Abstract
Antibody-based epidemiologic surveillance to determine population-level exposure to sexually transmitted infections could help inform public health fertility preservation strategies. We compared the performance of three platforms to detect antibodies against the Chlamydia trachomatis (CT) antigen Pgp3—multiplex bead array (MBA), enzyme-linked immunosorbent assay (ELISA), and lateral flow assay (LFA)—on serum from adolescents and young adults (AYA) with pelvic inflammatory disease (PID). Ninety-five of 118 AYA diagnosed with PID (80.5%) had positive antibody response to Pgp3 antigen by at least one test, and 78 (66.1%) tested positive by all three tests. Among 27 individuals with infection detected using nucleic acid amplification testing, 92.6% were positive by MBA (25/27), 77.8% (21/27) were positive by ELISA, and 74.07% (20/27) were positive by LFA. These data suggest that the MBA was the most sensitive of the three tests and could be useful in sero-epidemiologic studies designed to assess population-level exposure to CT.
1.0 Introduction
Interest in the use of antibody testing for surveillance of both urogenital and ocular chlamydia infection has increased in recent years. Long-lived antibody responses provide a measure of cumulative infection and thereby can inform estimates of exposure in a population1, 2, 3, 4, 5, 6, 7. The Pgp3 antigen has been studied extensively as an immunodominant antigen that, as a plasmid encoded protein8, is unique to Chlamydia trachomatis and has been a major focus of surveillance studies for both urogenital and ocular infection. Studies evaluating the use of serologic surveillance for population-level exposure to urogenital infection have used ELISA testing almost exclusively2, 4, 7, 9, while studies evaluating the use of sero-surveillance for trachoma have used both multiplex bead array (MBA) and ELISA6, 10, 11, 12, 13). Recently, a lateral flow-based rapid test (LFA) to detect antibodies against Pgp3 was developed for use in trachoma-endemic areas14. Studies using sera from trachoma endemic communities have demonstrated good concordance between the MBA, ELISA, and LFA tests and high sensitivity (>90%) and specificity (>95%) for all tests evaluated15.
Pelvic inflammatory disease (PID) is a common reproductive health disorder commonly caused by C. trachomatis that if left untreated results in significant adverse reproductive health outcomes including tubal infertility, ectopic pregnancy, and chronic pelvic pain .16, 17, 18 Despite the documented health impacts on women, both routine STI screening among adolescent and young adult (AYA) women to prevent PID19 and treatment of PID in clinical settings20, 21 are subpar. Further, PID is not a reportable condition, so it is difficult to assess the burden of disease at the population level using public health databases and/or link PID diagnoses to historical or current C. trachomatis infections to inform the design and evaluation of effective public health strategies. Seroprevalence data may be useful measure the impact of STI prevention programs on reproductive health outcomes in AYA women with symptomatic disease and/or asymptomatic women with unexplained morbidity that may be due to C. trachomatis infection. We compared the performance of MBA, ELISA, and LFA tests for antibodies against Pgp3 among AYA women with mild-moderate PID.
2.0 Methods
2.1 Ethics Statement
The Johns Hopkins Medicine Institutional Review Board approved the study (trial registration number: NCT01640379). CDC investigators were determined to be non-engaged in the study and did not have access to patient identifying information.
2.2 Patient Recruitment and Screening
The TECH-N Study is a large randomized controlled clinical trial of a text messaging and community health nursing intervention to improve short-term adherence and longitudinal health outcomes for AYA women diagnosed with mild-moderate acute pelvic inflammatory disease (PID) that was conducted between 2012-2017. The design and preliminary outcomes of this study have previously been published22, 23, 24. Briefly, trained research assistants recruited patients aged 13-25 years with mild-moderate acute PID from outpatient clinics and emergency departments (adult and pediatric) within a large urban academic medical center in Baltimore, Maryland (United States) at the time of PID diagnosis. PID diagnoses were based on the Centers for Disease Control and Prevention guidance for clinical diagnosis (https://www.cdc.gov/std/tg2015/pid.htm).
Enrolled participants completed an audio computerized assisted self-interview (ACASI) to collect baseline demographic and sexual and reproductive health information, such as prior STI/PID history. Participants were randomized to either the intervention or control group using a permutated block design25. Participants provided vaginal specimens for STI testing (including C. trachomatis) as well as blood samples.
