Abstract
Genes and gene products interact with each other to form signal transduction networks in the cell. The interactome networks are under intricate regulation in physiological conditions, but could go awry upon genome instability caused by genetic mutations. In the past decade with next-generation sequencing technologies, an increasing number of genomic mutations have been identified in a variety of disease patients and healthy individuals. As functional and systematic studies on these mutations leap forward, they begin to reveal insights into cellular homeostasis and disease mechanisms. In this review, we discuss recent advances in the field of network biology and signaling pathway perturbations upon genomic changes, and highlight the success of various omics datasets in unraveling genotype-to-phenotype relationships.
Keywords: Network perturbations, Protein interactions, Signaling pathways, Genetic mutations, Interactome and regulome
1. Introduction
With the development of high-throughput sequencing technologies, significant advances have been made to understand various types of cancer genomes. Large-scale collaborative projects, such as The Cancer Genome Atlas (TCGA) [1] and the International Cancer Genome Consortium (ICGC) [2], have revealed the molecular complexity of cancer genomes. Over a million genetic variants have been identified across cancer types [3]. The main challenge is to distinguish driver genetic variants from passengers and explain the functional consequences of such mutations.
Genotype-to-phenotype relationships are often modeled under the assumption that most genetic mutations lead to the loss of protein function. Missense mutations are the most common sequence alterations in human disease [4]. These mutations were previously thought to cause radical changes, resulting in protein instability/misfolding or abolishing gene expression [5]. However, it becomes more and more appreciated that different mutations of the same gene may cause different functional effects [6].
Genes do not function in isolation within the cell but interact with each other [7, 8]. Accordingly, genetic mutations may not only disrupt the functions of single genes but impair the biological pathway activities within cellular signaling networks [9–13]. In such networks, nodes represent entities such as genes, transcripts or proteins, and links represent interactions between entities. Network analysis thus provides a framework to model the extent to which changes at the gene level could influence the local or global structure of the network. Here, in this review, we focus on the molecular perturbations in key cellular signaling pathways. Especially, we review the studies on how genetic alterations, such as variants and gene fusions, rewire these pathways.
2. Perturbations in cellular signaling networks
Signaling genes and proteins communicate with each other to transduce environmental cues to direct a cellular response. Protein-protein interaction (PPI) networks therefore provide a framework for visualizing and inferring the informational flow. Methods that integrate interactome information could complement gene-based statistical methods to identify new biomarkers [14–16].
2.1. Protein-protein interaction networks
Human interactome can be broadly defined as networks that contain physical and functional interactions. Physical interactions usually involve actual biophysical contact between participating proteins. Currently, two commonly used approaches exist for identifying physical interactions at proteome scale (Figure 2): binary mapping and protein complex mapping [17]. Yeast two-hybrid (Y2H) systems are routinely used to screen possible binary interactions. Several approaches exist for identifying interactions based on protein complex mapping, including affinity purification (AP) followed by mass spectrometry (MS) and co-fractionation (CoFrac) followed by MS. These methods have been applied to human proteome and have generated several human interactome maps, such as HI-II-14 [18], BioPlex [19] and BioPlex2 [8], QUBIC [20] and CoFrac [21].
Figure 2.
The physical and functional interactome.
On the other hand, functional interactions refer to any kind of biologically relevant relationships among genes [22–24]. For example, genes can be connected if their expression patterns are significantly correlated [25]. Specially, the important functional interactions are ‘genetic interactions’, where mutations in two genes produce a synergistic or profound functional effect compared to each mutation’s individual effects. There are in general two major types of genetic interactions, including synthetic lethality and synthetic viability [26, 27]. Synthetic lethality refers to a combined loss of two genes leading to cell death, while the loss of each individual gene does not. Synthetic viability, on the other hand, occurs when the lethal effect of one gene loss is rescued by the simultaneous loss of another gene.
While physical and functional interactome networks provide invaluable information for discovering the principles of disease-related network perturbations [28–30], some issues need to be considered. Interactome networks containing protein interactions were likely identified using various techniques and under different biological conditions. How to integrate these interactome networks is still a challenge in interactome-based approaches to human diseases. In addition, these interactome networks often do not account for the tissue- or cell-type specific manifestations. The majority of current approaches only provide a snapshot of highly dynamic protein interactions. There is a scarcity of context-specific interactome network, although these could be approximated by integrating the transcriptome and proteome information [31].
2.2. Node removal and edgetic perturbations of interactome
Genetic alterations (including mutations and gene fusions) have been shown to alter human interactome networks by several different means [6, 32]. They could either grossly impair the protein function (quasi-null or node removal), or perturb some but not all interactions with other proteins (edgetic) (Figure 3) [33]. For instance, truncating mutations, such as out-of-frame indels and nonsense mutations, are most likely to perturb the interactome by node removal, while small in-frame indels likely cause edgetic perturbations. Missense mutations could disrupt the interactome by either node removal or disrupting protein interactions [33]. In addition, emerging evidence has shown that node removal and edgetic mutations could result in distinct phenotypic outcomes [33].
Figure 3.
Node removal and edgetic perturbation of network by genetic alterations.
In addition to genetic mutations, alternative splicing (AS) changes are frequently observed in cancer and are important signatures for tumor progression and therapy [34]. However, their functional impact and relevance to cancer development and progression remains mostly unknown. Together with gene duplication and recombination, AS plays a major role in increasing proteome diversity. An emerging concept is that AS events can mediate new protein interactions [35], and hence change the context in which the molecular functions are carried out by the protein (Figure 3). Through an integrated analysis of expression and protein-protein interactions, it was found that genes containing tissue-specific exons tend to occupy central positions in the interactome and display distinct interaction partners in the respective tissues, and are enriched in signaling, development, and disease genes [36]. More importantly, genes that contain tissue specific exons tend to make interactions that are distinct in different tissues. These observations suggest that tissue-specific splicing may perturb the interactome in a tissue-specific manner and systematic analysis of alternative splicing mediated interactome perturbations will provide novel insights into the identification of specific therapeutic targets for human cancer.
Moreover, gene fusions have been viewed as common driver mutations in various types of malignancies [37, 38]. However, the molecular functions of gene fusions, and the fusion proteins they encode, remain poorly understood. Recently, by an integrative analysis of the structural and regulatory properties of thousands of putative fusion events, Latysheva et al. found that fusion-forming proteins occupy central positions in human interactome [39]. In addition, fusion products disproportionately connect proteins that did not previously interact in the interactome [40]. In this manner, fusion proteins can escape cellular regulation and constitutively rewire the interactome (Figure 3). These findings provide insights into how fusion proteins could rewire human interactome in cancer.
