Abstract
Lithium is the mainstay treatment in bipolar disorder (BD) for its effectiveness in the acute phases of illness and in prevention of recurrences. Lithium’s mechanism of action is complex, and while it modulates the function of hundreds of molecular targets, most of these effects could be unspecific and not relevant for its clinical efficacy. In this study, we applied an integrated analytical approach using genome-wide expression and genotyping data from BD patients characterized for lithium response to identify lithium-responsive genes that may serve as biomarkers of its efficacy. To this purpose, we tested the effect of treatment with LiCl 1mM on the transcriptome of lymphoblasts from 10 lithium responders (LR) and 10 non-responders (NR) patients and identified genes significantly influenced by the treatment exclusively in LR. These findings were integrated with gene-based analysis on genome-wide genotyping data from an extended sample of 205 BD patients characterized for lithium response. The expression of 29 genes was significantly changed by lithium exclusively in LR. Gene-based analysis showed that two of these genes, zinc finger protein 429 (ZNF429) and zinc finger protein 493 (ZNF493), were also significantly associated with lithium response. Validation of transcriptomic data with quantitative real-time PCR confirmed the lithium-induced downregulation of ZNF493 in LR (p=0.036).
Using convergent analyses of genome-wide expression and genotyping data, we identified ZNF493 as a potential lithium-responsive target that may be involved in modulating lithium efficacy in BD. To our knowledge, this is the first evidence supporting the involvement of zinc finger proteins in lithium response.
Keywords: lithium, bipolar disorder, pharmacogenomics, biomarker, ZNF493
1. Introduction
Lithium has been used for over 70 years in the management of bipolar disorder (BD) for its effectiveness in the acute phases of illness (manic and depressive) and in the prevention of manic and depressive recurrences (Gitlin and Frye, 2012; Malhi et al., 2012). Response to lithium is highly variable, and although 30% of patients under chronic treatment are excellent responders, 70% show partial or no response (Grof et al., 2002). Part of this variability is explained by genetic variance, as also suggested by the evidence that lithium response is heritable (Grof et al., 2002). However, the complexity of lithium’s mechanism of action has hampered our understanding of the molecular networks involved in its clinical efficacy. Lithium has been shown to influence multiple molecular processes, including ion transport, neurotransmitter signaling, neuronal survival and many others. Nevertheless, the majority of these molecular modifications appear to be unrelated to its clinical effects (Alda, 2015). In this respect, the management of therapeutic intervention, side effects and toxicity would greatly benefit from the identification of biomarkers of response (McKnight et al., 2012).
Recently, findings from the largest genome-wide association (GWA) study conducted by the International Consortium on Lithium Genetics (ConLiGen) suggested the involvement of two non-coding RNA (lncRNA) genes in lithium response (Hou et al., 2016). This result is promising and with potentially high impact, but further exploration using different molecular techniques and possibly animal models is warranted. GWA allow exploring the genome for genetic variants associated with the phenotypic trait, but their power is limited in that the function of many variants is not known. On the other hand, transcriptomic studies allow investigating the role of differentially expressed genes in the phenotype under study. To this regard, deeper and more detailed information on the genetics of lithium response would be provided by the integration of data from multiple genome-wide datasets (Hunsberger et al., 2015; Niculescu and Kelsoe, 2001). Recently, Witt and coworkers (2014) applied a convergent approach to identify new candidate genes for BD. In this study, combination of GWA and genome-wide gene expression (GWGE) data measured during euthymic and manic state identified Stabilin 1 gene (STAB1) as a potential trait marker of BD (Witt et al., 2014). Another study recently compared microRNA (miRNA) and messenger RNA (mRNA) profiling in lymphoblastoid cell lines (LCLs) derived from BD patients characterized for lithium response (Hunsberger et al., 2015) to construct treatment specific gene regulatory networks, suggesting that the Let-7 family of miRNAs could be involved in modulating lithium response. These studies support the notion that convergent approaches could constitute powerful tools to identify genes and pathways involved in lithium response and possibly in its mechanism of action.
Here we present findings from the first study to date using genome-wide genotyping and genome wide expression profiles from a sample of BD patients characterized for lithium response, with the aim of identifying lithium-regulated genes possibly involved in its clinical efficacy.
2. Material and Methods
2.1. Sampling and genotyping
A study flow diagram is reported in Figure 1. The genotyped sample included 205 patients with BD recruited at the Lithium Clinic of the Clinical Psychopharmacology Centre of the University Hospital of Cagliari, Italy. Clinical characteristics of this sample have been previously described (Squassina et al., 2016). Briefly, the sample included 140 female and 65 male patients [mean age at sampling, years±standard deviation (SD): 47.9 ± 14.5)] with a diagnosis of BD type I (n = 154) or BD type II (n = 51), based on Research Diagnostic Criteria (RDC) (Spitzer et al., 1978) and DSM-IV (APA) criteria, obtained using personal semi-structured interviews [Schedule for Affective Disorder and Schizophrenia Lifetime Version (SADS-L)] (Endicott and Spitzer, 1978) and a systematic review of patients’ medical records. Clinical response to lithium treatment was assessed using the “Retrospective Criteria of Long-Term Treatment Response in Research Subjects with Bipolar Disorder”, described in Grof et al. (2002) and Manchia et al. (2013). The scale measures the degree of improvement in the course of treatment (score A) weighted against a number of possible confounders (score B). The degree of response for each patient is quantified with a score from 0 to 10 [total score (TS)], obtained by subtracting the score B from the score A. Patients with TS equal to 7 or higher are defined as lithium responders (LR) (Grof et al., 2002; Manchia et al., 2013). Our sample included 59 LR and 146 non-responders (NR).