2.3 Laboratory Testing
2.3.1 Nucleic Acid Amplification Test (NAAT)
Vaginal swabs were self-collected3 utilizing the APTIMA Vaginal Swab Specimen Collection Kit (Hologic Inc., San Diego, CA) and delivered to the Johns Hopkins University International STD (JHU STD) Laboratory for testing. All vaginal swab samples were tested for C. trachomatis and Neisseria gonorrhoeae using the APTIMA Combo 2 (AC2) Assay (Hologic Inc., San Diego, CA) according to manufacturer’s instructions. Vaginal swab specimens were also tested for Trichomonas vaginalis (TV) and Mycoplasma genitalium (MG) however, only C. trachomatis data are presented in this publication. Vaginal swab samples were tested for TV using the APTIMA Trichomonas vaginalis Assay (Hologic Inc., San Diego, CA) and MG testing was performed using an analyte specific reagent (ASR) assay (Hologic Inc., San Diego, CA).
2.3.2 Blood Sample Processing
Blood was received at JHU STD Laboratory in a 3mL serum separator tube. Blood was processed at 1300 RCF for 10 minutes to separate the serum; serum was separated and stored at −80°C until processing. Serum was tested for antibodies against the CT antigen Pgp3 only. Antibody testing for the other three pathogens (GC, TV, and MG) was not performed in this study.
2.3.3 Multiplex Bead Array (MBA)
Antigen selection, purification, and coupling to beads have been previously described3. Briefly, sera (2.5uL) were diluted 1:400 into 1 mL of sample buffer containing 3ug/mL of E.coli extract and then incubated with Luminex® SeroMAP™ microspheres (Luminex Corp., Austin, TX) coupled to Pgp3 antigen. Beads were washed using a solution of 1X phosphate-buffered saline (PBS) and 0.05% Tween-20 wash buffer (PBST1) then incubated with 50ng mouse anti-human IgG (Southern Biotech, Birmingham, AL) and 40ng mouse anti-human IgG4 (Life Technologies Corp., Carlsbad, CA) biotinylated detection antibodies; unbound antibody was removed by washing with PBST1. Bound antibody was detected using streptavidin conjugated to R-phycoerythrin (SAPE), (Life Technologies Corp., Carlsbad, CA). The fluorescent signal emitted by bound SAPE was read using a BioRad Luminex 100 instrument and reported as median fluorescence intensity (MFI). Data were analyzed by the BioPlex Manager 6.0 software (BioRad Laboratories Inc., Richmond, CA). Data are reported as MFI with background from the blank well subtracted out (MFI-BG).The cutoff value for positivity was determined to be 869 MFI using a receiver operator characteristic (ROC) analysis with a panel of previously classified positive and negative sera as controls. Samples were run in duplicate with blank, positive, and negative controls on each plate.
2.3.4 Enzyme-linked Immunosorbent Assay (ELISA)
Plates were sensitized with 50 μl of Pgp3 antigen (500 ng/ml in 0.1M NaHCO3, pH 9.6) overnight at 4°C. Antigen was decanted, plates washed 2x with PBS with 0.3% Tween-20 (Sigma Aldrich, St. Louis, MO, PBST2), and 50 μl of serum samples diluted 1:50 in 250 μl PBST2 in 5% milk buffer solution, was added to wells in duplicate. Positive and negative control samples were added to each plate. After shaking at room temperature (RT) for 2 hours, samples were decanted and plate washed 4x with PBST2. Fifty μl of anti-human IgG-horseradish peroxidase (HRP) conjugate (1:10,000 dilution in PBST, Southern Biotech, Birmingham AL) was added to each well. After shaking at RT for 1h, antibody was decanted, plates washed 4x with PBST, and 50 μl 3, 3, 5, 5-tetramethlybenzidine (TMB) was added to each well for 6 minutes. The reaction was stopped with 50 μl of H2SO4 and the optical density (OD) was read at 450 nm using a SpectraMax M5 Microplate Reader (Molecular Devices, LLC, Sunnyvale, CA). All data were normalized to the 200 unit (U) control at which point an OD of >0.582 was considered positive for antibodies to Pgp3 based on ROC analysis of a panel of previously classified positive and negative sera.
2.3.5 Pgp3 Lateral Flow Assay (LFA)
The LFA was run as previously described14. Ten μl of serum was added to the sample port of the Pgp3 LFA cassette, followed by 200 μl of chase buffer (PBST2). Samples were recorded as positive, negative, or invalid (if a control line was not present or the test line was non-continuous) after 30 minutes. No tests were invalid in this study.