3. Cellular pathway perturbations in cancer
3.1. MAPK pathway perturbations
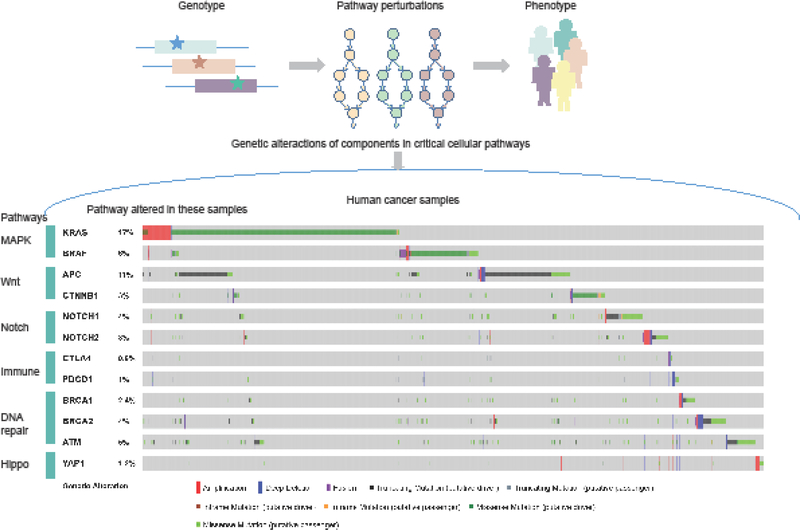
The mitogen-activated protein kinase (MAPK) cascade (also known as the Ras-Raf-MEK-ERK pathway) is a critical pathway that links extracellular signals to the intracellular machinery [41–43]. This signaling pathway controls fundamental cellular processes, including proliferation, differentiation, survival and cell death. Consistent with the critical roles in key cellular activities, deviation in the MAPK signaling pathway has been implicated in the development and progression of various types of cancer. The ERK pathway is one of the best characterized MAPK pathways in mammals and its dysregulation is associated with many types of human cancers [44]. ERK signaling can be activated by numerous extracellular signals, out of which growth factors and mitogens are of particular relevance to cancer. In addition, genetic mutations that lead to constitutive activation of ERK signaling frequently affect Ras and B-Raf [45, 46]. High-frequency mutations are centered on the Ras-Raf axis, suggesting that these genes are the regulatory hotspot of this pathway (Figure 1).
Figure 1.
Pathway-based model for understanding the genotype-to-phenotype relationships and the genetic alterations of critical cellular pathways in cancer.
Ras mutations.
Ras proteins are proto-oncogenes that are frequently mutated across human cancers. Three RAS genes (HRAS, NRAS and KRAS) constitute the most frequently mutated oncogene family in human cancers. Despite a high similarity between these isoforms, KRAS mutations are more frequently observed in cancer. Genetic mutations are invariably found at codons 12, 13 or 61, which prevent efficient GTP hydrolysis [47]. Thus these mutations render Ras in a GTP-bound state, which facilitates Ras to bind and activate their effectors, such as Raf. Such interaction between Ras and Raf is likely to play an important role in regulating downstream signaling involved in cancer. In addition, mutated Ras also recruits other molecules, such as KSR and SUR-8/SHOC-2 to modulate the activation of Raf [48]. These observations suggest that the genetic mutations not only disrupt the function of Ras itself, but also the regulatory interactions between Ras and Raf.
Raf mutations.
B-Raf has attracted enormous interest and is found mutated in different types of cancer [49], such as melanoma, colon cancer, thyroid cancer and serous ovarian cancer. Mutations in BRAF may result in ERK activation, a common cause in human cancers. For instance, the V600E change in the activation loop drastically induces the activation of its catalytic activity [50]. Moreover, increasing evidence has indicated that mutations in B-Raf that do not affect its kinase activity could also increase MEK-ERK signaling as a result of the formation of a heterodimer between the mutant BRaf and Raf-1 [51]. These mutations can promote cell proliferation, an essential process in tumor growth. In contrast to BRAF, mutations in ARAF and Raf-1 are very rare [52]. One Raf-1 mutation has been shown to elevate kinase activity, but the mutation fails to transform cells [52].
Because of its importance in cancer, the MAPK pathway has been a focus for drug discovery. Many compounds can inhibit signaling steps in the MAP/ERK pathway, and therefore are potential drugs for cancer treatment. Given that genetic mutations disrupt the MAPK pathway activity, systems-level knowledge of mutation-induced pathway rewiring might provide clues for the development of new therapeutic drugs targeting mutant proteins for human cancer.
3.2. Wnt/β-catenin pathway perturbations
The Wnt signaling pathway is a conserved pathway that regulates key cellular functions including proliferation, differentiation and migration [53, 54]. Specifically, the Wnt pathway controls these biological processes by a canonical or non-canonical pathway, depending on the involvement of β-catenin or not in signal transduction. Since its initial discovery, Wnt/β-catenin pathway is intricately involved in the pathogenesis of several types of cancer [55].
The critical role of the Wnt/β-catenin pathway in cancer was first observed in the context of APC gene mutation (Figure 1) [56]. APC interacts with β-catenin in the Wnt pathway, and genetic ablation of APC results in the upregulation of β-catenin that in combination with TCF/Lef1 shuttles to the nucleus and promotes cellular proliferation. Mutations in APC occur in a high proportion (up to 80%) of colorectal cancer patients, and these mutations are typically truncating the APC protein [57]. As APC mutations are detected early in the adenoma-carcinoma transformation, it has been suggested that loss of APC plays critical roles in the progression of cancer. Indeed, several in vivo studies in mice have demonstrated that APC inactivation is sufficient to initiate tumor development.
The central region of the 2,843 amino acid APC protein has been implicated in Wnt signaling regulation. APC mutations often generate stop codons or frameshift changes that lead to the deletion of the C-terminal part of the APC protein. It is known that the APC mutations lead to the removal of the interaction sites with axin [58]. Thus, the functional destruction complex cannot be assembled, which ultimately results in the constitutive stabilization of β-catenin. Beyond APC mutations, mutations in the β-catenin gene (CTNNb1) make it refractory to APC regulation by affecting the amino-terminal region [59]. Importantly, β-catenin mutations are exclusive to those that inactivate APC. These findings suggest the interaction between APC and β-catenin plays critical roles in regulating the activity of Wnt/β-catenin pathway.
3.3. Notch pathway perturbations
The Notch signaling is a highly evolutionarily conserved pathway which is critical for cell proliferation, differentiation and cell fate acquisition. Deregulated Notch signaling is found in various types of cancer [60], induced by genetic mutations and copy number variation. Genetic mutations in genes from the Notch pathway have been observed in a number of human cancer types including hematopoietic and solid tumors [61]. The majority of genetic alterations of Notch receptors in human cancer are observed in the NOTCH1 gene (Figure 1). It has been reported that a chromosome translocation in NOTCH1 creates a constitutively active and oncogenic NOTCH1 protein in leukemia cells by fusing T-cell receptor-β to the intracellular cleaved form of NOTCH1 (ICN1) [62]. The activating NOTCH1 mutations are found in various types of leukemia [63, 64], further supporting the oncogenic role of Notch pathway in cancer.
In contrast with the oncogenic roles of Notch signaling pathway observed in cancer, tumor-suppressive roles of this pathway have been recently observed in multiple cancer types. For example, activation of Notch signaling pathway was reported in a remission of acute myeloid leukemia [65]. The tumor-suppressor role of Notch pathway activation was also reported in B-cell acute lymphoblastic leukemia [66]. Interestingly, several studies have reported that mutations in NOTCH1 can also lead to NOTCH1 inactivation in head and neck squamous cell carcinoma [67]. The tumor suppressor role of the Notch pathway has important implications for cancer therapies, as several Notch pathway inhibitors have been discovered [68].
Among the four expressed receptors (NOTCH1–4) in human, NOTCH2 is another widely investigated player in cancer. The dysregulation of NOTCH2 has been reported in human hematological malignancies and various solid tumors. Mutations in NOTCH2 have been found in lymphoma and are associated with poor prognoses [69]. In addition, a truncating mutation in NOTCH2 was reported to promote cell proliferation in diffuse large B-cell lymphoma [70]. The overexpression of NOTCH2 was found in lung adenocarcinoma, glioma and cervical cancer, but with down-regulated expression in bladder cancer, lung cancer and breast cancer [71]. These results suggest that the Notch signaling pathway might play a diverse role in cancer progression.
