Abstract
It has been suggested that traditional ecological knowledge (TEK) may play a key role in forest conservation. However, empirical studies assessing to what extent TEK is associated with forest conservation compared with other variables are rare. Furthermore, to our knowledge, the spatial overlap of TEK and forest conservation has not been evaluated at fine scales. In this paper, we address both issues through a case study with Tsimane’ Amerindians in the Bolivian Amazon. We sampled 624 households across 59 villages to estimate TEK and used remote sensing data to assess forest conservation. We ran statistical and spatial analyses to evaluate whether TEK was associated and spatially overlapped with forest conservation at the village level. We find that Tsimane’ TEK is significantly and positively associated with forest conservation although acculturation variables bear stronger and negative associations with forest conservation. We also find a very significant spatial overlap between levels of Tsimane’ TEK and forest conservation. We discuss the potential reasons underpinning our results, which provide insights that may be useful for informing policies in the realms of development, conservation, and climate. We posit that the protection of indigenous cultural systems is vital and urgent to create more effective policies in such realms.
Keywords: Biocultural conservation, Bolivian lowlands, Ethnobotanical knowledge, Forest fragmentation, Indigenous knowledge systems, Indigenous acculturation
Introduction
Major concerns for global environmental change are the rapid rate at which biodiversity is being lost (Ceballos et al. 2015) along with cultural and linguistic diversity loss (Harmon 1996; Sutherland 2003; Reyes-García et al. 2013a; Kikvidze and Tevzadze 2015). Both processes are driven by similar human-induced pressures such as agricultural expansion, logging, and large-scale infrastructure development, which are particularly worrying in tropical regions (Lambin et al. 2003). Researchers have noted a significant spatial overlap between areas of high biological diversity and areas of high cultural and linguistic diversity, highlighting that such co-occurrence takes place mostly in areas inhabited by indigenous peoples across the tropics (Maffi 2005). The reasons underlying this spatial overlap are complex, differ among localities, and vary at different scales; yet, recent research suggests there could be some form of functional connection between such diversities (Gorenflo et al. 2012). Though this fact remains poorly understood, it seems clear that certain indigenous cultural systems and practices favor the conservation of species and the ecosystems that host them, and vice versa (Sobrevila 2008).
In Amazonia, indigenous peoples have been actively managing forests for hundreds if not thousands of years (May 1984; Mann 2008), often safeguarding (and sometimes enhancing) the continuous availability of forest resources through different management strategies adapted to local ecological conditions and shaped by culture throughout centuries (Posey 1985; Dufour 1990). Indigenous forest utilization may create a forest-culture continuum within villages resulting in a biodiversity-rich domesticated landscape characterized by managed forests and agroforestry systems (Wiersum 1997). For instance, swidden cultivation-fallow management systems are agroforestry systems often found among native Amazonian groups, which have significant ecological and economic benefits (Coomes et al. 2000). Intrinsically tied to such management practices, indigenous Amazonians have developed an in-depth local environmental knowledge and a comprehensive set of beliefs as part of their cosmology (Balée 2003; Huanca 2008). This practice-knowledge-belief complex, typical of indigenous and traditional societies worldwide, is what has been coined as traditional ecological knowledge (TEK) (Berkes 1999) and underpins most claims about the role of indigenous peoples in conservation (e.g., Gadgil et al. 1993; Berkes et al. 2000).
Several authors have emphasized the key role that indigenous territories play in forest conservation across Amazonia (e.g., Nepstad et al. 2006; Paneque-Gálvez et al. 2013a; Blackman et al. 2017) and the indigenous role in conservation due to factors such as managing their forestlands more efficiently, with lower intensity than nonindigenous peoples (Rudel et al. 2002; Lu et al. 2010) and a cosmology interwoven with forests and nonhuman nature (Descola 1998; Rival 1998; Huanca 2008). Nonetheless, the question of whether forest conservation across indigenous lands is typically the result of low population density, lack of technology, and absence of markets due to isolation, rather than of a real indigenous conservation ethic, remains controversial (Raymond 2007). In contributing to this debate, empirical studies assessing the potential role of indigenous TEK in forest conservation—compared with other factors—may be particularly clarifying. Some recent studies suggest that indigenous TEK—broadly defined sensu Berkes (1999) to include not just knowledge but also practices and beliefs—may make an important contribution to forest management and conservation in indigenous territories (e.g., Berkes and Davidson-Hunt 2006; Posey and Balick 2006; Herrmann and Torri 2009). Studies addressing the alleged importance of indigenous TEK for forest conservation have been carried out in one or a few indigenous villages, however, which may make it difficult to extract general conclusions even for the entire indigenous society studied. Spatially explicit estimates of both TEK and forest conservation at local scales are also necessary for the analysis of their spatial patterns to assess the degree of co-occurrence within a specific indigenous society, which is essential to better understand connections between TEK and conservation (Zent 2009). Nevertheless, to our knowledge all the studies evaluating the spatial overlap of biological and cultural or linguistic diversities have been made at regional (e.g., Nabhan et al. 2002), continental (e.g., Moore et al. 2002) or global scales (e.g., Harmon 1996; Gorenflo et al. 2012).
To address the knowledge gaps identified regarding the association of indigenous TEK and tropical forest conservation, this study set out with two objectives: (1) to test whether there exists an association between the level of indigenous TEK and the level of tropical forest conservation at the village level; and (2) to evaluate whether the spatial patterns of indigenous TEK match with those of tropical forest conservation at the local level. We hypothesize that indigenous villages with higher levels of TEK will have around them higher proportions of forest which in addition will be more conserved. We conduct our study using TEK data from the Tsimane’ Amerindians (Bolivian Amazon), who constitute an ideal indigenous group because their TEK has been extensively studied (e.g., Reyes-García et al. 2003, 2013a; Díaz-Reviriego et al. 2016), and their villages exhibit a large gradient in regard to TEK and cultural change (Reyes-García et al. 2014b) and also in relation to forest conservation around them (Pérez-Llorente et al. 2013). We focus on old-growth forests since they occupy much of the extent of the study area (Paneque-Gálvez et al. 2013a) and because they are of much greater importance for biodiversity and carbon conservation than early growth or disturbed forests (Luyssaert et al. 2008; Gibson et al. 2011).
Factors associated with Tsimane’ land use and forest conservation
The Tsimane’ Amerindians are native to the Amazon and live in the lowland forests of the southwest of the Beni and the east of La Paz departments, Bolivia. They number 10 000–12 000 people settled in 125 villages, mostly along the Maniqui, Quiquibey and Apere Rivers, and logging roads (Reyes-García et al. 2014a). The Tsimane’ economy centers on hunting, fishing, plant foraging and slash-and-burn farming for subsistence (Godoy et al. 2009). However, Tsimane’ living in villages close to the main towns (San Borja, Yucumo, and Rurrenabaque) are increasingly engaging in market-oriented activities such as cash cropping and wage labor, and depend on nonTsimane’ for purchasing goods and receiving credits (Reyes-García et al. 2012). The increasing integration into the market economy of the Tsimane’ society has led to important socioeconomic and cultural changes; this process affects the way the Tsimane’ manage and use their forest resources (Godoy et al. 2005), which is reflected in their landscapes (Pérez-Llorente et al. 2013). Thus, although outsiders such as cattle ranchers, colonist farmers and logging companies are mostly responsible for the deforestation and forest degradation caused in previous decades in the area inhabited by the Tsimane’, Tsimane’ themselves are increasingly responsible for clearing and degrading their forests (Godoy et al. 1998; Bottazzi and Dao 2013; Paneque-Gálvez et al. 2015). The main reasons are the expansion of the area that Tsimane’ cultivate with rice and other cash crops, their engagement in timber and nontimber forest products extraction for sale and barter, and—to a lesser extent—their involvement in cattle ranching (Vadez et al. 2008; Paneque-Gálvez et al. 2015).
Besides factors associated with market integration, researchers have pointed out other socioeconomic, political, and cultural factors that may be associated with the way Tsimane’ households use their forests at present. For instance, Godoy et al. (1998) examined the roles that tenure insecurity and household heads’ time preference might have in deforestation; Bottazzi and Dao (2013) looked at the role of land-use allocation, existing institutional arrangements and property rights in forest clearance; Paneque-Gálvez et al. (2013a) investigated the effects of land tenure—compared with other factors—in relation to trends in forest cover change and fragmentation across the four tenure arrangements in which Tsimane’ villages are settled, finding a high association between forest conservation and indigenous presence—in protected areas, forest logging concessions and their own titled territories, but not on private lands, which are inhabited by colonists; Godoy and Contreras (2001) assessed the association between the level of household education attainment and forest clearance.
Specifically about the association of TEK with forest conservation, Reyes-García et al. (2007, 2011) found negative relations between the level of TEK of the male household head and the amount of forest cleared by households, although results differed depending on the type of forest cleared (old-growth versus early growth). Although both studies shed light into the potential role of TEK in forest conservation, they were carried out at the household level, without accounting for potential differences at the village level, and without controlling for the spatial distribution of TEK and forest cover. In addition, both studies focused on the relation between TEK and old-growth and fallow forest clearance for agriculture, but did not consider other important ecological variables such as the extent of forest surrounding each village, or how well those forests were preserved (e.g., their level of fragmentation), both of which are key features in forest biodiversity conservation. Hence, in this study we focus on such measures to more comprehensively assess forest conservation at the village level and therefore its potential association with TEK.