2.4 Statistical Analysis
The percent antibody positive by each test was determined both as a proportion of the overall number of participants with PID and of those with a positive NAAT result. Cohen’s Kappa statistic was used to estimate the agreement between antibody positivity and a self-reported history of C. trachomatis infection, and between antibody positivity and a self-reported history of C. trachomatis infection or positive NAAT results at study entry. Cohen’s Kappa ranges from −1 to 1, where an estimate less than 0.2 indicates slight agreement, and between 0.2 and 0.4 fair, 0.4 to 0.6 moderate, and greater than 0.6 strong agreement26. We compared the intensity of the antibody response (MFI-BG) among specimens that were positive for antibodies against Pgp3 using MBA, ELISA, and LFA stratified by the number of positive assays using Wilcoxon rank-signed test (GraphPad Prism).
3.0 Results
3.1 Demographic characteristics of study participants
As reported elsewhere24, women enrolled in this study (N=118) were between the ages of 14 and 25, with an average age of 18.8 years. Most were African-American (105/118, 88.9%). Of those reporting a prior STI history (67/115, 58.2%), 48 (71.6%) specifically reported a history of C. trachomatis infection.
3.2 Anti-Pgp3 antibody responses in all AYA
Of 118 AYA enrolled, 95 (80.5%) had an antibody response by MBA. Seventy-eight (66.1%) were positive by all three tests, 2 (1.7%) were positive by only MBA and ELISA, and 15 (12.7%) were only positive by MBA (Table 1, left).
Table 1.
Detection of Antibodies against Pgp3 by Multiplex Bead Array (MBA), Enzyme linked immunosorbent assay (ELISA), and Lateral Flow Assay (LFA). Data shown are number and percent antibody positive total and of those with nucleic acid amplification testing (NAAT) data. Data show the number represented in each group testing positive for antibody responses to the C. trachomatis antigen Pgp3 of the total. Percentages represent percentage of anti-Pgp3+ samples testing positive by each test. Ab = antibody.
| N | anti-Pgp3 Ab+ | MBA+ | ELISA+ | LFA+ | |
|---|---|---|---|---|---|
|
|
|||||
| Total | 118 | 95 | 95 (100%) | 80 (84.2%) | 78 (82.1%) |
| NAAT+ | 27 | 25 | 25 (100%) | 21 (77.8%) | 20 (74.1%) |
3.3 Anti-Pgp3 antibody responses in NAAT-positive AYA
NAAT data were available for 112 women, of whom 27 (24.1%) were NAAT-positive for C. trachomatis. Of the 27 NAAT-positive individuals, 25 (92.6%) tested positive for anti-Pgp3 antibody by MBA. 77.8% (21/27) were positive by ELISA, and, 74.1% (20/27) were positive by LFA (Table 1).
The intensity of the antibody response for the MBA (Figure 1, left panel) and ELISA (Figure 1, right panel) are shown. The MFI-BG for specimens with only one antibody positive test (i.e. MBA+ELISA-LFA-) was lower than those specimens positive by all 3 tests (Figure 1, left panel). An insufficient number of specimens were positive by only two tests (MBA+/ELISA+/LFA-) to do further statistical analysis.
Figure 1.
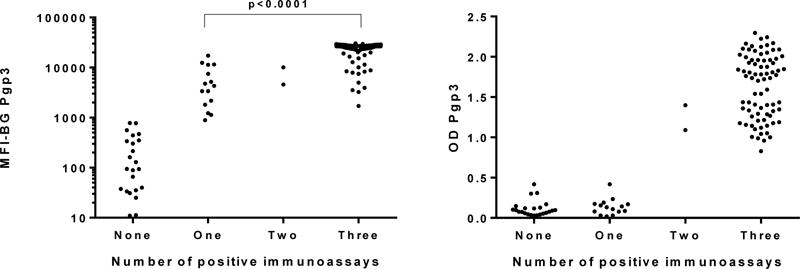
Intensity of antibody responses to Pgp3 antigen stratified by the number of positive immunoassays. Y-axis shows median fluorescence intensity with background subtracted out (MFI-BG) for the multiplex bead array (left panel) or the absorbance at 405 nm (OD Pgp3) for the ELISA (right panel). X-axis shows data stratified by the number of assays in which a sample tested positive. Each dot represents an individual specimen. A p-value of <0.05 was considered statistically significant.