Figure 1. Study flow diagram.
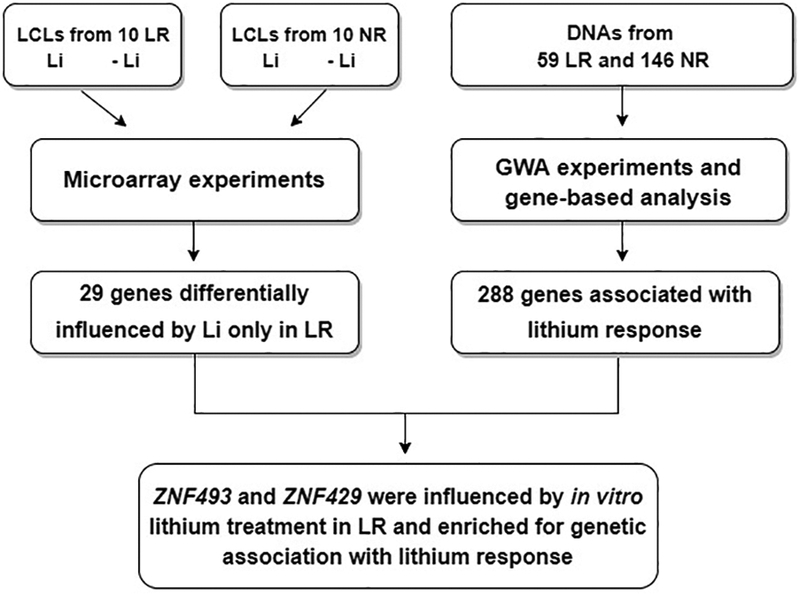
LR, lithium responders; GWA, genome-wide association; LCLs, lymphoblastoid cell lines; Li, lithium; NR, non-responders.
The research protocol followed the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the University of Cagliari, Italy. All participants signed informed written consent after a detailed description of the study procedures.
DNA was extracted from peripheral blood samples. The sample is part of the Consortium on Lithium Genetics (ConLiGen) (Hou et al., 2016; Schulze et al., 2010), and genome-wide genotyping was carried out at the National Institute of Mental Health (Bethesda, MD, USA), with Illumina 2.5 M Omni Chip arrays according to the manufacturers’ protocols.
2.2. Genome-wide gene expression
For the genome-wide expression study we selected 10 patients who showed excellent response to lithium (TS ≥ 8) and 10 NR (TS = 0) from the genotyped sample. Details on selection criteria and experimental procedures were described elsewhere (Squassina et al., 2013). All patients included in this sample had a diagnosis of BD type I. LR and NR did not differ for sex (M/F, LR: 5/5; NR: 4/6), age at sampling (years ± SD, LR: 46.6 ± 17.5; NR: 40.7 ± 10.0, Mann–Whitney test p-value = ns) or years of frozen storage of LCLs (years ± SD, LR: 8.8 ± 4.7; NR: 13.1 ± 5.2, Mann–Whitney test p-value = ns).
Briefly, Epstein-Barr virus immortalized LCLs were established from lymphocytes collected from each patient when enrolled in our molecular studies and stored in liquid nitrogen (Neitzel, 1986). At the time of the experiments, LCLs were thawed and cultured using a standard protocol previously described (Squassina et al., 2013). Once LCLs reached the required cell count (6–9 ×106 cells), two equivalent aliquots were transferred into separate flasks. One aliquot was cultured with medium supplemented with 1 mM lithium chloride (LiCl), while the other one was cultured with drug-free medium, under identical conditions. After seven days of treatment, cells were harvested for RNA extraction using TRI reagent solution (Ambion, Austin, TX). The quality of RNA was considered to be adequate when the A260/A280 ratio assessed using a NanoDrop ND-1000 spectrophotometer was in the range of 1.8–2.0, and the RNA integrity number (RIN) assessed using the Agilent®2100 Bioanalyzer was in the range of 7–10.
For the microarray experiments, 100 ng of total RNA were amplified and used to generate a sense-strand complementary DNA (cDNA) with incorporated dUTP, using the Ambion® WT Expression Kit (Applied Biosystems, Foster City, CA, USA). In a second step, cDNA fragmentation and labeling were carried out using the Affymetrix GeneChip WT Terminal Labeling Kit. Samples were hybridized to GeneChip Human Gene ST 1.0 arrays (Affymetrix, CA, USA) that comprise 764,885 probes representing 28,869 annotated genes. The arrays were placed in the hybridization oven for 17h at 45°C, and then washed and stained in the GeneChip Fluidics Station 450 (Affymetrix, CA, USA). Finally, the arrays were scanned using the GeneChip Scanner 3000 7G AutoLoader (Affymetrix, CA, USA). All samples were processed randomly by a technician blind to the clinical variables and the treatment status.