Material and methods
Assessment of old-growth forest conservation
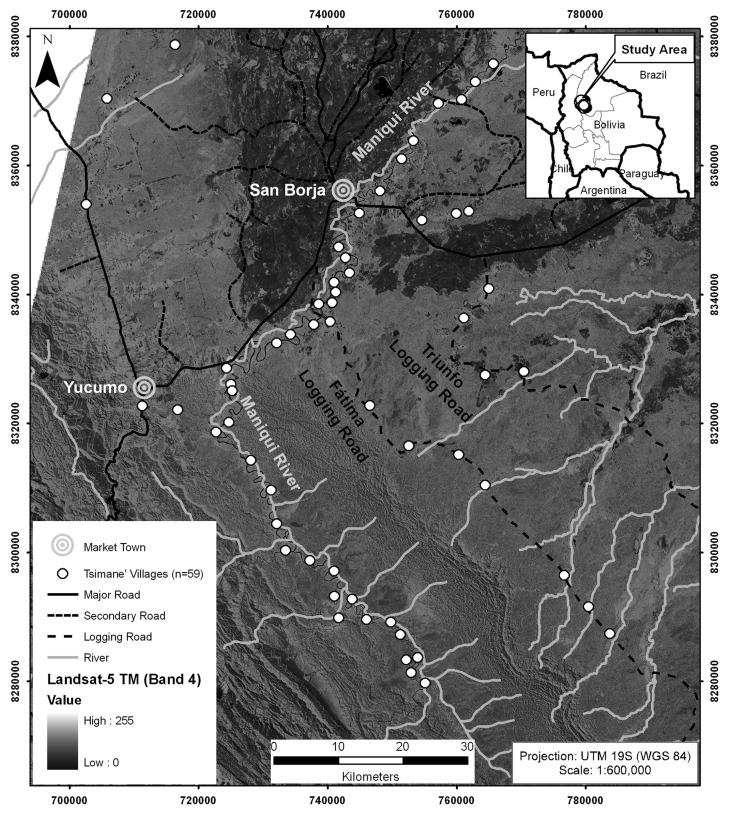
To assess old-growth forest conservation at the village level, we used two Landsat-5 TM satellite images from 2009 that had been previously classified into eight different broad land-use/cover classes (including old-growth forests) using support vector machines with reflectance and textural information (see Paneque-Gálvez et al. 2013b for details on the classification process). We masked out the old-growth forest class and used FRAGSTATS software to assess its degree of conservation per village. To do so we created a 5-km circular buffer around the center of each sampled village (n = 59, almost half of all Tsimane’ villages), because that is roughly the area the Tsimane’ use for subsistence activities like hunting and foraging (Cruz-Burga et al. 2013). Specifically, for each 5-km buffer we retrieved old-growth forest area and three standard variables to estimate forest fragmentation, which were the four outcome variables we used in statistical models (Table 1). We chose a conservative distance of 300 m to define the edge size that relates to old-growth forest fragmentation because (1) most disturbance processes associated with edges take place within 200 m (e.g., invasion of disturbance-adapted organisms, reduced understory-bird abundance, higher air temperature, lower soil-moisture content and relative air humidity, etc.)—see Broadbent et al. 2008 and Laurance et al. 2002—and (2) Tsimane’ land uses do not cause edges greater than 300 m (e.g., average agricultural plots are squared, and consist of 0.5–1 ha).
Table 1.
Landscape metrics used to assess the extent and fragmentation of old-growth forests at the village level (see McGarigal et al. 2002 for metric equations and further details). These metrics are used as outcome variables in ordinary least squares regression analysis. Landscape here refers to the 5-km buffer around each village school
| Class metric | Metric type | Range and units | Description and significance for conservation |
|---|---|---|---|
| Percentage of landscape (PLAND) | Area (extent) | (%) | Quantifies the percentage of landscape covered by old-growth forest. Here, 100% means that old-growth forest coverage is maximum, thus signaling good conservation status |
| Edge density (ED) | Edge (fragmentation) | (m/ha) | Quantifies the total length of old-growth forest edgesa in relation to landscape area. The greater this value, the more fragmented is the extent of old-growth forest, which indicates poorer conservation status |
| Cora area percent of landscape (CPLAND) | Core area (fragmentation) | (%) | Quantifies the percentage of landscape covered by core areasb of old-growth forest. A value of 100% indicates that all old-growth forest extent is not fragmented (i.e., well conserved) |
| Perimeter-area fractal dimension (PAFRAC) | Shape (fragmentation) | (None) | Reflects shape complexity across a range of old-growth forest patch sizes. PAFRAC approaches 1 for old-growth forest simple patch shapes (e.g., squares) and diverges toward 2 as old-growth forest patches are more complex. The simpler the patches are, the less they are fragmented and the more conserved |
aIn landscape ecology, an “edge” refers to a contact zone between two (or more) habitat types. Here, an edge refers to the boundary between an old-growth forest patch and other land cover patches (usually early growth forest and/or agricultural patches)
bIn landscape ecology, a “core area” refers to the area within a patch that is further than the specified edge distance from the patch perimeter (300 m in this study). In other words, it is the area within a patch that is unaffected by the edge effects that occur within the distance specified by the edge size. Here, core areas consist of areas of conserved old-growth forest
Assessment of TEK and retrieval of control variables
We used household data (n = 624) from a cross-sectional survey conducted in Tsimane’ villages throughout 2008 and 2009 to assess TEK and retrieve data on control variables (Table 2). Villages were selected considering their spatial location so that they reflected differences in their social and environmental attributes (Fig. 1). Survey data were collected at each village in ten households randomly selected out of a census provided by the highest-rank authority of the village. In villages with less than ten households, we collected data in all the households willing to participate in the study, while in villages with more than 40 households we collected data in 25% of them. Free, prior and informed consent was always obtained from each participant and refusal rate was < 5%. We interviewed male household heads unless they were absent (then female household heads were interviewed) because men tend to display larger variations in relation to TEK and other attributes potentially associated with TEK (e.g., formal education, acculturation, health, economic activities) (Reyes-García et al. 2013b). In addition to household data, to produce maps and retrieve a geographical variable (distance to closest market town, see Table 2), we used GPS points collected at the center of the studied villages (i.e., school) and GIS layers (roads, rivers, main towns) obtained from several governmental agencies.
Table 2.
Definition of explanatory and control variables used in regression analysis. Variables refer to villages
| Variables | Definition |
|---|---|
| Traditional ecological knowledge | |
| TEK | Mean total number of plant uses known, i.e., ethnobotanical knowledge |
| Acculturation | |
| Schooling | Mean higher level of schooling (grades 0–13) of respondents |
| Spanish | Proportion of informants that speak Spanish fluently |
| TV | Number of TVs |
| Population | |
| HH | Number of households |
| Spatial location | |
| DistSBY | Linear distancea to the closest main market town (San Borja or Yucumo) |
| Market integration | |
| Cows | Number of cows |
| TravelSB | Mean number of trips to the main market town (San Borja) during previous year of respondents |
| RiceEarnings | Mean total household earnings from the sale of rice since last harvest |
| RiceSold | Meant total amount of rice sold/bartered by households since last harvest |
| Forest cleared for agriculture | |
| Def | Mean total area of forest (old-growth and early growth) cut over the previous year for agriculture |
aLinear distance does not correspond to the real distance that exists either by road or river between a village and the closest market town
Fig. 1.
Landsat-5 TM mosaic (17/04/2009) used to classify old-growth forests across the study area, overlaid with the Tsimane’ villages surveyed, roads, rivers and main market towns
We proxied TEK (our explanatory variable) with informants’ ethnobotanical knowledge (Reyes-García et al. 2003, 2011) and restricted our analysis to adults (> 16 years) in accordance with previous studies. We used a questionnaire to ask respondents to categorize all the uses they knew from a list of 20 plants that had been randomly selected from a previous free-listing exercise and which uses had been verified by scan observations (Reyes-García et al. 2006). Specifically, for each plant in our list, whose name in Tsimane’ language was provided to respondents, we asked if the plant could be used for medicine, food, firewood, canoe building, house building, and/or other uses (Reyes-García et al. 2013b). To assess TEK at the village level, for each plant we averaged the total uses known by informants within each sampled village.
Data analysis
We first built a spatially explicit database containing all the variables used in this study and retrieved their descriptive statistics at the village level. We then sought linear correlations between the four forest conservation variables and TEK. By calculating Pearson correlation coefficient (Pearson’s r) we obtained an estimate of the strength of the association between each pair of forest conservation-TEK variables. Subsequently, we performed ordinary least squares regression analysis to assess the potential importance of associations between our four outcome variables (forest conservation) and the explanatory variable (TEK) while controlling for the influence of other variables (Eq. 1):
| 1 |
where for village i, F is an outcome variable, T is the explanatory variable, C is a control variable, ε is a random error term, α is a constant (intercept), and β and γ are the regression coefficients associated with the explanatory and control variables, respectively. We ran three regression models for each of our four outcome variables: one without controls, another controlling for distance to market towns and another one controlling for population density (we controlled for just one additional variable in the last two regression models because our sample of villages was relatively low for regression analysis (n = 59)). In addition, for each outcome variable we performed a robustness analysis using Eq. (1) and controlled for other village-level variables that may potentially affect forest conservation in tropical areas (e.g., related to agricultural expansion, market integration, acculturation) (Lambin et al. 2003). The robustness analysis allowed us to better estimate the regression coefficient (β) for the explanatory variable (TEK) and the coefficient of determination (R2) as we could infer ranges rather than single values for them. Finally, we mapped each of our four pairs of forest conservation-TEK variables alongside to unravel differences among villages according to their spatial location and to assess whether the spatial patterns of forest conservation metrics and TEK overlapped. To evaluate this potential overlap in statistical terms, we categorized the four forest variables and TEK as above/below average and applied Chi-squared tests. That way we could assess whether villages with high levels of forest conservation had also high levels of TEK (and vice versa), and the statistical significance of forest conservation-TEK overlapping spatial patterns.