4.0 Discussion
This is the first report of using bead-based or lateral flow assays to test for antibodies against the Ct antigen Pgp3. In this study, approximately 80% of AYA women with PID of mild to moderate severity and 92% of those with positive NAAT for Ct tested positive for antibody responses to the Pgp3 antigen using multiplex bead array, while a lower proportion tested positive using either Pgp3 ELISA or Pgp3 LFA. There is increased interest in seroepidemiology for evaluating community- or population-level exposure to Ct infection for both urogenital infection and trachoma. These data point to the use of bead-based assays as superior to ELISA or LFA testing for surveillance of urogenital infection, whereas the three platforms perform similarly in trachoma-endemic settings15 (Wiegand, submitted for publication). Using NAAT-positivity as a gold standard as previously done for urogenital infection7, 9, the sensitivity of the MBA was 92.6% compared to 77.8% for ELISA and 74.1% for the LFA. The ELISA sensitivity in this study aligns well with that seen from other studies in which Pgp3 ELISA sensitivities of 73.8%7 and 82.9%9 were observed. In these previous studies the sensitivities of the Pgp3 ELISA were much lower for specimens obtained from men; it may be worth exploring if use of the Pgp3 MBA would facilitate increased detection of Pgp3-specific antibody responses in men.
Testing samples from trachoma-endemic communities on the MBA, ELISA, and LFA yields generally high sensitivity and specificity (>90%) against gold standard tests and by latent class analysis (15, Wiegand et al, manuscript submitted). The differences in the sensitivities of the different assays depending on the route of entry of urogenital infection—urogenital or ocular—suggests possible immunological differences between the intensity of antibody responses to ocular and urogenital C. trachomatis infection, revealed by the lower sensitivity of the Pgp3 ELISA and LFA as well as the lower agreement between the MBA, ELISA, and LFA with samples from urogenital compared to ocular infection14. Repeated ocular infection with C. trachomatis in trachoma-endemic areas may result in higher antibody levels within an individual that are more readily detected in ELISA and LFA tests. This is supported by the data shown in Figure 1, in which individuals testing positive only with the MBA have statistically lower MFI-BG levels than individuals testing positive by all three tests.
There are several limitations to this study. First, there is a relatively small sample size of women with NAAT-confirmed infection to draw strong conclusions about the differences in sensitivities of the assays. A larger sample size would also have allowed us to determine if sociodemographic data affects antibody testing. Inclusion of men in future studies will be valuable to determine if the ability of the MBA to pick up low-titer antibody responses will improve detection of responses in males. Future studies utilizing these serological testing methods on other populations may provide useful and additional information in regards to CT antibody responses. Additional populations could include men and women who are asymptomatic that test positive for CT infection as well as males who have epididymitis. Another potential population would be men who have sex with men (MSM) however, these methods may not differentiate between those with rectal Lymphogranuloma venereum (LGV) infections and those with genital infections. Data gathered from testing additional populations could provide information on how best to use CT antibody serology testing as a surveillance tool.
Sero-epidemiology for CT exposure would be valuable considering our data suggest that a high proportion of urban AYA women diagnosed with PID have prior exposures to C. trachomatis infection beyond those reported on ACASI. One-third of the young women with mild-to-moderate PID in this study had no self-reported previous STI and no current C. trachomatis infection, but had a positive Pgp3 antibody response. This could be due to self-clearance of a previous asymptomatic infection27, or under self-reporting of a prior infection. Others have shown that only about half of AYA PID patients typically report a history of prior STI diagnoses at the time of PID diagnosis24, 28. Our data suggest that a substantial proportion of study participants with PID may have had a prior C. trachomatis infection that either went undiagnosed or was not self-reported.
Since PID is not a nationally reportable condition in most jurisdictions (whereas C. trachomatis infection is reportable), additional population surveillance to assess what fraction of impaired fecundity can be attributed to prior C. trachomatis infection in both individual clinical outcomes and overall population health may be of particular interest. Furthermore, the data presented here suggest that surveillance for CT would be best accomplished using bead-based MBA, which provide enhanced sensitivity compared to the standardly-used ELISA or the recently-developed LFA.
Acknowledgments
Funding Source: The TECH-N Study is supported by The National Institute of Nursing Research NINR [grant number: R01NR013507]. Additional work in Dr. Gaydos laboratory was supported by the NIBIB, NIH [grant U54EB007958] and the NIH, NIAID grant [U-01068613]. Funding for LFA tests was provided to CDC from an interagency agreement with USAID. The funding agencies had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Conflict of Interest Statement:
The authors declare no conflict of interest.