2.3. Validation with qRT-PCR
Genes showing convergent evidence for involvement in lithium response were validated with quantitative real-time PCR (qRT-PCR). Total RNA was incubated with DNAse I (ThermoFisher, Waltham MA, USA) to avoid genomic DNA contamination. Subsequently, cDNA was obtained using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems; Foster City, CA, USA). The reactions were performed in a 10 μl final volume using 40 ng of total RNA under the following conditions: 2 min at 50°C, 10 min at 95°C and 45 cycles at 95°C for 15 s and 60°C for 1 min). qRT-PCR experiments were run in triplicate using the TaqMan assays ‘on demand’ Hs03007122_m1 and Hs00703379_s1 (ThermoFisher Scientific, Waltham, MA, USA) in a StepOnePlus instrument (Applied Biosystems; Foster City, CA, USA). Target genes were normalized using Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) as a housekeeping gene.
2.4. Statistical analysis
2.4.1. Genotyping and gene-based analysis
Quality control of GWA data was performed with PLINK v. 1.09 (Purcell et al., 2007). Single nucleotide polymorphisms (SNPs) were excluded in case of minor allele frequency (MAF) < 0.05 or deviation from Hardy-Weinberg equilibrium with p < 0.0001. Individuals were excluded in case of genotype missingness > 0.03 or autosomal heterozygosity rate ≥ ±3 SD from the mean. The existence of discrepancies between reported sex and sex as indicated by genotypes on chromosome × was checked. After linkage disequilibrium (LD) pruning, cryptically related pairs (Pi_hat > 0.10) were identified and only one member of pairs was kept for subsequent analysis. Principal component analysis (PCA) using the smartpca program of EIGENSOFT (Price et al., 2006) with default options (maximum number of outlier removal iterations = 5; number of principal components to remove outliers during outlier removal iteration = 10) identified no population outliers.
Only autosomal SNPs were used for the analyses. Association with lithium response at a gene-based level was tested with the regression model implemented in MAGMA (de Leeuw et al., 2015). In the annotation step, MAGMA annotates SNP to genes if the SNP’s location is inside the region comprised between the 5’ and 3’ UTR of a gene. In a second step, gene-based p-values are computed using an F-test, taking into account LD between SNPs (de Leeuw et al., 2015).
2.4.2. Genome-wide gene expression
GeneChip data quality control was performed using Expression Console Software (Affymetrix, CA, USA). Transcriptome data were normalized using the Robust Multi-array Average algorithm (RMA) (Irizarry et al., 2003) and duplicated or missing Entrez Ids were removed. Genes were tested for differential expression after in vitro lithium treatment in both LR and NR using the paired t-test implemented in limma (Smyth, 2004). For each gene, estimates of the log2 Fold Change (FC) corresponding to the effect of lithium treatment were calculated by limma as the difference of average log2-transformed expression values [average (log2-post-treatment arrays) - average (log2-pre-treatment arrays] in both LR and NR. In a second step, linear FCs were calculated (FC = 2^logFC).
Based on findings from our previous work (Squassina et al., 2013), suggesting that LiCl in vitro has a small-medium effect on gene expression modifications in LCLs, significance was defined based on a less stringent false discovery rate (FDR) threshold of 20%. This threshold allowed us to include the largest possible number of genes at this step at the same time guaranteeing to reach 80% power to identify a differential expression with a medium effect size (Cohen’s dz = 0.7). Based on this criterion, we created a list of genes affected by lithium in LR and a list of genes affected by lithium in NR and selected those altered by lithium exclusively in LR, as these genes could be involved in modulating clinical efficacy of lithium. The analyses were performed using the limma package implemented in R (R Core Team, 2014), which is available as part of the Bioconductor project (Gentleman et al., 2004).
2.4.3. Integration of expression and gene-based data
Gene-based analysis on the GWA dataset was run to check for further evidence on the involvement in lithium response of genes showing LiCl-induced differential expression in LR (n = 29). Gene-based statistics was computed by MAGMA and a Bonferroni corrected threshold was calculated based on the number of genes of interest (Bonferroni p = 0.0017; nominal p = 0.05/n. of genes = 29).
2.4.4. Validation with qRT-PCR
Genes for validation with qRT-PCR were selected among those showing significantly differential expression after lithium treatment in LR but not in NR and association with lithium response in the gene-based analysis performed on the genotyping data. Relative expression levels were measured with the comparative Ct method (ΔΔCt) using GAPDH as the endogenous reference and an external control as the calibrator. Differences between baseline and lithium-treated samples were analyzed using the paired t-test. The significance threshold was set at p < 0.05. Statistical tests were carried out using SPSS statistical software package v20 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism v. 5.00 (GraphPad Software, San Diego, CA USA).
3. Results
Analyses of genome-wide expression data showed that lithium altered the expression of 33 genes in LR and 15 genes in NR with FDR < 20%. Four genes were modified by lithium in both groups and were excluded from subsequent analyses. In total, 29 genes were altered by lithium exclusively in LR (Table 1): 17 genes were downregulated and 12 upregulated.
Table 1.