3.4. Immune-related pathway perturbations
Identification of aberrant cell signaling pathways has long dominated the field of cancer biology. Increasing appreciation of cancer genomics has highlighted the critical functions of the immune system in cancer. Several studies have revealed the roles of immune-related pathways and opened new therapeutic interventions in cancer [72, 73]. It has been reported that tumor cells effectively suppress immune responses by activating negative regulatory pathways associated with immune homeostasis. Most studies currently focus on the Cytotoxic T-lymphocyte protein 4 (CTLA4) and programmed cell death protein 1 (PD-1) checkpoints [74]. Immunotherapy against CTLA4 and PD-1/PD-L1 has proved to be an effective therapeutic approach in a variety of cancer types (Figure 1).
CTLA4 is a member of the immunoglobulin superfamily and encodes a protein which transmits an inhibitory signal to T cells. CTLA4 competes with the co-stimulatory molecule CD28 for binding its ligand B7 on antigen presenting cells and thereby induces T-cell cycle arrest. Several genetic mutations in this gene have been reported to be associated with immune-related diseases. For example, A>G mutation in exon 1 (rs231775) is one of the most widely investigated variants, and has been reported to have negative effects on hepatocellular carcinoma by modifying the expression and function of CTLA4 [75]. The mutation rs231775 was also reported to be responsible for the increased susceptibility to colorectal cancer in Asian population [76]. Another checkpoint takes the form of the cell-surface receptor PD-1, which is expressed by T cells and binds to one of two ligands, PD-L1 and PD-L2. The binding of PD-L1 or PD-L2 to PD-1 generates an inhibitory signal that attenuates the activity of T cells. The PD-1/PD-L1 signaling pathway plays crucial roles in dampening immune surveillance for tumor cells. Tumor cells can escape host immune surveillance by expressing PD-L1, which negatively regulates immune responses by interacting with PD-1 on T cells. Considering their important roles in immune regulation, checkpoint inhibitors against CTLA4 and PD-1 have demonstrated remarkable effectiveness as treatments against cancers [77].
3.5. Hippo pathway perturbation in cancer
The Hippo pathway is a key signaling pathway that controls organ size in diverse species [78]. Deregulation of this pathway drives multiple aspects of tumor development and progression [79]. The Hippo pathway consists of a large network of proteins, such as the macrophage stimulating proteins MST1 and MST2, the large tumor suppressor kinases LATS1 or LATS2, and the transcriptional regulators YAP and TAZ. Studies reporting Hippo pathway deregulation have mostly relied on the detection of YAP in the nucleus of cancer tissues and its general absence in normal tissues [80, 81]. This subcellular translocation induces a growth-promoting transcriptional program, which has been reported in hepatocellular carcinoma, ovarian cancer and lung cancer. Although it is thought that the Hippo pathway regulates YAP and TAZ activity, the potential regulatory mechanisms are still unknown.
Compared with other cellular signaling pathways, few somatic or germline mutations have so far been discovered in genes of this pathway across cancer types. High-throughput sequencing studies are likely to identify an increasing number of mutations in key genes in this pathway. For example, hundreds of mutations have been reported in NF2 and FAT4. In addition, mutations in DCHS1 and DCHS2 have been reported in ovarian cancer and colorectal cancer. Regarding somatic mutations, copy number variation of YAP1 has been reported in various types of cancer (Figure 1) [82]. Functional studies have supported an oncogenic role of YAP amplification in cancer. Moreover, chromosomal translocation of TAZ and CAMTA1 has been reported. The TAZ-CAMTA1 fusion gene is highly expressed in cancer. Although the oncogenic mechanism of this fusion event is unclear, it is suggested that the transcriptional regulatory perturbation of CAMTA1 and TAZ might be the potential mechanism [83, 84]. Together, these results suggest that investigation of the pathway-network perturbation is essential for our understanding of the oncogenic mechanisms in the Hippo pathway.
3.6. DNA damage repair pathway perturbations
Maintenance of genomic integrity is critical for the viability and proper function of cells. The most toxic lesions encountered by cells are DNA double-strand breaks (DSBs). Deficiencies in DSB repair (Figure 1) can result in DNA mutations and chromosomal rearrangements, which are characteristic of cancer. For example, mutations in breast cancer genes 1 and 2 (BRCA1/BRCA2) were found to be associated with familial breast, ovarian, and prostate cancers. The majority of mutations associated with disease phenotypes evade correction by the homologous recombination repair pathway. Precise details of all potential DNA repair mechanisms have been fully reviewed elsewhere [85]. Here, we focus on how the loss of protein-protein interactions throughout this signaling cascade results in disease phenotypes.
Double strand breaks are initially recognized by the MRN (MRE11-Rad50-NBN) complex, which can then recruit and activate ATM at DSBs. Mutations in any of these complex members produce disease phenotypes, including various malignancies [86]. While MRE11 has nuclease activity, structural analysis has shown disorders that primarily arise from mutations compromising binding to Rad50 and NBN [87]. Likewise, mutations in Rad50 have also been reported to impair Rad50-MRE11 binding [88]. The eukaryotic-specific NBN (previously known as NBS1) binds MRE11 to link the MRN complex with ATM and is required for ATM binding and activation [89, 90]. This interaction is mediated by a FXY motif in the C-terminal of NBN binding to N-terminal HEAT (Huntingtin, elongation factor 3, protein phosphatase 2A, and yeast kinase TOR1) repeats within ATM [91]. Mutations within these HEAT repeats are prevalent in malignancies [92], suggesting this interaction is key to preventing oncogenesis.
In normal cells, following binding and activation of ATM by the MRN complex, ATM acts as a signal transducer through multiple mediators, such as CHEK2 and BRCA1, which then signal to effectors including RAD51 and BRCA2. Notably, mutations in ATM as well as the aforementioned downstream factors are all risk factors for familial breast cancers [93]. In the case of ATM’s primary downstream target, CHEK2, this susceptibility is linked with the inability of CHEK2 to properly homodimerize for complete activation, such as seen with the common 1100delC mutation [94]. The most common risk factor for breast cancer is truncating mutations in BRCA1 or BRCA2 [95]. Despite sequence similarity and relative risks for hereditary cancers, these proteins show distinct binding partners and functional roles. BRCA1 acts as a signal mediator to activate both DNA repair and cell cycle checkpoints, whereas BRCA2 predominately acts as a DSB repair scaffolding protein. In vivo, BRCA1 and BRCA2 proteins are directly linked via PALB2 [96], which when mutated is also a risk factor for familial breast cancer. While the amino terminus of BRCA2 contains the PALB2 binding site and is not likely to be lost by most truncating mutations, PALB2 binds the coiled-coil domains (residues 1393–1424) near the carboxyl domain of BRCA1, which is typically lost following protein truncation [97]. This BRCA1-PALB2-BRCA2 complex is critical for the recruitment of RAD51 recombinase via the BRC repeats within BRCA2 [98]. Mutations within these BRC repeats can prevent RAD51 recruitment to BRCA2 to inhibit repair [99].
In summary, compromised interactions between key DNA double-strand break sensors (MRE11/RAD50/NBN), transducers (ATM), mediators (CHEK2/BRCA1), and effectors (BRCA2/RAD51) can induce numerous disease phenotypes. Future studies on approaches to stabilize these interactions for cancer prevention, as well as leverage these deficiencies for cancer therapies stand to greatly improve patient prognosis, as seen by the resounding clinical success of PARP inhibitors in BRCA1/BRCA2 mutated breast and ovarian cancers [100].
4. Noncoding variants and regulome perturbations
Approximately 90% of disease-related genetic variants identified in cancer reside in noncoding regions [3], such as intergenic or intronic regions. Investigating the functional effects of these noncoding variants presents another challenge for unravelling genotype-to-phenotype relationships. These noncoding mutations could play critical regulatory roles in cancer [101], which might function through perturbations of the binding of transcription factors (TFs) [102], microRNAs (miRNAs) [103] or RNA binding proteins (RBPs) [104], as well as through disruption of chromatin interactions [105]. However, studies investigating these types of mutations have traditionally been low-throughput.