References
- 1.Comanducci M, Manetti R, Bini L, Santucci A, Pallini V, Cevenini R, Sueur JM, Orfila J, Ratti G. Humoral immune response to plasmid protein pgp3 in patients with Chlamydia trachomatis infection. Infect Immun. 1994;62:5491–7. doi: 10.1128/iai.62.12.5491-5497.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donati M, Laroucau K, Storni E, Mazzeo C, Magnino S, Di Francesco A, Baldelli R, Ceglie L, Renzi M, Cevenini R. Serological response to pgp3 protein in animal and human chlamydial infections. Vet Microbiol. 2009;135:181–5. doi: 10.1016/j.vetmic.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 3.Goodhew EB, Priest JW, Moss DM, Zhong G, Munoz B, Mkocha H, Martin DL, West SK, Gaydos C, Lammie PJ. CT694 and pgp3 as serological tools for monitoring trachoma programs. PLoS Negl Trop Dis. 2012;6:e1873. doi: 10.1371/journal.pntd.0001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horner P, Soldan K, Vieira SM, Wills GS, Woodhall SC, Pebody R, Nardone A, Stanford E, McClure MO. C. trachomatis Pgp3 antibody prevalence in young women in England, 1993-2010. PLoS One. 2013;8:e72001. doi: 10.1371/journal.pone.0072001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin DL, Bid R, Sandi F, Goodhew EB, Massae PA, Lasway A, Philippin H, Makupa W, Molina S, Holland MJ, Mabey DC, Drakeley C, Lammie PJ, Solomon AW. Serology for trachoma surveillance after cessation of mass drug administration. PLoS Negl Trop Dis. 2015;9:e0003555. doi: 10.1371/journal.pntd.0003555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin DL, Wiegand R, Goodhew B, Lammie P, Black CM, West S, Gaydos CA, Dize L, Mkocha H, Kasubi M, Gambhir M. Serological Measures of Trachoma Transmission Intensity. Sci Rep. 2015;5:18532. doi: 10.1038/srep18532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wills GS, Horner PJ, Reynolds R, Johnson AM, Muir DA, Brown DW, Winston A, Broadbent AJ, Parker D, McClure MO. Pgp3 antibody enzyme-linked immunosorbent assay, a sensitive and specific assay for seroepidemiological analysis of Chlamydia trachomatis infection. Clin Vaccine Immunol. 2009;16:835–43. doi: 10.1128/CVI.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comanducci M, Cevenini R, Moroni A, Giuliani MM, Ricci S, Scarlato V, Ratti G. Expression of a plasmid gene of Chlamydia trachomatis encoding a novel 28 kDa antigen. J Gen Microbiol. 1993;139:1083–92. doi: 10.1099/00221287-139-5-1083. [DOI] [PubMed] [Google Scholar]
- 9.Horner PJ, Wills GS, Reynolds R, Johnson AM, Muir DA, Winston A, Broadbent AJ, Parker D, McClure MO. Effect of time since exposure to Chlamydia trachomatis on chlamydia antibody detection in women: a cross-sectional study. Sex Transm Infect. 2013;89:398–403. doi: 10.1136/sextrans-2011-050386. [DOI] [PubMed] [Google Scholar]
- 10.Cocks N, Rainima-Qaniuci M, Yalen C, Macleod C, Nakolinivalu A, Migchelsen S, Roberts CH, Butcher R, Kama M, Mabey D, Marks M. Community seroprevalence survey for yaws and trachoma in the Western Division of Fiji. Trans R Soc Trop Med Hyg. 2016;110:582–587. doi: 10.1093/trstmh/trw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migchelsen SJ, Martin DL, Southisombath K, Turyaguma P, Heggen A, Rubangakene PP, Joof H, Makalo P, Cooley G, Gwyn S, Solomon AW, Holland MJ, Courtright P, Willis R, Alexander ND, Mabey DC, Roberts CH. Defining Seropositivity Thresholds for Use in Trachoma Elimination Studies. PLoS Negl Trop Dis. 2017;11:e0005230. doi: 10.1371/journal.pntd.0005230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West SK, Munoz B, Weaver J, Mrango Z, Dize L, Gaydos C, Quinn TC, Martin DL. Can We Use Antibodies to Chlamydia trachomatis as a Surveillance Tool for National Trachoma Control Programs? Results from a District Survey. PLoS Negl Trop Dis. 2016;10:e0004352. doi: 10.1371/journal.pntd.0004352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zambrano AI, Sharma S, Crowley K, Dize L, Munoz BE, Mishra SK, Rotondo LA, Gaydos CA, West SK. The World Health Organization Recommendations for Trachoma Surveillance, Experience in Nepal and Added Benefit of Testing for Antibodies to Chlamydia trachomatis pgp3 Protein: NESTS Study. PLoS Negl Trop Dis. 2016;10:e0005003. doi: 10.1371/journal.pntd.0005003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gwyn S, Mitchell A, Dean D, Mkocha H, Handali S, Martin DL. Lateral flow-based antibody testing for Chlamydia trachomatis. J Immunol Methods. 