Results
Descriptive statistics
Most selected variables showed a large variation among Tsimane’ villages, including the explanatory and outcome variables (Table 3). We found that forest cover was relatively low on average (~ 66%) though it greatly varied (SD ~ 25%), ranging from 16.70 to 98.40%, thus indicating that some villages may have undergone important deforestation rates while others have their forest cover still relatively intact. We also found that Tsimane’ villages display large variations regarding forest fragmentation: (1) edge density peaked at ~ 30 m/ha in seven villages and was < 8 m/ha in five villages, (2) mean core forest area was ~ 45% (SD = 26.52%) but 14 villages had < 20% core forest left whereas seven had over 80% core forest left, and (3) forest shape-complexity showed a mean value of 1.25 (SD = 0.04), which indicates that forest cover is still relatively simple regarding shape. The average village-level of TEK ranged from 14 to 41 plant uses known for our list of 20 common plants, with an average value of 22 plant uses known per village (SD = 5.34).
Table 3.
Descriptive statistics of the village-level variables used in the regression analysis. Variables’ names as given in Tables 1 and 2
| Variables | N | Unit | Mean | SD | Min | Max |
|---|---|---|---|---|---|---|
| Outcome variables | ||||||
| Forest conservation | ||||||
| PLAND | 59 | % | 65.87 | 24.80 | 16.70 | 98.40 |
| ED | 59 | m/ha | 18.52 | 7.00 | 3.35 | 33.31 |
| CPLAND | 59 | % | 45.46 | 26.52 | 3.65 | 91.47 |
| PAFRAC | 56 | – | 1.25 | 0.04 | 1.11 | 1.32 |
| Explanatory variable | ||||||
| Traditional ecological knowledge | ||||||
| TEK | 59 | n | 22.17 | 5.34 | 13.93 | 40.86 |
| Control variables | ||||||
| Acculturation | ||||||
| Schooling | 59 | n years | 1.86 | 1.22 | 0 | 4.62 |
| Spanish | 58 | %/100 | 0.20 | 0.22 | 0 | 0.91 |
| TV | 59 | n | 1.36 | 1.32 | 0 | 7 |
| Population | ||||||
| HH | 59 | n | 27.03 | 28.44 | 3 | 190 |
| Spatial location | ||||||
| DistSBY | 59 | km | 30.42 | 18.93 | 2.81 | 80.16 |
| Market integration | ||||||
| Cows | 59 | n | 4.49 | 8.93 | 0 | 41 |
| TravelSB | 59 | n | 18.71 | 16.11 | 2.14 | 99.11 |
| RiceEarningsa | 59 | Bs | 871.57 | 2180.65 | 0 | 16 651.25 |
| RiceSoldb | 58 | arroba | 22.18 | 25.60 | 0 | 166 |
| Forest cleared for agriculture | ||||||
| Defc | 59 | tarea | 10.19 | 5.60 | 3.08 | 43 |
a1 $US ~ 7 Bs (Bolivianos)
b1 arroba = 11.5 kg
c10 Tareas = 1 ha
Associations between Tsimane’ traditional ecological knowledge and forest conservation
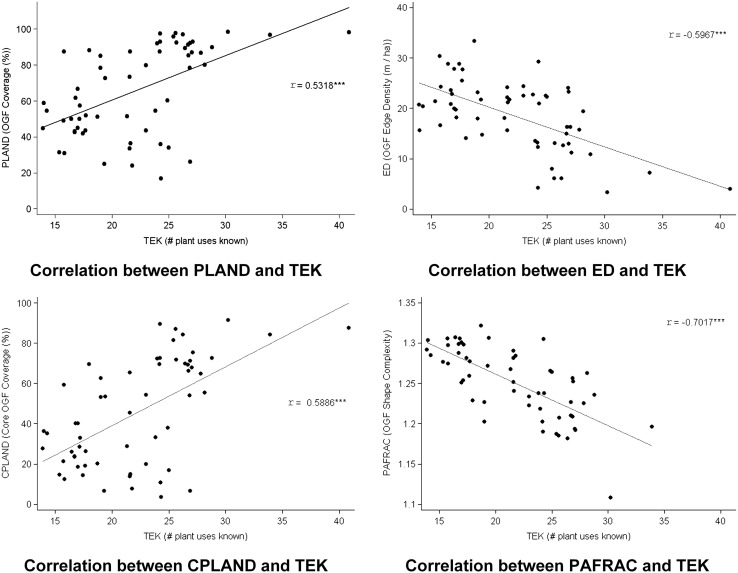
We found moderate to strong significant correlations (p ~ 0) between each pair of forest conservation-TEK variables (Fig. 2). We observed a positive correlation between the average TEK in a village and both the extent of forest and core forest area in the same village (Pearson’s r = 0.53 and r = 0.59, respectively). In contrast, we found a negative correlation between TEK and both forest edge density and forest shape-complexity (Pearson’s r = − 0.60 and r = − 0.70, respectively). The association between TEK and the four forest conservation variables was very significant (p ~ 0) in all regression models with no control variables, and remained so after controlling for distance to the closest market town and for population density (Table 4). However, the coefficient of determination (R2) was much higher in all models that included distance as a control variable than in models with no controls and in models controlling for population density, thus indicating the important association between distance and forest conservation variables.
Fig. 2.
Linear correlations between TEK and the four forest variables (Pearson’s r correlation coefficient shown; *** significant at 99.9% (p < 0.001)). Variables: TEK traditional ecological knowledge, PLAND percentage of landscape, ED edge density, CPLAND core percentage of land, PAFRAC perimeter-area fractal dimension. Forest variables are referred to old-growth forest (OGF) (see Tables 1, 2 for definitions)
Table 4.
Regression analysis showing the associations between the explanatory variable (traditional ecological knowledge—TEK) and the outcome variables (old-growth forest extent and fragmentation—PLAND, ED, CPLAND, PAFRAC). [x] models include TEK as unique explanatory variable, while [x′] models control for distance to the closest market town (DistSBY) and [x″] models control for population density (HH). R2 values give an estimate of the global fit of each model and the figures for TEK, DistSBY, HH represent the values of regression coefficients associated with the variables included in each model, in addition to the constant (see Eq. [1] for details). p values are shown in brackets and ^ refers to a variable purposely omitted in a regression model
| Outcome variables | Forest extent | Forest fragmentation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forest cover (PLAND) | Forest edge density (ED) | Core forest cover (CPLAND) | Forest shape-complexity (PAFRAC) | |||||||||
| Explanatory variable | [1] | [1′] | [1″] | [2] | [2′] | [2″] | [3] | [3′] | [3″] | [4] | [4′] | [4″] |
| TEK | 2.47 (0.000) | 1.09 (0.010) | 2.47 (0.000) | − 0.78 (0.000) | − 0.49 (0.000) | − 0.77 (0.000) | 2.92 (0.000) | 1.46 (0.001) | 2.91 (0.000) | − 0.006 (0.000) | − 0.004 (0.000) | − 0.006 (0.000) |
| Control variable | ||||||||||||
| DistSBY | ^ | 0.88 (0.000) | ^ | ^ | − 0.18 (0.000) | ^ | ^ | 0.93 (0.000) | ^ | ^ | − 0.001 (0.000) | ^ |
| HH | ^ | ^ | 0.001 (0.989) | ^ | ^ | 0.02 (0.556) | ^ | ^ | − 0.04 (0.702) | ^ | ^ | 0.000 (0.094) |
| N | 59 | 59 | 59 | 59 | 59 | 59 | 59 | 59 | 59 | 56 | 56 | 56 |
| Constant | 11.09 (0.355) | 14.80 (0.084) | 11.04 (0.384) | 35.87 (0.000) | 35.09 (0.000) | 35.27 (0.000) | − 19.36 (0.116) | − 15.42 (0.066) | − 17.88 (0.168) | 1.39 (0.000) | 1.38 (0.000) | 1.38 (0.000) |
| R 2 | 0.283 | 0.648 | 0.28 | 0.345 | 0.558 | 0.360 | 0.346 | 0.705 | 0.348 | 0.492 | 0.646 | 0.519 |
In robustness analysis, we found that TEK remained significantly associated with all four forest conservation variables while controlling for variables related to acculturation, population density, spatial location, market integration, and forest cleared for agriculture over the previous year, some of which did not affect the association between TEK and forest variables whatsoever (Table 5). We estimated the coefficient for the association between TEK and forest extent to range between 1.09 and 2.52, TEK and forest edge density between − 0.79 and − 0.49, TEK and core forest extent between 1.46 and 2.97, and TEK and forest shape-complexity between − 0.0065 and − 0.0045 (p < 0.01 in all cases). The range values of the associations between TEK and both forest and core forest extent are particularly relevant given the relatively large magnitude of their coefficients.
