Abstract
Objective
Research has highlighted the importance of reward-based processes in binge eating (BE). However, both increased and decreased activation have been observed in reward related brain regions for BE. Differences may be similar to addiction research, where the reward system is initially hyper-responsive at early stages of use, but becomes hypo-responsive with prolonged drug/alcohol use. This study was the first to examine differences in reward system responsivity at early versus chronic BE stages.
Method
Using an animal model, Sprague-Dawley female rats were identified as BE prone (BEP) or BE resistant (BER) and randomly assigned to an early or chronic stage group. Neural activation (via Fos) was quantified in the nucleus accumbens core (NAC) and shell (NAS).
Results
Early stage BEP rats had the highest levels of Fos expression of all of the study groups. By contrast, chronic stage BEP rats exhibited decreased activation in the NAS and NAC that was similar to the activation in chronic stage BER rats.
Discussion
Findings are significant in suggesting hyper-neural activation to reward in the early stages of BE and decreased activation in later stages of BE. Additional studies are needed to elucidate how these differences may impact risk for and maintenance of BE.
Extant data show that reward system functioning is related to binge eating (BE) (e.g., Davis et al., 2009; Doucette, Khokhar, & Green, 2015; Schienle, Schäfer, Hermann, & Vaitl, 2009), but the direction of the association (i.e., hyper-/hypo-responsivity) varies. Increased activation in reward-related brain structures (e.g., caudate, Wang et al., 2012) has been observed in women with binge-related disorders (e.g., bulimia nervosa, BE disorder) when consuming palatable food (PF), but decreased reward-related activity in response to PF (e.g., insula, Bohon & Stice, 2011) has also been found.
Some hypothesize that duration of BE might contribute to discrepant findings (Bohon & Stice, 2011; Wang et al., 2001). Individuals prone to BE might experience initial hyper-responsivity of the reward system to PF leading to BE (e.g., Bohon & Stice, 2011). Chronic BE could result in down-regulation of the reward system (i.e., hypo-responsivity) and more frequent and/or severe BE to achieve the same rewarding response experienced in earlier stages (a pattern observed for drugs/alcohol - see Boileau et al., 2003; Dawe, Gullo, & Loxton, 2004; Nestler, 2005; Willner, James, & Morgan, 2005).
This pilot study was the first to directly compare differences in neural responsivity at early and chronic stages of BE using a well-established animal model of BE (i.e., BE Resistant/BE Prone (BER/BEP)). This model identifies BER/BEP rats by examining naturally occurring differences in PF consumption during intermittent feeding tests, and has strong face validity for BE in humans. BEP rats only binge on PF (no over-consumption of chow), and do not differ in obesity proneness from non-BE rats (Boggiano et al., 2007). Sex differences are observed in this model (females>males; Klump, Racine, Hildebrandt, & Sisk, 2013), and BE proneness emerges during puberty (Klump, Suisman, Culbert, Kashy, & Sisk, 2011).
We focused on the nucleus accumbens (NA) core (NAC) and shell (NAS), as these reward-related structures are activated by both natural (e.g., food) and artificial (e.g., alcohol) rewards (e.g., Alsiö et al., 2010; Peciña & Berridge, 2000, 2005). We measured responsivity by quantifying Fos, the protein product of c-fos (an immediate early gene), a neural activation marker (Herrera & Robertson, 1996; Sinclair et al., 2015). Higher Fos expression indicates increased engagement/activation of the brain region following stimulus exposure (i.e., PF intake), and vice versa. We hypothesized that early stage BEP animals would show greater responsivity in the NA to PF consumption compared to BER rats (replication of Sinclair et al., 2015). However, we anticipated that chronic stage BEP animals would have lower responsivity compared to early stage BEP rats, suggesting a downregulation in responsivity of the NA that is specific BEP animals after chronic, long-term consumption of PF.
Methods
Animals
Sprague-Dawley female rats (N = 120) were obtained from Harlan (Madison, Wisconsin) on ~postnatal day 70. Animals were given ad libitum access to chow (Rodent diet 8640; Harlan Teklad Global Diets, Madison, Wisconsin) and water. Animals were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and protocols were approved by the Michigan State University Institutional Animal Care and Use Committee.