Most of these studies were focused on the perturbation of TF regulation. Although great progress has been made to identify TF binding, such as ChIP-Seq and consecutive affinity-purification systematic evolution of ligands by exponential enrichment (CAP-SELEX) technology [106], the coverage of the available datasets has been limited to about 20% of human TFs [107]. Another potential issue is that since most TFs were tested in a single cell type or condition, it is difficult to deal with tissue or cell-type specific regulation. A recent study has developed enhanced yeast one-hybrid assays to increase the screening throughput of TF binding differences between wild-type and mutant noncoding alleles [108]. The study identified differential TF regulation of disease-related mutations and observed that mutations could also lead to gain of new interactions in disease.
Beyond perturbing transcriptional regulation, noncoding mutations can also disrupt posttranscriptional and translational regulation. Recent studies have identified multiple cancer-related mutations within miRNA gene regions and within the miRNA binding sites in target genes or long non-coding RNAs (lncRNAs) [109]. In addition, several mutations were also found to affect the binding of RBPs to their target genes. However, there are few studies that have systematically analyzed mutation-mediated miRNAgene or RBP-gene regulatory network rewiring in cancer. Future studies should take advantage of the integration of multiple levels of regulation/interactions when characterizing the functional consequences of noncoding mutations.
5. Conclusions
Network-based analysis of complex diseases is gaining recognition as a crucial new tool in the development of preventive and therapeutic strategies. However, investigating the impact of diverse genetic alterations on the interactome and regulome is just underway. A number of issues remain challenging and still need to be addressed – first, the majority of network perturbation studies thus far exclusively focus on protein-protein interaction networks. Ideally, full cellular networks – including the transcription regulatory network, post-transcription regulatory network and metabolic networks – should be considered simultaneously. New mathematical methods are required to satisfactorily integrate these networks. Second, the development and progression of cancer is a dynamic process, yet most current studies have employed single end-point studies. It will be essential to fully dissect time series datasets to understand cancer developmental dynamics. Finally, analysis of network perturbations should be performed not only at a cell population level, but also at the single cell level. With significant impact of network perturbations in cellular signaling, modulation of protein– protein interactions is becoming increasingly important in drug discovery and chemical biology. Taken together, functional characterization of the effects of genetic variants on cellular signaling pathways will undoubtedly facilitate our understanding of the relationships between genotypes and phenotypes.
Acknowledgments
The authors would like to acknowledge the following grants: the Cancer Prevention and Research Institute of Texas (CPRIT) New Investigator Grant RR160021 (N.S.), the University of Texas System Rising STARs award (N.S.), the AASLD Foundation Pinnacle Research Award in Liver Disease (N.S.), the University Center Foundation via the Institutional Research Grant program at the University of Texas MD Anderson Cancer Center (N.S.), and NIH/NCI Transition Career Development Award Grant 1K22CA214765 (S.Y.). Y.L. is supported by a postdoctoral fellowship from the Harold C. and Mary L. Daily Endowment Fund. Additional funding was provided by Susan G. Komen PDF17483544 to D.J.M.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].N. Cancer Genome Atlas Research, Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, Ellrott K, Shmulevich I, Sander C, Stuart JM, The Cancer Genome Atlas Pan-Cancer analysis project, Nature genetics 45(10) (2013) 1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].C. International Cancer Genome, Hudson TJ, Anderson W, Artez A, Barker AD, Bell C, Bernabe RR, Bhan MK, Calvo F, Eerola I, Gerhard DS, Guttmacher A, Guyer M, Hemsley FM, Jennings JL, Kerr D, Klatt P, Kolar P, Kusada J, Lane DP, Laplace F, Youyong L, Nettekoven G, Ozenberger B, Peterson J, Rao TS, Remacle J, Schafer AJ, Shibata T, Stratton MR, Vockley JG, Watanabe K, Yang H, Yuen MM, Knoppers BM, Bobrow M, Cambon-Thomsen A, Dressler LG, Dyke SO, Joly Y, Kato K, Kennedy KL, Nicolas P, Parker MJ, Rial-Sebbag E, Romeo-Casabona CM, Shaw KM, Wallace S, Wiesner GL, Zeps N, Lichter P, Biankin AV, Chabannon C, Chin L, Clement B, de Alava E, Degos F, Ferguson ML, Geary P, Hayes DN, Hudson TJ, Johns AL, Kasprzyk A, Nakagawa H, Penny R, Piris MA, Sarin R, Scarpa A, Shibata T, van de Vijver M, Futreal PA, Aburatani H, Bayes M, Botwell DD, Campbell PJ, Estivill X, Gerhard DS, Grimmond SM, Gut I, Hirst M, Lopez-Otin C, Majumder P, Marra M, McPherson JD, Nakagawa H, Ning Z, Puente XS, Ruan Y, Shibata T, Stratton MR, Stunnenberg HG, Swerdlow H, Velculescu VE, Wilson RK, Xue HH, Yang L, Spellman PT, Bader GD, Boutros PC, Campbell PJ, Flicek P, Getz G, Guigo R, Guo G, Haussler D, Heath S, Hubbard TJ, Jiang T, Jones SM, Li Q, Lopez-Bigas N, Luo R, Muthuswamy L, Ouellette BF, Pearson JV, Puente XS, Quesada V, Raphael BJ, Sander C, Shibata T, Speed TP, Stein LD, Stuart JM, Teague JW, Totoki Y, Tsunoda T, Valencia A, Wheeler DA, Wu H, Zhao S, Zhou G, Stein LD, Guigo R, Hubbard TJ, Joly Y, Jones SM, Kasprzyk A, Lathrop M, Lopez-Bigas N, Ouellette BF, Spellman PT, Teague JW, Thomas G, Valencia A, Yoshida T, Kennedy KL, Axton M, Dyke SO, Futreal PA, Gerhard DS, Gunter C, Guyer M, Hudson TJ, McPherson JD, Miller LJ, Ozenberger B, Shaw KM, Kasprzyk A, Stein LD, Zhang J, Haider SA, Wang J, Yung CK, Cros A, Liang Y, Gnaneshan S, Guberman J, Hsu J, Bobrow M, Chalmers DR, Hasel KW, Joly Y, Kaan TS, Kennedy KL, Knoppers BM, Lowrance WW, Masui T, Nicolas P, Rial-Sebbag E, Rodriguez LL, Vergely C, Yoshida T, Grimmond SM, Biankin AV, Bowtell DD, Cloonan N, deFazio A, Eshleman JR, Etemadmoghadam D, Gardiner BB, Kench JG, Scarpa A, Sutherland RL, Tempero MA, Waddell NJ, Wilson PJ, McPherson JD, Gallinger S, Tsao MS, Shaw PA, Petersen GM, Mukhopadhyay D, Chin L, DePinho RA, Thayer S, Muthuswamy L, Shazand K, Beck T, Sam M, Timms L, Ballin V, Lu Y, Ji J, Zhang X, Chen F, Hu X, Zhou G, Yang Q, Tian G, Zhang L, Xing X, Li X, Zhu Z, Yu Y, Yu J, Yang H, Lathrop M, Tost J, Brennan P, Holcatova I, Zaridze D, Brazma A, Egevard L, Prokhortchouk E, Banks RE, Uhlen M, Cambon-Thomsen A, Viksna J, Ponten F, Skryabin K, Stratton MR, Futreal PA, Birney E, Borg A, Borresen-Dale AL, Caldas C, Foekens JA, Martin S, Reis-Filho JS, Richardson AL, Sotiriou C, Stunnenberg HG, Thoms G, van de Vijver M, van’t Veer L, Calvo F, Birnbaum D, Blanche H, Boucher P, Boyault S, Chabannon C, Gut I, Masson-Jacquemier JD, Lathrop M, Pauporte I, Pivot