Genes significantly altered by in vitro lithium treatment exclusively in lithium responders in the microarray dataset
| Gene | FC | 95% CI | p | q |
|---|---|---|---|---|
| FLJ36840 | 0.80 | 0.75–0.86 | 2.80E-06 | 0.006 |
| FAM46C | 1.24 | 1.16–1.33 | 3.10E-06 | 0.006 |
| KLF6 | 1.28 | 1.19–1.39 | 3.80E-06 | 0.006 |
| B4GALT6 | 0.82 | 0.76–0.88 | 1.30E-05 | 0.015 |
| NUDT9P1 | 0.76 | 0.69–0.85 | 3.20E-05 | 0.028 |
| SNORD12C | 1.22 | 1.13–1.32 | 4.60E-05 | 0.036 |
| MIRLET7E | 1.23 | 1.13–1.34 | 0.0001 | 0.054 |
| MYO15B | 1.21 | 1.12–1.31 | 0.0001 | 0.055 |
| SRSF6 | 1.18 | 1.10–1.27 | 0.0001 | 0.085 |
| LINC01152 | 0.80 | 0.72–0.88 | 0.0002 | 0.113 |
| ZNF493 | 0.84 | 0.77–0.91 | 0.0002 | 0.113 |
| ZNF429 | 0.81 | 0.74–0.89 | 0.0003 | 0.114 |
| CETN3 | 0.78 | 0.70–0.88 | 0.0003 | 0.114 |
| NEDD1 | 0.86 | 0.81–0.92 | 0.0003 | 0.114 |
| PSG6 | 0.86 | 0.80–0.92 | 0.0003 | 0.114 |
| ZNF667-AS1 | 1.25 | 1.13–1.40 | 0.0003 | 0.114 |
| PRRX1 | 0.84 | 0.77–0.91 | 0.0003 | 0.114 |
| ZBED8 | 0.78 | 0.69–0.87 | 0.0003 | 0.117 |
| ATF4 | 1.18 | 1.09–1.29 | 0.0004 | 0.137 |
| ZNF724P | 0.83 | 0.76–0.91 | 0.0005 | 0.156 |
| TRIB1 | 1.16 | 1.08–1.26 | 0.0005 | 0.165 |
| IMPAD1 | 0.82 | 0.74–0.90 | 0.0005 | 0.165 |
| CR1L | 0.84 | 0.77–0.92 | 0.0006 | 0.17 |
| LOC100131860 | 0.81 | 0.73–0.90 | 0.0006 | 0.17 |
| LDHC | 1.17 | 1.08–1.26 | 0.0006 | 0.17 |
| SNORA19 | 1.20 | 1.09–1.32 | 0.0006 | 0.171 |
| MIR30E | 0.81 | 0.72–0.90 | 0.0007 | 0.172 |
| FBXO9 | 0.82 | 0.73–0.91 | 0.0008 | 0.189 |
| SNORD59B | 1.33 | 1.15.1–54 | 0.0008 | 0.189 |
CI, confidence interval; FC, fold change. Linear FCs were calculated as FC = 2^logFC using the estimates of the log2-fold-change corresponding to the effect of lithium treatment provided by limma. The significance threshold was set at q < 0.2 at this step.
Genes reported in bold were also found to be associated with lithium response in the genotyped dataset.
In the GWA analysis, after quality control, a total of 197 patients (56 LR and 141 NR) and 1,051,764 SNPs remained for further analysis. Of the 29 genes altered by lithium in LR, two genes were also significantly associated with lithium response in the gene-based analysis (ZNF429, Z statistics = 3.56, gene-based p = 0.0002; ZNF493, Z statistics = 3.54, gene-based p = 0.0002). A regional association plot of the region on chromosome 19 in which these two genes are located was created using Locus Zoom (http://locuszoom.org/, Figure 2).
Figure 2. Regional association plot.
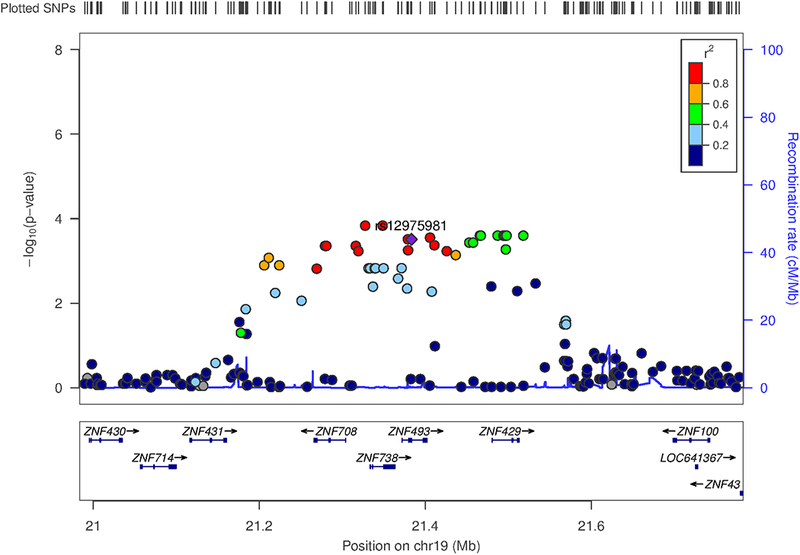
Regional association plot of the region on chromosome 19 in which the two genes (ZNF493, ZNF429) associated with lithium response and significantly affected by lithium treatment are located. The index SNP is the top-SNP (rs12975981) of ZNF493 and is shown as a purple diamond. Association p-values of SNPs are plotted as points. SNPs are colored based on linkage disequilibrium with the index SNP. cM, centimorgan; Mb, megabase; SNP, single nucleotide polymorphism.
Validation with qRT-PCR conformed the lithium-induced underexpression of ZNF493 in LR [fold change (FC) = 0.66, t = 2.46, p = 0.036), Figure 3], while ZNF429 showed a trend for downregulation (FC = 0.83, t = 2.24; p = 0.06).