Experimental Design
Animals were tested in two cohorts (n = 60/cohort) following BER/BEP procedures (e.g., Boggiano et al., 2007; Klump et al., 2011). PF (i.e., Betty Crocker Creamy Vanilla Frosting, General Mills, Inc., Minneapolis, MN) was hung in a petri dish inside the cage at dark onset (1200h) on feeding test days (Monday, Wednesday, Friday). Body weight was measured daily, and PF and chow were measured at the end of the 4-hour feeding test. For Cohort 1, PF was accidentally left in the cages for 8 hours during feeding test 12, so we exposed animals in Cohort 2 to 8-hours of PF exposure as well. Animals scoring in the top tertile of 4-hour PF intake for their cohort on at least 50% of feeding tests (i.e., ≥3 out of 6 feeding tests), and never in the bottom, were classified as BEP. Animals scoring in the bottom tertile ≥50% of the time, and never in the top, were classified as BER. Tertiles were calculated after the first 6 feeding tests in each cohort to randomize approximately equal numbers of BER/BEP rats to the early (sacrificed after the 6th feeding test – see below) and chronic (continued feeding tests – see below) stage groups.
We based early stage classifications on data showing increased activation in the NA in BEP rats after 6–8 feeding tests (Sinclair et al., 2015). Thus, early stage rats were sacrificed after the 6th feeding test. Animals randomized to the chronic stage completed 18 additional feeding tests and were sacrificed after the 24th test. Because no criteria exist for defining chronic stages of BE, we chose an extended schedule that was 4x longer than the typical number of tests used in BER/BEP studies (e.g., Klump et al., 2013).
After completion of all feeding tests, additional BER/BEP classifications were conducted to arrive at the final sample for analysis (see Supplemental Table S1). First, we combined data from the two cohorts of rats (n = 60 animals/cohort) and calculated “Combined Cohort” tertiles using PF intake from the first six feeding tests in the combined sample (N = 120). This ensured that rats classified as BER/BEP in individual cohorts still met BER/BEP criteria when including all rats from the study. Findings were very similar when using original cohort tertiles as compared to the Combined Cohort tertiles (Supplemental Table S3).
Second, given our interest in the neural responsivity after consistent engagement in BE over an extended period of time, we evaluated all chronic stage animals for their consistency in meeting BER/BEP criteria across the extended testing period. We required that rats included in the final chronic group for analyses met criteria for their respective phenotypes (i.e., highest/lowest tertile in ≥3 out of first 6 feeding tests) in at least one additional feeding test block (i.e., feeding tests 7–11, 13–18, or 19–24) during the chronic stage. Criteria were selected to maximize sample sizes while still examining animals that demonstrated continued BE. This mimics long-term human BE where individuals often have periods of recovery/relapse over 6 months to 9 years (e.g., recovery/relapse rates of 27–35%; Keel, Dorer, Franko, Jackson, & Herzog, 2005; Olmsted, MacDonald, McFarlane, Trottier, & Colton, 2015) rather than consistent BE across longer durations of illness.
Procedures for induction of Fos, tissue processing and Fos quantification followed methods used by previous studies (see Supplemental Materials). Animals were exposed to PF for one hour to induce Fos expression, and were sacrificed 30 minutes later to allow for peak translation of the Fos protein (i.e., 60–120 minutes after PF exposure) (e.g., Gaykema et al., 2014). Immunohistochemistry (for Fos detection) and cresyl violet staining (for anatomical tracing) of NAC and NAS tissue were then completed, and Fos cells were quantified using Neurolucida software, an Olympus BX51 microscope, and Q-Imaging Color 12 bit camera. Animals with poor tissue quality and/or outliers in Fos densities (Hoaglin & Iglewicz, 1987) were excluded from analyses. Final sample sizes were: NAC: early (BER n = 9, BEP n = 5), chronic (BER n = 6, BEP n = 6); NAS: early (BER n = 10, BEP n = 5), chronic (BER n = 6, BEP n = 6).
Results
Analysis of covariance (ANCOVA) was used to compare differences in Fos expression (in Fos density = Fos cells/mm2) across early/chronic stages and BER/BEP phenotype. The amount of PF consumed during the 1-hour exposure prior to sacrifice was included as a covariate to ensure that Fos differences were related to underlying neural differences in response to PF (versus amount of PF consumed before sacrifice). There was a significant main effect of BER/BEP phenotype in the NAS (p < 0.01) and NAC (p < .001), with increased responsivity in BEP as compared to BER rats, regardless of BE stage. There were no significant main effects of stage in the NAS (p = .14) or NAC (p = .27) (Table 1).
Table 1.