X, Vincent-Salomon A, Tabone E, Theillet C, Thomas G, Tost J, Treilleux I, Calvo F, Bioulac-Sage P, Clement B, Decaens T, Degos F, Franco D, Gut I, Gut M, Heath S, Lathrop M, Samuel D, Thomas G, Zucman-Rossi J, Lichter P, Eils R, Brors B, Korbel JO, Korshunov A, Landgraf P, Lehrach H, Pfister S, Radlwimmer B, Reifenberger G, Taylor MD, von Kalle C, Majumder PP, Sarin R, Rao TS, Bhan MK, Scarpa A, Pederzoli P, Lawlor RA, Delledonne M, Bardelli A, Biankin AV, Grimmond SM, Gress T, Klimstra D, Zamboni G, Shibata T, Nakamura Y, Nakagawa H, Kusada J, Tsunoda T, Miyano S, Aburatani H, Kato K, Fujimoto A, Yoshida T, Campo E, Lopez-Otin C, Estivill X, Guigo R, de Sanjose S, Piris MA, Montserrat E, Gonzalez-Diaz M, Puente XS, Jares P, Valencia A, Himmelbauer H, Quesada V, Bea S, Stratton MR, Futreal PA, Campbell PJ, Vincent-Salomon A, Richardson AL, Reis-Filho JS, van de Vijver M, Thomas G, Masson-Jacquemier JD, Aparicio S, Borg A, Borresen-Dale AL, Caldas C, Foekens JA, Stunnenberg HG, van’t Veer L, Easton DF, Spellman PT, Martin S, Barker AD, Chin L, Collins FS, Compton CC, Ferguson ML, Gerhard DS, Getz G, Gunter C, Guttmacher A, Guyer M, Hayes DN, Lander ES, Ozenberger B, Penny R, Peterson J, Sander C, Shaw KM, Speed TP, Spellman PT, Vockley JG, Wheeler DA, Wilson RK, Hudson TJ, Chin L, Knoppers BM, Lander ES, Lichter P, Stein LD, Stratton MR, Anderson W, Barker AD, Bell C, Bobrow M, Burke W, Collins FS, Compton CC, DePinho RA, Easton DF, Futreal PA, Gerhard DS, Green AR, Guyer M, Hamilton SR, Hubbard TJ, Kallioniemi OP, Kennedy KL, Ley TJ, Liu ET, Lu Y, Majumder P, Marra M, Ozenberger B, Peterson J, Schafer AJ, Spellman PT, Stunnenberg HG, Wainwright BJ, Wilson RK, Yang H, International network of cancer genome projects, Nature 464(7291) (2010) 993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].MacArthur J, Bowler E, Cerezo M, Gil L, Hall P, Hastings E, Junkins H, McMahon A, Milano A, Morales J, Pendlington ZM, Welter D, Burdett T, Hindorff L, Flicek P, Cunningham F, Parkinson H, The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog), Nucleic acids research 45(D1) (2017) D896–D901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN, The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine, Hum. Genet. 133(1) (2014) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Subramanian S, Kumar S, Evolutionary anatomies of positions and types of disease-associated and neutral amino acid mutations in the human genome, BMC Genomics 7 (2006) 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhong Q, Simonis N, Li QR, Charloteaux B, Heuze F, Klitgord N, Tam S, Yu H, Venkatesan K, Mou D, Swearingen V, Yildirim MA, Yan H, Dricot A, Szeto D, Lin C, Hao T, Fan C, Milstein S, Dupuy D, Brasseur R, Hill DE, Cusick ME, Vidal M, Edgetic perturbation models of human inherited disorders, Mol. Syst. Biol. 5 (2009) 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vidal M, Cusick ME, Barabasi AL, Interactome networks and human disease, Cell 144(6) (2011) 986–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Huttlin EL, Bruckner RJ, Paulo JA, Cannon JR, Ting L, Baltier K, Colby G, Gebreab F, Gygi MP, Parzen H, Szpyt J, Tam S, Zarraga G, Pontano-Vaites L, Swarup S, White AE, Schweppe DK, Rad R, Erickson BK, Obar RA, Guruharsha KG, Li K, Artavanis-Tsakonas S, Gygi SP, Harper JW, Architecture of the human interactome defines protein communities and disease networks, Nature 545(7655) (2017) 505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., Kinzler KW, Cancer genome landscapes, Science 339(6127) (2013) 1546–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell 144(5) (2011) 646–74. [DOI] [PubMed] [Google Scholar]
- [11].Polivka J Jr., Janku F, Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway, Pharmacology & therapeutics 142(2) (2014) 164–75. [DOI] [PubMed] [Google Scholar]
- [12].Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E, PI3K/AKT signaling pathway and cancer: an updated review, Annals of medicine 46(6) (2014) 372–83. [DOI] [PubMed] [Google Scholar]
- [13].Amakye D, Jagani Z, Dorsch M, Unraveling the therapeutic potential of the Hedgehog pathway in cancer, Nature medicine 19(11) (2013) 1410–22. [DOI] [PubMed] [Google Scholar]
- [14].Horn H, Lawrence MS, Chouinard CR, Shrestha Y, Hu JX, Worstell E, Shea E, Ilic N, Kim E, Kamburov A, Kashani A, Hahn WC, Campbell JD, Boehm JS, Getz G, Lage K, NetSig: network-based discovery from cancer genomes, Nature methods 15(1) (2018) 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li Y, Li L, Wang Z, Pan T, Sahni N, Jin X, Wang G, Li J, Zheng X, Zhang Y, Xu J, Yi S, Li X, LncMAP: Pan-cancer atlas of long noncoding RNA-mediated transcriptional network perturbations, Nucleic acids research (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Creixell P, Reimand J, Haider S, Wu G, Shibata T, Vazquez M, Mustonen V, Gonzalez-Perez A, Pearson J, Sander C, Raphael BJ, Marks DS, Ouellette BFF, Valencia A, Bader GD, Boutros PC, Stuart JM, Linding R, Lopez-Bigas N, Stein LD, Mutation C, Pathway C Analysis Working Group of the International Cancer Genome, Pathway and network analysis of cancer genomes, Nature methods 12(7) (2015) 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Luck K, Sheynkman GM, Zhang I, Vidal M, Proteome-Scale Human Interactomics, Trends in biochemical sciences 42(5) (2017) 342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rolland T, Tasan M, Charloteaux B, Pevzner SJ, Zhong Q, Sahni N, Yi S, Lemmens I, Fontanillo C, Mosca R, Kamburov A, Ghiassian SD, Yang X, Ghamsari L, Balcha D, Begg BE, Braun P, Brehme M, Broly MP, Carvunis AR, Convery-Zupan D, Corominas R, Coulombe-Huntington J, Dann E, Dreze M, Dricot A, Fan C, Franzosa E, Gebreab F, Gutierrez BJ, Hardy MF, Jin M, Kang S, Kiros R, Lin GN, Luck K, MacWilliams A, Menche J, Murray RR, Palagi A, Poulin MM, Rambout X, Rasla J, Reichert P, Romero V, Ruyssinck E, Sahalie JM, Scholz A, Shah AA, Sharma A, Shen Y, Spirohn K, Tam S, Tejeda AO, Trigg SA, Twizere JC, Vega K, Walsh J, Cusick ME, Xia Y, Barabasi AL, Iakoucheva LM, Aloy P, De Las Rivas J, Tavernier J, Calderwood MA, Hill DE, Hao T, Roth FP, Vidal M, A proteome-scale map of the human interactome network, Cell 159(5) (2014) 1212–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Huttlin EL, Ting L, Bruckner RJ, Gebreab F, Gygi MP, Szpyt J, Tam S, Zarraga G, Colby G, Baltier K, Dong R, Guarani V, Vaites LP, Ordureau A, Rad R, Erickson BK, Wuhr M, Chick J, Zhai B, Kolippakkam D, Mintseris J, Obar RA, Harris T, Artavanis-Tsakonas S, Sowa ME, De Camilli P, Paulo JA, Harper JW, Gygi SP, The BioPlex Network: A Systematic Exploration of the Human Interactome, Cell 162(2) (2015) 425–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hein MY, Hubner NC, Poser I, Cox J, Nagaraj N, Toyoda Y, Gak IA, Weisswange I, Mansfeld J, Buchholz F, Hyman AA, Mann M, A human interactome in three quantitative dimensions organized by stoichiometries and abundances, Cell 163(3) (2015) 712–23. [DOI] [PubMed] [Google Scholar]
