disorders. Anesth Analg. 2009;109:164–167. doi: 10.1213/ane.0b013e3181a1f708. [DOI] [PubMed] [Google Scholar]
- 17.Gregoretti C, Confalonieri M, Navalesi P, Squadrone V, Frigerio P, Beltrame F, et al. Evaluation of patient skin breakdown and comfort with a new face mask for non-invasive ventilation: a multi-center study. Intensive Care Med. 2002;28:278–284. doi: 10.1007/s00134-002-1208-7. [DOI] [PubMed] [Google Scholar]
- 18.Esteban A, Frutos-Vivar F, Ferguson ND, Arabi Y, Apezteguia C, Gonzalez M, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350:2452–2460. doi: 10.1056/NEJMoa032736. [DOI] [PubMed] [Google Scholar]
- 19.Kang BJ, Koh Y, Lim C-M, Huh JW, Baek S, Han M, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41:623–632. doi: 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- 20.Nagata K, Morimoto T, Fujimoto D, Otoshi T, Nakagawa A, Otsuka K, et al. Efficacy of high-flow nasal cannula therapy in acute hypoxemic respiratory failure: decreased use of mechanical ventilation. Respir Care. 2015;60:1390–1396. doi: 10.4187/respcare.04026. [DOI] [PubMed] [Google Scholar]
- 21.Mauri T, Turrini C, Eronia N, Grasselli G, Volta CA, Bellani G, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195:1207–1215. doi: 10.1164/rccm.201605-0916OC. [DOI] [PubMed] [Google Scholar]
- 22.Ritchie JE, Williams AB, Gerard C, Hockey H. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesth Intensive Care. 2011;39:1103–1110. doi: 10.1177/0310057X1103900620. [DOI] [PubMed] [Google Scholar]
- 23.Wagstaff TAJ, Soni N. Performance of six types of oxygen delivery devices at varying respiratory rates. Anaesthesia. 2007;62:492–503. doi: 10.1111/j.1365-2044.2007.05026.x. [DOI] [PubMed] [Google Scholar]
- 24.Sahin-Yilmaz A, Naclerio RM. Anatomy and physiology of the upper airway. Proc Am Thorac Soc. 2011;8:31–39. doi: 10.1513/pats.201007-050RN. [DOI] [PubMed] [Google Scholar]
- 25.Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103:1400–1405. doi: 10.1016/j.rmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Shepard JWJ, Thawley SE. Localization of upper airway collapse during sleep in patients with obstructive sleep apnea. Am Rev Respir Dis. 1990;141:1350–1355. doi: 10.1164/ajrccm/141.5_Pt_1.1350. [DOI] [PubMed] [Google Scholar]
- 27.Gold AR, Smith PL, Schwartz AR. Effect of alae nasi activation on maximal nasal inspiratory airflow in humans. J Appl Physiol (1985) 1998;84:2115–2122. doi: 10.1152/jappl.1998.84.6.2115. [DOI] [PubMed] [Google Scholar]
- 28.Fontanari P, Burnet H, Zattara-Hartmann MC, Jammes Y. Changes in airway resistance induced by nasal inhalation of cold dry, dry, or moist air in normal individuals. J Appl Physiol (1985). 1996;81:1739–1743. doi: 10.1152/jappl.1996.81.4.1739. [DOI] [PubMed] [Google Scholar]
- 29.On LS, Boonyongsunchai P, Webb S, Davies L, Calverley PM, Costello RW. Function of pulmonary neuronal M(2) muscarinic receptors in stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1320–1325. doi: 10.1164/ajrccm.163.6.2002129. [DOI] [PubMed] [Google Scholar]
- 30.Kallstrom TJAARC. Clinical practice guideline: oxygen therapy for adults in the acute care facility--2002 revision & update. Respir Care. 2002;47:717–720. [PubMed] [Google Scholar]
- 31.Chanques G, Constantin J-M, Sauter M, Jung B, Sebbane M, Verzilli D, et al. Discomfort associated with underhumidified high-flow oxygen therapy in critically ill patients. Intensive Care Med. 2009;35:996–1003. doi: 10.1007/s00134-009-1456-x. [DOI] [PubMed] [Google Scholar]
- 32.Salah B, Dinh Xuan AT, Fouilladieu JL, Lockhart A, Regnard J. Nasal mucociliary transport in healthy subjects is slower when breathing dry air. Eur Respir J. 1988;1:852–855. [PubMed] [Google Scholar]
- 33.Kilgour E, Rankin N, Ryan S, Pack R. Mucociliary function deteriorates in the clinical range of inspired air temperature and humidity. Intensive Care Med. 2004;30:1491–1494. doi: 10.1007/s00134-004-2235-3. [DOI] [PubMed] [Google Scholar]