Figure 3. Relative expression levels of ZNF493.
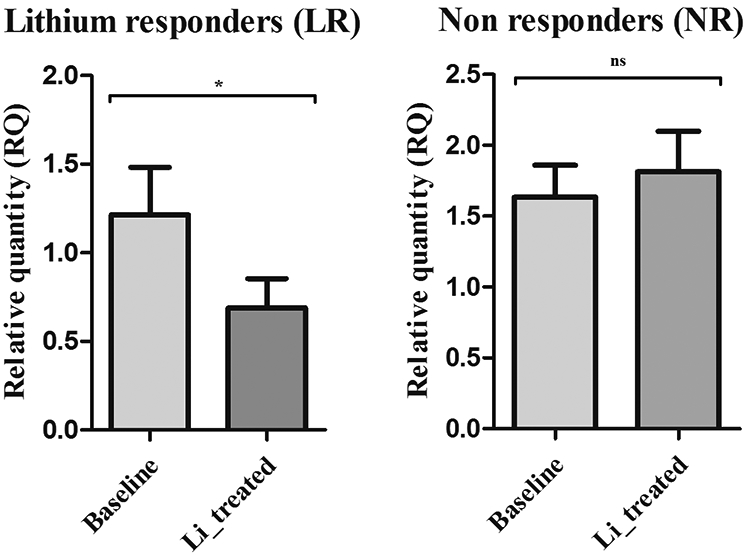
ZNF493 expression levels are significantly different after lithium treatment in lithium responders (* = p < 0.05) but not in non-responders (p > 0.05).
4. Discussion
Using data from genome-wide gene expression and genotyping, our study shows for the first time that zinc finger proteins could be involved in modulating lithium response. Zinc finger proteins are a large group of small functional domains (23–28 aminoacids) that contain multiple residues of cysteine (Cys) and/or histidine (His) and require one or more zinc ions to stabilize their structure (Laity et al., 2001; Wolfe et al., 2000). These domains are abundant in eukaryotic genomes and are involved in several functions including transcriptional activation, regulation of apoptosis and protein folding (Laity et al., 2001). Zinc finger proteins are classified in different families on the basis of the number and order of Cys/His residues, e.g. Cys2His2, Cys4, and Cys6 (Sun et al., 2015; Wolfe et al., 2000). Domains containing the Cys2His2 residues are the most represented. These proteins bind to major groove of the DNA and are involved in transcriptional regulation (Sun et al., 2015; Wolfe et al., 2000). Both ZNF429 and ZNF493 codify for Cys2His2 zinc finger proteins according to public databases (UniProtKB/Swiss-Prot). Although a possible role of these specific genes in BD has not been suggested yet, another member of the Cys2His2 zinc finger class, zinc finger protein 804A (ZNF804A), was recently associated with BD and schizophrenia (Williams et al., 2011; Zhang et al., 2016; Zhang et al., 2011). As for ZNF429 and ZNF493, the role of ZNF804A is not well characterized. However, a recent study using a gene knockdown approach in neural progenitor cells suggested ZNF804A to be involved in modulation of transcription of a large number of genes (Chen et al., 2015). Specifically, this study showed that genes involved in interferon-signaling were significantly enriched among those that were downregulated after ZNF804A knockdown, suggesting that this gene might be able to impact response to inflammatory cytokines. Much less is known about ZNF429 and ZNF493. Both genes are located at 19p12, a region previously associated with telomere homeostasis in humans (Mangino et al., 2012). Specifically, the genetic variant rs412658, that is located near zinc finger protein 676 (ZNF676), has been associated with telomere variation (Mangino et al., 2012). Although the mechanism through which ZNF676 could impact telomere variation is not known, zinc finger proteins could either modulate the expression of genes involved in telomere maintenance through their interaction with DNA or influence the posttranslational expression of a gene via binding with RNA or proteins (Mangino et al., 2012). Moreover, zinc finger proteins can stabilize the G-quadruplex structure formed by telomeric DNA (Isalan et al., 2001; Ladame et al., 2006; Mangino et al., 2012) and engineered zinc fingers are able to inhibit human telomerase activity (Patel et al., 2004). A recent preclinical study reported intriguing results supporting the role of another member of the zinc finger protein family, zinc finger protein 637 (Zfp637), in telomere maintenance (Gao et al., 2014). In this study, the endogenous expression levels of Zfp637 and mouse telomerase reverse transcriptase (mTERT) were found to be downregulated during oxidative stress-induced premature senescence. Conversely, overexpression of Zfp637 was found to increase telomerase activity and reduction of oxidative stress via binding to the mTERT promoter (Gao et al., 2014). Interestingly, several studies suggest that lithium might normalize telomere dysfunction (Martinsson et al., 2013; Squassina et al., 2016; Squassina et al., 2015; Wei et al., 2015), although the exact mechanisms are not known. It could be speculated that the impact that lithium could exert on telomere length could be partly mediated by its action on zinc finger proteins. However, to date no evidence is available to support this hypothesis. As no previous study reported a possible role of ZNF429 and ZNF493 in lithium’s mechanism of action, further investigation is required to confirm and extend our findings.