- [21].Wan C, Borgeson B, Phanse S, Tu F, Drew K, Clark G, Xiong X, Kagan O, Kwan J, Bezginov A, Chessman K, Pal S, Cromar G, Papoulas O, Ni Z, Boutz DR, Stoilova S, Havugimana PC, Guo X, Malty RH, Sarov M, Greenblatt J, Babu M, Derry WB, Tillier ER, Wallingford JB, Parkinson J, Marcotte EM, Emili A, Panorama of ancient metazoan macromolecular complexes, Nature 525(7569) (2015) 339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu G, Feng X, Stein L, A human functional protein interaction network and its application to cancer data analysis, Genome biology 11(5) (2010) R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xu J, Li Y, Lu J, Pan T, Ding N, Wang Z, Shao T, Zhang J, Wang L, Li X, The mRNA related ceRNA-ceRNA landscape and significance across 20 major cancer types, Nucleic acids research 43(17) (2015) 8169–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li Y, Xu J, Chen H, Bai J, Li S, Zhao Z, Shao T, Jiang T, Ren H, Kang C, Li X, Comprehensive analysis of the functional microRNA-mRNA regulatory network identifies miRNA signatures associated with glioma malignant progression, Nucleic acids research 41(22) (2013) e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang B, Horvath S, A general framework for weighted gene co-expression network analysis, Statistical applications in genetics and molecular biology 4 (2005) Article17. [DOI] [PubMed] [Google Scholar]
- [26].Kaelin WG Jr., The concept of synthetic lethality in the context of anticancer therapy, Nature reviews. Cancer 5(9) (2005) 689–98. [DOI] [PubMed] [Google Scholar]
- [27].Ashworth A, Lord CJ, Reis-Filho JS, Genetic interactions in cancer progression and treatment, Cell 145(1) (2011) 30–8. [DOI] [PubMed] [Google Scholar]
- [28].Menche J, Sharma A, Kitsak M, Ghiassian SD, Vidal M, Loscalzo J, Barabasi AL, Disease networks. Uncovering disease-disease relationships through the incomplete interactome, Science 347(6224) (2015) 1257601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xu J, Li CX, Li YS, Lv JY, Ma Y, Shao TT, Xu LD, Wang YY, Du L, Zhang YP, Jiang W, Li CQ, Xiao Y, Li X, MiRNA-miRNA synergistic network: construction via co-regulating functional modules and disease miRNA topological features, Nucleic acids research 39(3) (2011) 825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Xu J, Feng L, Han Z, Li Y, Wu A, Shao T, Ding N, Li L, Deng W, Di X, Wang J, Zhang L, Li X, Zhang K, Cheng S, Extensive ceRNA-ceRNA interaction networks mediated by miRNAs regulate development in multiple rhesus tissues, Nucleic acids research 44(19) (2016) 9438–9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Basha O, Barshir R, Sharon M, Lerman E, Kirson BF, Hekselman I, Yeger-Lotem E, The TissueNet v.2 database: A quantitative view of protein-protein interactions across human tissues, Nucleic acids research 45(D1) (2017) D427–D431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Charloteaux B, Zhong Q, Dreze M, Cusick ME, Hill DE, Vidal M, Protein-protein interactions and networks: forward and reverse edgetics, Methods in molecular biology 759 (2011) 197–213. [DOI] [PubMed] [Google Scholar]
- [33].Sahni N, Yi S, Taipale M, Fuxman Bass JI, Coulombe-Huntington J, Yang F, Peng J, Weile J, Karras GI, Wang Y, Kovacs IA, Kamburov A, Krykbaeva I, Lam MH, Tucker G, Khurana V, Sharma A, Liu YY, Yachie N, Zhong Q, Shen Y, Palagi A, San-Miguel A, Fan C, Balcha D, Dricot A, Jordan DM, Walsh JM, Shah AA, Yang X, Stoyanova AK, Leighton A, Calderwood MA, Jacob Y, Cusick ME, Salehi-Ashtiani K, Whitesell LJ, Sunyaev S, Berger B, Barabasi AL, Charloteaux B, Hill DE, Hao T, Roth FP, Xia Y, Walhout AJ, Lindquist S, Vidal M, Widespread macromolecular interaction perturbations in human genetic disorders, Cell 161(3) (2015) 647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Oltean S, Bates DO, Hallmarks of alternative splicing in cancer, Oncogene 33(46) (2014) 5311–8. [DOI] [PubMed] [Google Scholar]
- [35].Buljan M, Chalancon G, Dunker AK, Bateman A, Balaji S, Fuxreiter M, Babu MM, Alternative splicing of intrinsically disordered regions and rewiring of protein interactions, Current opinion in structural biology 23(3) (2013) 443–50. [DOI] [PubMed] [Google Scholar]
- [36].Buljan M, Chalancon G, Eustermann S, Wagner GP, Fuxreiter M, Bateman A, Babu MM, Tissue-specific splicing of disordered segments that embed binding motifs rewires protein interaction networks, Molecular cell 46(6) (2012) 871–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Watson IR, Takahashi K, Futreal PA, Chin L, Emerging patterns of somatic mutations in cancer, Nature reviews. Genetics 14(10) (2013) 703–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Latysheva NS, Babu MM, Discovering and understanding oncogenic gene fusions through data intensive computational approaches, Nucleic acids research 44(10) (2016) 4487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Latysheva NS, Oates ME, Maddox L, Flock T, Gough J, Buljan M, Weatheritt RJ, Babu MM, Molecular Principles of Gene Fusion Mediated Rewiring of Protein Interaction Networks in Cancer, Molecular cell 63(4) (2016) 579–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Frenkel-Morgenstern M, Gorohovski A, Tagore S, Sekar V, Vazquez M, Valencia A, ChiPPI: a novel method for mapping chimeric protein-protein interactions uncovers selection principles of protein fusion events in cancer, Nucleic acids research (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dhillon AS, Hagan S, Rath O, Kolch W, MAP kinase signalling pathways in cancer, Oncogene 26(22) (2007) 3279–90. [DOI] [PubMed] [Google Scholar]
- [42].Burotto M, Chiou VL, Lee JM, Kohn EC, The MAPK pathway across different malignancies: a new perspective, Cancer 120(22) (2014) 3446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Modos D, Brooks J, Fazekas D, Ari E, Vellai T, Csermely P, Korcsmaros T, Lenti K, Identification of critical paralog groups with indispensable roles in the regulation of signaling flow, Scientific reports 6 (2016) 38588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Roberts PJ, Der CJ, Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer, Oncogene 26(22) (2007) 3291–310. [DOI] [PubMed] [Google Scholar]
- [45].Fernandez-Medarde A, Santos E, Ras in cancer and developmental diseases, Genes & cancer 2(3) (2011) 344–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hou W, Liu J, Chen P, Wang H, Ye BC, Qiang F, Mutation analysis of key genes in RAS/RAF and PI3K/PTEN pathways in Chinese patients with hepatocellular carcinoma, Oncology letters 8(3) (2014) 1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Burd CE, Liu W, Huynh MV, Waqas MA, Gillahan JE, Clark KS, Fu K, Martin BL, Jeck WR, Souroullas GP, Darr DB, Zedek DC, Miley MJ, Baguley BC, Campbell SL, Sharpless NE, Mutation-specific RAS oncogenicity explains NRAS codon 61 selection in melanoma, Cancer discovery 4(12) (2014) 1418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Li W, Han M, Guan KL, The leucine-rich repeat protein SUR-8 enhances MAP kinase activation and forms a complex with Ras and Raf, Genes & development 14(8) (2000) 895–900. [PMC free article] [PubMed] [Google Scholar]