- 34.Mundel T, Feng S, Tatkov S, Schneider H. Mechanisms of nasal high flow on ventilation during wakefulness and sleep. J Appl Physiol (1985) 2013;114:1058–1065. doi: 10.1152/japplphysiol.01308.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth. 2009;103:886–890. doi: 10.1093/bja/aep280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spahija J, de Marchie M, Grassino A. Effects of imposed pursed-lips breathing on respiratory mechanics and dyspnea at rest and during exercise in COPD. Chest. 2005;128:640–650. doi: 10.1378/chest.128.2.640. [DOI] [PubMed] [Google Scholar]
- 37.Nakos G, Lachana A, Prekates A, Pneumatikos J, Guillaume M, Pappas K, et al. Respiratory effects of tracheal gas insufflation in spontaneously breathing COPD patients. Intensive Care Med. 1995;21:904–912. doi: 10.1007/BF01712331. [DOI] [PubMed] [Google Scholar]
- 38.Vargas F, Saint-Leger M, Boyer A, Bui NH, Hilbert G. Physiologic effects of high-flow nasal cannula oxygen in critical care subjects. Respir Care. 2015;60:1369–1376. doi: 10.4187/respcare.03814. [DOI] [PubMed] [Google Scholar]
- 39.Parke RL, Bloch A, McGuinness SP. Effect of very-high-flow nasal therapy on airway pressure and end-expiratory lung impedance in healthy volunteers. Respir Care. 2015;60:1397–1403. doi: 10.4187/respcare.04028. [DOI] [PubMed] [Google Scholar]
- 40.Moller W, Celik G, Feng S, Bartenstein P, Meyer G, Oliver E, et al. Nasal high flow clears anatomical dead space in upper airway models. J Appl Physiol (1985) 2015;118:1525–1532. doi: 10.1152/japplphysiol.00934.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braunlich J, Beyer D, Mai D, Hammerschmidt S, Seyfarth H-J, Wirtz H. Effects of nasal high flow on ventilation in volunteers, COPD and idiopathic pulmonary fibrosis patients. Respiration. 2013;85:319–325. doi: 10.1159/000342027. [DOI] [PubMed] [Google Scholar]
- 42.Pisani L, Fasano L, Corcione N, Comellini V, Musti MA, Brandao M, et al. Change in pulmonary mechanics and the effect on breathing pattern of high flow oxygen therapy in stable hypercapnic COPD. Thorax. 2017;72:373–375. doi: 10.1136/thoraxjnl-2016-209673. [DOI] [PubMed] [Google Scholar]
- 43.Fraser JF, Spooner AJ, Dunster KR, Anstey CM, Corley A. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomised crossover trial. Thorax. 2016;71:759–761. doi: 10.1136/thoraxjnl-2015-207962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mauri T, Alban L, Turrini C, Cambiaghi B, Carlesso E, Taccone P, et al. Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: effects of increasing flow rates. Intensive Care Med. 2017;43:1453–1463. doi: 10.1007/s00134-017-4890-1. [DOI] [PubMed] [Google Scholar]
- 45.Mauri T, Galazzi A, Binda F, Masciopinto L, Corcione N, Carlesso E, et al. Impact of flow and temperature on patient comfort during respiratory support by high-flow nasal cannula. Crit Care. 2018;22:120. doi: 10.1186/s13054-018-2039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016;61:529–541. doi: 10.4187/respcare.04577. [DOI] [PubMed] [Google Scholar]
- 47.Maitra S, Som A, Bhattacharjee S, Arora MK, Baidya DK. Comparison of high-flow nasal oxygen therapy with conventional oxygen therapy and noninvasive ventilation in adult patients with acute hypoxemic respiratory failure: a meta-analysis and systematic review. J Crit Care. 2016;35:138–144. doi: 10.1016/j.jcrc.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 48.Lee CC, Mankodi D, Shaharyar S, Ravindranathan S, Danckers M, Herscovici P, et al. High flow nasal cannula versus conventional oxygen therapy and non-invasive ventilation in adults with acute hypoxemic respiratory failure: a systematic review. Respir Med. 2016;121:100–108. doi: 10.1016/j.rmed.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Lenglet H, Sztrymf B, Leroy C, Brun P, Dreyfuss D, Ricard J-D. Humidified high flow nasal oxygen during respiratory failure in the emergency department: feasibility and efficacy. Respir Care. 2012;57:1873–1878. doi: 10.4187/respcare.01575. [DOI] [PubMed] [Google Scholar]