ANCOVA Results for Differences in Fos Density in the Nucleus Accumbens Core and Shell by BER/BEP Phenotype and Stage of BE.
| M (SE) | F (df,df) | p | Partial eta squared | |
|---|---|---|---|---|
| Nucleus Accumbens Core | ||||
|
| ||||
| Phenotype | F (1,21) = 20.06 | <.001 | .49 | |
| BER | 23.43 (2.02) | |||
| BEP | 37.29 (2.31) | |||
| Stage | F (1,21) = 1.28 | .27 | .06 | |
| Early | 32.07 (2.11) | |||
| Chronic | 28.65 (2.18) | |||
| Phenotype x Stage | F (1,21) = 3.24 | .08 | .13 | |
| BER Early | 22.35 (2.52) | |||
| BEP Early | 41.78 (3.38) | |||
| BER Chronic | 24.50 (3.15) | |||
| BEP Chronic | 32.79 (3.14) | |||
|
| ||||
| Nucleus Accumbens Shell | ||||
|
| ||||
| Phenotype | F (1,22) = 7.81 | .01 | .26 | |
| BER | 39.16 (3.59) | |||
| BEP | 54.70 (4.19) | |||
| Stage | F (1,22) = 2.13 | .14 | .10 | |
| Early | 51.09 (3.77) | |||
| Chronic | 42.77 (3.98) | |||
| Phenotype x Stage | F (1,22) = 2.69 | .10 | .12 | |
| BER Early | 38.57 (4.36) | |||
| BEP Early | 63.62 (6.16) | |||
| BER Chronic | 39.76 (5.76) | |||
| BEP Chronic | 45.78 (5.70) | |||
Note. ANCOVA models covaried 1-hour palatable food intake amounts prior to sacrifice. BER = binge eating resistant, BEP = binge eating prone. Partial eta squared (partial η2) values reflect effect sizes (i.e., standardized measure of the magnitude of mean differences between groups; effect size interpretation: small, partial η2 = .01; medium, partial η2 = .06; large, partial η2 = .14). Sample sizes for nucleus accumbens core: early stage BER n = 9, early stage BEP n = 5, chronic stage BER n = 6, chronic stage BEP n = 6. Sample sizes for nucleus accumbens shell: early stage BER n = 10, early stage BEP n = 5, chronic stage BER n = 6, chronic stage BEP n = 6.
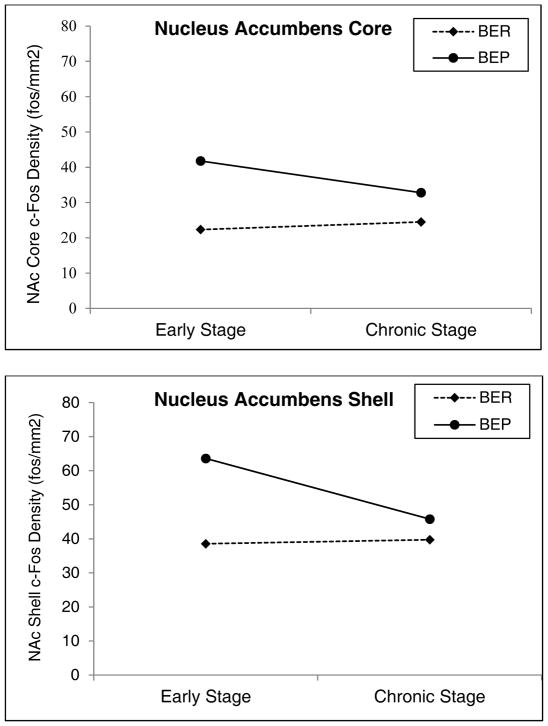
Trends towards significant phenotype x stage interactions in both the NAS (p = .10) and NAC (p = .08) were of large effect sizes (ηp2= 0.12–.13). Therefore, follow-up t-tests were conducted to further elucidate potential differences in responsivity across phenotype and stage. Early stage BEP rats had significantly higher Fos cell densities than early stage BER rats in both the NAS (p = .01) and NAC (p < .001) (large effect sizes d = 1.82–2.57) (Figure 1). By contrast, BER/BEP differences in Fos densities were attenuated in chronic stage animals, where group differences were non-significant (p’s = .16–.91) and effect sizes were decreased (d = 0.43–1.08) (Figure 1). We also examined differences in Fos densities between early and chronic stage BEP rats to see if decreased responsivity is observed within the same phenotype. Chronic stage BEP rats showed lower Fos densities in the NAS (p = 0.01) and NAC (p = 0.21) as compared to early stage BEP rats (Figure 1), and these group differences were large in magnitude (d = 1.18–1.29). Interestingly, there were very modest differences in Fos densities between early and chronic stage BER rats (p’s = .46–.90; d = 0.09–0.28), suggesting that stage-related changes in neural activation were specific to BEP rats.