- [49].Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA, Mutations of the BRAF gene in human cancer, Nature 417(6892) (2002) 949–54. [DOI] [PubMed] [Google Scholar]
- [50].Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, Marais R, Cancer Genome P, Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF, Cell 116(6) (2004) 855–67. [DOI] [PubMed] [Google Scholar]
- [51].Garnett MJ, Rana S, Paterson H, Barford D, Marais R, Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization, Molecular cell 20(6) (2005) 963–9. [DOI] [PubMed] [Google Scholar]
- [52].Emuss V, Garnett M, Mason C, Marais R, Mutations of C-RAF are rare in human cancer because C-RAF has a low basal kinase activity compared with B-RAF, Cancer research 65(21) (2005) 9719–26. [DOI] [PubMed] [Google Scholar]
- [53].Stewart DJ, Wnt signaling pathway in non-small cell lung cancer, Journal of the National Cancer Institute 106(1) (2014) djt356. [DOI] [PubMed] [Google Scholar]
- [54].Tai D, Wells K, Arcaroli J, Vanderbilt C, Aisner DL, Messersmith WA, Lieu CH, Targeting the WNT Signaling Pathway in Cancer Therapeutics, The oncologist 20(10) (2015) 1189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Duchartre Y, Kim YM, Kahn M, The Wnt signaling pathway in cancer, Critical reviews in oncology/hematology 99 (2016) 141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Schneikert J, Behrens J, The canonical Wnt signalling pathway and its APC partner in colon cancer development, Gut 56(3) (2007) 417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kwong LN, Dove WF, APC and its modifiers in colon cancer, Advances in experimental medicine and biology 656 (2009) 85–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Polakis P, Wnt signaling in cancer, Cold Spring Harbor perspectives in biology 4(5) (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Morin PJ, Kinzler KW, Sparks AB, beta-Catenin Mutations: Insights into the APC Pathway and the Power of Genetics, Cancer research 76(19) (2016) 5587–5589. [DOI] [PubMed] [Google Scholar]
- [60].Yuan X, Wu H, Xu H, Xiong H, Chu Q, Yu S, Wu GS, Wu K, Notch signaling: an emerging therapeutic target for cancer treatment, Cancer letters 369(1) (2015) 20–7. [DOI] [PubMed] [Google Scholar]
- [61].Mao L, NOTCH mutations: multiple faces in human malignancies, Cancer prevention research 8(4) (2015) 259–61. [DOI] [PubMed] [Google Scholar]
- [62].Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, Sklar J, TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms, Cell 66(4) (1991) 649–61. [DOI] [PubMed] [Google Scholar]
- [63].Weng AP, Ferrando AA, Lee W, J.P.t. Morris, L.B. Silverman, C. Sanchez-Irizarry, S.C. Blacklow, A.T. Look, J.C. Aster, Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia, Science 306(5694) (2004) 269–71. [DOI] [PubMed] [Google Scholar]
- [64].Fabbri G, Rasi S, Rossi D, Trifonov V, Khiabanian H, Ma J, Grunn A, Fangazio M, Capello D, Monti S, Cresta S, Gargiulo E, Forconi F, Guarini A, Arcaini L, Paulli M, Laurenti L, Larocca LM, Marasca R, Gattei V, Oscier D, Bertoni F, Mullighan CG, Foa R, Pasqualucci L, Rabadan R, Dalla-Favera R, Gaidano G, Analysis of the chronic lymphocytic leukemia coding genome: role of NOTCH1 mutational activation, The Journal of experimental medicine 208(7) (2011) 1389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kannan S, Sutphin RM, Hall MG, Golfman LS, Fang W, Nolo RM, Akers LJ, Hammitt RA, McMurray JS, Kornblau SM, Melnick AM, Figueroa ME, Zweidler-McKay PA, Notch activation inhibits AML growth and survival: a potential therapeutic approach, The Journal of experimental medicine 210(2) (2013) 321–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zweidler-McKay PA, He Y, Xu L, Rodriguez CG, Karnell FG, Carpenter AC, Aster JC, Allman D, Pear WS, Notch signaling is a potent inducer of growth arrest and apoptosis in a wide range of B-cell malignancies, Blood 106(12) (2005) 3898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, Fakhry C, Xie TX, Zhang J, Wang J, Zhang N, El-Naggar AK, Jasser SA, Weinstein JN, Trevino L, Drummond JA, Muzny DM, Wu Y, Wood LD, Hruban RH, Westra WH, Koch WM, Califano JA, Gibbs RA, Sidransky D, Vogelstein B, Velculescu VE, Papadopoulos N, Wheeler DA, Kinzler KW, Myers JN, Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1, Science 333(6046) (2011) 1154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, Yang SX, Ivy SP, Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update, Nature reviews. Clinical oncology 12(8) (2015) 445–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kiel MJ, Velusamy T, Betz BL, Zhao L, Weigelin HG, Chiang MY, Huebner-Chan DR, Bailey NG, Yang DT, Bhagat G, Miranda RN, Bahler DW, Medeiros LJ, Lim MS, Elenitoba-Johnson KS, Whole-genome sequencing identifies recurrent somatic NOTCH2 mutations in splenic marginal zone lymphoma, The Journal of experimental medicine 209(9) (2012) 1553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang X, Shi Y, Weng Y, Lai Q, Luo T, Zhao J, Ren G, Li W, Pan H, Ke Y, Zhang W, He Q, Wang Q, Zhou R, The truncate mutation of Notch2 enhances cell proliferation through activating the NF-kappaB signal pathway in the diffuse large B-cell lymphomas, PloS one 9(10) (2014) e108747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wang C, Li Q, Liu F, Chen X, Liu B, Nesa EU, Guan S, Han L, Tan B, Wang N, Wang X, Song Q, Jia Y, Wang J, Lu M, Cheng Y, Notch2 as a promising prognostic biomarker for oesophageal squamous cell carcinoma, Scientific reports 6 (2016) 25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Law AMK, Lim E, Ormandy CJ, Gallego-Ortega D, The innate and adaptive infiltrating immune systems as targets for breast cancer immunotherapy, Endocrine-related cancer 24(7) (2017) X1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Liu XS, Mardis ER, Applications of Immunogenomics to Cancer, Cell 168(4) (2017) 600–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chen DS, Mellman I, Elements of cancer immunity and the cancer-immune set point, Nature 541(7637) (2017) 321–330. [DOI] [PubMed] [Google Scholar]
- [75].Liu Z, Song Z, Sun J, Sun F, Li C, Sun J, Xu L, Association between CTLA-4 rs231775 polymorphism and hepatocellular carcinoma susceptibility, International journal of clinical and experimental pathology 8(11) (2015) 15118–22. [PMC free article] [PubMed] [Google Scholar]
- [76].Wang Y, Wang X, Zhao R, The association of CTLA-4 A49G polymorphism with colorectal cancer risk in a Chinese Han population, International journal of immunogenetics 42(2) (2015) 93–9. [DOI] [PubMed] [Google Scholar]
- [77].Postow MA, Callahan MK, Wolchok JD, Immune Checkpoint Blockade in Cancer Therapy, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 33(17) (2015) 1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pan D, The hippo signaling pathway in development and cancer, Developmental cell 19(4) (2010) 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Harvey KF, Zhang X, Thomas DM, The Hippo pathway and human cancer, Nature reviews. Cancer 13(4) (2013) 246–57. [DOI] [PubMed] [Google Scholar]