- 50.Schwabbauer N, Berg B, Blumenstock G, Haap M, Hetzel J, Riessen R. Nasal high–flow oxygen therapy in patients with hypoxic respiratory failure: effect on functional and subjective respiratory parameters compared to conventional oxygen therapy and non-invasive ventilation (NIV) BMC Anesthesiol. 2014;14:66. doi: 10.1186/1471-2253-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griesdale DEG, Bosma TL, Kurth T, Isac G, Chittock DR. Complications of endotracheal intubation in the critically ill. Intensive Care Med. 2008;34:1835–1842. doi: 10.1007/s00134-008-1205-6. [DOI] [PubMed] [Google Scholar]
- 52.Mort TC. Preoxygenation in critically ill patients requiring emergency tracheal intubation. Crit Care Med. 2005;33:2672–2675. doi: 10.1097/01.CCM.0000187131.67594.9E. [DOI] [PubMed] [Google Scholar]
- 53.Raineri SM, Cortegiani A, Accurso G, Procaccianti C, Vitale F, Caruso S, et al. Efficacy and safety of using high-flow nasal oxygenation in patients undergoing rapid sequence intubation. Turk J Anaesthesiol Reanim. 2017;45:335–339. doi: 10.5152/TJAR.2017.47048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaber S, Monnin M, Girard M, Conseil M, Cisse M, Carr J, et al. Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-Centre, blinded, randomised controlled OPTINIV trial. Intensive Care Med. 2016;42:1877–1887. doi: 10.1007/s00134-016-4588-9. [DOI] [PubMed] [Google Scholar]
- 55.Mosier JM, Hypes CD, Sakles JC. Understanding preoxygenation and apneic oxygenation during intubation in the critically ill. Intensive Care Med. 2017;43:226–228. doi: 10.1007/s00134-016-4426-0. [DOI] [PubMed] [Google Scholar]
- 56.Gander S, Frascarolo P, Suter M, Spahn DR, Magnusson L. Positive end-expiratory pressure during induction of general anesthesia increases duration of nonhypoxic apnea in morbidly obese patients. Anesth Analg. 2005;100:580–584. doi: 10.1213/01.ANE.0000143339.40385.1B. [DOI] [PubMed] [Google Scholar]
- 57.Futier E, Constantin J-M, Pelosi P, Chanques G, Massone A, Petit A, et al. Noninvasive ventilation and alveolar recruitment maneuver improve respiratory function during and after intubation of morbidly obese patients: a randomized controlled study. Anesthesiology. 2011;114:1354–1363. doi: 10.1097/ALN.0b013e31821811ba. [DOI] [PubMed] [Google Scholar]
- 58.Jaber S, Michelet P, Chanques G. Role of non-invasive ventilation (NIV) in the perioperative period. Best Pract Res Clin Anaesthesiol. 2010;24:253–265. doi: 10.1016/j.bpa.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Badiger S, John M, Fearnley RA, Ahmad I. Optimizing oxygenation and intubation conditions during awake fibre-optic intubation using a high-flow nasal oxygen-delivery system. Br J Anaesth. 2015;115:629–632. doi: 10.1093/bja/aev262. [DOI] [PubMed] [Google Scholar]
- 60.Heinrich S, Horbach T, Stubner B, Prottengeier J, Irouschek A, Schmidt J. Benefits of heated and humidified high flow nasal oxygen for preoxygenation in morbidly obese patients undergoing bariatric surgery: a randomized controlled study. J Obes Bariatrics. 2014;1:7. [Google Scholar]
- 61.Constantin J-M, Cayot-Constantin S, Roszyk L, Futier E, Sapin V, Dastugue B, et al. Response to recruitment maneuver influences net alveolar fluid clearance in acute respiratory distress syndrome. Anesthesiology. 2007;106:944–951. doi: 10.1097/01.anes.0000265153.17062.64. [DOI] [PubMed] [Google Scholar]