Our results should be interpreted in light of their limitations. Firstly, the sample size of this study likely provided us a limited statistical power to detect small effect sizes. Due to the small-medium effect of LiCl on the modulation of gene expression in LCLs, as suggested by our previous work (Squassina et al., 2013), we decided to relax the FDR threshold to 20% in the transcriptome analysis. While this approach allowed us to increase the number of genes based on which we generated a hypothesis that we then validated in the further steps of the current study, this approach also increased the chance of false positives. Our findings will thus require replication in larger independent samples. Secondly, although LCLs represent a valuable model to test peripheral effects of medications (Viswanath et al., 2015), they do not constitute a brain model, as previous studies reported contrasting findings, with authors reporting significant correlation with brain (Sullivan et al., 2006) and others reporting little correlation (Rollins et al., 2010). The strengths of our study include the integration of genotyping and gene expression data from patients characterized for lithium response using a validated scale and the choice to include only subjects of Sardinian origin, a population with a high genetic homogeneity and a low level of stratification (Arcos-Burgos and Muenke, 2002).
5. Conclusion
Using a convergent approach, our study identified ZNF493 as a lithium-responsive gene possibly involved in modulating the clinical efficacy of lithium in BD. This gene codifies for a member of zinc finger proteins, a large family of functional domains involved in transcriptional activation, regulation of apoptosis and protein folding. To our knowledge, this is the first evidence supporting the involvement of these proteins in lithium mechanism of action and response. Further studies are warranted to explore the mechanistic link between zinc finger proteins and lithium mechanism of action.
Acknowledgements
The authors wish to thank the patients and their families for participating in this study.
Funding
This work was in part funded by Intramural Research Program of the National Institute of Mental Health (ZIA-MH00284311; NCT00001174), and by grant from the Sardinia Regional Government (P.O.R. Sardegna F.S.E. Operational Program of the Autonomous Region of Sardinia, European Social Fund 2007–2013—Axis IV Human Resources, Objective l.3, Line of Activity l.3.1.). TGS and UH received support from the Dr-Lisa-Oehler-Foundation (Kassel, Germany).
References
- Arcos-Burgos M, Muenke M. 2002. Genetics of population isolates. Clin Genet 61:233–247. [DOI] [PubMed] [Google Scholar]
- Chen J, Lin M, Hrabovsky A, Pedrosa E, Dean J, Jain S, Zheng D, Lachman HM. 2015. ZNF804A Transcriptional Networks in Differentiating Neurons Derived from Induced Pluripotent Stem Cells of Human Origin. PloS one 10:e0124597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw CA, Mooij JM, Heskes T, Posthuma D. 2015. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput Biol 11:e1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL. 1978. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry 35:837–844. [DOI] [PubMed] [Google Scholar]
- Gao B, Li K, Wei YY, Zhang J, Li J, Zhang L, Gao JP, Li YY, Huang LG, Lin P, Wei YQ. 2014. Zinc finger protein 637 protects cells against oxidative stress-induced premature senescence by mTERT-mediated telomerase activity and telomere maintenance. Cell Death Dis 5:e1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin M, Frye MA. 2012. Maintenance therapies in bipolar disorders. Bipolar Disord 14 Suppl 2:51–65. [DOI] [PubMed] [Google Scholar]
- Grof P, Duffy A, Cavazzoni P, Grof E, Garnham J, MacDougall M, O’Donovan C, Alda M. 2002. Is response to prophylactic lithium a familial trait? J Clin Psychiatry 63:942–947. [DOI] [PubMed] [Google Scholar]
- Hou L, Heilbronner U, Degenhardt F, Adli M, Akiyama K, Akula N, Ardau R, Arias B, Backlund L, Banzato CE, Benabarre A, Bengesser S, Bhattacharjee AK, Biernacka JM, Birner A, Brichant-Petitjean C, Bui ET, Cervantes P, Chen GB, Chen HC, Chillotti C, Cichon S, Clark SR, Colom F, Cousins DA, Cruceanu C, Czerski PM, Dantas CR, Dayer A, Etain B, Falkai P, Forstner AJ, Frisen L, Fullerton JM, Gard S, Garnham JS, Goes FS, Grof P, Gruber O, Hashimoto R, Hauser J, Herms S, Hoffmann P, Hofmann A, Jamain S, Jimenez E, Kahn JP, Kassem L, Kittel-Schneider S, Kliwicki S, Konig B, Kusumi I, Lackner N, Laje G, Landen M, Lavebratt C, Leboyer M, Leckband SG, Jaramillo CA, MacQueen G, Manchia M, Martinsson L, Mattheisen M, McCarthy MJ, McElroy SL, Mitjans M, Mondimore FM, Monteleone P, Nievergelt CM, Nothen MM, Osby U, Ozaki N, Perlis RH, Pfennig A, Reich-Erkelenz D, Rouleau GA, Schofield PR, Schubert KO, Schweizer BW, Seemuller