Figure 1. Differences in Fos Density Between BE Prone and BE Resistant Rats across Stage of BE in the Nucleus Accumbens Core and Shell.
Note. BER = binge eating resistant, BEP = binge eating prone, NAc = nucleus accumbens. Mean comparisons of Fos density (Fos cells/mm2) in the a controlling for 1-hour PF consumption prior to sacrifice. Early Stage = feeding tests 1–6, Chronic Stage = feeding tests 7–24. Error bars represent one standard error. Sample sizes for nucleus accumbens core: early stage BER n = 9, early stage BEP n = 5, chronic stage BER n = 6, chronic stage BEP n = 6. Sample sizes for nucleus accumbens shell: early stage BER n = 10, early stage BEP n = 5, chronic stage BER n = 6, chronic stage BEP n = 6.
Discussion
This pilot study was the first to directly investigate differences in neural responsivity to PF in the NA across chronicity of BE. Notably, chronic stage BEP rats had lower responsivity in the NAC and NAS as compared to early stage BEP rats. This down-regulation was specific to BEP rats as the responsivity of BER rats stayed relatively consistent across stages. Additionally, results replicated previous work (Sinclair et al., 2015), showing that NAC and NAS responsivity was higher in BEP rats vs. BER rats. All significant and trend-level significant effects were large in magnitude, providing further support for the patterns observed in the study. Findings suggest a progression of effects in the NA, such that there is an initial hyper-responsivity to PF, followed by a down-regulation after long-term engagement in BE. Given that this was a small pilot study, it will be critical to replicate findings in larger samples and translate the findings to human BE populations in future studies.
Similar patterns have been found in alcohol/drug research, where early increased reward responsivity is reinforcing/motivating leading to increased consumption/use, while decreased neural responsivity is found after chronic use (see Volkow & Li, 2005 for review). A similar process is likely in BE, where hyper-responsivity at the early stages might be an important risk factor for development of BE, while hypo-responsivity after chronic long-term BE might be a maintenance factor. Future studies should delineate the stages of BE, and target critical time points during which neural changes take place, to more clearly understand this potential mechanism underlying BE.
It is important to consider what might account for BE stage-related differences in neural activation. Opioid neurotransmission within the NAS is linked to changes “liking” (i.e., hedonic response when consuming an natural/artificial reward; Berridge, 1996, 2009; Peciña & Berridge, 2005), while dopamine in the NAC underlies “wanting” (i.e., motivation to seek a reward; e.g., Berridge, 1996, 2009; Robinson & Berridge, 1993). It may be that opioid/dopamine systems are more activated during early BE, leading to increased levels of “liking” and “wanting,” and increased levels of BE. At chronic stages, these systems may be downregulated, leading to decreased “liking” and “wanting” of PF, and increased engagement in BE in order to achieve the same initially rewarding response. Future work should investigate this possibility.
A few limitations should be noted. First, we used a cross-sectional design. Future studies using longitudinal techniques (e.g., fMRI) are needed to investigate potential changes in neural responsivity to PF over time in humans and animals. Second, this study only included female rats. Future work should determine if the same processes are present in male rats. While we did not monitor estrous cyclicity, it is unlikely this would significantly change findings in BER/BEP groups. Previous work has demonstrated that hormone removal (via ovariectomy) induces the expected effects (i.e., increased PF intake) in both BER and BEP rats (Klump, Suisman, Culbert, Kashy, Keel, et al., 2011).
Supplementary Material
Acknowledgments
This research was supported by a grant awarded from the Global Foundation for Eating Disorders awarded to BAH and KLK, and the National Institute of Mental Health (T32-MH016804, BAH). We would like to thank Jane Venier for her expert technical assistance with all aspects of the protocol.