- 62.Patel A, Nouraei SAR. Transnasal humidified rapid-insufflation Ventilatory exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. 2015;70:323–329. doi: 10.1111/anae.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papazian L, Corley A, Hess D, Fraser JF, Frat J-P, Guitton C, et al. Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. Intensive Care Med. 2016;42:1336–1349. doi: 10.1007/s00134-016-4277-8. [DOI] [PubMed] [Google Scholar]
- 64.Miguel-Montanes R, Hajage D, Messika J, Bertrand F, Gaudry S, Rafat C, et al. Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Crit Care Med. 2015;43:574–583. doi: 10.1097/CCM.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 65.Vourc'h M, Asfar P, Volteau C, Bachoumas K, Clavieras N, Egreteau P-Y, et al. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med. 2015;41:1538–1548. doi: 10.1007/s00134-015-3796-z. [DOI] [PubMed] [Google Scholar]
- 66.Simon M, Wachs C, Braune S, de Heer G, Frings D, Kluge S. High-flow nasal cannula versus bag-valve-mask for Preoxygenation before intubation in subjects with hypoxemic respiratory failure. Respir Care. 2016;61:1160–1167. doi: 10.4187/respcare.04413. [DOI] [PubMed] [Google Scholar]
- 67.Strandberg Å, Tokics L, Brismar B, Lundquist H, Hedenstierna G. Atelectasis during anaesthesia and in the postoperative period. Acta Anaesthesiol Scand. 1986;30:154–158. doi: 10.1111/j.1399-6576.1986.tb02387.x. [DOI] [PubMed] [Google Scholar]
- 68.Duggan M, Kavanagh BP. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology. 2005;102:838–854. doi: 10.1097/00000542-200504000-00021. [DOI] [PubMed] [Google Scholar]
- 69.Futier E, Marret E, Jaber S. Perioperative positive pressure ventilation: an integrated approach to improve pulmonary care. Anesthesiology. 2014;121:400–408. doi: 10.1097/ALN.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 70.Magnusson L, Spahn DR. New concepts of atelectasis during general anaesthesia. Br J Anaesth. 2003;91:61–72. doi: 10.1093/bja/aeg085. [DOI] [PubMed] [Google Scholar]
- 71.Cabrini L, Moizo E, Nicelli E, Licini G, Turi S, Landoni G, et al. Noninvasive ventilation outside the intensive care unit from the patient point of view: a pilot study. Respir Care. 2012;57:704–709. doi: 10.4187/respcare.01474. [DOI] [PubMed] [Google Scholar]
- 72.Lee JH, Rehder KJ, Williford L, Cheifetz IM, Turner DA. Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literature. Intensive Care Med. 2013;39:247–257. doi: 10.1007/s00134-012-2743-5. [DOI] [PubMed] [Google Scholar]
- 73.Futier E, Paugam-Burtz C, Godet T, Khoy-Ear L, Rozencwajg S, Delay JM, et al. Effect of early postextubation high-flow nasal cannula vs conventional oxygen therapy on hypoxaemia in patients after major abdominal surgery: a French multicentre randomised controlled trial (OPERA) Intensive Care Med. 2016;42:1888–1898. doi: 10.1007/s00134-016-4594-y. [DOI] [PubMed] [Google Scholar]
- 74.Nava S, Navalesi P, Gregoretti C. Interfaces and humidification for noninvasive mechanical ventilation. Respir Care. 2009;54:71–84. [PubMed] [Google Scholar]
- 75.Hernandez G, Vaquero C, Gonzalez P, Subira C, Frutos-Vivar F, Rialp G, et al. Effect of Postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315:1354–1361. doi: 10.1001/jama.2016.2711. [DOI] [PubMed] [Google Scholar]
- 76.Hernandez G, Vaquero C, Colinas L, Cuena R, Gonzalez P, Canabal A, et al. Effect of Postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and Postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316:1565–1574. doi: 10.1001/jama.2016.14194. [DOI] [PubMed] [Google Scholar]
- 77.Stephan F, Barrucand B, Petit P, Rezaiguia-Delclaux S, Medard A, Delannoy B, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA. 2015;313:2331–2339. doi: 10.1001/jama.2015.5213. [DOI] [PubMed] [Google Scholar]
- 78.Gregoretti C, Cortegiani A, Accurso G, Raineri SM, Giarratano A. Noninvasive ventilation for acute hypoxemic respiratory failure/ARDS: the show must go on. Turk J Anaesth Reanim. 2018;46:1–2 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