F, Severino G, Shekhtman T, Shilling PD, Shimoda K, Simhandl C, Slaney CM, Smoller JW, Squassina A, Stamm T, Stopkova P, Tighe SK, Tortorella A, Turecki G, Volkert J, Witt S, Wright A, Young LT, Zandi PP, Potash JB, DePaulo JR, Bauer M, Reininghaus EZ, Novak T, Aubry JM, Maj M, Baune BT, Mitchell PB, Vieta E, Frye MA, Rybakowski JK, Kuo PH, Kato T, Grigoroiu-Serbanescu M, Reif A, Del Zompo M, Bellivier F, Schalling M, Wray NR, Kelsoe JR, Alda M, Rietschel M, McMahon FJ, Schulze TG. 2016. Genetic variants associated with response to lithium treatment in bipolar disorder: a genome-wide association study. Lancet 387:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsberger JG, Chibane FL, Elkahloun AG, Henderson R, Singh R, Lawson J, Cruceanu C, Nagarajan V, Turecki G, Squassina A, Medeiros CD, Del Zompo M, Rouleau GA, Alda M, Chuang DM. 2015. Novel integrative genomic tool for interrogating lithium response in bipolar disorder. Transl Psychiatry 5:e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. 2003. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264. [DOI] [PubMed] [Google Scholar]
- Isalan M, Patel SD, Balasubramanian S, Choo Y. 2001. Selection of zinc fingers that bind single-stranded telomeric DNA in the G-quadruplex conformation. Biochemistry 40:830–836. [DOI] [PubMed] [Google Scholar]
- Ladame S, Schouten JA, Roldan J, Redman JE, Neidle S, Balasubramanian S. 2006. Exploring the recognition of quadruplex DNA by an engineered Cys2-His2 zinc finger protein. Biochemistry 45:1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laity JH, Lee BM, Wright PE. 2001. Zinc finger proteins: new insights into structural and functional diversity. Curr Opin Struct Biol 11:39–46. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Tanious M, Das P, Berk M. 2012. The science and practice of lithium therapy. Aust N Z J Psychiatry 46:192–211. [DOI] [PubMed] [Google Scholar]
- Manchia M, Adli M, Akula N, Ardau R, Aubry JM, Backlund L, Banzato CE, Baune BT, Bellivier F, Bengesser S, Biernacka JM, Brichant-Petitjean C, Bui E, Calkin CV, Cheng AT, Chillotti C, Cichon S, Clark S, Czerski PM, Dantas C, Zompo MD, Depaulo JR, Detera-Wadleigh SD, Etain B, Falkai P, Frisen L, Frye MA, Fullerton J, Gard S, Garnham J, Goes FS, Grof P, Gruber O, Hashimoto R, Hauser J, Heilbronner U, Hoban R, Hou L, Jamain S, Kahn JP, Kassem L, Kato T, Kelsoe JR, Kittel-Schneider S, Kliwicki S, Kuo PH, Kusumi I, Laje G, Lavebratt C, Leboyer M, Leckband SG, Lopez Jaramillo CA, Maj M, Malafosse A, Martinsson L, Masui T, Mitchell PB, Mondimore F, Monteleone P, Nallet A, Neuner M, Novak T, O’Donovan C, Osby U, Ozaki N, Perlis RH, Pfennig A, Potash JB, Reich-Erkelenz D, Reif A, Reininghaus E, Richardson S, Rouleau GA, Rybakowski JK, Schalling M, Schofield PR, Schubert OK, Schweizer B, Seemuller F, Grigoroiu-Serbanescu M, Severino G, Seymour LR, Slaney C, Smoller JW, Squassina A, Stamm T, Steele J, Stopkova P, Tighe SK, Tortorella A, Turecki G, Wray NR, Wright A, Zandi PP, Zilles D, Bauer M, Rietschel M, McMahon FJ, Schulze TG, Alda M. 2013. Assessment of Response to Lithium Maintenance Treatment in Bipolar Disorder: A Consortium on Lithium Genetics (ConLiGen) Report. PloS one 8:e65636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangino M, Hwang SJ, Spector TD, Hunt SC, Kimura M, Fitzpatrick AL, Christiansen L, Petersen I, Elbers CC, Harris T, Chen W, Srinivasan SR, Kark JD, Benetos A, El Shamieh S, Visvikis-Siest S, Christensen K, Berenson GS, Valdes AM, Vinuela A, Garcia M, Arnett DK, Broeckel U, Province MA, Pankow JS, Kammerer C, Liu Y, Nalls M, Tishkoff S, Thomas F, Ziv E, Psaty BM, Bis JC, Rotter JI, Taylor KD, Smith E, Schork NJ, Levy D, Aviv A. 2012. Genome-wide meta-analysis points to CTC1 and ZNF676 as genes regulating telomere homeostasis in humans. Hum Mol Genet 21:5385–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinsson L, Wei Y, Xu D, Melas PA, Mathe AA, Schalling M, Lavebratt C, Backlund L. 2013. Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Transl Psychiatry 3:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight RF, Adida M, Budge K, Stockton S, Goodwin GM, Geddes JR. 2012. Lithium toxicity profile: a systematic review and meta-analysis. Lancet 379:721–728. [DOI] [PubMed] [Google Scholar]
- Neitzel H 1986. A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum Genet 73:320–326. [DOI] [PubMed] [Google Scholar]
- Niculescu AB 3rd, Kelsoe JR. 2001. Convergent functional genomics: application to bipolar disorder. Ann Med 33:263–271. [DOI] [PubMed] [Google Scholar]
- Patel SD, Isalan M, Gavory G, Ladame S, Choo Y, Balasubramanian S. 2004. Inhibition of human telomerase activity by an engineered zinc finger protein that binds G-quadruplexes. Biochemistry 43:13452–13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. 2006. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38:904–909. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL http://www.R-project.org/. [Google Scholar]