Table 5.
Robustness analysis showing results from regression models using different control variables. Core models refer to regressions without any control variable (like [x] models in Table 4). Values in “TEK” columns refer to the regression coefficient of our main explanatory variable (traditional ecological knowledge—TEK); Values in “Control” columns refer to the regression coefficient of control variables; R2 values give an estimate of the global fit of each model. The symbols *, **, *** indicate that regressions are significant at p < 0.1, p < 0.05, p < 0.01, respectively
| Forest extent | Forest fragmentation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLAND | ED | CPLAND | PAFRAC | |||||||||
| TEK | Control | R 2 | TEK | Control | R 2 | TEK | Control | R 2 | TEK | Control | R 2 | |
| Core | ||||||||||||
| (without control variable) | 2.47*** | – | 0.283 | − 0.78*** | – | 0.356 | 2.92*** | – | 0.346 | − 0.0064*** | – | 0.492 |
| Acculturation | ||||||||||||
| Schooling | 1.94*** | − 7.83*** | 0.418 | − 0.67*** | 1.71*** | 0.437 | 2.37*** | − 8.18*** | 0.476 | − 0.0058*** | 0.01*** | 0.561 |
| Spanish | 1.70*** | − 34.45** | 0.343 | − 0.70*** | 4.14 | 0.369 | 2.21*** | − 32.55** | 0.393 | − 0.0051*** | 0.05** | 0.536 |
| TV | 2.52*** | − 6.35*** | 0.397 | − 0.79*** | 1.46*** | 0.432 | 2.97*** | − 6.88*** | 0.464 | − 0.0065*** | 0.01** | 0.534 |
| Population | ||||||||||||
| HH | 2.47*** | 0.01 | 0.283 | − 0.78*** | 0.02 | 0.361 | 2.91*** | − 0.03 | 0.347 | − 0.0064*** | 0.00 | 0.516 |
| Spatial location | ||||||||||||
| DistSBY | 1.09** | 0.88*** | 0.648 | − 0.49*** | − 0.18*** | 0.558 | 1.46*** | 0.93*** | 0.705 | − 0.0045*** | − 0.00*** | 0.646 |
| Market integration | ||||||||||||
| Cows | 2.29*** | − 0.58* | 0.324 | − 0.74*** | 0.13 | 0.385 | 2.73*** | − 0.61* | 0.387 | − 0.0061*** | 0.00* | 0.527 |
| TravelSB | 1.51*** | − 0.77*** | 0.494 | − 0.60*** | 0.15*** | 0.456 | 1.88*** | − 0.84*** | 0.565 | − 0.0055*** | 0.00** | 0.547 |
| RiceEarnings | 2.38*** | − 0.00 | 0.291 | − 0.75*** | 0.00 | 0.367 | 2.82*** | − 0.00 | 0.357 | − 0.0062*** | 0.00 | 0.509 |
| RiceSold | 2.37*** | − 0.11 | 0.293 | − 0.74*** | 0.02 | 0.364 | 2.80*** | − 0.11 | 0.354 | − 0.0058*** | 0.00*** | 0.576 |
| Forest cleared for agriculture | ||||||||||||
| Def | 2.39*** | − 0.18 | 0.284 | − 0.74*** | 0.09 | 0.361 | 2.83*** | − 0.21 | 0.348 | − 0.0056*** | 0.00** | 0.528 |
Besides TEK, acculturation variables were strongly—but negatively—associated with forest conservation (Table 5). We found that villages with higher levels of fluency in Spanish were associated with a decrease of ~ 34 and ~ 33% of forest and core forest extent, respectively, and with an increase of edge density of ~ 4 m/ha, when controlling also for TEK. In addition, we observed that one year more of schooling in the village average was associated with the loss of ~ 10% of forest cover and ~ 11% of core forest cover without controlling for other explanatory variables and with a loss of ~ 8% in both forest cover and core forest cover when controlling for TEK (results not shown). The number of TVs in villages bore a significant and negative association with forest conservation (loss of ~ 6% of forest cover and core forest cover with and without controlling for TEK, respectively).
Spatial overlap between Tsimane’ traditional ecological knowledge and forest conservation
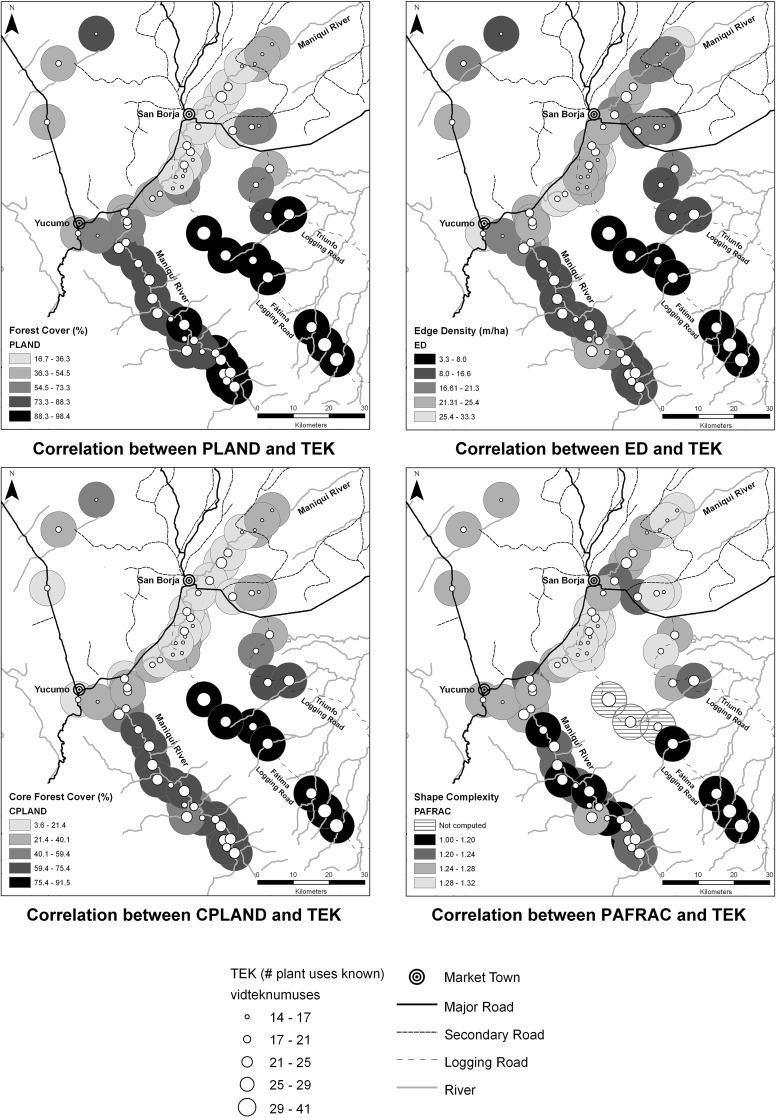
We found that Tsimane’ villages along the Fátima logging road have the highest levels of TEK and of forest conservation too (Fig. 3). We observed a gradient in TEK and forest conservation along the Maniqui River as upstream villages show high values for both variables and, barring few exceptions, downstream villages have low values. Tsimane’ villages close to San Borja (the main market town) have very little forests left, which moreover are highly fragmented. Regarding TEK in downstream villages, those with the lowest levels of ethnobotanical knowledge are between the two towns and very close to the road, as well as along the lowest part of the river. Finally, we observe two other clusters of villages; the three villages north of Yucumo display low levels of TEK and low to moderate values in forest extent and forest fragmentation, and the four villages along the Triunfo logging road show moderate to high levels of both TEK and forest fragmentation. Chi-squared tests demonstrated that villages that have conserved more forest have higher levels of TEK than villages that have conserved less forest: we found Pearson’s χ2 = 14.28, 12.51, 16.28, and 18.91, DF [1], p = 0 (in all cases), for forest cover, edge density, core forest cover, and perimeter-area fractal dimension vs TEK, respectively.
Fig. 3.
Spatial correlations between TEK and the four forest variables (n = 59 aside from PAFRAC n = 56). Buffers with darker colors indicate higher degrees of forest conservation, bigger dots signal higher levels of TEK; and vice versa. Variables: TEK traditional ecological knowledge, PLAND percentage of landscape, ED edge density, CPLAND core percentage of land, PAFRAC perimeter-area fractal dimension. Forest variables are referred to old-growth forest (see Tables 1, 2 for definitions)
Discussion
Three main findings stem from our research: (1) there exists very significant and strong associations between Tsimane’ TEK and forest conservation, (2) there is a very significant spatial overlap between high levels of Tsimane’ TEK and high levels of forest conservation (and vice versa), and (3) Tsimane’ acculturation appears to have more influence than TEK on forest conservation. We discuss each finding next following the same order. We ground our interpretations, to a great extent, on previous research we have conducted among the Tsimane’ and our knowledge of this indigenous society and their forests.