References
- Alsiö J, Olszewski PK, Norbäck AH, Gunnarsson ZEA, Levine AS, Pickering C, Schiöth HB. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience. 2010;171:779–787. doi: 10.1016/j.neuroscience.2010.09.046. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neuroscience & Biobehavioral Reviews. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-B. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Wanting and liking: Observations from the neuroscience and psychology laboratory. Inquiry. 2009;52:378–398. doi: 10.1080/00201740903087359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggiano MM, Artiga AI, Pritchett CE, Chandler-Laney PC, Smith ML, Eldridge AJ. High intake of palatable food predicts binge-eating independent of susceptibility to obesity: an animal model of lean vs obese binge-eating and obesity with and without binge-eating. International Journal of Obesity. 2007;31:1357–1367. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- Bohon C, Stice E. Reward abnormalities among women with full and subthreshold bulimia nervosa: a functional magnetic resonance imaging study. International Journal of Eating Disorders. 2011;44:585–595. doi: 10.1002/eat.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assaad J, Pihl RO, Benkelfat C, Leyton M, Diksic M, … Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Davis C, Levitan RD, Reid C, Carter JC, Kaplan AS, Patte KA, … Kennedy JL. Dopamine for “wanting” and opioids for “liking”: a comparison of obese adults with and without binge eating. Obesity. 2009;17:1220–1225. doi: 10.1038/oby.2009.52. [DOI] [PubMed] [Google Scholar]
- Dawe S, Gullo MJ, Loxton Nj. Reward drive and rash impulsiveness as dimensions of impulsivity: implications for substance misuse. Addictive Behaviors. 2004;29:1389–1405. doi: 10.1016/j.addbeh.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Doucette WT, Khokhar JY, Green AL. Nucleus accumbens deep brain stimulation in a rat model of binge eating. Translational Psychiatry. 2015;5:1–6. doi: 10.1038/tp.2015.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykema RPA, Nguyen XT, Boehret JM, Lambeth PS, Joy-Gaba J, Warthen DM, Scott MM. Characterization of excitatory and inhibitory neuron activation in the mouse medial prefrontal cortex following palatable food ingestion and food driven exploratory behavior. Frontiers in Neuroanatomy. 2014;8:60. doi: 10.3389/fnana.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA. Activation of c-fos in the brain. Progress in Neurobiology. 1996;50:83–107. doi: 10.1016/S0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Hoaglin DC, Iglewicz B. Fine-tuning some resistant rules for outlier labeling. Journal of the American Statistical Association. 1987;82:1147–1149. [Google Scholar]
- Keel PK, Dorer DJ, Franko DL, Jackson SC, Herzog DB. Postremission predictors of relapse in women with eating disorders. American Journal of Psychiatry. 2005;162:2263–2268. doi: 10.1176/appi.ajp.162.12.2263. [DOI] [PubMed] [Google Scholar]
- Klump KL, Racine SE, Hildebrandt B, Sisk CL. Sex differences in binge eating patterns in male and female adult rats. International Journal of Eating Disorders. 2013;46:729–736. doi: 10.1002/eat.22139. [DOI] [PubMed] [Google Scholar]
- Klump KL, Suisman JL, Culbert KM, Kashy DA, Keel PK, Sisk C. The effects of ovariectomy on binge eating proneness in adult female rats. Hormones and Behavior. 2011;59:585–593. doi: 10.1016/j.yhbeh.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Suisman JL, Culbert KM, Kashy DA, Sisk CL. Binge eating proneness emerges during puberty in female rats: a longitudinal study. Journal of Abnormal Psychology. 2011;120:948–955. doi: 10.1037/a0023600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nature Neuroscience. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Olmsted MP, MacDonald DE, McFarlane T, Trottier K, Colton P. Predictors of rapid relapse in bulimia nervosa. International Journal of Eating Disorders. 2015;48:337–340. doi: 10.1002/eat.22380. [DOI] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Research. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do μ-opioids cause increased hedonic impact of sweetness? The Journal of Neuroscience. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schäfer A, Hermann A, Vaitl D. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biological Psychiatry. 2009;65:654–661. doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Sinclair E, Culbert KM, Gradl D, Richardson K, Klump KL, Sisk CL. Differential mesocorticolimbic responses to palatable food in binge eating prone and binge eating resistant female rats. Physiology & Behavior. 2015;152:249–256. doi: 10.1016/j.physbeh.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drugs and alcohol: treating and preventing abuse, addiction and their medical consequences. Pharmacology & Therapeutics. 2005;108:3–17. doi: 10.1016/j.pharmthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Geliebter A, Volkow ND, Telang FW, Logan J, Jayne MC, … Zhu W. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity. 2012;19:1601–1608. doi: 10.1038/oby.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, James D, Morgan M. Excessive alcohol consumption and dependence on amphetamine are associated with parallel increases in subjective ratings of both ‘wanting’ and ‘liking’. Addiction. 2005;100:1487–1495. doi: 10.1111/j.1360-0443.2005.01222.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.