- Rollins B, Martin MV, Morgan L, Vawter MP. 2010. Analysis of whole genome biomarker expression in blood and brain. Am J Med Genet B Neuropsychiatr Genet 153B:919–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze TG, Alda M, Adli M, Akula N, Ardau R, Bui ET, Chillotti C, Cichon S, Czerski P, Del Zompo M, Detera-Wadleigh SD, Grof P, Gruber O, Hashimoto R, Hauser J, Hoban R, Iwata N, Kassem L, Kato T, Kittel-Schneider S, Kliwicki S, Kelsoe JR, Kusumi I, Laje G, Leckband SG, Manchia M, Macqueen G, Masui T, Ozaki N, Perlis RH, Pfennig A, Piccardi P, Richardson S, Rouleau G, Reif A, Rybakowski JK, Sasse J, Schumacher J, Severino G, Smoller JW, Squassina A, Turecki G, Young LT, Yoshikawa T, Bauer M, McMahon FJ. 2010. The International Consortium on Lithium Genetics (ConLiGen): an initiative by the NIMH and IGSLI to study the genetic basis of response to lithium treatment. Neuropsychobiology 62:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK. 2004. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3:Article3. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J, Robins E. 1978. Research diagnostic criteria: rationale and reliability. Arch Gen Psychiatry 35:773–782. [DOI] [PubMed] [Google Scholar]
- Squassina A, Costa M, Congiu D, Manchia M, Angius A, Deiana V, Ardau R, Chillotti C, Severino G, Calza S, Del Zompo M. 2013. Insulin-like growth factor 1 (IGF-1) expression is up-regulated in lymphoblastoid cell lines of lithium responsive bipolar disorder patients. Pharmacol Res 73:1–7. [DOI] [PubMed] [Google Scholar]
- Squassina A, Pisanu C, Congiu D, Caria P, Frau D, Niola P, Melis C, Baggiani G, Lopez JP, Cruceanu C, Turecki G, Severino G, Bocchetta A, Vanni R, Chillotti C, Del Zompo M. 2016. Leukocyte telomere length positively correlates with duration of lithium treatment in bipolar disorder patients. Eur Neuropsychopharmacol 26:1241–1247. [DOI] [PubMed] [Google Scholar]
- Squassina A, Pisanu C, Corbett N, Alda M. 2017. Telomere length in bipolar disorder and lithium response. Eur Neuropsychopharmacol 27:560–567. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Fan C, Perou CM. 2006. Evaluating the comparability of gene expression in blood and brain. Am J Med Genet B Neuropsychiatr Genet 141B:261–268. [DOI] [PubMed] [Google Scholar]
- Sun Y, Hu D, Liang J, Bao YP, Meng SQ, Lu L, Shi J. 2015. Association between variants of zinc finger genes and psychiatric disorders: systematic review and meta-analysis. Schizophr Res 162: 124–137. [DOI] [PubMed] [Google Scholar]
- Viswanath B, Jose SP, Squassina A, Thirthalli J, Purushottam M, Mukherjee O, Vladimirov V, Patrinos GP, Del Zompo M, Jain S. 2015. Cellular models to study bipolar disorder: A systematic review. J Affect Disord 184:36–50. [DOI] [PubMed] [Google Scholar]
- Wei YB, Backlund L, Wegener G, Mathe AA, Lavebratt C. 2015. Telomerase dysregulation in the hippocampus of a rat model of depression: normalization by lithium. Int J Neuropsychopharmacol 18:pyv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HJ, Norton N, Dwyer S, Moskvina V, Nikolov I, Carroll L, Georgieva L, Williams NM, Morris DW, Quinn EM, Giegling I, Ikeda M, Wood J, Lencz T, Hultman C, Lichtenstein P, Thiselton D, Maher BS; Molecular Genetics of Schizophrenia Collaboration (MGS) International Schizophrenia Consortium (ISC), SGENE-plus, GROUP, Malhotra AK, Riley B, Kendler KS, Gill M, Sullivan P, Sklar P, Purcell S, Nimgaonkar VL, Kirov G, Holmans P, Corvin A, Rujescu D, Craddock N, Owen MJ, O’Donovan MC. 2011. Fine mapping of ZNF804A and genome-wide significant evidence for its involvement in schizophrenia and bipolar disorder. Mol Psychiatry 16:429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt SH, Juraeva D, Sticht C, Strohmaier J, Meier S, Treutlein J, Dukal H, Frank J, Lang M, Deuschle M, Schulze TG, Degenhardt F, Mattheisen M, Brors B, Cichon S, Nothen MM, Witt CC, Rietschel M. 2014. Investigation of manic and euthymic episodes identifies state- and trait-specific gene expression and STAB1 as a new candidate gene for bipolar disorder. Transl Psychiatry 4:e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe SA, Nekludova L, Pabo CO. 2000. DNA recognition by Cys2His2 zinc finger proteins. Annu Rev Biophys Biomol Struct 29:183–212. [DOI] [PubMed] [Google Scholar]
- Zhang C, Wang Z, Hong W, Wu Z, Peng D, Fang Y. 2016. ZNF804A Genetic Variation Confers Risk to Bipolar Disorder. Mol Neurobiol 53:2936–2943. [DOI] [PubMed] [Google Scholar]
- Zhang R, Lu SM, Qiu C, Liu XG, Gao CG, Guo TW, Valenzuela RK, Deng HW, Ma J. 2011. Population-based and family-based association studies of ZNF804A locus and schizophrenia. Mol Psychiatry 16:360–361. [DOI] [PubMed] [Google Scholar]