Associations between Tsimane’ traditional ecological knowledge and forest conservation
Our first finding supports the long-standing view in ethnoecology which sustains that indigenous ecological knowledge may play an important role in tropical forest conservation (e.g., Posey 1985; Alcorn 1993). Overall, our case study suggests that Tsimane’ TEK may be an important determinant of forest conservation in the study area. But, what may be the mechanisms underlying this finding? A previous study among the Tsimane’ (Reyes-García et al. 2007) found a strong and significant negative association between individual levels of TEK and forest clearance (and therefore between TEK and forest extent), which in their opinion was explained because people who had higher TEK levels used more of the forest and were therefore less prone to cut it. In our study, carried out at the village level, this explanation seems appropriate too since villages with higher average levels of TEK may reflect a higher communal dependency on forest resources. Such dependency may result in higher forest coverage around them because villagers clear little to maintain their supply of forest resources. Villages with higher average levels of TEK are also likely to reflect a greater maintenance of traditional institutions, which may be better suited to foster sustainable forest management (Ostrom et al. 1999).
To explain the association found between Tsimane’ TEK and forest fragmentation, the combination of the two other aspects of TEK (practice and beliefs) may be more relevant than the knowledge component itself. For example, forest edges are created through Tsimane’ swidden-fallow systems, which in addition affect the extent of core forest and the shape-complexity of forests surrounding villages. We found that population density bears no significant association with these forest metrics and, consequently, forest fragmentation is not related to the number of swidden-fallow plots within a village. We thereby think that in villages in which people have more TEK on average, swidden-fallow systems must be more efficient so that they create less forest fragmentation. This could be achieved by different mechanisms such as cropping more useful plants in swidden fields, rotating crops more efficiently, arranging crops in layers according to their ecological needs to optimize space usage, lengthening the fallow, or clearing fallow forest rather than old-growth forest. All these techniques would improve crop yields without depleting soil nutrients, and would reduce the need for clearing and fragmenting more old-growth forest than strictly needed for household self-subsistence. This hypothesis is consistent with the findings of Reyes-García et al. (2008), who found a positive association between the level of TEK of male household heads and crop diversity in their swidden fields. Similarly, it fits well with the results of Reyes-García et al. (2011), who claimed that the more TEK Tsimane’ individuals had (1) the more selective and efficient they were in clearing old-growth forest (because they practiced joint production while clearing), and (2) the less fallow forest they cleared (which suggests they lengthened the fallow because they used these forests more efficiently too).
We argue that villages with higher levels of TEK may not only have more efficient cultivators and forest managers, but also more experienced foragers who can gather edible wild food in the forest, as well as more skilled hunters and fishers. All these traditional subsistence-oriented activities are carried out without affecting forest structure, at least as much as market-oriented activities like logging, cash cropping, and ranching, and may make people less dependent on the market, thus reducing the time and area of cleared forest needed for agricultural production and timber extraction. In addition, based on our ethnographic knowledge of the Tsimane’, we can affirm that individuals with more ecological knowledge tend to hold more onto ancestral beliefs than those who have little TEK (Reyes-García et al. 2014b). Tsimane’ traditional beliefs unravel the existence of a variety of spirits living in forests, which translates into conservative rules of management of certain trees and other places (i.e., water sources, salt springs). Similarly, Tsimane’ traditional beliefs convey a series of taboos and prohibitions that effectively safeguard certain forest areas because no human activities are allowed, and underpin social norms and practices that limit the use of the forest (Huanca 2008). Therefore, traditional beliefs may have had significant effects on the extent and conservation of forest around those Tsimane’ villages where TEK is still high.
Spatial overlap between Tsimane’ traditional ecological knowledge and forest conservation
Our second finding indicates that, with few exceptions, villages with above-average TEK show above-average forest conservation (and vice versa), and that this association is highly significant in Pearson’s Chi-squared tests between TEK and all forest conservation variables. We also observed a clear spatial pattern in those associations. Visual interpretation of such spatial patterns and our regression analyses suggest that TEK and the four forest variables are also associated with distance to the main market towns and accessibility. For instance, villages with the highest levels of TEK and forest conservation are found along the Fátima logging road. This road becomes unusable every year during several months due to the abundant rain that carries away the artisanal bridges built to cross some tributaries of the Maniqui River. This constitutes a real barrier to the movement of people and goods (e.g., timber), which has led these villages to remain relatively isolated. Contrarily, access to villages along the other logging road is much easier; yet, though more subtle, there is a gradient in TEK and forest conservation with distance and accessibility. Regarding the majority of villages, which are settled along the Maniqui River, the highest levels of TEK and forest conservation are found in the upper section, i.e., the farthest from towns. Although the most remote villages upstream take 2.5–3 days to be reached by canoe, access is possible even in the dry season, which may explain why both TEK and forest conservation are not as high as in villages along the Fátima logging road.
As expected, villages close to towns show low levels of forest conservation and, in general, of TEK too. This can be explained because these villages are relatively integrated into the market economy and have severely changed their lifestyles as a consequence, thus transforming their productive system to sell cash crops such as rice (Vadez et al. 2008), rear cattle, and extract timber (Paneque-Gálvez et al. 2015). These villages have also suffered from encroachment upon their land since colonists started to arrive in the area in the 1970s and settled close to the main roads (Reyes-García et al. 2012). Such encroachers have cleared much forestland because clearing was regarded as a requirement to claim land ownership under national colonization plans and agricultural laws (Bottazzi and Dao 2013; Paneque-Gálvez et al. 2013a). Finally, we observed that some villages (the two upper ones north to Yucumo and the last four in the lower section of the Maniqui River) had very low levels of TEK and yet, their forests were relatively well preserved. We believe this may reflect a limitation in our TEK variable because the average ethnobotanical knowledge in these villages is probably not that low; rather, people living in such villages may not know as many plant uses from our 20-species list owing to local floristic differences. For instance, the four villages downstream the Maniqui River are settled within the Beni Biological Station, a Biosphere Reserve that includes different forest types not present upstream (e.g., swampy forests); therefore, their floristic composition might be significantly different from the rest of the forests present in the territory sampled.
Associations between Tsimane’ acculturation and forest conservation
We found that the three acculturation measures used as controls in regressions (schooling, Spanish fluency, and number of TVs) bore larger associations with forest conservation than TEK and they were negative. Our finding contrasts with previous results obtained among the Tsimane’. Godoy and Contreras (2001), for example, showed that having more formal education and being fluent in Spanish allow Tsimane’ individuals to work as laborers outside their villages, something associated with a decrease in deforestation. It is also contrary to the long-lasting view of Kuznets curves regarding forest conservation, which states that when households drive themselves out of poverty through increasing their income—something usually associated with education attainment—they increasingly stop clearing forest. However, this view remains equivocal (Chowdhury and Moran 2012) and other studies have suggested that acculturation is associated with forest destruction because of agricultural expansion and a more intense extraction of forest resources (Kingsbury 2001).
As regards our results, we believe that Tsimane’ acculturation negatively affects forest conservation through direct and indirect effects. On the one hand, the most acculturated villages largely coincide with those that are more integrated into the market economy and produce more cash crops, which causes extensive forest clearance (Vadez et al. 2008). We have also observed that Tsimane’ individuals who engage in logging activities tend to live in villages near towns and pursue less traditional lifestyles. For instance, some Tsimane’ school teachers engage in selective logging because they have the economic resources to purchase a chainsaw and the ability to negotiate in Spanish to sell their harvest to nonindigenous intermediaries. On the other hand, previous findings indicate the existence of an inverse significant relation between TEK and formal education among the Tsimane’, which is largely explained because the more time children spend at school the less time they spend acquiring TEK (Reyes-García et al. 2010). Hence, we posit that aside from the direct effects that formal education can have on forest conservation, it may also pose indirect threats to the preservation of forests in the study area through the loss of TEK, as it may entail the loss of knowledge, skills and beliefs that are likely to underpin Tsimane’ sustainable forest practices.
Conclusions
In this paper we have shown, with quantitative and spatial analyses, that TEK and old-growth forest conservation are strongly associated and that there is a high spatial overlap between them across Tsimane’ territory in the Bolivian Amazon. We have also provided evidence of a strong and negative association between Tsimane’ acculturation and forest conservation, which in our opinion can be partly attributed to the negative association between acculturation and TEK found for Tsimane’ villages (Reyes-García et al. 2014b) and the rapid ongoing process of loss of TEK estimated for this indigenous group (Reyes-García et al. 2013a). Although there are other factors that also seem to play an important role in forest conservation across the study area (e.g., village distance to market towns, accessibility), the existence of some form of functional connection between TEK and forest conservation at the scale of our analysis seems plausible. In that respect, we have provided some insights into the mechanisms that may underlie such a connection when discussing our results, and contributed to research that acknowledges the importance of indigenous peoples’ TEK for their own livelihoods as well as for biodiversity conservation and climate change mitigation (Simpson 2004; Sobrevila 2008; Salick and Ross 2009).
Future research to further our understanding of the potential role of TEK in Amazonian tropical forest conservation and elsewhere may include other dimensions of TEK not accounted for specifically in our study (e.g., beliefs), local institutions that regulate TEK and forest commons (e.g., resource use norms), the temporal dimension in forest conservation assessment—particularly regarding degradation and regrowth given their increasing coverage worldwide (Skutsch et al. 2017)—, and direct measures of biological diversity (e.g., fauna, flora). In addition, it is particularly important to better understand the drivers of TEK loss to tackle its negative effects. Long-term studies coupled with innovative methods that allow for post-evaluation measures that can reveal whether TEK is being lost or recovered are likewise necessary, notwithstanding the dynamic nature of TEK and the inherent difficulties of taking on this task.