- [80].Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, Montgomery EA, Anders RA, Expression of Yes-associated protein in common solid tumors, Human pathology 39(11) (2008) 1582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, Lowe SW, Poon RT, Luk JM, Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma, Cancer 115(19) (2009) 4576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, Mu D, Lucito R, Powers S, Lowe SW, Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach, Cell 125(7) (2006) 1253–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Tanas MR, Sboner A, Oliveira AM, Erickson-Johnson MR, Hespelt J, Hanwright PJ, Flanagan J, Luo Y, Fenwick K, Natrajan R, Mitsopoulos C, Zvelebil M, Hoch BL, Weiss SW, Debiec-Rychter M, Sciot R, West RB, Lazar AJ, Ashworth A, Reis-Filho JS, Lord CJ, Gerstein MB, Rubin MA, Rubin BP, Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma, Science translational medicine 3(98) (2011) 98ra82. [DOI] [PubMed] [Google Scholar]
- [84].Errani C, Zhang L, Sung YS, Hajdu M, Singer S, Maki RG, Healey JH, Antonescu CR, A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites, Genes, chromosomes & cancer 50(8) (2011) 644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ciccia A, Elledge SJ, The DNA Damage Response: Making It Safe to Play with Knives, Molecular cell 40 (2010) 179–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kobayashi J, Antoccia A, Tauchi H, Matsuura S, Komatsu K, NBS1 and its functional role in the DNA damage response, DNA Repair 3 (2004) 855–861. [DOI] [PubMed] [Google Scholar]
- [87].Stewart GS, Maser RS, Stankovic T, Bressan DA, Kaplan MI, Jaspers NGJ, Raams A, Byrd PJ, Petrini JHJ, Taylor AMR, The DNA Double-Strand Break Repair Gene hMRE11 Is Mutated in Individuals with an Ataxia-Telangiectasia-like Disorder, Cell 99 (1999) 577–587. [DOI] [PubMed] [Google Scholar]
- [88].Waltes R, Kalb R, Gatei M, Kijas AW, Stumm M, Sobeck A, Wieland B, Varon R, Lerenthal Y, Lavin MF, Schindler D, Dörk T, Human RAD50 Deficiency in a Nijmegen Breakage Syndrome-like Disorder, The American Journal of Human Genetics 84 (2009) 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].D’Amours D, Jackson SP, The mre11 complex: at the crossroads of dna repair and checkpoint signalling, Nature Reviews Molecular Cell Biology 3 (2002) 317–327. [DOI] [PubMed] [Google Scholar]
- [90].Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, Shiloh Y, Requirement of the MRN complex for ATM activation by DNA damage., The EMBO journal 22 (2003) 5612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].You Z, Chahwan C, Bailis J, Hunter T, Russell P, ATM Activation and Its Recruitment to Damaged DNA Require Binding to the C Terminus of Nbs1, Molecular and Cellular Biology 25 (2005) 5363–5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Choi M, Kipps T, Kurzrock R, ATM Mutations in Cancer: Therapeutic Implications, Molecular Cancer Therapeutics 15 (2016). [DOI] [PubMed] [Google Scholar]
- [93].Lalloo F, Evans DG, Familial Breast Cancer, Clinical Genetics 82 (2012) 105–114. [DOI] [PubMed] [Google Scholar]
- [94].Wasielewski M, Vasen H, Wijnen J, Hooning M, Dooijes D, Tops C, Klijn JGM, Meijers-Heijboer H, Schutte M, Wokolowczyk D, Matuszewski M, Sun P, Lubinski J, Narod S, Chekmariova E, Sokolenko A, Imyanitov E, Hamann U, Rashid M, Brauch H, Justenhoven C, Ashworth A, Peto J, Eccles D, Eeles R, Evans D, Houlston R, Murday V, Narod S, Peretz T, Peto J, Phelan C, Zhang H, Szabo C, Devilee P, Goldgar D, Futreal P, Nathanson K, Weber B, Rahman N, Stratton M, CHEK2 1100delC is a susceptibility allele for HNPCC-related colorectal cancer., Clinical cancer research : an official journal of the American Association for Cancer Research 14 (2008) 4989–94. [DOI] [PubMed] [Google Scholar]
- [95].Fackenthal JD, Olopade OI, Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations, Nature Reviews Cancer 7 (2007) 937–948. [DOI] [PubMed] [Google Scholar]
- [96].Sy SMH, Huen MSY, Chen J, PALB2 is an integral component of the BRCA complex required for homologous recombination repair., Proceedings of the National Academy of Sciences of the United States of America 106 (2009) 7155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zhang F, Fan Q, Ren K, Andreassen PR, PALB2 Functionally Connects the Breast Cancer Susceptibility Proteins BRCA1 and BRCA2, Molecular Cancer Research 7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Carreira A, Hilario J, Amitani I, Baskin RJ, Shivji MKK, Venkitaraman AR, Kowalczykowski SC, The BRC Repeats of BRCA2 Modulate the DNA-Binding Selectivity of RAD51, Cell 136 (2009) 1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Venkitaraman AR, Linking the Cellular Functions of BRCA Genes to Cancer Pathogenesis and Treatment, Annual Review of Pathology: Mechanisms of Disease 4 (2009) 461–487 [DOI] [PubMed] [Google Scholar]
- [100].Weil MK, Chen AP, PARP inhibitor treatment in ovarian and breast cancer., Current problems in cancer 35 (2011) 7–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ma M, Ru Y, Chuang LS, Hsu NY, Shi LS, Hakenberg J, Cheng WY, Uzilov A, Ding W, Glicksberg BS, Chen R, Disease-associated variants in different categories of disease located in distinct regulatory elements, BMC genomics 16 Suppl 8 (2015) S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Melton C, Reuter JA, Spacek DV, Snyder M, Recurrent somatic mutations in regulatory regions of human cancer genomes, Nature genetics 47(7) (2015) 710–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lin S, Gregory RI, MicroRNA biogenesis pathways in cancer, Nature reviews. Cancer 15(6) (2015) 321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kapeli K, Martinez FJ, Yeo GW, Genetic mutations in RNA-binding proteins and their roles in ALS, Human genetics (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kim K, Jang K, Yang W, Choi EY, Park SM, Bae M, Kim YJ, Choi JK, Chromatin structure-based prediction of recurrent noncoding mutations in cancer, Nature genetics 48(11) (2016) 1321–1326. [DOI] [PubMed] [Google Scholar]
- [106].Jolma A, Yin Y, Nitta KR, Dave K, Popov A, Taipale M, Enge M, Kivioja T, Morgunova E, Taipale J, DNA-dependent formation of transcription factor pairs alters their binding specificity, Nature 527(7578) (2015) 384–8. [DOI] [PubMed] [Google Scholar]
- [107].Consortium EP, An integrated encyclopedia of DNA elements in the human genome, Nature 489(7414) (2012) 57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Fuxman Bass JI, Sahni N, Shrestha S, Garcia-Gonzalez A, Mori A, Bhat N, Yi S, Hill DE, Vidal M, Walhout AJ, Human gene-centered transcription factor networks for enhancers and disease variants, Cell 161(3) (2015) 661–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bhattacharya A, Cui Y, SomamiR 2.0: a database of cancer somatic mutations altering microRNA-ceRNA interactions, Nucleic Acids Res. 44(D1) (2016) D1005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]