2016;435:27–31. doi: 10.1016/j.jim.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Gwyn S, Cooley G, Goodhew B, Kohlhoff S, Banniettis N, Wiegand R, Martin DL. Comparison of Platforms for Testing Antibody Responses against the Chlamydia trachomatis Antigen Pgp3. Am J Trop Med Hyg. 2017;97:1662–1668. doi: 10.4269/ajtmh.17-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trent M, Lehmann HP, Qian Q, Thompson CB, Ellen JM, Frick KD. Adolescent and parental utilities for the health states associated with pelvic inflammatory disease. Sex Transm Infect. 2011;87:583–7. doi: 10.1136/sextrans-2011-050187. [DOI] [PubMed] [Google Scholar]
- 17.Westrom L. Effect of pelvic inflammatory disease on fertility. Venereology. 1995;8:219–22. [PubMed] [Google Scholar]
- 18.Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185–92. [PubMed] [Google Scholar]
- 19.Goyal M, Hersh A, Luan X, Localio R, Trent M, Zaoutis T. National trends in pelvic inflammatory disease among adolescents in the emergency department. J Adolesc Health. 2013;53:249–52. doi: 10.1016/j.jadohealth.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shih TY, Gaydos CA, Rothman RE, Hsieh YH. Poor provider adherence to the Centers for Disease Control and Prevention treatment guidelines in US emergency department visits with a diagnosis of pelvic inflammatory disease. Sex Transm Dis. 2011;38:299–305. doi: 10.1097/OLQ.0b013e31820b8bb4. [DOI] [PubMed] [Google Scholar]
- 21.Trent M. Status of adolescent pelvic inflammatory disease management in the United States. Curr Opin Obstet Gynecol. 2013;25:350–6. doi: 10.1097/GCO.0b013e328364ea79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butz AM, Gaydos C, Chung SE, Johnson BH, Huettner S, Trent M. Care-Seeking Behavior After Notification Among Young Women With Recurrent Sexually Transmitted Infections After Pelvic Inflammatory Disease. Clin Pediatr (Phila) 2016;55:1107–12. doi: 10.1177/0009922816662863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz Buchanan CR, Chung SE, Butz A, Perin J, Gaydos C, Trent M. Perceived Social Support, Parental Notification, and Parental Engagement after Pelvic Inflammatory Disease among Urban Adolescent and Young Adults. Pediatr Neonatal Nurs. 2016;4:12–16. doi: 10.17140/pnnoj-4-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trent M, Chung SE, Gaydos C, Frick KD, Anders J, Huettner S, Rothman R, Butz A. Recruitment of Minority Adolescents and Young Adults into Randomised Clinical Trials: Testing the Design of the Technology Enhanced Community Health Nursing (TECH-N) Pelvic Inflammatory Disease Trial. Eur Med J Reprod Health. 2016;2:41–51. [PMC free article] [PubMed] [Google Scholar]
- 25.Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol. 2004;4:26. doi: 10.1186/1471-2288-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 27.Morre SA, van den Brule AJ, Rozendaal L, Boeke AJ, Voorhorst FJ, de Blok S, Meijer CJ. The natural course of asymptomatic Chlamydia trachomatis infections: 45% clearance and no development of clinical PID after one-year follow-up. Int J STD AIDS. 2002;13(Suppl 2):12–8. doi: 10.1258/095646202762226092. [DOI] [PubMed] [Google Scholar]
- 28.Trent M, Chung SE, Burke M, Walker A, Ellen JM. Results of a randomized controlled trial of a brief behavioral intervention for pelvic inflammatory disease in adolescents. J Pediatr Adolesc Gynecol. 2010;23:96–101. doi: 10.1016/j.jpag.2009.06.005. [DOI] [PubMed] [Google Scholar]