As the world’s cultural and biological diversities are being rapidly eroded, often by the very same forces (Sutherland 2003), research in the field of biocultural conservation provides clues that may allow for the development of a broader research agenda on indigenous livelihoods, conservation, and climate change. Previous research have demonstrated the value of TEK for science and management (e.g., Berkes et al. 2000; Huntington 2000) and indigenous cultural systems—TEK being an important component—are likely to underpin the effectiveness of indigenous territories to inhibit deforestation, forest degradation, and fires compared with protected areas and other land tenure systems (e.g.,Nepstad et al. 2006; Nelson and Chomitz 2011; Nolte et al. 2013; Paneque-Gálvez et al. 2013a; Vergara-Asenjo and Potvin 2014; Ceddia et al. 2015; Blackman et al. 2017).
We suggest that to create more effective policies in the realms of development, conservation, and climate, States should incorporate direct and indirect measures to protect indigenous cultural systems. A critical action in our opinion is to provide contextualized education to indigenous peoples so that their languages and TEK are valued and revitalize; although there are various challenges ahead (Reyes-García et al. 2010; McCarter et al. 2014), there have been proposals to integrate science and TEK in formal education (e.g., Eijck and Roth 2007). Another key concern should be securing indigenous land tenure given its potential connection to preserving their ties to land and cosmology coupled with the ever-greater threats looming over indigenous customary lands (Finer et al. 2008; Paneque-Gálvez et al. 2017). In addition, we argue that conservation policies that foster the preservation and revitalization of cultural systems should be prioritized across indigenous territories over typical conservation policies. For instance, classic fortress conservation schemes have frequently resulted in the violent displacement of indigenous peoples, thus posing severe ethical issues (West et al. 2006; Dowie, 2009). Community-based conservation strategies have been suggested to address ethical concerns and to be more effective than fortress conservation (Porter-Bolland et al. 2012); yet, these initiatives are usually externally driven and may not have the flexibility to incorporate bottom-up approaches to conservation so that livelihood goals are integrated. New market-based mechanisms advanced by the neoliberal conservation agenda prioritize capital accumulation (Büscher and Fletcher 2015) and have been criticized for being forms of green grabbing (Fairhead et al. 2012). Overall, conservation policies have failed to acknowledge the importance of indigenous TEK, which in our view partly explains why they tend to be not only ineffective, but also socially unjust.
Acknowledgements
This work was funded through a FBBVA research grant (BIOCON_06_106-07) to the project Conservación del Bosque Amazónico y Territorios Indígenas: del Conflicto a la Colaboración. Estudio de Caso en la Amazonía Boliviana. J. Paneque-Gálvez is grateful to Ricardo Godoy, Tomás Huanca, Pablo Domínguez, and Gerardo Bocco for providing comments on the original manuscript, to Juan Carlos Ledezma for providing GIS data, to TAPS for their assistance with data collection and logistics while conducting fieldwork in the study area, and to the Tsimane’ for their help and friendship in the forest.
Biographies
Jaime Paneque-Gálvez
is an Assistant Professor at the National Autonomous University of Mexico, the Center of Research in Environmental Geography (Morelia, Mexico). His research interests include citizen science and grassroots innovation, biocultural conservation, and the political ecology of land change in tropical forests and peri-urban areas.
Irene Pérez-Llorente
is a PhD candidate at the National Autonomous University of Mexico, the Center of Research in Environmental Geography (Morelia, Mexico). Her research focuses on the role of territory in environmental conflicts, the political ecology of indigenous resistance, and the integration of different disciplines and data to study complex environmental problems.
Ana Catarina Luz
is a Postdoctoral researcher at the Centre for Ecology, Evolution and Environmental Changes (cE3c). Her research focuses on the sustainability and resilience of urban systems, mapping the use of green spaces and ecosystem services, and biocultural interactions, toward contributing to advanced urban green infrastructure planning and management.
Maximilien Guèze
is an interdisciplinary researcher working at the interface of plant ecology and social sciences. He has conducted doctoral and postdoctoral research at ICTA-UAB on indigenous knowledge, forest practices, and cultural change and their interactions with sustainability science and conservation. He is currently a member of the scientific and technical support team for the IPBES global assessment of biodiversity and ecosystem services.
Jean-François Mas
is a tenured Professor at the National Autonomous University of Mexico, the Center of Research in Environmental Geography (Morelia, Mexico). He specializes in remote sensing, geographical information science, and spatial modeling. His research interests include land-use/land-cover change monitoring and modeling, accuracy assessment of spatial data, forest inventory, and vegetation cartography.
Manuel J. Macía
is an Associate Professor at the Universidad Autónoma de Madrid, Spain. He is a plant ecologist working in tropical forests—mainly in the Amazon and Andes—and local human population to understand their role in the current ecosystems.
Martí Orta-Martínez
is an Assistant Professor at the Central Calalonia University/University of Vic (Vic, Spain). His research has focused, in a broad sense, on socioenvironmental conflicts, petroleum extraction, and the conservation of tropical rainforests.
Victoria Reyes-García
received her Ph.D in Antropology in 2001, from the University of Florida. She is an ICREA Researcher at the Institute of Environmental Science and Technology. Her research addresses local knowledge systems and biocultural conservation.
Contributor Information
Jaime Paneque-Gálvez, Phone: (+52) 443 322 2777, Email: jpanequegalvez@ciga.unam.mx.
Irene Pérez-Llorente, Email: ireneperezllorente@gmail.com.
Ana Catarina Luz, Email: acluz@fc.ul.pt.
Maximilien Guèze, Email: maximilien.gueze@gmail.com.
Jean-François Mas, Email: jfmas@ciga.unam.mx.
Manuel J. Macía, Email: manuel.macia@uam.es
Martí Orta-Martínez, Email: martiorta@gmail.com.
Victoria Reyes-García, Email: victoria.reyes@uab.cat.
References
- Alcorn JB. Indigenous peoples and conservation. Conservation Biology. 1993;7:424–426. doi: 10.1046/j.1523-1739.1993.07020424.x. [DOI] [Google Scholar]
- Balée W. Native views of the environment in Amazonia. In: Selin H, editor. Nature Across Cultures: Views of Nature and the Environment in Non-Western Cultures. Dordrecht: Kluwer Academic Publishers; 2003. pp. 277–288. [Google Scholar]
- Berkes F. Sacred Ecology. Traditional Ecological Knowledge and Resource Management. Philadelphia: Taylor and Francis; 1999. [Google Scholar]
- Berkes F, Coldin J, Folke C. Rediscovery of traditional ecological knowledge as adaptive management. Ecological Applications. 2000;10:1251–1262. doi: 10.1890/1051-0761(2000)010[1251:ROTEKA]2.0.CO;2. [DOI] [Google Scholar]
- Berkes F, Davidson-Hunt IJ. Biodiversity, traditional management systems, and cultural landscapes: Examples from the boreal forest of Canada. International Social Science Journal. 2006;58:35–47. doi: 10.1111/j.1468-2451.2006.00605.x. [DOI] [Google Scholar]
- Blackman A, Corral L, Lima ES, Asner GP. Titling indigenous communities protects forests in the Peruvian Amazon. Proceedings of the National academy of Sciences of the United States of America. 2017;114:4123–4128. doi: 10.1073/pnas.1603290114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottazzi P, Dao H. On the road through the Bolivian Amazon: A multi-level land governance analysis of deforestation. Land Use Policy. 2013;30:137–146. doi: 10.1016/j.landusepol.2012.03.010. [DOI] [Google Scholar]
- Broadbent EN, Asner GP, Keller M, Knapp DE, Oliveira PJC, Silva JN. Forest fragmentation and edge effects from deforestation and selective logging in the Brazilian Amazon. Biological Conservation. 2008;141:1745–1757. doi: 10.1016/j.biocon.2008.04.024. [DOI] [Google Scholar]
- Büscher B, Fletcher R. Accumulation by conservation. New Political Economy. 2015;20:273–298. doi: 10.1080/13563467.2014.923824. [DOI] [Google Scholar]
- Ceballos G, Ehrlich PR, Barnosky AD, García A, Pringle RM, Palmer TM. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Science Advances. 2015;1:e1400253. doi: 10.1126/sciadv.1400253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceddia MG, Gunter U, Corriveau-Bourque A. Land tenure and agricultural expansion in Latin America: The role of Indigenous Peoples’ and local communities’ forest rights. Global Environmental Change. 2015;35:316–322. doi: 10.1016/j.gloenvcha.2015.09.010. [DOI] [Google Scholar]
- Coomes OT, Grimard F, Burt GJ. Tropical forests and shifting cultivation: Secondary forest fallow dynamics among traditional farmers of the Peruvian Amazon. Ecological Economics. 2000;32:109–124. doi: 10.1016/S0921-8009(99)00066-X. [DOI] [Google Scholar]
- Cruz-Burga, Z., V. Reyes-García, J. Alarcón Novoa, J. Paneque-Gálvez, and A.C. Luz. 2013. Uso de territorio e integración a la economía de mercado. Estudio de caso en la amazonía boliviana. Natura@Economía 1: 105–121 (in Spanish, English summary).
- Chowdhury RR, Moran EF. Turning the curve: A critical review of Kuznets approaches. Applied Geography. 2012;32:3–11. doi: 10.1016/j.apgeog.2010.07.004. [DOI] [Google Scholar]
- Descola P. The Spears of Twilight: Life and Death in the Amazon Jungle. New York: The New Press; 1998. [Google Scholar]
- Díaz-Reviriego I, Fernández-Llamazares Á, Salpeteur M, Howard PL, Reyes-García V. Gendered medicinal plant knowledge contributions to adaptive capacity and health sovereignty in Amazonia. Ambio. 2016;45:263–275. doi: 10.1007/s13280-016-0826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowie M. Conservation Refugees: The Hundred-Year Conflict Between Global Conservation and Native Peoples. Cambridge: MIT Press; 2009. [Google Scholar]
- Dufour DL. Use of tropical rainforests by native Amazonians. BioScience. 1990;40:652–659. doi: 10.2307/1311432. [DOI] [Google Scholar]
- Eijck MV, Roth W-M. Keeping the local local: Recalibrating the status of science and traditional ecological knowledge (TEK) in education. Science Education. 2007;91:926–947. doi: 10.1002/sce.20227. [DOI] [Google Scholar]
- Fairhead J, Leach M, Scoones I. Green grabbing: A new appropriation of nature? Journal of Peasant Studies. 2012;39:237–261. doi: 10.1080/03066150.2012.671770. [DOI] [Google Scholar]
- Finer M, Jenkins CN, Pimm SL, Keane B, Ross C. Oil and gas projects in the Western Amazon: Threats to wilderness, biodiversity, and indigenous peoples. PLoS ONE. 2008;3:e2932. doi: 10.1371/journal.pone.0002932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadgil M, Berkes F, Folke C. Indigenous knowledge for biodiversity conservation. Ambio. 1993;22:151–156. doi: 10.1007/s13280-020-01478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson L, Lee TM, Koh LP, Brook BW, Gardner TA, Barlow J, Peres CA, Bradshaw CJA, et al. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature. 2011;478:378–381. doi: 10.1038/nature10425. [DOI] [PubMed] [Google Scholar]
- Godoy R, Contreras M. A comparative study of education and tropical deforestation among lowland Bolivian Amerindians: Forest values, environmental externality, and school subsidies. Economic Development and Cultural Change. 2001;49:555–574. doi: 10.1086/452515. [DOI] [Google Scholar]
- Godoy R, Jacobson M, De Castro J, Aliaga V, Romero J, Davis A. The role of tenure security and private time preference in neotropical deforestation. Land Economics. 1998;74:162–170. doi: 10.2307/3147048. [DOI] [Google Scholar]
- Godoy R, Reyes-García V, Byron E, Leonard WR, Vadez V. The effect of market economies on the well-being of indigenous peoples and on their use of renewable natural resources. Annual Review of Anthropology. 2005;34:121–138. doi: 10.1146/annurev.anthro.34.081804.120412. [DOI] [Google Scholar]
- Godoy R, Reyes-García V, Vadez V, Leonard WR, Tanner S, Huanca T, Wilkie D, TAPS Bolivia Study Team The relation between forest clearance and household income among native Amazonians: Results from the Tsimane’ Amazonian panel study, Bolivia. Ecological Economics. 2009;68:1864–1871. doi: 10.1016/j.ecolecon.2009.01.001. [DOI] [Google Scholar]
- Gorenflo LJ, Romaine S, Mittermeier RA, Walker-Painemilla K. Co-occurrence of linguistic and biological diversity in biodiversity hotspots and high biodiversity wilderness areas. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8032–8037. doi: 10.1073/pnas.1117511109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon D. Losing species, losing languages: Connections between biological and linguistic diversity. Southwest journal of Linguistics. 1996;15:89–108. [Google Scholar]
- Herrmann TM, Torri M-C. Changing forest conservation and management paradigms: traditional ecological knowledge systems and sustainable forestry: Perspectives from Chile and India. International Journal of Sustainable Development & World Ecology. 2009;16:392–403. doi: 10.1080/13504500903346404. [DOI] [Google Scholar]
- Huanca T. Tsimane’ Oral Tradition. Landscape and Identity in Tropical Forest. La Paz: Wa-Gui; 2008. [Google Scholar]
- Huntington HP. Using traditional ecological knowledge in science: Methods and applications. Ecological Applications. 2000;10:1270–1274. doi: 10.1890/1051-0761(2000)010[1270:UTEKIS]2.0.CO;2. [DOI] [Google Scholar]
- Kikvidze Z, Tevzadze G. Loss of traditional knowledge aggravates wolf–human conflict in Georgia (Caucasus) in the wake of socio-economic change. Ambio. 2015;44:452–457. doi: 10.1007/s13280-014-0580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury ND. Impacts of land use and cultural change in a fragile environment: Indigenous acculturation and deforestation in Kavanayén, Gran Sabana, Venezuela. Interciencia. 2001;26:327–336. [Google Scholar]
- Lambin EF, Geist HJ, Lepers E. Dynamics of land-use and land-cover change in tropical regions. Annual Review of Environment and Resources. 2003;28:205–241. doi: 10.1146/annurev.energy.28.050302.105459. [DOI] [Google Scholar]
- Laurance WF, Lovejoy TE, Vasconcelos HL, Bruna EM, Didham RK, Stouffer PC, Gascon C, Bierregaard RO, et al. Ecosystem decay of Amazonian forest fragments: A 22-year investigation. Conservation Biology. 2002;16:605–618. doi: 10.1046/j.1523-1739.2002.01025.x. [DOI] [Google Scholar]
- Lu F, Gray C, Bilsborrow RE, Mena CF, Erlien CM, Bremner J, Barbieri A, Walsh SJ. Contrasting colonist and indigenous impacts on Amazonian Forests. Conservation Biology. 2010;24:881–885. doi: 10.1111/j.1523-1739.2010.01463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyssaert S, Schulze ED, Borner A, Knohl A, Hessenmoller D, Law BE, Ciais P, Grace J. Old-growth forests as global carbon sinks. Nature. 2008;455:213–215. doi: 10.1038/nature07276. [DOI] [PubMed] [Google Scholar]
- Maffi L. Linguistic, cultural and biological diversity. Annual Review of Anthropology. 2005;29:599–617. doi: 10.1146/annurev.anthro.34.081804.120437. [DOI] [Google Scholar]
- Mann CC. Ancient earthmovers of the Amazon. Science. 2008;321:1148–1152. doi: 10.1126/science.321.5893.1148. [DOI] [PubMed] [Google Scholar]
- May RM. Anthropology: Prehistory of Amazonian Indians. Nature. 1984;312:19–20. doi: 10.1038/312019a0. [DOI] [PubMed] [Google Scholar]
- McCarter J, Gavin MC, Baereleo S, Love M. The challenges of maintaining indigenous ecological knowledge. Ecology and Society. 2014;19(3):39. doi: 10.5751/ES-06741-190339. [DOI] [Google Scholar]
- McGarigal, K., S.A. Cushman, M.C. Neel, and E. Ene. 2002. FRAGSTATS: Spatial Pattern Analysis Program for Categorical Maps. Amherst: University of Massachusetts. Retrieved from: http://www.umass.edu/landeco/research/fragstats/fragstats.html.
- Moore JL, Manne L, Brooks T, Burgess ND, Davies R, Rahbek C, Williams P, Balmford A. The distribution of cultural and biological diversity in Africa. Proceedings of the Royal Society of London Series B: Biological Sciences. 2002;269:1645–1653. doi: 10.1098/rspb.2002.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhan GP, Pynes P, Joe T. Safeguarding Species, languages, and cultures in the time of diversity loss: From the Colorado Plateau to Global Hotspots. Annals of the Missouri Botanical Garden. 2002;89:164–175. doi: 10.2307/3298561. [DOI] [Google Scholar]
- Nelson A, Chomitz KM. Effectiveness of strict vs. multiple use protected areas in reducing tropical forest fires: A global analysis using matching methods. PLoS ONE. 2011;6:e22722. doi: 10.1371/journal.pone.0022722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepstad D, Schwartzman S, Bamberger B, Santilli M, Ray D, Schlesinger P, Lefebvre P, Alencar A, et al. Inhibition of Amazon deforestation and fire by parks and indigenous lands. Conservation Biology. 2006;20:65–73. doi: 10.1111/j.1523-1739.2006.00351.x. [DOI] [PubMed] [Google Scholar]
- Nolte C, Agrawal A, Silvius KM, Soares-Filho BS. Governance regime and location influence avoided deforestation success of protected areas in the Brazilian Amazon. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4956–4961. doi: 10.1073/pnas.1214786110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom E, Burger J, Field CB, Norgaard RB, Policansky D. Revisiting the commons: Local Lessons, global challenges. Science. 1999;284:278–282. doi: 10.1126/science.284.5412.278. [DOI] [PubMed] [Google Scholar]
- Paneque-Gálvez, J., A.C. Luz, P. Bottazzi, M. Guèze, and V. Reyes-García. 2015. Breve historia del pueblo Tsimane’: Territorio, recursos naturales y gobernanza indígena. In Cambio global, cambio local. La Sociedad Tsimane’ ante la globalización, Amazonía Boliviana, ed. V. Reyes-García, and T. Huanca, 39–64. Barcelona: Icaria & Institut Català d’Antropologia (in Spanish).
- Paneque-Gálvez J, Mas J-F, Guèze M, Luz AC, Macía MJ, Orta-Martínez M, Pino J, Reyes-García V. Land tenure and forest cover change. The case of southwestern Beni, Bolivian Amazon, 1986–2009. Applied Geography. 2013;43:113–126. doi: 10.1016/j.apgeog.2013.06.005. [DOI] [Google Scholar]
- Paneque-Gálvez J, Mas J-F, Moré G, Cristóbal J, Orta-Martínez M, Luz AC, Guèze M, Macía MJ, Reyes-García V. Enhanced land use/cover classification of heterogeneous tropical landscapes using support vector machines and textural homogeneity. International Journal of Applied Earth Observation and Geoinformation. 2013;23:372–383. doi: 10.1016/j.jag.2012.10.007. [DOI] [Google Scholar]
- Paneque-Gálvez J, Vargas-Ramírez N, Napoletano MB, Cummings A. Grassroots innovation using drones for indigenous mapping and monitoring. Land. 2017;6(4):86. doi: 10.3390/land6040086. [DOI] [Google Scholar]
- Pérez-Llorente I, Paneque-Gálvez J, Luz AC, Macía MJ, Guèze M, Domínguez-Gómez JA, Reyes-García V. Changing indigenous cultures, economies and landscapes: The case of the Tsimane’, Bolivian Amazon. Landscape and Urban Planning. 2013;120:147–157. doi: 10.1016/j.landurbplan.2013.08.015. [DOI] [Google Scholar]
- Porter-Bolland L, Ellis EA, Guariguata MR, Ruiz-Mallen I, Negrete-Yankelevich S, Reyes-García V. Community managed forests and forest protected areas: An assessment of their conservation effectiveness across the tropics. Forest Ecology and Management. 2012;268:6–17. doi: 10.1016/j.foreco.2011.05.034. [DOI] [Google Scholar]
- Posey DA. Indigenous management of tropical forest ecosystems: the case of the Kayapó indians of the Brazilian Amazon. Agroforestry Systems. 1985;3:139–158. doi: 10.1007/BF00122640. [DOI] [Google Scholar]
- Posey DA, Balick MJ. Human Impacts on Amazonia: The Role of Traditional Ecological Knowledge in Conservation and Development. New York: Columbia University Press; 2006. [Google Scholar]
- Raymond H. The ecologically noble savage debate. Annual Review of Anthropology. 2007;36:177–190. doi: 10.1146/annurev.anthro.35.081705.123321. [DOI] [Google Scholar]
- Reyes-García V, Godoy R, Vadez V, Apaza L, Byron E, Huanca T, Leonard WR, Perez E, et al. Ethnobotanical knowledge shared widely among Tsimane’ Amerindians, Bolivia. Science. 2003;299:1707. doi: 10.1126/science.1080274. [DOI] [PubMed] [Google Scholar]
- Reyes-García V, Guèze M, Luz AC, Paneque-Gálvez J, Macía MJ, Orta-Martínez M, Pino J, Rubio-Campillo X. Evidence of traditional knowledge loss among a contemporary indigenous society. Evolution and Human Behavior. 2013;34:249–257. doi: 10.1016/j.evolhumbehav.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-García V, Huanca T, Vadez V, Leonard W, Wilkie D. Cultural, practical, and economic value of wild plants: A quantitative study in the Bolivian Amazon. Economic Botany. 2006;60:62–74. doi: 10.1663/0013-0001(2006)60[62:CPAEVO]2.0.CO;2. [DOI] [Google Scholar]
- Reyes-García V, Kightley E, Ruiz-Mallén I, Fuentes-Peláez N, Demps K, Huanca T, Martínez-Rodríguez MR. Schooling and local environmental knowledge: Do they complement or substitute each other? International Journal of Educational Development. 2010;30:305–313. doi: 10.1016/j.ijedudev.2009.11.007. [DOI] [Google Scholar]
- Reyes-García V, Ledezma JC, Paneque-Gálvez J, Orta M, Gueze M, Lobo A, Guinart D, Luz AC. Presence and purpose of nonindigenous peoples on indigenous lands: A descriptive account from the Bolivian Lowlands. Society & Natural Resources. 2012;25:270–284. doi: 10.1080/08941920.2010.531078. [DOI] [Google Scholar]
- Reyes-García V, Luz AC, Gueze M, Paneque-Gálvez J, Macía MJ, Orta-Martínez M, Pino J. Secular trends on traditional ecological knowledge: An analysis of changes in different domains of knowledge among Tsimane’ men. Learning and Individual Differences. 2013;27:206–212. doi: 10.1016/j.lindif.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-García V, Paneque-Gálvez J, Bottazzi P, Luz AC, Gueze M, Macía MJ, Orta-Martínez M, Pacheco P. Indigenous land reconfiguration and fragmented institutions: A historical political ecology of Tsimane’ lands (Bolivian Amazon) Journal of Rural Studies. 2014;34:282–291. doi: 10.1016/j.jrurstud.2014.02.007. [DOI] [Google Scholar]
- Reyes-García V, Paneque-Gálvez J, Luz A, Gueze M, Macía MJ, Orta-Martínez M, Pino J. Cultural change and traditional ecological knowledge: An empirical analysis from the Tsimane’ in the Bolivian Amazon. Human Organization. 2014;73:162–173. doi: 10.17730/humo.73.2.31nl363qgr30n017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-García V, Pascual U, Vadez V, Huanca T, TAPS Bolivia Study Team The role of ethnobotanical skills and agricultural labor in forest clearance: Evidence from the Bolivian Amazon. Ambio. 2011;40:310–321. doi: 10.1007/s13280-010-0107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-García V, Vadez V, Marti N, Huanca T, Leonard WR, Tanner S. Ethnobotanical knowledge and crop diversity in swidden fields: A study in a native Amazonian society. Human Ecology. 2008;36:569–580. doi: 10.1007/s10745-008-9177-2. [DOI] [Google Scholar]
- Reyes-García V, Vadez V, Tanner S, Huanca T, Leonard WR, McDade T. Ethnobotanical skills and clearance of tropical rain forest for agriculture: A case study in the lowlands of Bolivia. Ambio. 2007;36:406–408. doi: 10.1579/0044-7447(2007)36[406:ESACOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rival L. Domestication as a historical and symbolic process: wild gardens and cultivated forests in the Ecuadorian Amazon. In: Balée W, editor. Advances in Historical Ecology. New York: Columbia University Press; 1998. pp. 232–250. [Google Scholar]
- Rudel TK, Bates D, Machinguiashi R. Ecologically noble Amerindians? Cattle ranching and cash cropping among Shuar and colonists in Ecuador. Latin American Research Review. 2002;37:144–159. [Google Scholar]
- Salick J, Ross N. Traditional peoples and climate change. Global Environmental Change. 2009;19:137–139. doi: 10.1016/j.gloenvcha.2009.01.004. [DOI] [Google Scholar]
- Simpson LR. Anticolonial strategies for the recovery and maintenance of Indigenous knowledge. The American Indian Quarterly. 2004;28:373–384. doi: 10.1353/aiq.2004.0107. [DOI] [Google Scholar]
- Skutsch M, Paneque-Gálvez J, Ghilardi A, Balderas Torres A, Morfin-Rios J, Michel-Fuentes JM, Carrillo O, Ross D. Adapting REDD + policy to sink conditions. Forest Policy and Economics. 2017;80:160–166. doi: 10.1016/j.forpol.2017.03.016. [DOI] [Google Scholar]
- Sobrevila C. The Role of Indigenous Peoples in Biodiversity Conservation. The Natural but Often Forgotten Partners. Washington: The International Bank for Reconstruction and Development/The World Bank; 2008. [Google Scholar]
- Sutherland WJ. Parallel extinction risk and global distribution of languages and species. Nature. 2003;423:276–279. doi: 10.1038/nature01607. [DOI] [PubMed] [Google Scholar]
- Vadez V, Reyes-García V, Huanca T, Leonard WR. Cash cropping, farm technologies, and deforestation: What are the connections? A model with empirical data from the Bolivian Amazon. Human Organization. 2008;67:384–396. doi: 10.17730/humo.67.4.45164623415rp7n8. [DOI] [Google Scholar]
- Vergara-Asenjo G, Potvin C. Forest protection and tenure status: The key role of indigenous peoples and protected areas in Panama. Global Environmental Change. 2014;28:205–215. doi: 10.1016/j.gloenvcha.2014.07.002. [DOI] [Google Scholar]
- West P, Igoe J, Brockington D. Parks and peoples: The social impact of protected areas. Annual Review of Anthropology. 2006;35:251–277. doi: 10.1146/annurev.anthro.35.081705.123308. [DOI] [Google Scholar]
- Wiersum KF. Indigenous exploitation and management of tropical forest resources: An evolutionary continuum in forest-people interactions. Agriculture, Ecosystems & Environment. 1997;63:1–16. doi: 10.1016/S0167-8809(96)01124-3. [DOI] [Google Scholar]
- Zent S. Traditional ecological knowledge (TEK) and biocultural diversity: A close-up look at linkages, delearning trends & changing patterns of transmission. In: Bates P, Chiba M, Kube S, Nakashima D, editors. Learning and Knowing in Indigenous Societies Today. Paris: UNESCO; 2009. pp. 39–57. [Google Scholar]